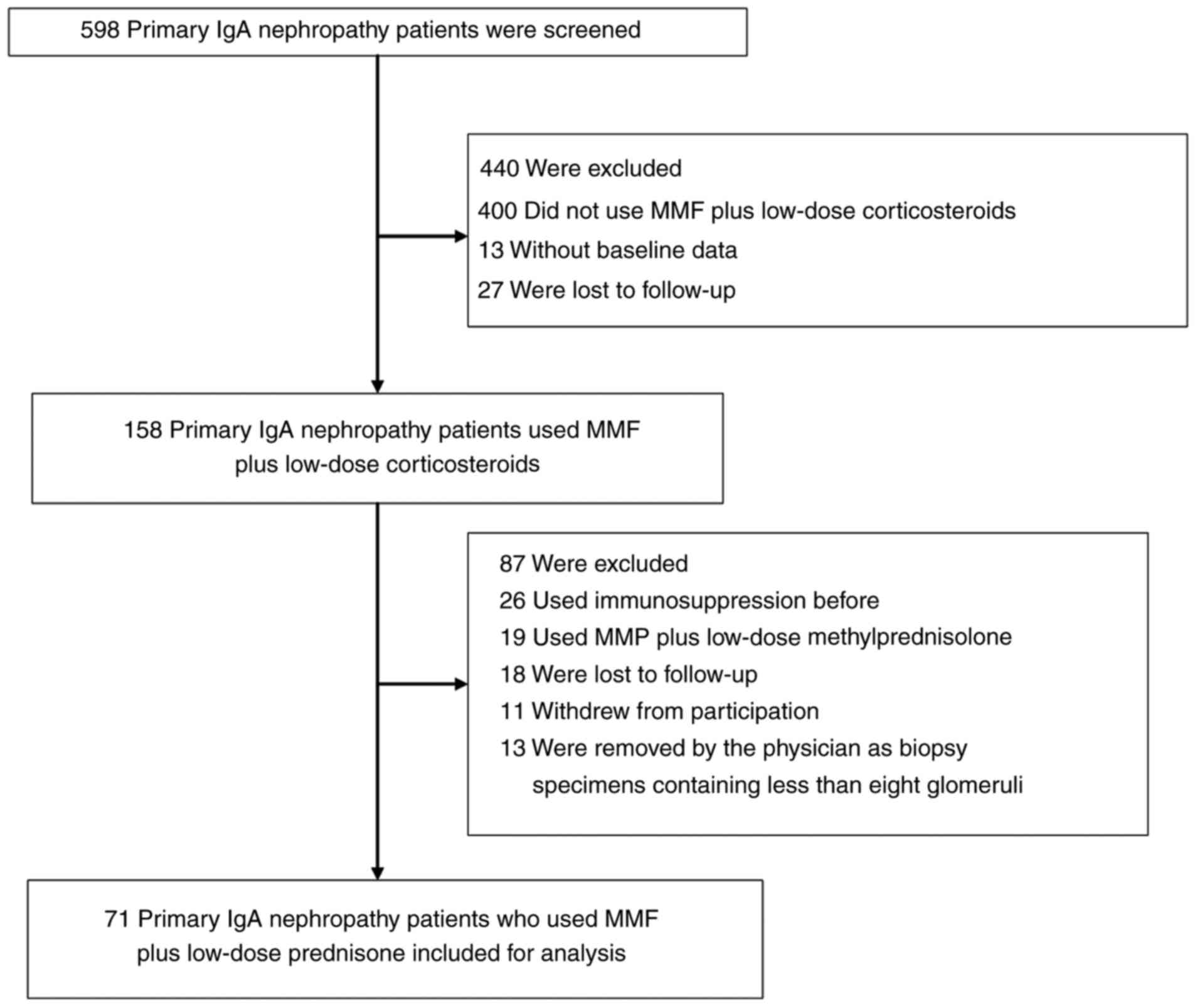
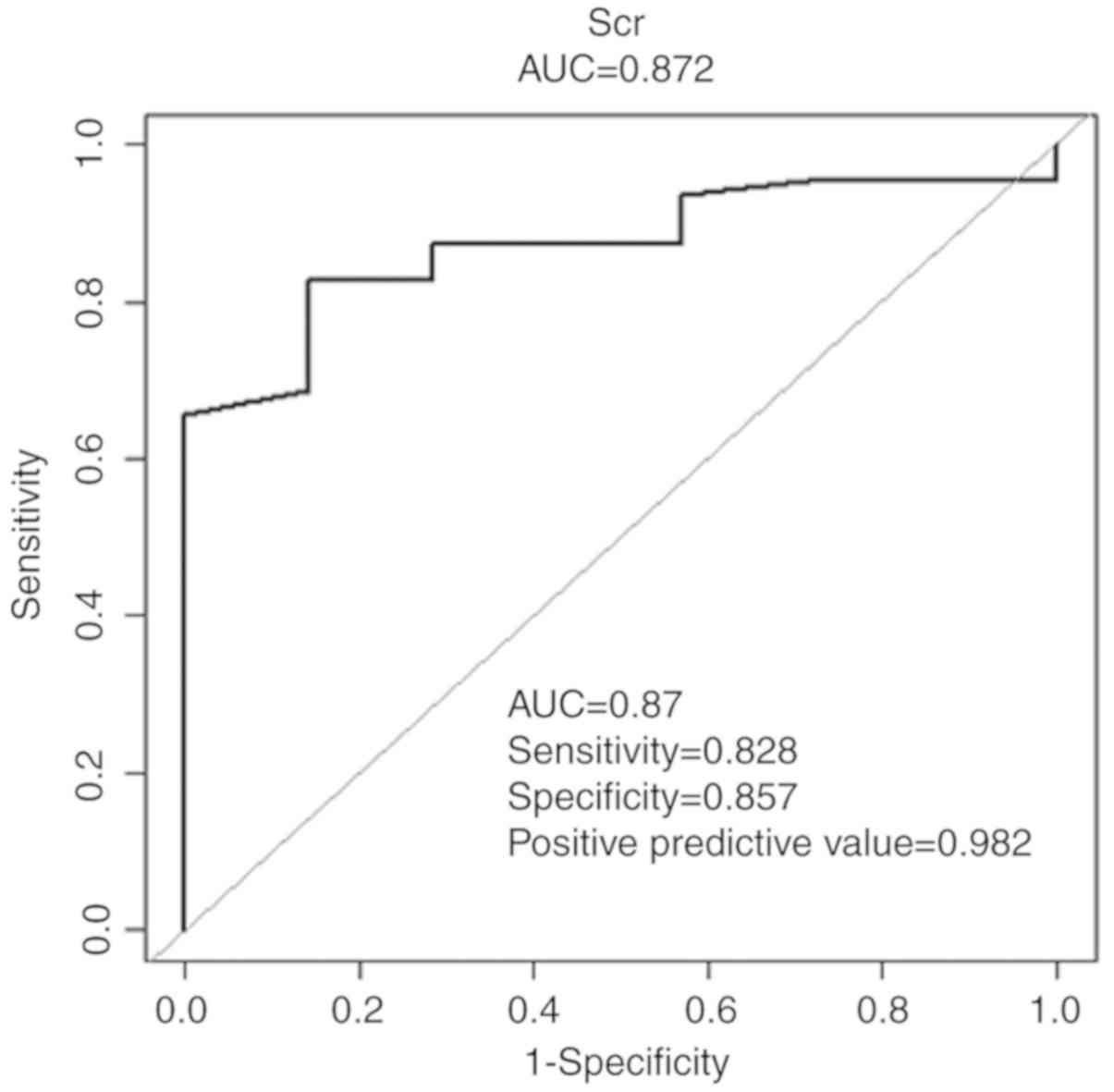
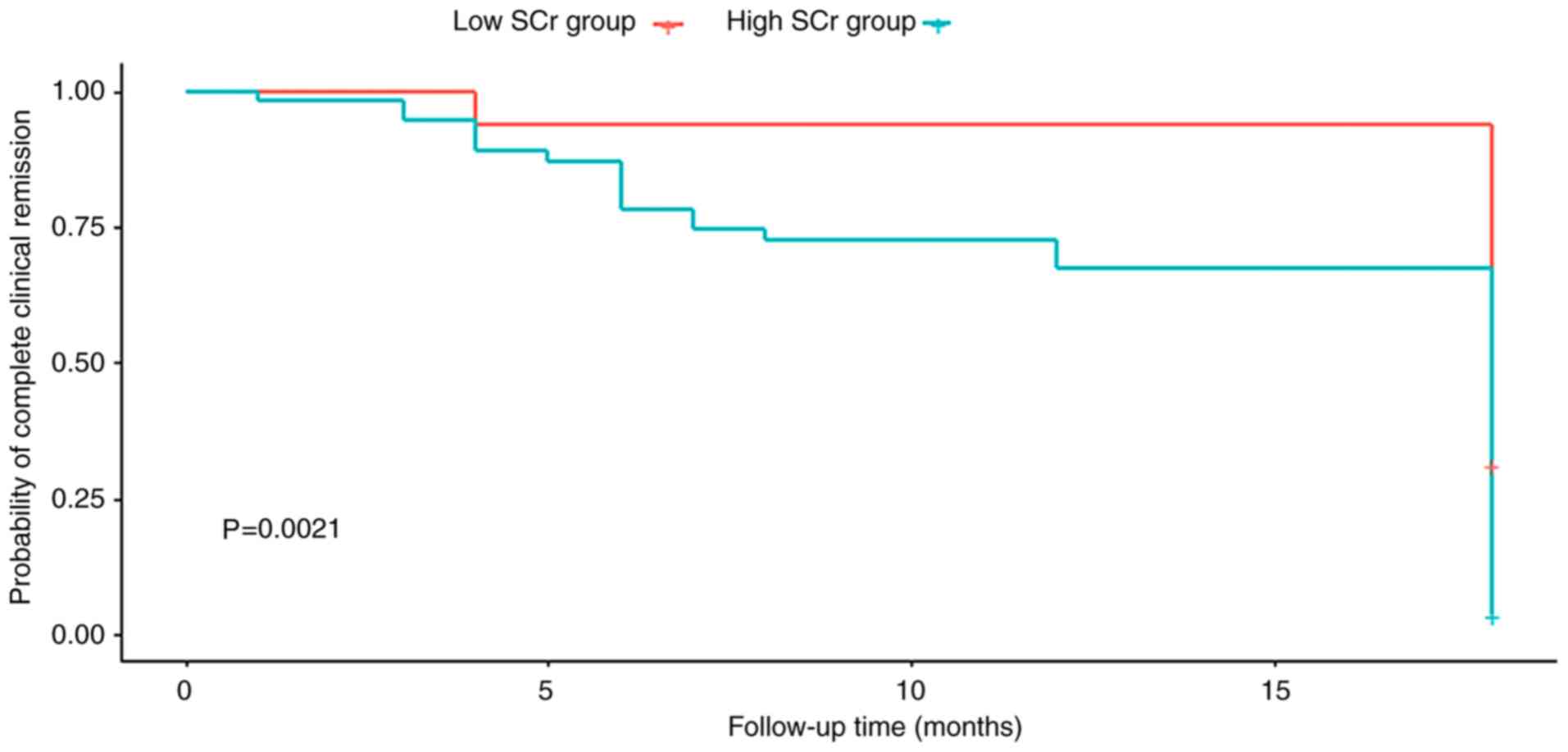
|
1
|
D'Amico G: Natural history of idiopathic
IgA nephropathy and factors predictive of disease outcome. Semin
Nephrol. 24:179–196. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li LS and Liu ZH: Epidemiologic data of
renal diseases from a single unit in China: Analysis based on
13,519 renal biopsies. Kidney Int. 66:920–923. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Koyama A, Igarashi M and Kobayashi M:
Natural history and risk factors for immunoglobulin A nephropathy
in Japan. Research Group on Progressive Renal Diseases. Am J Kidney
Dis. 29:526–532. 1997.PubMed/NCBI View Article : Google Scholar
|
|
4
|
D'Amico G: Natural history of idiopathic
IgA nephropathy: Role of clinical and histological prognostic
factors. Am J Kidney Dis. 36:227–237. 2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Donadio JV and Grande JP: IgA nephropathy.
N Engl J Med. 347:738–748. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Collins AJ, Foley RN, Gilbertson DT and
Chen SC: United States Renal Data System public health surveillance
of chronic kidney disease and end-stage renal disease. Kidney Int
Suppl (2011). 5:2–7. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jin DC, Yun SR, Lee SW, Han SW, Kim W,
Park J and Kim YK: Lessons from 30 years' data of Korean end-stage
renal disease registry, 1985-2015. Kidney Res Clin Pract.
34:132–139. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ginzler EM, Dooley MA, Aranow C, Kim MY,
Buyon J, Merrill JT, Petri M, Gilkeson GS, Wallace DJ, Weisman MH
and Appel GB: Mycophenolate mofetil or intravenous cyclophosphamide
for lupus nephritis. N Engl J Med. 353:2219–2228. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rasche FM, Keller F, von Muller L, Sailer
LK, Karges W and Czock D: Mycophenolic acid therapy after
cyclophosphamide pulses in progressive IgA nephropathy. J Nephrol.
19:465–472. 2006.PubMed/NCBI
|
|
10
|
Nowack R, Birck R and van der Woude FJ:
Mycophenolate mofetil for systemic vasculitis and IgA nephropathy.
Lancet. 349(774)1997.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tang S, Leung JC, Chan LY, Lui YH, Tang
CS, Kan CH, Ho YW and Lai KN: Mycophenolate mofetil alleviates
persistent proteinuria in IgA nephropathy. Kidney Int. 68:802–812.
2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen X, Chen P, Cai G, Wu J, Cui Y, Zhang
Y, Liu S and Tang L: A randomized control trial of mycophenolate
mofetil treatment in severe IgA nephropathy. Zhonghua Yi Xue Za
Zhi. 82:796–801. 2002.PubMed/NCBI(In Chinese).
|
|
13
|
Langhoff E, Olgaard K and Ladefoged J: The
immunosuppressive potency in vitro of physiological and synthetic
steroids on lymphocyte cultures. Int J Immunopharmacol. 9:469–473.
1987.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wan QJ, Hu HF, He YC, Luan SD, Chen HT,
Liu HP, Li T, Xu Y, Xu HL and Liao Y: Severe pneumonia in
mycophenolate mofetil combined with low-dose
corticosteroids-treated patients with immunoglobulin A nephropathy.
Kaohsiung J Med Sci. 31:42–46. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Levey AS, Stevens LA, Schmid CH, Zhang YL,
Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene
T, et al: A new equation to estimate glomerular filtration rate.
Ann Intern Med. 150:604–612. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Working Group of the International IgA
Nephropathy Network and the Renal Pathology Society;. Cattran DC,
Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE,
Amore A, Barratt J, et al: The Oxford classification of IgA
nephropathy: Rationale, clinicopathological correlations, and
classification. Kidney Int. 76:534–545. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rauen T, Eitner F, Fitzner C, Sommerer C,
Zeier M, Otte B, Panzer U, Peters H, Benck U, Mertens PR, et al:
Intensive supportive care plus immunosuppression in IgA
nephropathy. N Engl J Med. 373:2225–2236. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Moresco RN, Speeckaert MM and Delanghe JR:
Diagnosis and monitoring of IgA nephropathy: The role of biomarkers
as an alternative to renal biopsy. Autoimmun Rev. 14:847–853.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Barbour SJ, Cattran DC, Kim SJ, Levin A,
Wald R, Hladunewich MA and Reich HN: Individuals of pacific Asian
origin with IgA nephropathy have an increased risk of progression
to end-stage renal disease. Kidney Int. 84:1017–1024.
2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Allison AC and Eugui EM: Mycophenolate
mofetil and its mechanisms of action. Immunopharmacology.
47:85–118. 2000.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Allison AC and Eugui EM: Purine metabolism
and immunosuppressive effects of mycophenolate mofetil (MMF). Clin
Transpl. 10:77–84. 1996.PubMed/NCBI
|
|
22
|
Rosselli JL, Thacker SM, Karpinski JP and
Petkewicz KA: Treatment of IgA nephropathy: An update. Nephrology.
45:1284–1296. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Huang Y, Liu Z, Huang H, Liu H and Li L:
Effects of mycophenolic acid on endothelial cells. Int
Immunopharmacol. 5:1029–1039. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rathi M, Goyal A, Jaryal A, Sharma A,
Gupta PK, Ramachandran R, Kumar V, Kohli HS, Sakhuja V, Jha V, et
al: Comparison of low-dose intravenous cyclophosphamide with oral
mycophenolate mofetil in the treatment of lupus nephritis. Kidney
Int. 89:235–242. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zheng XY, Wei RB, Tang L, Li P and Zheng
XD: Meta-analysis of combined therapy for adult hepatitis B
virus-associated glomerulonephritis. World J Gastroenterol.
18:821–832. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhu B, Chen N, Lin Y, Ren H, Zhang W, Wang
W, Pan X and Yu H: Mycophenolate mofetil in induction and
maintenance therapy of severe lupus nephritis: A meta-analysis of
randomized controlled trials. Nephrol Dial Transplant.
22:1933–1942. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ong LM, Hooi LS, Lim TO, Goh BL, Ahmad G,
Ghazalli R, Teo SM, Wong HS, Tan SY Shaariah W, et al: Randomized
controlled trial of pulse intravenous cyclophosphamide versus
mycophenolate mofetil in the induction therapy of proliferative
lupus nephritis. Nephrology. 10:504–510. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Laskari K, Mavragani CP, Tzioufas AG and
Moutsopoulos HM: Mycophenolate mofetil as maintenance therapy for
proliferative lupus nephritis: A longterm observational prospective
study. Arthritis Res Ther. 12(R208)2010.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Maes BD, Oyen R, Claes K, Evenepoel P,
Kuypers D, Vanwalleghem J, Van Damme B and Vanrenterghem YF:
Mycophenolate mofetil in IgA nephropathy: Results of a 3-year
prospective placebo-controlled randomized study. Kidney Int.
65:1842–1919. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Frisch G, Lin J, Rosenstock J, Markowitz
G, D'Agati V, Radhakrishnan J, Preddie D, Crew J, Valeri A and
Appel G: Mycophenolate mofetil (MMF) vs placebo in patients with
moderately advanced IgA nephropathy: A doubleblind randomized
controlled trial. Nephrol Dial Transplant. 20:2139–2145.
2005.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hogg RJ, Bay RC, Jennette JC, Sibley R,
Kumar S, Fervenza FC, Appel G, Cattran D, Fischer D, Hurley RM, et
al: Randomised controlled trial of mycophenolate mofetil in
children, adolescents, and adults with IgA nephropathy. Am J Kidney
Dis. 66:783–791. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tang SC, Tang AW, Wong SS, Leung JC, Ho YW
and Lai KN: Long-term study of mycophenolate mofetil treatment in
IgA nephropathy. Kidney Int. 77:543–549. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Roccatello D, Rossi D, Marletto F, Naretto
C, Sciascia S, Baldovino S, Piras D and Giachino O: Long term
effects of methylprednisolone pulses and mycophenolate mofetil in
IgA nephropathy patients at risk of progression. J Nephrol.
25:198–203. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu X, Dewei D, Sun S, Xu G, Liu H, He L
and Zhang P: Treatment of severe IgA nephropathy: Mycophenolate
mofetil/prednisone compared to cyclophosphamide/prednisone. Int J
Clin Pharmacol Ther. 52:95–102. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
35
|
Hou JH, Le WB Chen N, Wang WM, Liu ZS, Liu
D, Chen JH, Tian J, Fu P, Hu ZX, et al: Mycophenolate mofetil
combined with prednisone versus full-dose prednisone in IgA
nephropathy with active proliferative lesions: A randomized
controlled trial. Am J Kidney Dis. 69:788–795. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wyatt RJ and Julian BA: IgA nephropathy. N
Engl J Med. 368:2402–2414. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Maixnerova D, Bauerova L, Skibova J,
Rysava R, Reiterova J, Merta M, Honsova E and Tesar V: The
retrospective analysis of 343 Czech patients with IgA
nephropathy-one centre experience. Nephrol Dial Transplant.
27:1492–1498. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Reich HN, Troyanov S, Scholey JW and
Cattran DC: Toronto Glomerulonephritis Registry: Remission of
proteinuria improves prognosis in IgA nephropathy. J Am Soc
Nephrol. 18:3177–3183. 2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Alamartine E, Sauron C, Laurent B, Sury A,
Seffert A and Mariat C: The use of Oxford classification of IgA
nephropathy to predict renal survival. Clin J Am Soc Nephrol.
6:2384–2388. 2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Barbour SJ, Coppo R, Zhang H, Liu ZH,
Suzuki Y, Matsuzaki K, Katafuchi R, Er L, Espino-Hernandez G, Kim
SJ, et al: Evaluating a new international risk-prediction tool in
IgA nephropathy. JAMA Intern Med. 179:942–952. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chen T, Li X, Li Y, Xia E, Qin Y, Liang S,
Xu F, Liang D, Zeng C and Liu Z: Prediction and risk stratification
of kidney outcomes in IgA nephropathy. Am J Kidney Dis. 74:300–309.
2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Syrjänen J, Mustonen J and Pasternack A:
Hypertriglyceridaemia and hyperuricaemia are risk factors for
progression of IgA nephropathy. Nephrol Dial Transplant. 15:34–42.
2000.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shu D, Xu F, Su Z, Zhang J, Chen C, Zhang
J, Ding X, Lv Y, Lin H and Huang P: Risk factors of progressive IgA
nephropathy which progress to end stage renal disease within ten
years: A case-control study. BMC Nephrol. 18(11)2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lv J, Yang Y, Zhang H, Chen W, Pan X, Guo
Z, Wang C, Li S, Zhang J, Zhang J, et al: Prediction of outcomes in
crescentic IgA nephropathy in a multicenter cohort study. J Am Soc
Nephrol. 24:2118–2125. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ni Z, Yuan Y, Wang Q, Cao L, Che XJ, Zhang
MF, Xie YY, Qi CJ and Mou S: Time-averaged albumin predicts the
long-term prognosis of IgA nephropathy patients who achieved
remission. J Transl Med. 12(194)2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Haroun MK, Jaar BG, Hoffman SC, Comstock
GW, Klag MJ and Coresh J: Risk factors for chronic kidney disease:
A prospective study of 23,534 men and women in Washington County,
Maryland. J Am Soc Nephrol. 14:2934–2941. 2003.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zoppini G, Targher G, Chonchol M, Perrone
F, Lippi G and Muggeo M: Higher HDL cholesterol levels are
associated with a lower incidence of chronic kidney disease in
patients with type 2 diabetes. Nutr Metab Cardiovasc Dis.
19:580–586. 2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Herzenberg AM, Fogo AB, Reich HN, Troyanov
S, Bavbek N, Massat AE, Hunley TE, Hladunewich MA, Julian BA,
Fervenza FC, et al: Validation of the Oxford classification of IgA
nephropathy. Kidney Int. 80:310–317. 2011.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Liu XW, Li DM, Xu GS and Sun SR:
Comparison of the therapeutic effects of leflunomide and
mycophenolate mofetil in the treatment of immunoglobulin a
nephropathy manifesting with nephrotic syndrome. Int J Clin
Pharmacol Ther. 48:509–513. 2010.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Du B, Jia Y, Zhou W, Min X, Miao L and Cui
W: Efficacy and safety of mycophenolate mofetil in patients with
IgA nephropathy: An update meta-analysis. BMC Nephrol.
18(245)2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Trimarchi H, Barratt J, Cattran DC, Cook
HT, Coppo R, Haas M, Liu ZH, Roberts IS, Yuzawa Y, Zhang H, et al:
Oxford Classification of IgA nephropathy 2016-the role of
crescentic lesions: An update from the IgA Nephropathy
Classification Working Group. Kidney Int. 91:1014–1021.
2017.PubMed/NCBI View Article : Google Scholar
|