Introduction
IgA nephropathy (IgAN) is the most common type of
primary glomerulonephritis worldwide and is particularly prevalent
in Asia (1). IgAN accounts for 45%
of primary glomerulonephritis cases in China (2). IgAN causes end-stage renal disease
(ESRD) in a significant proportion of patients (3-5).
Given the devastating medical and socioeconomic burden for patients
with chronic kidney disease, diverse therapeutic options should be
considered to slow the progression of kidney disease (6,7). As the
pathogenic mechanisms of IgAN remain incompletely understood, no
specific treatments remain unavailable. Most of the current
treatment strategies include the regulation of blood pressure (BP)
using angiotensin-converting enzyme inhibitors or angiotensin
receptor blockers and amelioration of proteinuria.
Mycophenolate mofetil (MMF) is used as an
immunosuppressant in patients undergoing renal transplantation. It
is also used for immunosuppressive treatment in patients with
autoimmune diseases (8,9). MMF was first used to treat IgAN in
1997(10). Certain prospective
randomized controlled trials have proved the superior effectiveness
of MMF to that of other immunosuppressive drugs or placebo in
Chinese patients with less advanced IgAN (11,12).
Although similar studies have been performed in IgAN, little is
known regarding which indicators are able to predict the efficacy
of MMF combined with low-dose prednisone in IgAN. Therefore, the
aim of the present study was to identify predictive indicators of
the efficacy of MMF combined with low-dose prednisone in IgAN.
Patients and methods
Study population
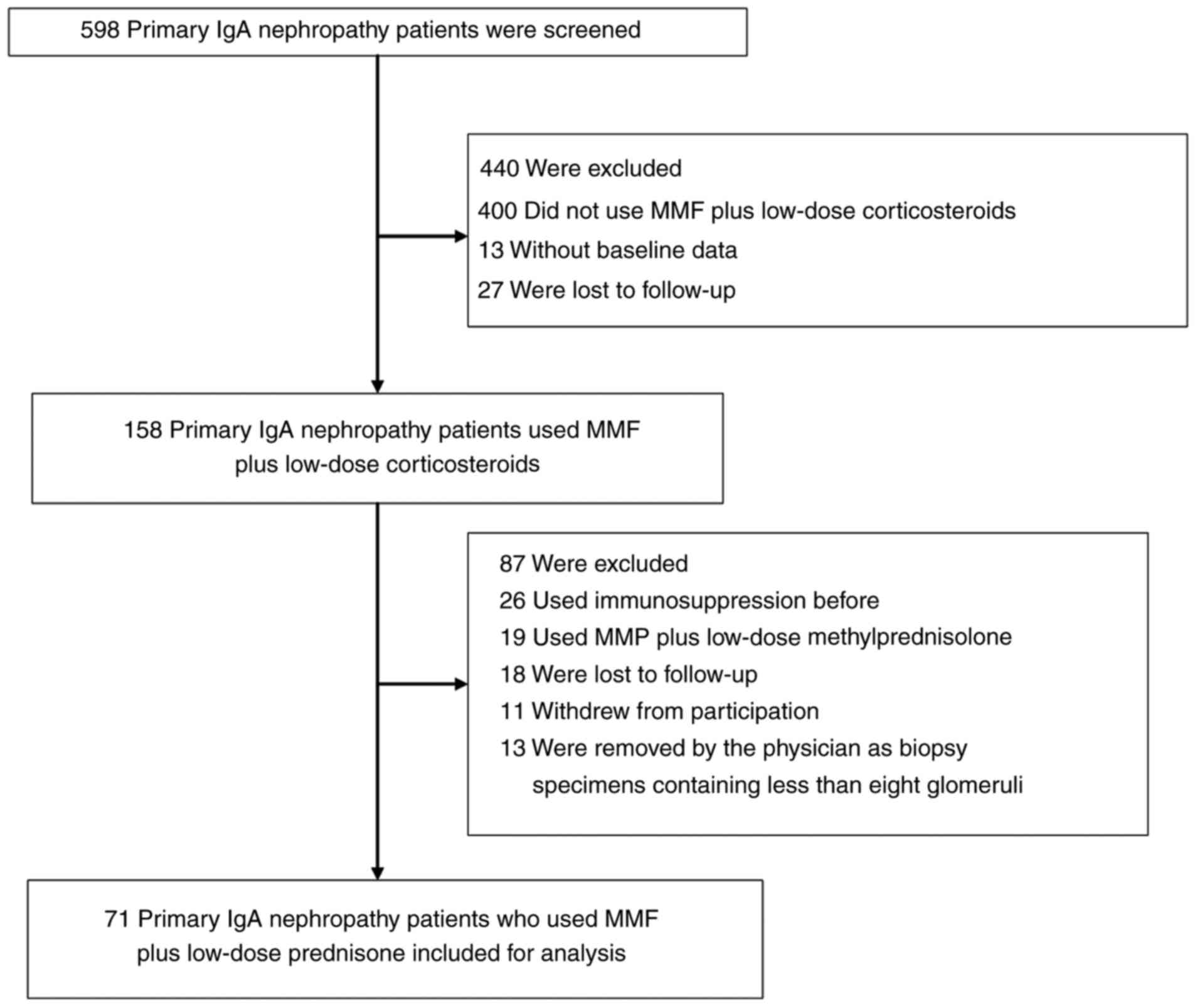
From September 2009 to October 2017, 598 patients
with primary IgAN were screened at the Department of Nephrology of
Shenzhen Second People's Hospital (Shenzhen, China). A total of 440
patients were excluded (comprising 400 patients who did not use MMF
plus low-dose corticosteroids, 13 patients without baseline data
and 27 patients were lost to follow-up). Subsequently, 158 patients
using MMF plus low-dose corticosteroids were enrolled in the study.
According to the literature (13),
in vitro, the relative immunosuppressive potency of
prednisone is zero, whereas that of methylprednisolone is 11. Thus,
patients treated with MMF plus methylprednisolone may be more
susceptible to infection than those treated with MMF combined with
prednisone. A retrospective study indicated that chronically
impaired renal function and methylprednisolone treatment are risk
factors for severe pneumonia. In patients with patients Lee Class
III-V IgAN, MMF plus prednisone is safer than MMF plus
methylprednisolone (14). In the
present study, only indicators for predicting the efficacy of using
MMF combined with low-dose prednisone in IgA nephropathy were
investigated, and the 19 patients who used MMF plus low-dose
methylprednisolone were therefore excluded. Furthermore, 26
patients who previously used immunosuppressive therapy were
excluded, 18 patients who were lost to follow-up, 11 patients who
withdrew from participation, 13 patients who were removed by the
physician due to biopsy specimens containing <8 glomeruli.
Finally, 71 patients with primary IgAN using MMF plus low-dose
prednisone were included. The study selection process is depicted
in Fig. 1. The key inclusion
criteria were primary IgAN confirmed by biopsy and patient age of
18-70 years. The major exclusion criteria were as follows: i)
Biopsy specimens containing <8 glomeruli; ii) secondary IgAN
(e.g., caused by lupus, liver cirrhosis, Henoch-Schonlein purpura);
and iii) any prior immunosuppressive therapy.
Study design and clinical, biochemical
and histological data collection
A retrospective cohort study was performed. All
patients enrolled underwent kidney biopsy from 2009 to 2017.
Patients were followed up for 18 months unless they progressed to
ESRD or severe pneumonia (within 18 months). Using a database from
the Shenzhen Second People's Hospital, the demographic, clinical
and biochemical data at the time of renal biopsy, including age,
sex, systolic blood pressure (SBP) and diastolic blood pressure
(DBP), were retrieved and considered baseline data. The following
biochemical laboratory data were also collected: Proteinuria and
concentrations of serum creatinine (SCr), hemoglobin, serum
albumin, serum uric acid, serum triglycerides, serum total
cholesterol, high-density lipoprotein cholesterol and low-density
lipoprotein cholesterol. The estimated glomerular filtration rate
was calculated using the Chronic Kidney Disease Epidemiology
Collaboration equation (15).
The histological diagnosis of IgAN was based upon
the demonstration of mesangial proliferative changes by light
microscopy and the concomitant presence of predominant or
codominant mesangial deposition of IgA. The pathological diagnosis
was made by the same pathologist from Guangzhou King Medical Centre
of Clinical Laboratory (Guangzhou, China). Renal lesions were
histopathologically evaluated and scored using the Oxford
classification (16). This
classification identifies four pathological components: Mesangial
hypercellularity (M), endocapillary hypercellularity (E), segmental
glomerulosclerosis (S) and tubular atrophy/interstitial fibrosis
(T). According to this classification, the following scoring system
apply: i) Mesangial score ≤0.5 (M0) or >0.5 (M1); ii)
endocapillary hypercellularity absent (E0) or present (E1); iii)
Segmental glomerulosclerosis absent (S0) or present (S1); iv)
presence or absence of podocyte hypertrophy/tip lesions in biopsy
specimens with S1; and v) Tubular atrophy/interstitial fibrosis
≤25% (T0), 26-50% (T1), or >50% (T2) (16).
Treatments
The treatment regimen was MMF plus low-dose
prednisone (0.5 mg/kg/day). The initial dose of MMF was 1.0 or 1.5
g/day in patients with a body weight of <50 or ≥50 kg,
respectively. MMF therapy was initiated at 1-1.5 g/day for six
months, 0.5-1.0 g/day for the next six months and then 0.25-0.5
g/day for the final six months. Prednisone was administered at 0.5
mg/kg/day for two months and then slowly tapered by 5 mg every two
weeks until discontinuation. Patient records were reviewed from the
start of treatment through to the time-point when the chart was
reviewed and until the drug was discontinued or severe infection
occurred (final time-point).
Follow-up and outcome measures
The data collected included age, sex, SBP, DBP,
proteinuria, serum uric acid concentration, serum triglyceride
concentration, hemoglobin concentration, serum albumin
concentration, serum total cholesterol concentration and the Oxford
classification. The information on the patients was retrieved from
a database from Shenzhen Second People's Hospital and confirmed by
a telephone call at the end of the study. The end-point was
complete clinical remission (defined as proteinuria with a
protein-to-creatinine ratio of <0.2 and stable renal function
with a decrease in the estimated glomerular filtration rate (eGFR)
of <5 ml per minute per 1.73 m2 from baseline at the
end of the 18-month follow-up period) (17).
Statistical analysis
Continuous variables are expressed as the mean ±
standard deviation or median (range) as appropriate. The
association of the initial SCr concentration was examined as a
continuous variable categorized into quartiles with outcomes of
incomplete clinical remission. Cox proportional hazards models were
used to evaluate these associations without adjustment and with
adjustment for confounding variables. In the adjusted regression
model, age, sex, SBP, DBP, proteinuria, serum uric acid
concentration, hemoglobin concentration, serum albumin
concentration, serum triglyceride concentration, serum total
cholesterol concentration and the Oxford classification were
included. Hazard ratios (HRs) and 95% CIs were calculated. The
lowest quartile was the reference for the initial SCr
concentration.
Logistic regression analyses with the initial SCr
concentration as a continuous variable were performed for the
overall population. Interaction and stratified analyses were
conducted according to sex (male and female), hypertension status
at baseline (hypertension or no hypertension), endocapillary
proliferation (E0 and E1) and segmental glomerulosclerosis/adhesion
(S0 and S1). To examine the association between the initial SCr
concentration and outcome, the Cox models were re-established using
the initial SCr concentration as a continuous variable. HRs were
calculated per 1 mg/dl increase in the initial SCr concentration
and the risk change for incomplete clinical remission. The cutoff
value for the initial SCr concentration was determined using
receiver operating characteristics (ROC) curve analysis.
Kaplan-Meier survival curves were used to compare complete clinical
remission rates. All P-values were calculated using two-tailed
tests of statistical significance with a type I error rate of 5%. P
interaction in the results section represents the P-value for
interaction. All statistical analyses were performed using Empower
(R) (www.empowerstats.com; X&Y Solutions,
Inc.) and R (http://www.R-project.org).
Results
From September 2009 to October 2017, a total of 598
patients with primary IgAN presenting at the Department of
Nephrology of Shenzhen Second People's Hospital (Shenzhen, China)
were initially screened. As presented in the flow chart in Fig. 1, 440 patients were excluded
(comprising 400 patients who did not use MMF plus low-dose
corticosteroids, 13 patients without baseline data and 27 patients
lost to follow-up). A total of 158 patients using MMF plus low-dose
corticosteroids were retained. Of these, 87 patients were excluded
(comprising 26 patients who previously used immunosuppressive
therapy, 19 patients taking MMF plus low-dose methylprednisolone,
18 patients lost to follow-up, 11 patients who withdrew from
participation and 13 patients removed by the physician as biopsy
specimens contained <8 glomeruli). Finally, 71 patients with
primary IgAN using MMF plus low-dose prednisone were included
(Fig. 1).
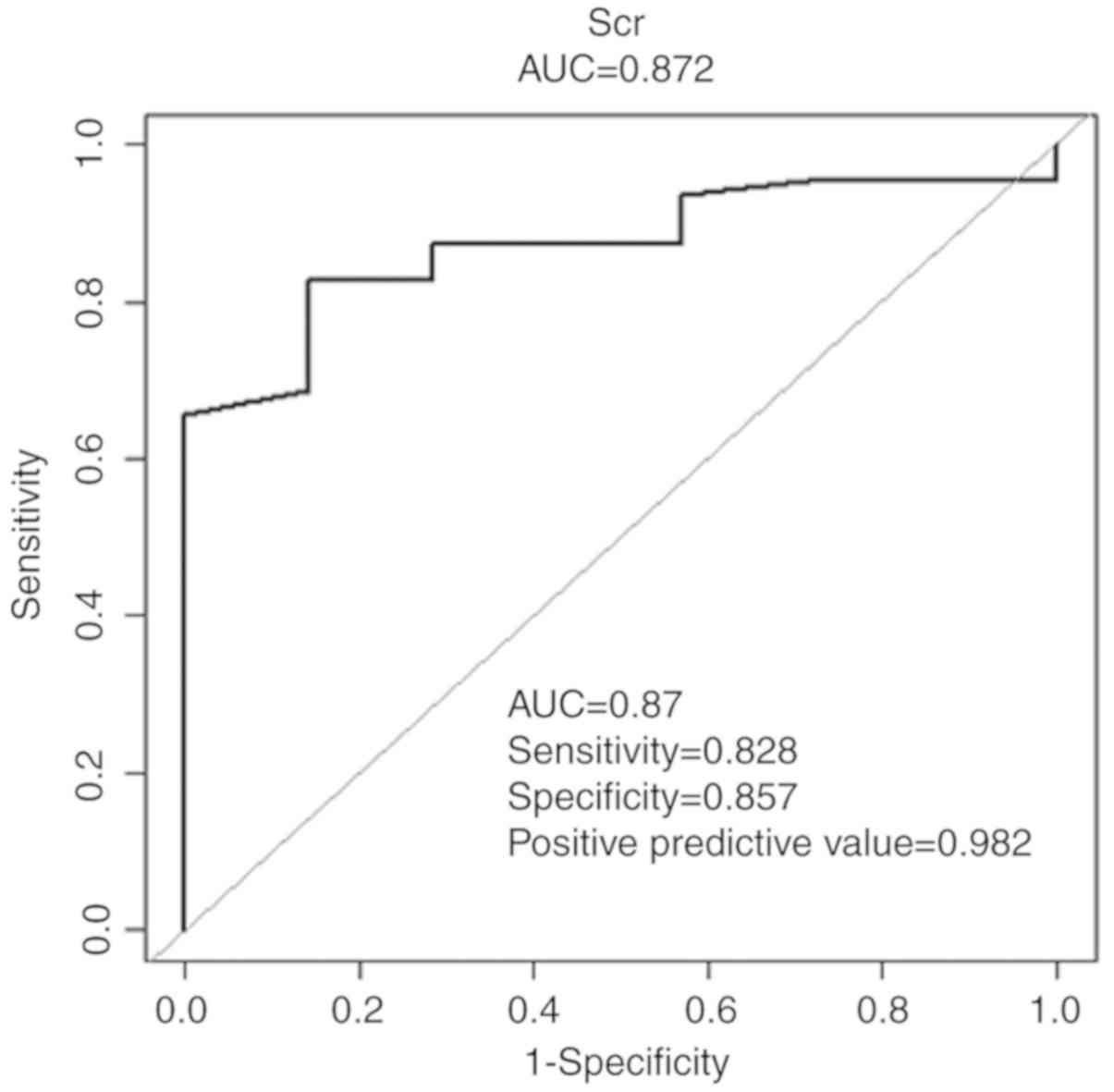
The initial SCr concentration was the patient's SCr
value at biopsy. The initial SCr concentration was the measurement
prior to treatment with MMF combined with low-dose prednisone. The
cutoff value for the initial SCr concentration determined using ROC
curve analysis was 0.89 (Fig. 2).
Therefore, this level was selected to group patients. A total of 16
patients with an initial SCr concentration of <0.89 mg/dl were
assigned to the low SCr group, whereas 55 patients with an initial
SCr concentration of ≥0.89 mg/dl were assigned to the high SCr
group.
The 18-month trial was completed by 52 patients
(73.2%) in this study. In the full-analysis set, 7 of 71 patients
(9.86%) had achieved complete clinical remission at the final
visit. Table I presents the baseline
demographic, clinical and biochemical characteristics of the study
subjects. The majority of the patients (54.9%) were female. The
mean age was 34.3±9.9 years (range, 20-70 years). The mean SCr
concentration was 1.4±0.7 mg/dl. The mean eGFR level was 71.0±36.0
ml/min/1.73 m2. A total of 41 patients had an eGFR of at
least 60 ml/min/1.73 m2 and 21 patients had an eGFR
between 30 and 59 ml/min/1.73 m2. In addition, patients
in the low SCr group had less proteinuria, higher eGFR, lower
triglyceride concentrations and lower uric acid concentrations
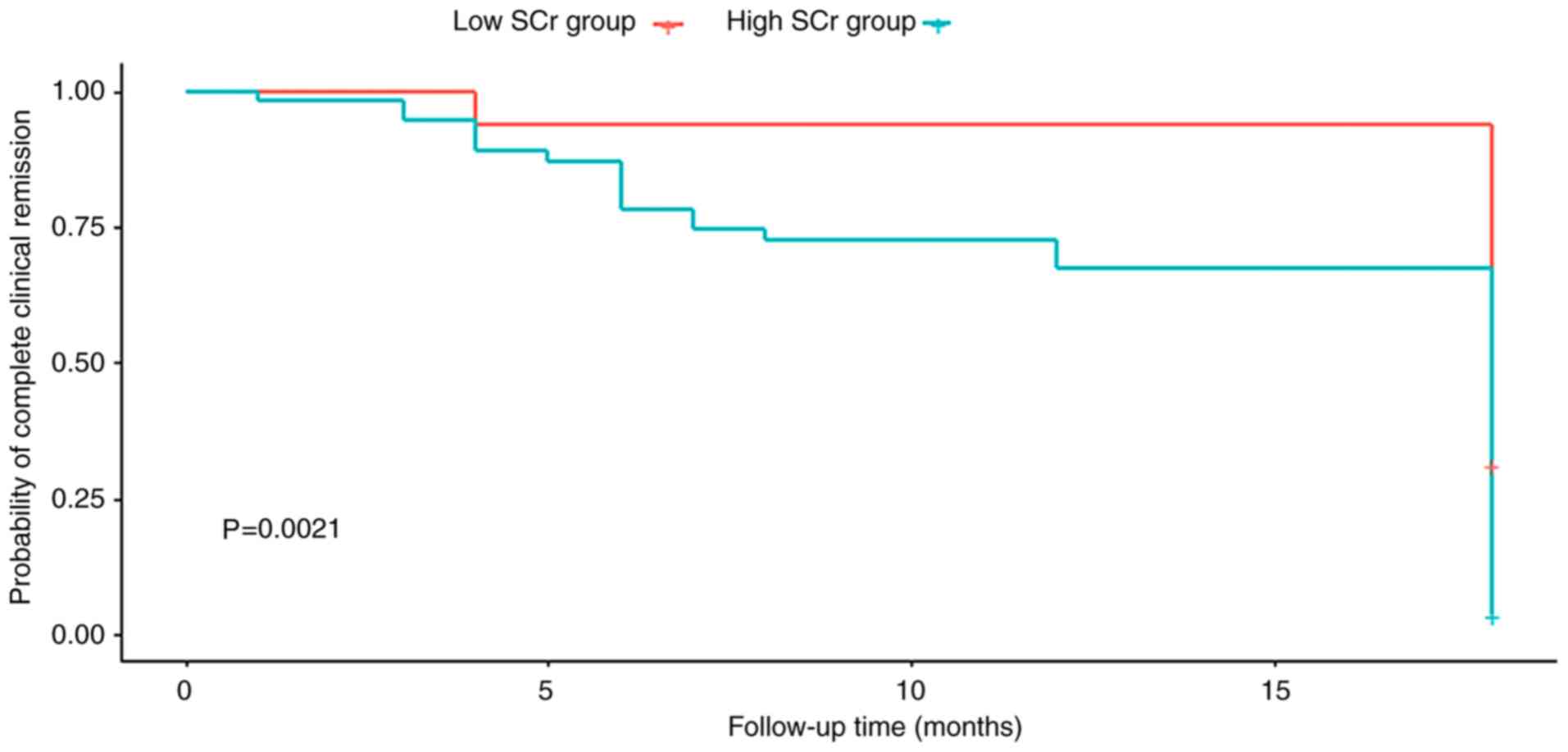
compared with patients in the high SCr group (Table I). Kaplan-Meier survival analysis
revealed that patients in the high SCr group had a significantly
lower rate of complete clinical remission than those in the low SCr
group (Fig. 3). Table II presents the results of the
logistic regression analysis using univariate and multivariate Cox
proportional hazards models. In the univariate analysis, the HR for
incomplete clinical remission significantly increased as the
quartiles of the initial SCr concentration increased. The HR for
quartile 4 was significantly higher than the HR for quartile 1
(quartile 4 vs. quartile 1: HR, 2.51; 95% CI, 1.20-5.21; P=0.01).
Additional adjustment for confounding variables did not reduce the
HRs for the association between the initial SCr concentration and
incomplete clinical remission (quartile 4 vs. quartile 1: HR, 7.27;
95% CI, 1.21-43.63; P=0.03). The total data were analyzed, where
each unit increase in the initial SCr concentration was associated
with a 67 and 194% increase in the risk of incomplete clinical
remission based on model 1 (95% CI, 1.02-2.73; P=0.04) and model 2
(95% CI, 1.01-8.60; P=0.048), respectively. Following adjustment
for age, sex, proteinuria, Oxford classification, SBP and DBP
during follow-up, the HRs for the association between the initial
SCr concentration and incomplete clinical remission also
increased.
 | Table IPatient characteristics at
baseline. |
Table I
Patient characteristics at
baseline.
| Variable | Low SCr group
(<0.89; n=16) | High SCr group
(≥0.89; n=55) | Total (n=71) | P-value |
|---|
| Age (years) | 34.25±9.57 | 34.27±10.05 | 34.27±9.87 | 0.994 |
| Male | 1 (6.25) | 31 (56.36) | 32 (45.07) | 0.063 |
| Hypertension | 12 (75.00) | 29 (52.73) | 41 (57.75) | 0.112 |
| SBP (mmHg) | 128.75±17.89 | 139.91±20.84 | 137.39±20.63 | 0.038 |
| DBP (mmHg) | 77.81±9.84 | 88.60±14.17 | 86.17±14.01 | 0.006 |
| Serum creatinine
(mg/dl) | 0.69±0.09 | 1.60±0.65 | 1.40±0.69 | <0.001 |
| eGFR (ml/min per
1.73 m2) | 122.03±26.62 | 56.16±22.05 | 71.00±35.99 | <0.001 |
| Proteinuria
(g/24h) | 1.04±0.74 | 2.71±2.67 | 2.26±2.42 | 0.017 |
| Serum albumin
(g/l) | 37.71±4.66 | 37.71±6.17 | 37.71±5.83 | 0.996 |
| Uric acid
(µmol/l) | 306.29±74.35 | 447.06±102.19 | 414.89±112.99 | <0.001 |
| Hemoglobin
(g/l) | 121.12±22.78 | 126.57±22.41 | 125.34±22.45 | 0.397 |
| Triglycerides
(mmol/l) | 1.17±0.68 | 1.90±1.34 | 1.72±1.25 | 0.040 |
| Total cholesterol
(mmol/l) | 4.59±0.88 | 5.40±1.59 | 5.20±1.49 | 0.057 |
| HDLc (mmol/l) | 1.30±0.26 | 1.19±0.32 | 1.22±0.31 | 0.224 |
| LDLc (mmol/l) | 2.66±0.69 | 3.25±1.16 | 3.11±1.09 | 0.061 |
| The Oxford
classificationa |
|
M1 | 15 (93.75) | 49 (89.09) | 64 (90.14) | 0.582 |
|
E1 | 11 (68.75) | 36 (65.45) | 47 (66.20) | 0.806 |
|
S1 | 11 (68.75) | 22 (40.00) | 33 (46.48) | 0.051 |
|
T1 | 2 (12.50) | 27 (49.09) | 29 (40.85) | <0.001 |
|
T2 | 0 (0.00) | 9 (16.36) | 9 (12.68) | <0.001 |
 | Table IILogistic regression analysis of the
association between the initial serum creatinine concentration and
the risk of incomplete clinical remission. |
Table II
Logistic regression analysis of the
association between the initial serum creatinine concentration and
the risk of incomplete clinical remission.
| Group | Unadjusted Hazard
ratio (95% CI) | P-value | Model
1a Hazard ratio (95%
CI) | P-value | Model
2b Hazard ratio (95%
CI) | P-value |
|---|
| Total | 1.40
(1.01-1.94) | 0.041 | 1.67
(1.02=2.73) | 0.040 | 2.94
(1.01=8.60) | 0.048 |
| Quartiles |
|
Quartile 1
(<0.90) | 1.0 | | 1.0 | | 1.0 | |
|
Quartile 2
(≥0.90-<1.25) | 1.70
(0.80-3.58) | 0.167 | 2.16
(0.73=6.39) | 0.166 | 1.69
(0.45=6.31) | 0.435 |
|
Quartile 3
(≥1.25-<1.64) | 1.84
(0.89=3.83) | 0.101 | 2.22
(0.78=6.27) | 0.133 | 2.29
(0.63=8.31) | 0.209 |
|
Quartile 4
(≥1.64) | 2.51
(1.204=5.210) | 0.014 | 6.18
(1.71=22.36) | 0.006 | 7.27
(1.21=43.63) | 0.030 |
To determine the consistency in the association
between increased initial SCr concentration and the risk of
incomplete clinical remission, interaction and stratified analyses
were performed (Table III). For
each unit increase in the initial SCr concentration, the HR for
incomplete clinical remission was 1.16 in males (P=0.60) and 1.55
in females (P=0.04), and the difference was not statistically
significant (P interaction =0.41). The HR for incomplete clinical
remission for patients in the E1 subgroup was 2.09 (P=0.02)
compared with 1.33 (P=0.15) for patients in the E0 subgroup (P
interaction =0.24). The HR for patients in the S1 subgroup was 1.95
(P=0.007) and that for patients in the S0 subgroup was 1.10
(P=0.70). There were no statistically significant differences in
patients stratified by M or T (data not shown). In addition, for
each unit increase in the initial SCr concentration, the HR for
incomplete clinical remission was 1.44 (P=0.06) and 1.35 (P=0.19)
for hypertensive and normotensive patients, respectively.
 | Table IIIHazard ratios with 95% CIs for
incomplete clinical remission per unit increase in the initial
serum creatinine concentration in subgroups by sex, hypertension, E
and S. |
Table III
Hazard ratios with 95% CIs for
incomplete clinical remission per unit increase in the initial
serum creatinine concentration in subgroups by sex, hypertension, E
and S.
| Item | Cases (n) | Hazard ratio (95%
CI) | P-value | P for
interaction |
|---|
| Sex | | | | 0.414 |
|
Male | 32 | 1.16
(0.67-2.02) | 0.601 | |
|
Female | 39 | 1.55
(1.02-2.36) | 0.042 | |
| Hypertension | | | | 0.850 |
|
Yes | 41 | 1.44
(0.85-2.45) | 0.061 | |
|
No | 30 | 1.35
(0.86-2.10) | 0.189 | |
| E | | | | 0.242 |
|
E0 | 24 | 1.33
(0.90-1.97) | 0.151 | |
|
E1 | 47 | 2.09
(1.12-3.91) | 0.020 | |
| S | | | | 0.096 |
|
S0 | 38 | 1.10
(0.69-1.75) | 0.697 | |
|
S1 | 33 | 1.95
(1.20-3.17) | 0.007 | |
Discussion
IgAN is the leading cause of ESRD (18). There are substantial ethnic
variations and Asian populations have an increased incidence of
IgAN and an elevated risk of renal function decline (19). The predominant characteristic of IgAN
is galactose-deficient IgA1 deposition in the mesangial area.
However, the pathogenesis of IgAN is still not fully understood and
clinical and pathological phenotypes of IgAN are variable. Thus,
treatments for IgAN have not been effective. MMF acts by releasing
mycophenolic acid, which leads to apoptosis in cytotoxic
T-lymphocytes and reduction in antibody synthesis via the selective
inhibition of T- and B-lymphocyte proliferation (20,21),
induction of intercellular adhesion molecule-1 mRNA expression and
interleukin-6 secretion, and inhibition of cell proliferation,
particularly in endothelial cells (22,23). MMF
is beneficial for IgAN secondary to systemic diseases, including
lupus nephritis and hepatitis B virus-associated glomerulonephritis
(24,25). Certain studies have indicated that in
diffuse proliferative lupus nephritis, MMF has a higher efficacy to
induce remission than pulsed intravenous therapy with
cyclophosphamide (CTX) (26-28).
To date, eight randomized controlled trials on MMF therapy for IgAN
have been performed. Certain studies demonstrated no benefit from
MMF therapy (29-31),
whereas other studies observed amelioration of proteinuria and
long-term renoprotection following treatment with MMF (11,32).
An Italian multicenter study indicated that in
patients with active disease, proteinuria >2.4 g/day and renal
failure (mean SCr concentration, 1.6 mg/dl), a combined regimen
with MMF and steroids may induce amelioration of proteinuria and
delay successive progression toward renal failure (33). A Chinese study compared combined
therapy with prednisone and MMF vs. prednisone and CTX for severe
IgAN. In the MMF group, amelioration of proteinuria and improvement
of renal function were observed, with fewer adverse events than in
the CTX group (34). However,
another study evaluated the efficacy and safety of MMF plus
prednisone vs. full-dose prednisone in patients with IgAN with
active proliferative lesions. At 6 months, there were no
significant differences between the groups regarding complete
remission or median time to achieve remission. In addition, during
the follow-up period, relapse rates and total adverse events did
not differ between the groups (35).
These mentioned studies enrolled patients with different clinical
and histological characteristics. For the various patients with
different clinical and histological characteristics, the efficacy
of MMF therapy exhibited certain differences. It is necessary to
identify indicators for predicting the efficacy of using MMF in
patients with IgAN. Therefore, in the present study, predictive
indicators of the efficacy of using mycophenolate mofetil combined
with low-dose prednisone in IgAN patients were determined.
In the present retrospective cohort study, ~10% of
patients (7/71) displayed complete clinical remission with MMF plus
prednisone. The rate of complete clinical remission for patients
using MMF plus low-dose prednisone was further determined and it
was revealed that it was possible to predict the efficacy of using
MMF combined with low-dose prednisone using the initial SCr
concentration. During the course of treatment with MMF plus
low-dose prednisone, the average SCr value of the patients with
complete clinical remission was relatively stable and its changes
occurred slowly. However, the variations in the average SCr value
of the patients with incomplete clinical remission were
significant. The variations in the average SCr value of the
patients with incomplete clinical remission were ~5 times higher
than those of the patients with complete clinical remission. A
higher initial SCr concentration was significantly associated with
an increased incidence of incomplete clinical remission. This
association persisted even after adjustment for demographic,
clinical and histopathology factors.
The following clinical predictors of renal outcome
in IgAN, determined at the time of diagnosis, have been assessed in
several clinical studies: Proteinuria, hypertension, decreased eGFR
(36-38)
and histological grading (39).
Certain risk-prediction tools indicated that in IgA nephropathy,
the risk factors included age, sex, the Oxford classification
histologic score, serum albumin and serum uric acid (40,41).
Furthermore, studies have indicated that serum triglyceride, serum
total cholesterol and hemoglobin were risk factors for the
prognosis of IgA nephropathy (42,43).
Therefore, these variables were adjusted to minimize the potential
impact of confounders. Of note, in the present study, the Oxford
classification was not independently associated with the occurrence
of incomplete clinical remission. The present results will thus be
important for determining therapeutic strategies in the clinic.
There has been a great interest in exploring the
association between clinical markers and renal outcomes. In a study
of 113 Chinese patients diagnosed with crescentic IgAN, the initial
SCr concentration was the strongest predictor of kidney failure
(44). Significant associations
between SCr concentration and renal prognosis in patients with IgAN
have also been demonstrated. However, there are limited data on the
association between the initial SCr concentration and the efficacy
of MMF combined with low-dose prednisone in IgAN. The present
results are consistent with those of a previous study (45), indicating that patients with a higher
initial SCr concentration had a higher risk of incomplete clinical
remission after adjustment for other risk factors. In the present
study, stratified analyses suggested that the risk of incomplete
clinical remission increased significantly with increasing initial
SCr concentrations for female patients but not for male patients.
These results were consistent with those of previous studies. A
number of large-scale studies have indicated that the rate of
progression of renal disease is greater in females than in males
(46,47). Furthermore, the risk of incomplete
clinical remission increased significantly with increasing initial
SCr concentrations for patients in the E1 and S1 subgroups. This
result was consistent with the Oxford classification, which may aid
in predicting outcomes for patients with IgAN (16,48).
Of note, the present study had certain important
limitations that should be noted. First, the number of observed
events is small and limits the statistical power of this
explorative study to a certain extent. However, according to a
recent meta-analysis, eight randomized controlled trials have
assessed the use of MMF in patients with IgA nephropathy (11,12,28-31,33,49,50).
Most of them were also small-sized and the study with the biggest
size included 84 patients (33). The
patients in these studies and the present study are similar in
number. Furthermore, statistical methods were applied to
retrospectively determine predictive indicators of the efficacy of
using MMF combined with low-dose prednisone in patients with IgAN.
Of note, the present results were statistically significant and the
sample size was sufficient to draw a conclusion. Furthermore, the
Oxford or MEST classification was used, while crescentic (C)
features were not considered. The IgA Nephropathy Classification
Working Group provided an update that C features should be added to
the MEST score and biopsy reporting should provide a MEST-C score
for the previous year (51). In
addition, the present study is based on a cohort from a single
center, and whether these observations may be extrapolated to other
populations of Chinese and non-Chinese IgAN patients using MMF plus
prednisone remains to be evaluated.
In conclusion, in the present cohort of patients
with IgAN treated with MMF plus low-dose prednisone, the initial
SCr concentration was an independent risk factor for the outcome of
incomplete clinical remission. The present study is important for
determining therapeutic strategies in the clinic. Further research
is required to replicate these results in other cohorts and to
compare their predictive ability with that of traditional factors,
including proteinuria, hypertension and the new Oxford
classification.
Acknowledgements
Not applicable.
Funding
This study was supported in part by the Basic
Research Project and Technology Development Projects of Shenzhen
Municipal Science and Technology Innovation Council (grant nos.
JCYJ20130329104904512, CXZZ20140421155346007 and
CXZZ20150601140615135) and the Research Project of the Health and
Family Planning Commission of Shenzhen Municipality (grant no.
SZFZ2018063).
Availability of data and materials
The datasets used and/or analyzed during the present
study available from the corresponding author on reasonable
request.
Authors' contributions
YH, QW, HS, HH and FT contributed to the study
conception and design, analyzed and interpreted the data and
drafted the manuscript. CC analyzed data and reviewed the
manuscript. HS and HH oversaw the project, contributed to the
discussion and reviewed the manuscript. YH and QW are the
guarantors of this work and, as such, had full access to all the
data in the study and take responsibility for the integrity of the
data and the accuracy of the data analysis. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Review Board and
Ethics Committee of Shenzhen Second People's Hospital (no.
20180110010; Shenzhen, China). Although all patients included in
this study were provided with the description of investigations,
the study was performed as a medical record-based retrospective
analysis and the included subjects were anonymized. Therefore, the
Review Board and Ethics Committee of Shenzhen Second People's
Hospital approved the exemption from obtaining written consent (no.
20180110010).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
D'Amico G: Natural history of idiopathic
IgA nephropathy and factors predictive of disease outcome. Semin
Nephrol. 24:179–196. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li LS and Liu ZH: Epidemiologic data of
renal diseases from a single unit in China: Analysis based on
13,519 renal biopsies. Kidney Int. 66:920–923. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Koyama A, Igarashi M and Kobayashi M:
Natural history and risk factors for immunoglobulin A nephropathy
in Japan. Research Group on Progressive Renal Diseases. Am J Kidney
Dis. 29:526–532. 1997.PubMed/NCBI View Article : Google Scholar
|
|
4
|
D'Amico G: Natural history of idiopathic
IgA nephropathy: Role of clinical and histological prognostic
factors. Am J Kidney Dis. 36:227–237. 2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Donadio JV and Grande JP: IgA nephropathy.
N Engl J Med. 347:738–748. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Collins AJ, Foley RN, Gilbertson DT and
Chen SC: United States Renal Data System public health surveillance
of chronic kidney disease and end-stage renal disease. Kidney Int
Suppl (2011). 5:2–7. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jin DC, Yun SR, Lee SW, Han SW, Kim W,
Park J and Kim YK: Lessons from 30 years' data of Korean end-stage
renal disease registry, 1985-2015. Kidney Res Clin Pract.
34:132–139. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ginzler EM, Dooley MA, Aranow C, Kim MY,
Buyon J, Merrill JT, Petri M, Gilkeson GS, Wallace DJ, Weisman MH
and Appel GB: Mycophenolate mofetil or intravenous cyclophosphamide
for lupus nephritis. N Engl J Med. 353:2219–2228. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rasche FM, Keller F, von Muller L, Sailer
LK, Karges W and Czock D: Mycophenolic acid therapy after
cyclophosphamide pulses in progressive IgA nephropathy. J Nephrol.
19:465–472. 2006.PubMed/NCBI
|
|
10
|
Nowack R, Birck R and van der Woude FJ:
Mycophenolate mofetil for systemic vasculitis and IgA nephropathy.
Lancet. 349(774)1997.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tang S, Leung JC, Chan LY, Lui YH, Tang
CS, Kan CH, Ho YW and Lai KN: Mycophenolate mofetil alleviates
persistent proteinuria in IgA nephropathy. Kidney Int. 68:802–812.
2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen X, Chen P, Cai G, Wu J, Cui Y, Zhang
Y, Liu S and Tang L: A randomized control trial of mycophenolate
mofetil treatment in severe IgA nephropathy. Zhonghua Yi Xue Za
Zhi. 82:796–801. 2002.PubMed/NCBI(In Chinese).
|
|
13
|
Langhoff E, Olgaard K and Ladefoged J: The
immunosuppressive potency in vitro of physiological and synthetic
steroids on lymphocyte cultures. Int J Immunopharmacol. 9:469–473.
1987.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wan QJ, Hu HF, He YC, Luan SD, Chen HT,
Liu HP, Li T, Xu Y, Xu HL and Liao Y: Severe pneumonia in
mycophenolate mofetil combined with low-dose
corticosteroids-treated patients with immunoglobulin A nephropathy.
Kaohsiung J Med Sci. 31:42–46. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Levey AS, Stevens LA, Schmid CH, Zhang YL,
Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene
T, et al: A new equation to estimate glomerular filtration rate.
Ann Intern Med. 150:604–612. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Working Group of the International IgA
Nephropathy Network and the Renal Pathology Society;. Cattran DC,
Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE,
Amore A, Barratt J, et al: The Oxford classification of IgA
nephropathy: Rationale, clinicopathological correlations, and
classification. Kidney Int. 76:534–545. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rauen T, Eitner F, Fitzner C, Sommerer C,
Zeier M, Otte B, Panzer U, Peters H, Benck U, Mertens PR, et al:
Intensive supportive care plus immunosuppression in IgA
nephropathy. N Engl J Med. 373:2225–2236. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Moresco RN, Speeckaert MM and Delanghe JR:
Diagnosis and monitoring of IgA nephropathy: The role of biomarkers
as an alternative to renal biopsy. Autoimmun Rev. 14:847–853.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Barbour SJ, Cattran DC, Kim SJ, Levin A,
Wald R, Hladunewich MA and Reich HN: Individuals of pacific Asian
origin with IgA nephropathy have an increased risk of progression
to end-stage renal disease. Kidney Int. 84:1017–1024.
2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Allison AC and Eugui EM: Mycophenolate
mofetil and its mechanisms of action. Immunopharmacology.
47:85–118. 2000.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Allison AC and Eugui EM: Purine metabolism
and immunosuppressive effects of mycophenolate mofetil (MMF). Clin
Transpl. 10:77–84. 1996.PubMed/NCBI
|
|
22
|
Rosselli JL, Thacker SM, Karpinski JP and
Petkewicz KA: Treatment of IgA nephropathy: An update. Nephrology.
45:1284–1296. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Huang Y, Liu Z, Huang H, Liu H and Li L:
Effects of mycophenolic acid on endothelial cells. Int
Immunopharmacol. 5:1029–1039. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rathi M, Goyal A, Jaryal A, Sharma A,
Gupta PK, Ramachandran R, Kumar V, Kohli HS, Sakhuja V, Jha V, et
al: Comparison of low-dose intravenous cyclophosphamide with oral
mycophenolate mofetil in the treatment of lupus nephritis. Kidney
Int. 89:235–242. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zheng XY, Wei RB, Tang L, Li P and Zheng
XD: Meta-analysis of combined therapy for adult hepatitis B
virus-associated glomerulonephritis. World J Gastroenterol.
18:821–832. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhu B, Chen N, Lin Y, Ren H, Zhang W, Wang
W, Pan X and Yu H: Mycophenolate mofetil in induction and
maintenance therapy of severe lupus nephritis: A meta-analysis of
randomized controlled trials. Nephrol Dial Transplant.
22:1933–1942. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ong LM, Hooi LS, Lim TO, Goh BL, Ahmad G,
Ghazalli R, Teo SM, Wong HS, Tan SY Shaariah W, et al: Randomized
controlled trial of pulse intravenous cyclophosphamide versus
mycophenolate mofetil in the induction therapy of proliferative
lupus nephritis. Nephrology. 10:504–510. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Laskari K, Mavragani CP, Tzioufas AG and
Moutsopoulos HM: Mycophenolate mofetil as maintenance therapy for
proliferative lupus nephritis: A longterm observational prospective
study. Arthritis Res Ther. 12(R208)2010.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Maes BD, Oyen R, Claes K, Evenepoel P,
Kuypers D, Vanwalleghem J, Van Damme B and Vanrenterghem YF:
Mycophenolate mofetil in IgA nephropathy: Results of a 3-year
prospective placebo-controlled randomized study. Kidney Int.
65:1842–1919. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Frisch G, Lin J, Rosenstock J, Markowitz
G, D'Agati V, Radhakrishnan J, Preddie D, Crew J, Valeri A and
Appel G: Mycophenolate mofetil (MMF) vs placebo in patients with
moderately advanced IgA nephropathy: A doubleblind randomized
controlled trial. Nephrol Dial Transplant. 20:2139–2145.
2005.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hogg RJ, Bay RC, Jennette JC, Sibley R,
Kumar S, Fervenza FC, Appel G, Cattran D, Fischer D, Hurley RM, et
al: Randomised controlled trial of mycophenolate mofetil in
children, adolescents, and adults with IgA nephropathy. Am J Kidney
Dis. 66:783–791. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tang SC, Tang AW, Wong SS, Leung JC, Ho YW
and Lai KN: Long-term study of mycophenolate mofetil treatment in
IgA nephropathy. Kidney Int. 77:543–549. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Roccatello D, Rossi D, Marletto F, Naretto
C, Sciascia S, Baldovino S, Piras D and Giachino O: Long term
effects of methylprednisolone pulses and mycophenolate mofetil in
IgA nephropathy patients at risk of progression. J Nephrol.
25:198–203. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu X, Dewei D, Sun S, Xu G, Liu H, He L
and Zhang P: Treatment of severe IgA nephropathy: Mycophenolate
mofetil/prednisone compared to cyclophosphamide/prednisone. Int J
Clin Pharmacol Ther. 52:95–102. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
35
|
Hou JH, Le WB Chen N, Wang WM, Liu ZS, Liu
D, Chen JH, Tian J, Fu P, Hu ZX, et al: Mycophenolate mofetil
combined with prednisone versus full-dose prednisone in IgA
nephropathy with active proliferative lesions: A randomized
controlled trial. Am J Kidney Dis. 69:788–795. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wyatt RJ and Julian BA: IgA nephropathy. N
Engl J Med. 368:2402–2414. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Maixnerova D, Bauerova L, Skibova J,
Rysava R, Reiterova J, Merta M, Honsova E and Tesar V: The
retrospective analysis of 343 Czech patients with IgA
nephropathy-one centre experience. Nephrol Dial Transplant.
27:1492–1498. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Reich HN, Troyanov S, Scholey JW and
Cattran DC: Toronto Glomerulonephritis Registry: Remission of
proteinuria improves prognosis in IgA nephropathy. J Am Soc
Nephrol. 18:3177–3183. 2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Alamartine E, Sauron C, Laurent B, Sury A,
Seffert A and Mariat C: The use of Oxford classification of IgA
nephropathy to predict renal survival. Clin J Am Soc Nephrol.
6:2384–2388. 2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Barbour SJ, Coppo R, Zhang H, Liu ZH,
Suzuki Y, Matsuzaki K, Katafuchi R, Er L, Espino-Hernandez G, Kim
SJ, et al: Evaluating a new international risk-prediction tool in
IgA nephropathy. JAMA Intern Med. 179:942–952. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chen T, Li X, Li Y, Xia E, Qin Y, Liang S,
Xu F, Liang D, Zeng C and Liu Z: Prediction and risk stratification
of kidney outcomes in IgA nephropathy. Am J Kidney Dis. 74:300–309.
2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Syrjänen J, Mustonen J and Pasternack A:
Hypertriglyceridaemia and hyperuricaemia are risk factors for
progression of IgA nephropathy. Nephrol Dial Transplant. 15:34–42.
2000.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shu D, Xu F, Su Z, Zhang J, Chen C, Zhang
J, Ding X, Lv Y, Lin H and Huang P: Risk factors of progressive IgA
nephropathy which progress to end stage renal disease within ten
years: A case-control study. BMC Nephrol. 18(11)2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lv J, Yang Y, Zhang H, Chen W, Pan X, Guo
Z, Wang C, Li S, Zhang J, Zhang J, et al: Prediction of outcomes in
crescentic IgA nephropathy in a multicenter cohort study. J Am Soc
Nephrol. 24:2118–2125. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ni Z, Yuan Y, Wang Q, Cao L, Che XJ, Zhang
MF, Xie YY, Qi CJ and Mou S: Time-averaged albumin predicts the
long-term prognosis of IgA nephropathy patients who achieved
remission. J Transl Med. 12(194)2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Haroun MK, Jaar BG, Hoffman SC, Comstock
GW, Klag MJ and Coresh J: Risk factors for chronic kidney disease:
A prospective study of 23,534 men and women in Washington County,
Maryland. J Am Soc Nephrol. 14:2934–2941. 2003.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zoppini G, Targher G, Chonchol M, Perrone
F, Lippi G and Muggeo M: Higher HDL cholesterol levels are
associated with a lower incidence of chronic kidney disease in
patients with type 2 diabetes. Nutr Metab Cardiovasc Dis.
19:580–586. 2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Herzenberg AM, Fogo AB, Reich HN, Troyanov
S, Bavbek N, Massat AE, Hunley TE, Hladunewich MA, Julian BA,
Fervenza FC, et al: Validation of the Oxford classification of IgA
nephropathy. Kidney Int. 80:310–317. 2011.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Liu XW, Li DM, Xu GS and Sun SR:
Comparison of the therapeutic effects of leflunomide and
mycophenolate mofetil in the treatment of immunoglobulin a
nephropathy manifesting with nephrotic syndrome. Int J Clin
Pharmacol Ther. 48:509–513. 2010.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Du B, Jia Y, Zhou W, Min X, Miao L and Cui
W: Efficacy and safety of mycophenolate mofetil in patients with
IgA nephropathy: An update meta-analysis. BMC Nephrol.
18(245)2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Trimarchi H, Barratt J, Cattran DC, Cook
HT, Coppo R, Haas M, Liu ZH, Roberts IS, Yuzawa Y, Zhang H, et al:
Oxford Classification of IgA nephropathy 2016-the role of
crescentic lesions: An update from the IgA Nephropathy
Classification Working Group. Kidney Int. 91:1014–1021.
2017.PubMed/NCBI View Article : Google Scholar
|