Introduction
Adenomyosis is an estrogen-dependent gynecological
condition that is characterized by pelvic pain, aberrant uterine
bleeding and infertility (1).
However, the clinical presentation of adenomyosis remains
ambiguous. Although magnetic resonance imaging has improved the
diagnostic accuracy of adenomyosis, this condition remains
asymptomatic with a prevalence of 21 to 33% in women of
reproductive age (2). Despite the
prevalence and severity of symptoms associated with adenomyosis,
little is known about the pathogenesis and etiology of this
condition. To date, two main theories have been proposed to explain
the origin of adenomyosis: i) Invagination of the endometrial
basalis as a result of the activation of tissue injury and repair
mechanism; and ii) metaplasia of displaced embryonic pluripotent
Mullerian remnants or differentiation of adult stem cells (3). At present, the main method for treating
symptomatic adenomyosis is to relieve symptoms and improve
fertility whilst minimizing side effects (4). Although there have been a number
randomized, double-blind clinical studies exploring the possible
pharmacological interventions for adenomyosis (5-7), no
such strategy is currently available for adenomyosis. In addition,
no specific guidelines exist for the optimal management of this
condition.
Similar to endometriosis, adenomyosis is closely
associated with inflammation and angiogenesis (8,9), to
which the prostaglandin cascade initiated by cyclooxygenases (COXs)
may serve an important role. There are two types of COXs, COX-1 and
COX-2, which can be differentially inhibited by a large number of
nonsteroidal anti-inflammatory drugs (NSAIDs). NSAIDs are
non-hormonal compounds that are commonly used as symptomatic
treatment for dysmenorrhea and heavy bleeding associated with
adenomyosis (10,11). Findings from recent studies have
suggested that NSAIDs are also capable of inhibiting angiogenesis,
proliferation, the expression of aromatase P450 and
epithelial-mesenchymal transition (EMT) in numerous cancer models,
such as colorectal cancer, prostate cancer and human glioblastoma
endothelial cells, highlighting their potentially novel properties
(12-14).
Additionally, the long-term use of NSAIDs has been proposed as a
therapeutic strategy for a number of malignancies, including
prostate, colon and breast cancer (15-18).
The NSAIDs that are currently available can be
classified into two main families: Non-selective NSAIDs (nsNSAIDs)
and selective COX-2 inhibitors (coxibs). According to their
half-lives, nsNSAIDs can be classified further into two groups:
Those with a short half-life (<6 h), including ibuprofen, and
those with longer half-lives, including naproxen (19). Ibuprofen and naproxen are commonly
used for dysmenorrhea in patients with adenomyosis. However, the
therapeutic application of nsNSAIDs is limited by gastrointestinal
and renal complications as a result of long-term COX-1 inhibition
(20). In contrast, coxibs
selectively act on the COX-2 isoenzyme which maintain the
anti-inflammatory properties of traditional NSAIDs without
effecting the activity of the COX-1 isoenzyme (21).
Therefore, the aim of the present study is to
examine the efficacy of celecoxib, a potent COX-2 inhibitor, on the
establishment of adenomyosis lesions in a murine model. In
addition, the extent of EMT, vascularization and estrogen
production in uterine tissue homogenates were evaluated.
Materials and methods
Preparation of animals
All experiments were performed under the guidelines
of the National Research Council's Guide for the Care and Use of
Laboratory Animals (22), and were
approved by the Institutional Experimental Animals Review Board of
Shanghai Obstetrics and Gynecology Hospital of Fudan University
(Shanghai, China). A total of 12, 19-day pregnant ICR mice,
weighing 40-50 g, were purchased from Laboratory Animal Center of
the Shanghai Institutes of Biological Sciences. Each dam and her
pups were housed in the same cage under a controlled environment
with 70-80% humidity at 22˚C with 12 h light/dark cycles and had
access to chow and freshwater ad libitum.
Mouse model of adenomyosis and animal
treatment
A total of 70 female neonatal mice were born from 12
pregnant ICR mice, where all the male neonatal progeny were
sacrificed by cervical dislocation. The neonates were randomly
divided into 7 groups: i) Control (n=10); ii) adenomyosis model
(ADE) (n=10); iii) low-dose celecoxib (celecoxib low) (n=10); iv)
high-dose celecoxib (celecoxib high) (n=10); v) naproxen (n=10);
vi) aspirin (n=10); and vii) ibuprofen (n=10). The mice in the
control group were dosed with the solvent [peanut
oil/lecithin/condensed milk mixture (10:1:15 v/v)] only, while
those in the ADE group were orally dosed with tamoxifen to induce
adenomyosis and the mice in the other groups were orally dosed with
tamoxifen and the corresponding drugs. Negative control group was
obtained from the control group which was incubated in PBS instead
of primary antibodies for the exclusion of non-specific
staining.
The female neonatal mice in the ADE and drug
treatment groups were treated orally with 1 mg/kg tamoxifen
(Shanghai Fudan Forward Pharmaceutical Co., Ltd.) suspended in a
peanut oil/lecithin/condensed milk mixture (10:1:15 v/v) at a dose
volume of 5 µl/g body weight, once daily on days 2 to 5 after birth
(day of birth=day 1), as reported previously (23). From day 6 after birth, mice were
administered orally with either a low dose of celecoxib (50 mg/kg;
Pfizer, Inc.), a high dose of celecoxib (100 mg/kg; Pfizer, Inc.),
naproxen (20 mg/kg; cat. no. N8280; Sigma-Aldrich; Merck KGaA),
aspirin (160 mg/kg; cat. no. A2093; Sigma-Aldrich; Merck KGaA) or
ibuprofen (30 mg/kg; cat. no. I4883; Sigma-Aldrich; Merck KGaA).
The mice in the control and ADE groups were orally administered
similarly with corresponding quantities of the vehicle. All mice
were weaned and separated from the dams upon reaching 3 weeks of
age, where the dams were sacrificed using cervical dislocation.
From aged 30 days, all mice were subjected to a hotplate test every
15 days until day 60. Uterine samples from the mice in the seven
groups aforementioned were harvested at 60 days of age.
Hotplate test procedure
The hotplate test was performed using a commercially
available Hot Plate Analgesia Meter (BME-480; Chinese Academy of
Medical Sciences) as described previously (24). The surface of the plate was heated to
and maintained at a constant temperature of 50.0±0.1˚C as measured
using a built-in digital thermometer. A plastic cylinder of 20 cm
in diameter and 18 cm in height was placed on the hotplate. All
mice were allowed to acclimatize under a controlled environment at
22-24˚C with 70-80% humidity in the testing room for 10 min prior
to test commencement. The latency response to thermal stimuli was
defined as the time (sec) elapsed from the moment the mouse was
placed inside the cylinder to the moment it licked its hind paws.
Each mouse was tested once per session. The latency was calculated
as the mean between two readings recorded between 24 h
intervals.
Specimen collection
After treatment for 60 days, all mice were
anaesthetized and perfused with warm (37˚C) saline followed by a
warm solution of 4% paraformaldehyde plus 0.2% picric acid
dissolved in 0.1 M phosphate buffer, which served as the fixative,
via the ascending aorta. The uteri were then excised, where half of
the samples were immediately fixed in 4% paraformaldehyde at room
temperature for 48 h and then embedded in paraffin for hematoxylin
and eosin (H&E)or immunohistochemical staining. All remaining
uterine tissue samples were placed immediately in liquid nitrogen
for subsequent determination of estrogen concentration in uterine
tissue homogenates.
Immunohistochemistry
Each paraffin-embedded tissue block was subjected to
serial 3 mm sectioning for H&E staining for the assessment of
lesion formation or subsequent immunohistochemical staining of
E-cadherin (cat. no. ab76055; 1:100; Abcam), N-cadherin (cat. no.
ab18203; 1:1,000; Abcam), CD31 (cat. no. ab28364; 1:50; Abcam),
aromatase P450 (cat. no. ab28146; 1:50; Abcam) or COX-2 (cat. no.
ab15191; 1:100; Abcam). Briefly, paraffin-embedded slides were
deparaffinized, rehydrated and washed in PBS (pH 7.4) three times
for 15 min each. Then the slices were subjected to antigen
retrieval with citric acid buffer (pH 6.0) at 98˚C for a total of
30 min. Next they were treated with 3% hydrogen peroxide (Beyotime
Institute of Biotechnology) and blocked with 10% goat serum
(Beyotime Institute of Biotechnology) for 1 h at room temperature
and incubated with the aforementioned primary antibodies or PBS
(negative group) overnight at 4˚C. After washing with PBS (pH 7.4)
three times for 15 min each, the sections were incubated with the
horseradish peroxidase-conjugated secondary antibody (cat. no.
ab6728; 1:2,000; Abcam) at room temperature for 60 min.
Subsequently, the sections were washed again with PBS and incubated
with 0.01% 3,3'-diaminobenzidine tetrahydrochloride (Beyotime
Institute of Biotechnology) for 2 min at room temperature. The
sections were then washed thoroughly in PBS three times for 5 min
each, stained in hematoxylin for 20 sec at room temperature,
dehydrated in an ascending absolute alcohol gradient, washed with
xylene and mounted in synthetic resin for microscopic examination
with a light microscope.
Quantification of immunoreactivity was performed
using Image Pro-Plus 6.0 (Media Cybernetics, Inc.) software, as
described previously (25). Vascular
microvessels were quantified with CD31, in the stromal compartment
of the uteri, where the CD31-labeled microvessel density was
quantified under x200 magnification. The average number was
calculated from 5 randomly selected images per tissue section, as
previously described (26).
Estradiol concentration in uterine
tissue homogenates
The mouse uterine tissues were homogenized in PBS
(pH 7.2-7.4) and centrifuged at 5,000 x g at 4˚C before
supernatants were collected. Estradiol (mouse) ELISA kit
(K3830-100, BioVision, Inc.) was used to determine the
concentration of estradiol in mouse uteri, according to
manufacturer's protocol. Each assay was performed in
triplicate.
Masson's trichrome staining
Masson's trichrome staining was used for the
detection of collagen fibers in tissue samples. Tissue sections
were deparaffinized in xylene, rehydrated in a descending graded
alcohol series and incubated in Bouin's solution at 37˚C for 2 h.
The Bouin's solution consisted of 75 ml saturated picric acid, 25
ml 10% formalin solution (v/v) and 5 ml acetic acid. Tissue
sections were then stained using the Masson's Trichrome Staining
kit (Baso Diagnostics, Inc.) according to manufacturer's protocols.
The areas of the collagen fiber layer stained in blue were obtained
by light microscope under a x200 magnification and quantified using
Image Pro-Plus 6.0 software (Media Cybernetics, Inc.).
Western blot analysis
The endometrial adenocarcinoma cell line Ishikawa
cells were purchased from the National Infrastructure of Cell Line
Resources and cultured at 37˚C in DMEM/F12 medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS. Ishikawa cells
were separately treated with DMSO, 1 µM β-Estradiol (cat. no.
E8875; Sigma-Aldrich; Merck KGaA), 0.1 µM β-Estradiol, 50 µM
celecoxib and 50 µM celecoxib +1 µM β-Estradiol at 37˚C for 48 h,
then cell proteins were extracted and western blot analysis was
performed as previously described (20). Primary antibodies used included
E-cadherin (cat. no. ab76055; 1:1,000; Abcam), anti-GAPDH (cat. no.
ab181602; 1:3,000; Abcam), pan-cytokeratin (cat. no. ab7753;
1:1,000; Abcam) and vimentin (cat. no. ab193555; 1:1,000; Abcam).
The secondary antibodies that were used included goat anti-rabbit
IgG (cat. no. ab6721; 1:1,000; Abcam) and goat anti-mouse IgG (cat.
no. ab6728; 1:1,000; Abcam). The densities of the bands were
quantified using Quantity One Analysis software v4.62 (Bio-Rad
Laboratories, Inc.) and normalized against those of GAPDH.
Statistical analysis
SPSS18.0 software (SPSS, Inc.) was used for all
statistical analyses. Comparisons of means from multiple groups
were performed using a one-way ANOVA followed by Dunnett's post hoc
test. Data is presented as the mean ± SEM. P<0.05 was considered
to indicate a statistically significant difference.
Results
Infiltration of myometrium by the
endometrium following celecoxib treatment
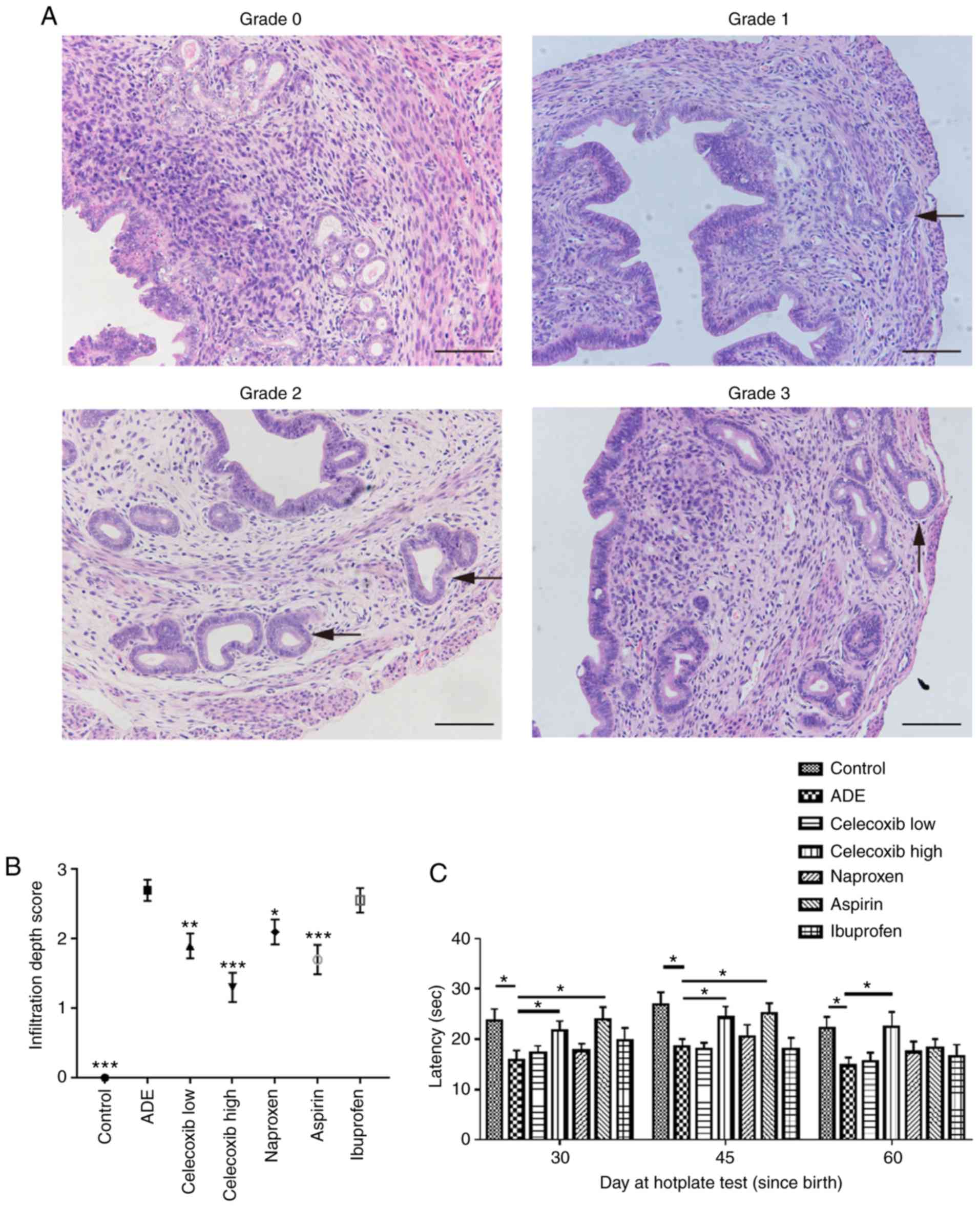
The depth of ectopic endometrial infiltration into
the myometrium was evaluated using the criteria previously
described by Bird et al (27): Grade 0, complete absence of any
ectopic endometrium in the myometrium; grade 1, penetration of the
ectopic endometrium into the superficial myometrium; grade 2,
penetration into the mid myometrium; and grade 3, penetration
beyond the mid myometrium (Fig. 1A;
representative images are from each group at 60 days post-birth to
demonstrate the detailed criteria that was used in the present
study). As demonstrated in Fig. 1B,
compared with the ADE group, mice in the control group exhibited
significantly decreased infiltration. Mice treated with either low
or high dose celecoxib, naproxen or aspirin demonstrated
significantly reduced myometrial infiltration compared with
untreated mice in the ADE group. There was no significant
difference in the depth of myometrium infiltration between mice
treated with ibuprofen and untreated mice from the ADE group.
Compared with mice treated with nsNSAIDs, naproxen, aspirin and
ibuprofen, mice treated with the selective COX-2 inhibitor
celecoxib exhibited less myometrium infiltration.
Effect of celecoxib treatment on
hotplate response latency following adenomyosis establishment
The hotplate test is a commonly used method for
measuring nociception and evaluating response threshold to thermal
stimuli in rodents (28). In the
present study, all mice were subjected to hotplate testing every 15
days from 30 days after birth (Fig.
1C). Hotplate response latency in mice from the ADE group was
significantly decreased compared with those in the control group.
On days 30 and 45 after birth, treatment with high-dose celecoxib
and aspirin significantly prolonged the response latency compared
with the ADE group. However, a significantly prolonged response
latency was not observed in the aspirin treatment group at 60 days
after birth (Fig. 1C).
Celecoxib treatment inhibits the
expression of COX-2 in the uterus following adenomyosis
establishment
Under physiological conditions, COX-1 is expressed
in almost all tissues and cells and it serves a protective role in
the gastrointestinal tract (29). In
contrast, COX-2 is not universally expressed in the majority of
normal tissues, but can be rapidly induced following stimulation by
proinflammatory factors, lipopolysaccharides or growth factors; in
turn leading to the occurrence and development of a variety of
diseases like atherogenesis, human macula densa and so on (30).
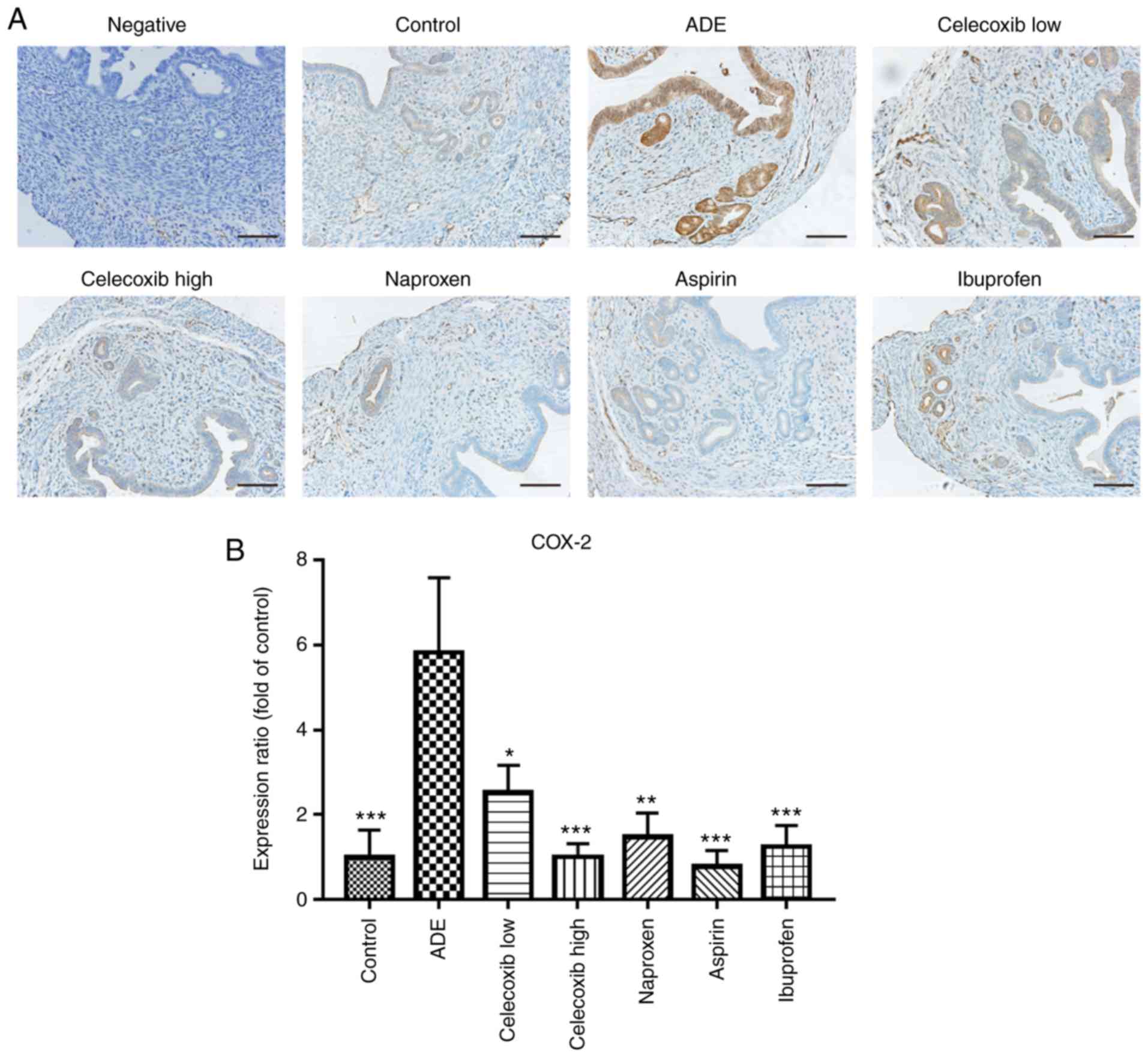
As reported by previous studies, COX-2 expression
increases significantly during the pathogenesis of adenomyosis
(31-33).
Therefore, immunohistochemical staining was used to assess the
expression of COX-2 following NSAIDs and celecoxib treatment. As
indicated in Fig. 2, compared with
the control group, mice in the ADE group exhibited significantly
increased COX-2 expression following adenomyosis induction.
Treatment with high and low dose celecoxib and nsNSAIDs
significantly reduced the expression of COX-2 compared with the ADE
group.
Celecoxib treatment reduces estrogen
production in the uterus following adenomyosis establishment
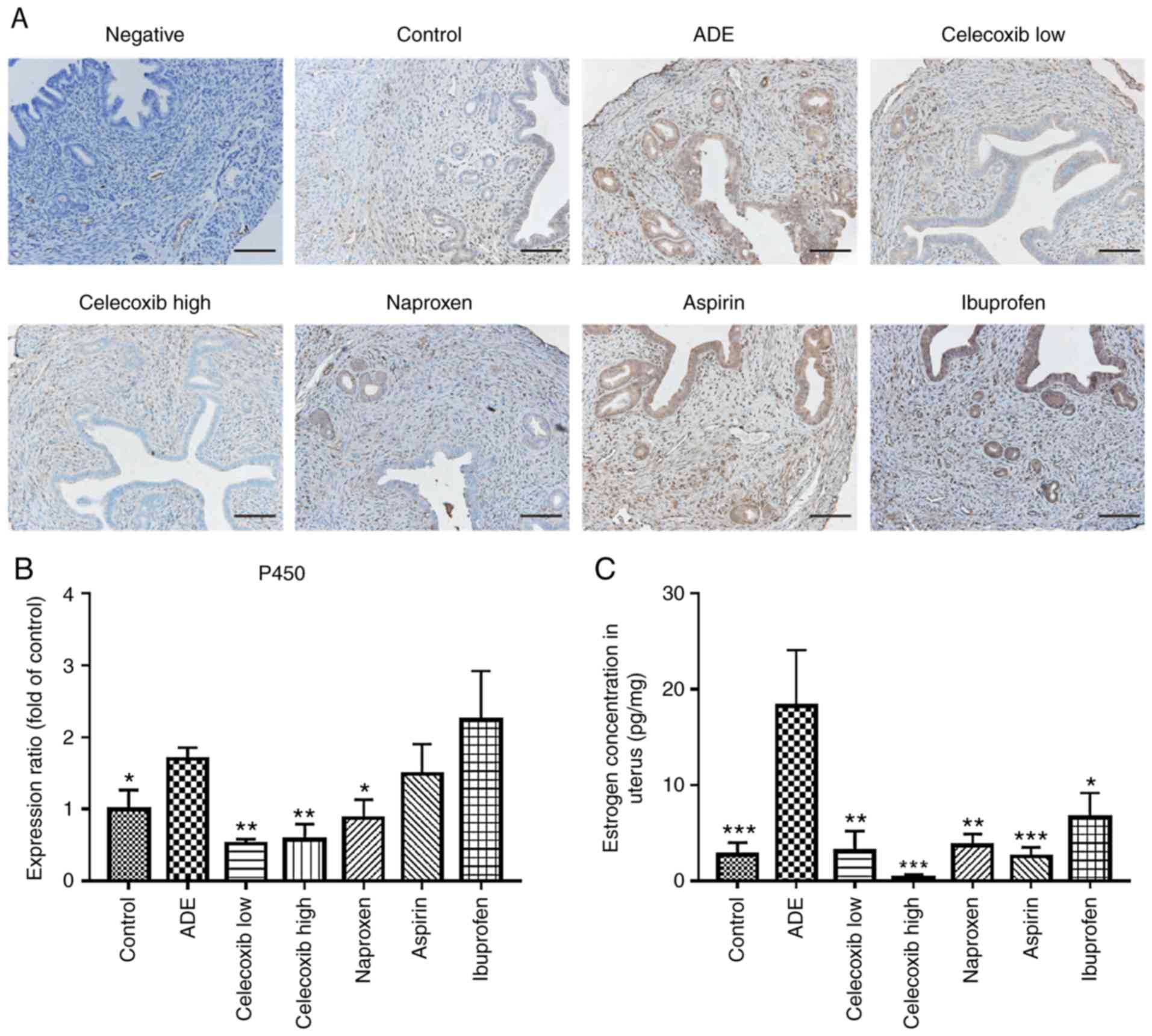
Aromatase P450 is considered to be the rate-limiting
enzyme during estrogen synthesis in the uterus (34). Therefore, in the present study the
expression of aromatase P450 was evaluated in uterine tissues. As
presented in Fig. 3A and B, aromatase P450 expression was
predominantly localized in the cytoplasm of glandular epithelial
cells in the eutopic endometrium and lesions within the myometrium.
The expression level of aromatase P450 in the uterus was
significantly higher in mice from the ADE group compared with the
control group. Following treatment with low-dose celecoxib,
high-dose celecoxib or naproxen, a significant decrease in
aromatase P450 was observed in the uterus compared with the
untreated ADE group. However, no significant difference was
observed following ibuprofen or aspirin treatment.
ELISA was subsequently performed to determine the
levels of estradiol (E2) in uterine tissue homogenates. The levels
of E2 in uterine tissue homogenates of untreated mice from the ADE
group were revealed to be significantly higher compared with the
control group (Fig. 3C). Following
celecoxib and nsNSAID treatment, the levels of E2 in the uterine
tissue homogenates were significantly lower compared with those of
untreated mice from the ADE group (Fig.
3C).
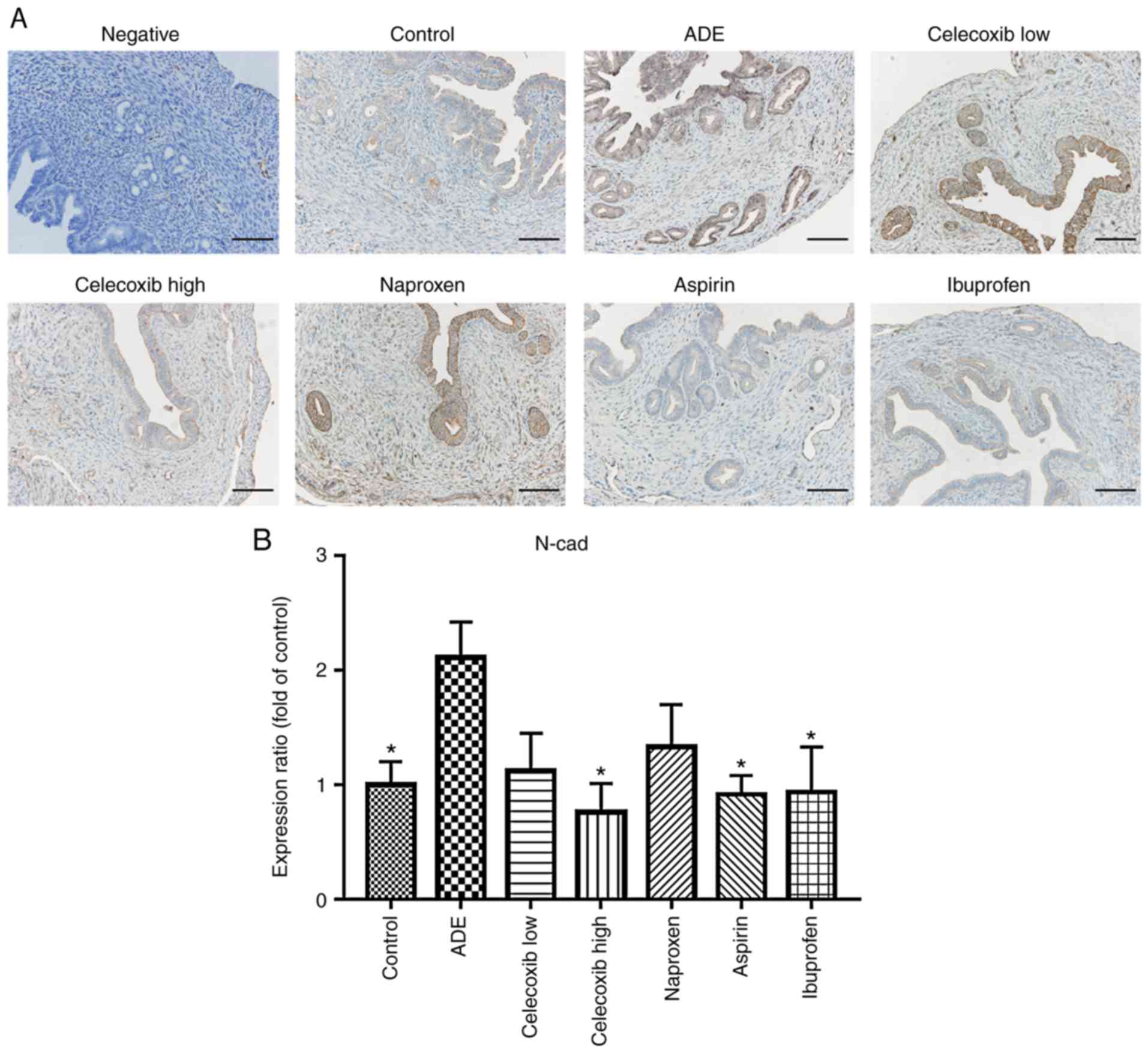
Celecoxib treatment reverses EMT
following the establishment of adenomyosis
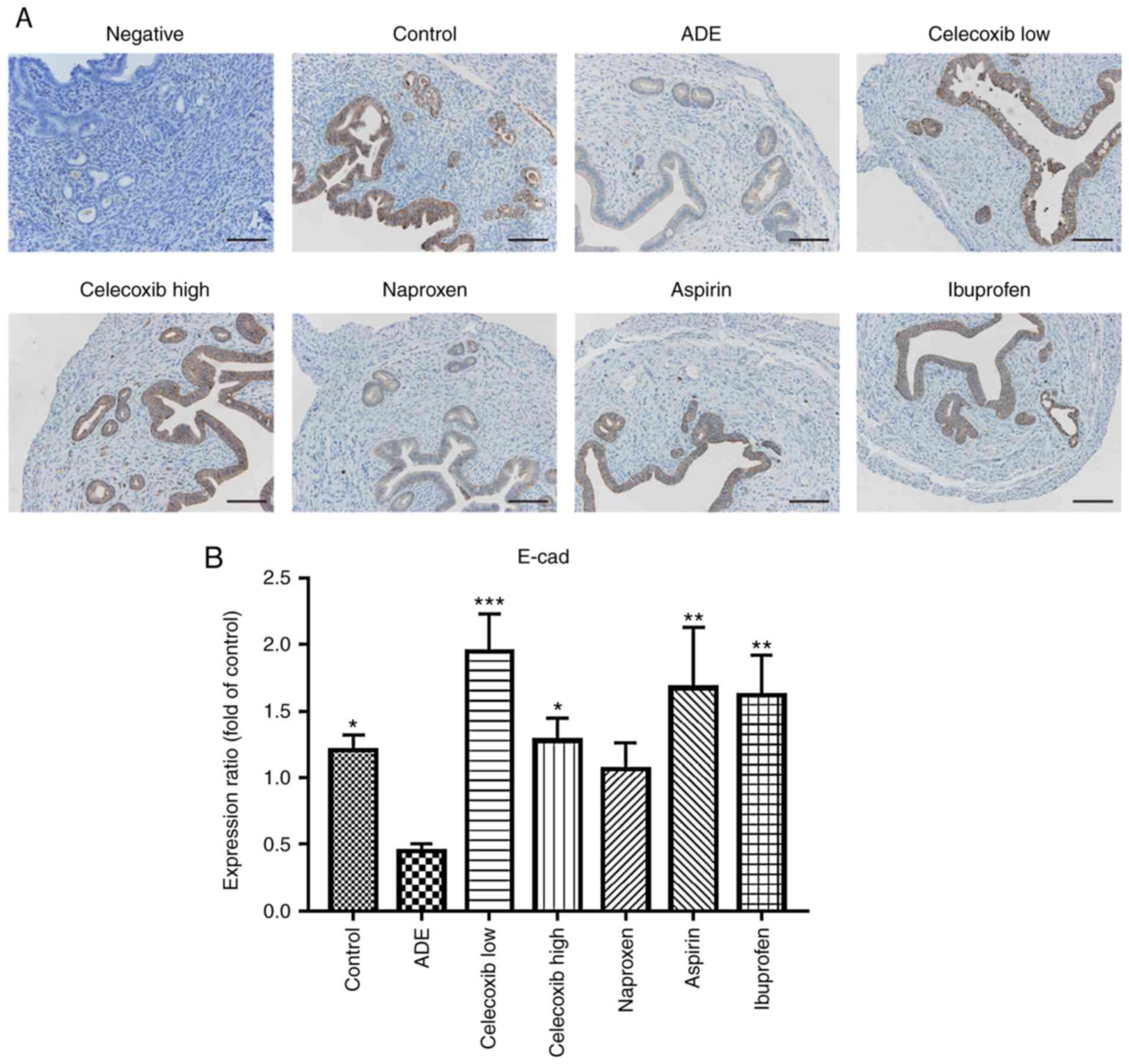
Immunohistochemistry was used to assess whether the
expression of N-cadherin and E-cadherin was affected by celecoxib
or NSAIDs treatment. As indicated in Fig. 4, E-cadherin was mainly expressed in
the membrane of epithelial cells, where its levels were
significantly reduced following adenomyosis induction. With the
exception of naproxen, treatment with ibuprofen, aspirin and
celecoxib significantly increased E-cadherin expression compared
with the ADE group. In contrast, the opposite effects were observed
regarding N-cadherin expression, which was significantly increased
following adenomyosis induction, but was reduced following
ibuprofen, aspirin or high celecoxib treatment, while low celecoxib
and naproxen didn't affect the N-cadherin expression significantly
(Fig. 5).
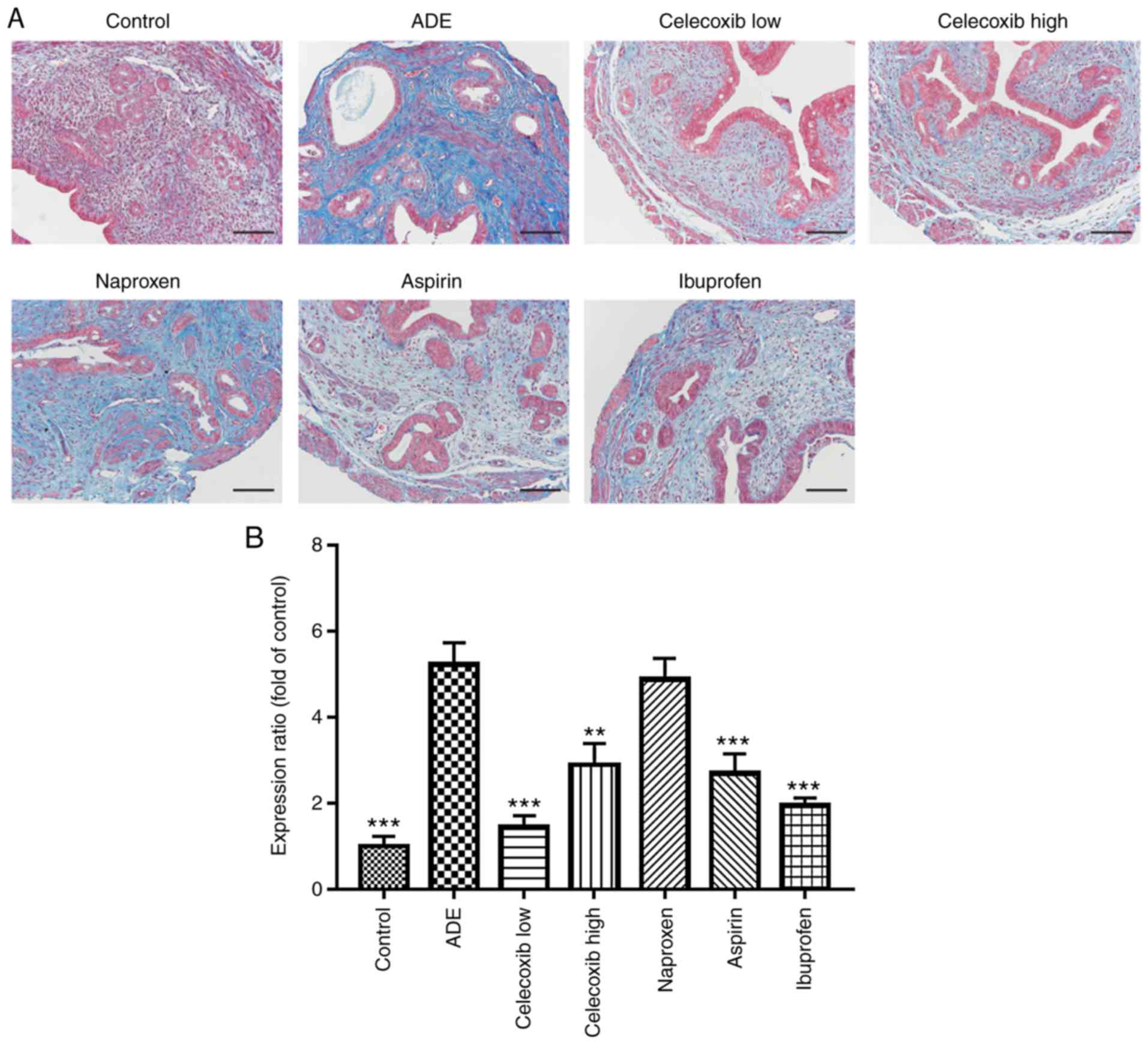
Progression of adenomyosis to fibrosis
is repressed by celecoxib treatment
Masson's trichrome staining was performed to
evaluate fibrosis in the mouse uterine tissues following treatments
with NSAIDs and celecoxib. Portions of the stromal cells, muscle
fibers or epithelial cells were stained red, whilst the collagen
fibers were stained blue.
Collagen staining was observed to be almost negative
in uterine tissues isolated from mice in the control group, whereas
advanced adenomyotic lesions obtained from mice from the ADE group
contained more collagen fibers (Fig.
6A and B). In addition, the
extent of fibrosis, as indicated by the expression of collagen, was
indicated to be significantly reduced by treatment with ibuprofen,
aspirin, low-dose and high-dose celecoxib compared with mice from
the ADE group (Fig. 6).
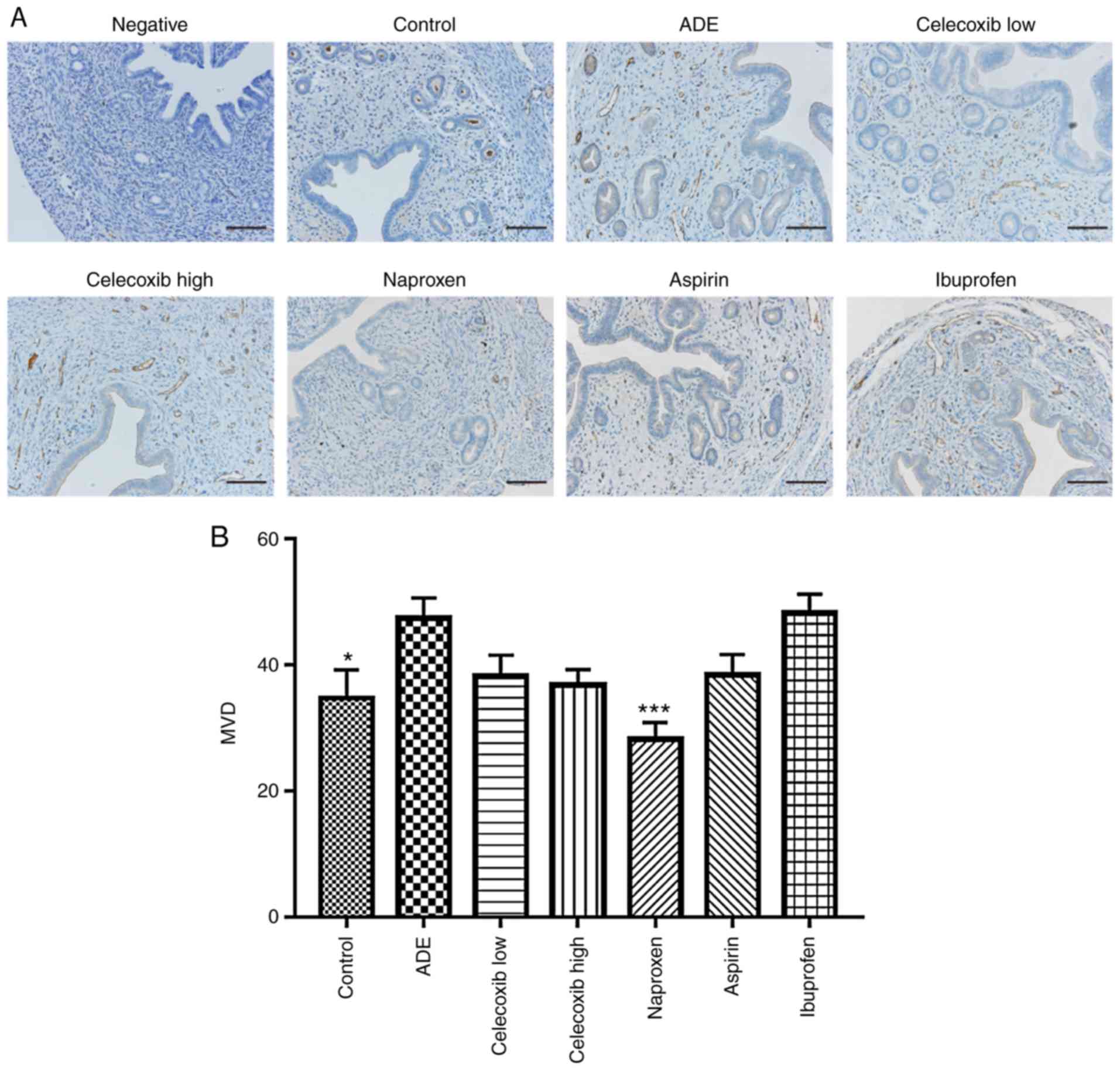
Effect of NSAIDs treatment on uterine
vascular density following adenomyosis establishment
To determine vascular density, blood vessels in the
uterine tissues were stained using the anti-CD31 antibody, where
the number of vessels per area was subsequently quantified
(Fig. 7). Compared with the control
group, there was a statistically significant increase in vessel
density detected in the ADE group. Although a statistically
significant reduction was observed in vessel density in the uterine
tissues following treatment with naproxen, no significant
differences were observed in the ibuprofen, aspirin, low-dose or
high-dose celecoxib-treated groups compared with the ADE group.
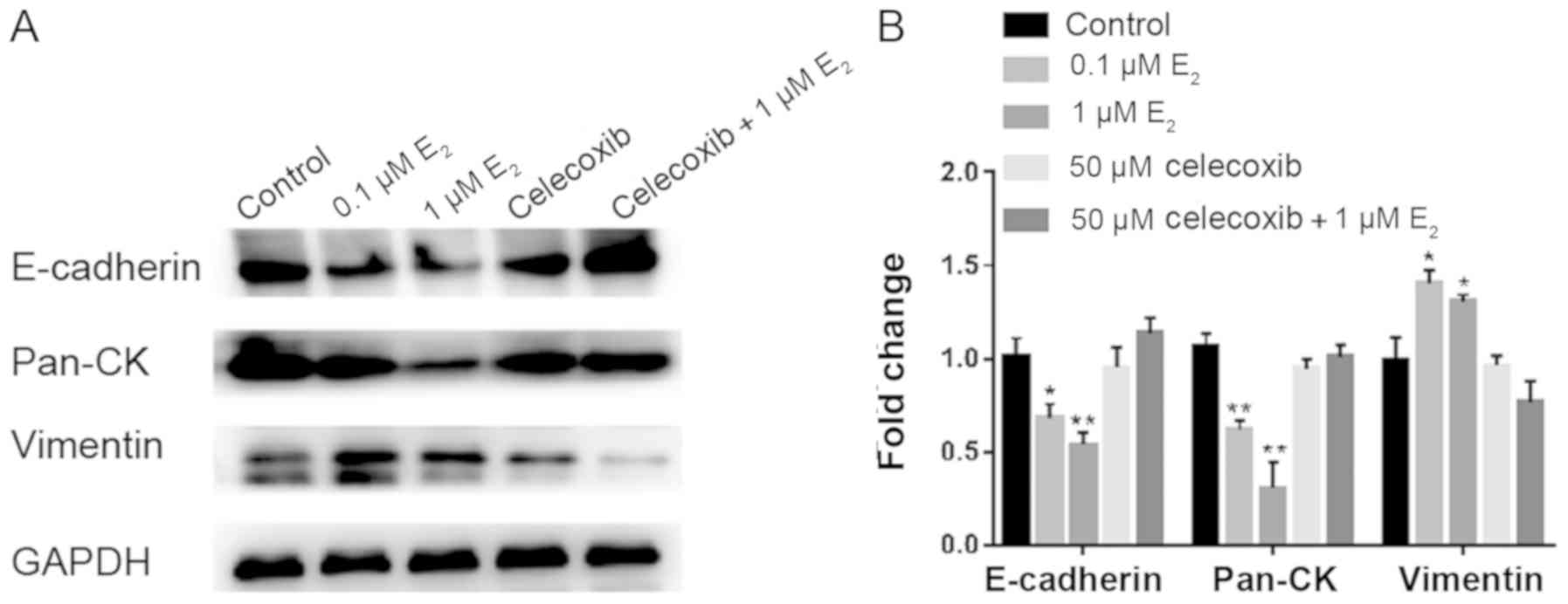
Celecoxib inhibits EMT in Ishikawa
cells following estradiol treatment
Previous reports have demonstrated that
E2 can induce EMT in endometrial epithelial cells during
adenomyosis (35-37).
To demonstrate if it is a direct effect of celecoxib on
epithelialization, Ishikawa cells were treated with DMSO, 0.1 µM
E2, 1 µM E2, 50 µM celecoxib or 50 µM
celecoxib+1 µM E2 for 48 h, following which western blot
analysis was performed to determine the expression of proteins
associated with EMT.
Following treatment with E2, the
expression of epithelial markers E-cadherin and pan-cytokeratin was
observed to be significantly downregulated in Ishikawa cells, which
was accompanied by the concomitant upregulation of the mesenchymal
marker vimentin (Fig. 8). Treatment
with Celecoxib reversed the aforementioned effects (Fig. 8).
Discussion
Adenomyosis is a major cause of pelvic pain and
infertility in women. Due to the lack of understanding in the
pathogenesis of adenomyosis, treatment options that are current
available remain inadequate, which mainly focus upon pain relief
and improving fertility (38). A
number of studies have previously reported that in addition to
containing anti-inflammatory and analgesic properties, NSAIDs also
serve important roles in a variety of biological functions
(39-41).
In the present study, the potential therapeutic effects of NSAIDs
were evaluated in a murine model of tamoxifen-induced adenomyosis,
and the results indicated that NSAIDs and the COX-2 selective
inhibitor celecoxib markedly reduced the depth of endometrial
infiltration into the myometrium, with celecoxib demonstrating the
greatest efficacy. In addition, high-dose celecoxib treatment
prolonged the thermal response latency, suggesting that COX-2
inhibition reduced pain sensitivity in mice with adenomyosis.
Steroid hormones are closely associated with the
etiology of adenomyosis. In particular, hyperestrogenism as a
result of aberrant local paracrine activity in the eutopic and
ectopic endometrium may serve as a preliminary risk factor for the
initiation of this disease (42).
This notion is supported by findings from a previous study that
elevated levels of E2 were frequently observed in the menstrual
blood of women with adenomyosis compared with peripheral blood
levels (43). Hyperestrogenism is
suggested to result from increased local aromatization of androgens
and reduced local estrogen metabolism in the eutopic and ectopic
endometrium (44). Notably, Kitawaki
et al (45) reported that the
expression aromatase cytochrome P450, which is a rate-limiting
enzyme in steroidogenesis, was not observed in the endometrium of
healthy uteri. In contrast, aromatase cytochrome P450 was present
in the eutopic endometrium of patients with adenomyosis, which
promoted estrogen biosynthesis and increased estrogen
bioavailability due to the local aromatization of circulating
androgens into E2.
In the present study, E2 and P450 expression levels
were increased in the uterus of mice in the ADE group. Following
celecoxib and naproxen treatment, the expression of P450 in the
uterus was significantly lower compared with untreated ADE mice,
which may explain the reduced E2 concentration in the corresponding
uterine tissue homogenates. Although the uterine levels of E2 were
reduced in the aspirin and ibuprofen group, the expression of P450
was not significantly decreased, suggesting that additional factors
may be involved, including the expression of 17-β hydroxysteroid
dehydrogenase type 2 enzyme, which has been previously revealed to
contribute to E2 degradation (46).
EMT is characterized by the loss of cell-cell
adhesions between the normally stationary polarized epithelial
cells, which transform into the highly motile mesenchymal cells. It
is a biological process involved in embryological development,
tissue repair and cancer cell migration and invasion (47). Consistent with previous studies
(48), reduced E-cadherin expression
and increased N-cadherin expression were detected in the epithelial
cells of adenomyotic lesions compared with normal endometrium in
the present study, suggesting that invasiveness and progression of
adenomyotic lesions could be regulated by EMT. Following celecoxib
treatment, the expression of E-cadherin in epithelial cells was
significantly increased, while that of N-cadherin was decreased.
The reversion of EMT was also observed in the nsNSAIDs-treated
groups, including aspirin and ibuprofen. Additionally, celecoxib
abrogated EMT in response to high concentrations of estrogen in
Ishikawa cells, suggesting that COX-2 inhibition may reverse EMT
during adenomyosis.
Tissue fibrosis is characterized by the accumulation
of extracellular matrix (ECM) and loss of cellular homeostasis
(49). In adenomyosis, each cyclic
bleed accompanied with vascular or tissue injury experienced by the
ectopic endometrium is followed by tissue repair (50). On tissue injury, evolutionarily
conserved mechanisms initiate the tissue repair process, which is
known to involve EMT (51). However,
with persistent cycles of tissue injury followed by repair, the
physiological wound-healing response can become aberrant, resulting
in excessive ECM deposition leading to fibrosis (52). In the present study, fibrosis was
also observed in the uteri of mice with ADE, which was reduced by
celecoxib treatment, suggesting an important role for COX-2 in
fibrotic progression during adenomyosis. Additionally, repression
of fibrosis was also observed in mice treated with nsNSAIDs,
including aspirin and ibuprofen.
To explore the uterine vascular responses to
celecoxib further, the present study also examined vessel density
and observed that celecoxib did not exert significant effects on
angiogenesis. The interpretation of this finding with regard to the
antiangiogenic effects of celecoxib in this model is limited. In
view of the angiogenesis effect of the increased expression or
activity of Cox-2, recently the inhibition of Cox-2 by celecoxib
has been explored to be used in the treatment of diverse cancers
such as breast cancer and hepatocellular carcinoma (18,53,54). So
further should be performed in larger sample populations or in
vitro to reveal the association between the inhibition of Cox-2
by celecoxib and angiogenesis, which may provide a more solid
theoretical basis for the application of celecoxib for the
treatment of adenomyosis.
In conclusion, nsNSAIDs and the COX-2 selective
inhibitor celecoxib markedly reduced the severity of adenomyosis in
the present study. In particular, celecoxib demonstrated the
greatest efficacy, which may serve as a promising therapeutic
intervention strategy due to previous reports of superior tolerance
in humans (54). The
celecoxib-mediated suppression of adenomyosis is likely to be
multifactorial, including estrogen production in the uterus,
fibrosis and EMT, which should enable the clinical use of celecoxib
to be extended beyond analgesia into adenomyosis. The present study
supports further investigation into the use of celecoxib as novel
non-hormonal, non-surgical alternative for the treatment of
adenomyosis.
Acknowledgements
The authors would like to thank Dr Zhu Zhiling
(Obstetrics and Gynecology Hospital of Fudan University, Shanghai,
China) for her technical support.
Funding
The present study was supported by funding from the
National Key R&D Program of China (grant no. 2016YFC1303100)
and the National Natural Science Foundation of China (grant nos.
31570803 and 81773090).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZJ performed the lab experiments, analyzed the data
and wrote the manuscript. XW contributed to the experimental
preparation. HL and CX contributed to the conception of the study
and critically revised the manuscript. CX supervised the research
project and gave final approval.
Ethics approval and consent to
participate
All experiments were performed under the guidelines
of the National Research Council's Guide for the Care and Use of
Laboratory Animals, and were approved by the Institutional
Experimental Animals Review Board of Shanghai Obstetrics and
Gynecology Hospital of Fudan University (Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
García-Solares J, Donnez J, Donnez O and
Dolmans MM: Pathogenesis of uterine adenomyosis: Invagination or
metaplasia? Fertil Steril. 109:371–379. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bazot M and Daraï E: Role of transvaginal
sonography and magnetic resonance imaging in the diagnosis of
uterine adenomyosis. Fertil Steril. 109:389–397. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Leyendecker G, Wildt L and Mall G: The
pathophysiology of endometriosis and adenomyosis: Tissue injury and
repair. Arch Gynecol Obstet. 280:529–538. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pontis A, D'Alterio MN, Pirarba S, de
Angelis C, Tinelli R and Angioni S: Adenomyosis: A systematic
review of medical treatment. Gynecol Endocrinol. 32:696–700.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Spitz IM: Clinical utility of progesterone
receptor modulators and their effect on the endometrium. Curr Opin
Obstet Gynecol. 21:318–24. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Badawy AM, Elnashar AM and Mosbah AA:
Aromatase inhibitors or gonadotropin-releasing hormone agonists for
the management of uterine adenomyosis: A randomized controlled
trial. Acta Obstet Gynecol Scand. 91:489–495. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fawzy M and Mesbah Y: Comparison of
dienogest versus triptorelin acetate in premenopausal women with
adenomyosis: A prospective clinical trial. Arch Gynecol Obstet.
292:1267–1271. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Vigano P, Corti L and Berlanda N: Beyond
infertility: Obstetrical and postpartum complications associated
with endometriosis and adenomyosis. Fertil Steril. 104:802–812.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Huang TS, Chen YJ, Chou TY, Chen CY, Li
HY, Huang BS, Tsai HW, Lan HY, Chang CH, Twu NF, et al:
Oestrogen-induced angiogenesis promotes adenomyosis by activating
the Slug-VEGF axis in endometrial epithelial cells. J Cell Mol Med.
18:1358–1371. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Marjoribanks J, Ayeleke RO, Farquhar C and
Proctor M: Nonsteroidal anti-inflammatory drugs for dysmenorrhoea.
Cochrane Database Syst Rev: CD001751, 2015.
|
|
11
|
Lethaby A, Duckitt K and Farquhar C:
Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding.
Cochrane Database Syst Rev: CD000400, 2013.
|
|
12
|
Navone SE, Guarnaccia L, Cordiglieri C,
Crisà FM, Caroli M, Locatelli M, Schisano L, Rampini P, Miozzo M,
La Verde N, et al: Aspirin affects tumor angiogenesis and
sensitizes human glioblastoma endothelial cells to temozolomide,
bevacizumab, and sunitinib, impairing vascular endothelial growth
factor-related signaling. World Neurosurg. 120:e380–e391.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang L, Li S, Li L, Chen Z and Yang Y:
COX2 inhibition in the endothelium induces glucose metabolism
normalization and impairs tumor progression. Mol Med Rep.
17:2937–2944. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hu W, Pei H, Sun F, Li P, Nie J, Li B, Hei
TK and Zhou G: Epithelial-mesenchymal transition in non-targeted
lung tissues of Kunming mice exposed to X-rays is suppressed by
celecoxib. J Radiat Res. 59:583–587. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gungor H, Ilhan N and Eroksuz H: The
effectiveness of cyclooxygenase-2 inhibitors and evaluation of
angiogenesis in the model of experimental colorectal cancer. Biomed
Pharmacother. 102:221–229. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sauer CM, Myran DT, Costentin CE, Zwisler
G, Safder T, Papatheodorou S and Mucci LA: Effect of long term
aspirin use on the incidence of prostate cancer: A systematic
review and meta-analysis. Crit Rev Oncol Hematol. 132:66–75.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gao F, Zafar MI, Jüttner S, Höcker M and
Wiedenmann B: Expression and molecular regulation of the Cox2 gene
in gastroenteropancreatic neuroendocrine tumors and
antiproliferation of nonsteroidal anti-inflammatory drugs (NSAIDs).
Med Sci Monit. 24:8125–8140. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li J, Hao Q, Cao W, Vadgama JV and Wu Y:
Celecoxib in breast cancer prevention and therapy. Cancer Manag
Res. 10:4653–4667. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Guo CG and Leung WK: Potential strategies
in the prevention of nonsteroidal anti-inflammatory
drugs-associated adverse effects in the lower gastrointestinal
tract. Gut Liver 2019 (Epub ahead of print).
|
|
20
|
Monteiro B and Steagall PV:
Antiinflammatory drugs. Vet Clin North Am Small Anim Pract.
49:993–1011. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Melcarne L, García-Iglesias P and Calvet
X: Management of NSAID-associated peptic ulcer disease. Expert Rev
Gastroenterol Hepatol. 10:723–733. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Granstrom DE: Agricultural (nonbiomedical)
animal research outside the laboratory: A review of guidelines for
institutional animal care and use committees. ILAR J. 44:206–210.
2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shen M, Liu X, Zhang H and Guo SW:
Transforming growth factor β1 signaling coincides with
epithelial-mesenchymal transition and fibroblast-to-myofibroblast
transdifferentiation in the development of adenomyosis in mice. Hum
Reprod. 31:355–369. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhu B, Chen Y, Zhang H, Liu X and Guo SW:
Resveratrol reduces myometrial infiltration, uterine hyperactivity,
and stress levels and alleviates generalized hyperalgesia in mice
with induced adenomyosis. Reprod Sci. 22:1336–1349. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jin Z, Wang L and Zhu Z: Effect of
GuiXiong Xiaoyi Wan in treatment of endometriosis on rats. Evid
Based Complement Alternat Med. 2015(208514)2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ding D, Liu X, Duan J and Guo SW:
Platelets are an unindicted culprit in the development of
endometriosis: Clinical and experimental evidence. Hum Reprod.
30:812–832. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bird CC, McElin TW and Manalo-Estrella P:
The elusive adenomyosis of the uterus-revisited. Am J Obstet
Gynecol. 112:583–593. 1972.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Plone MA, Emerich DF and Lindner MD:
Individual differences in the hotplate test and effects of
habituation on sensitivity to morphine. Pain. 66:265–270.
1996.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bjarnason I, Scarpignato C, Holmgren E,
Olszewski M, Rainsford KD and Lanas A: Mechanisms of damage to the
gastrointestinal tract from nonsteroidal anti-inflammatory drugs.
Gastroenterology. 154:500–514. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Parente L and Perretti M: Advances in the
pathophysiology of constitutive and inducible cyclooxygenases: Two
enzymes in the spotlight. Biochem Pharmacol. 65:153–159.
2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ota H, Igarashi S, Sasaki M and Tanaka T:
Distribution of cyclooxygenase-2 in eutopic and ectopic endometrium
in endometriosis and adenomyosis. Hum Reprod. 16:561–566.
2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang Y, Qu Y and Song W: Genetic variation
in COX-2-1195 and the risk of endometriosis and adenomyosis. Clin
Exp Obstet Gynecol. 42:168–172. 2015.PubMed/NCBI
|
|
33
|
Maia HJ Jr, Haddad C, Pinheiro N and Casoy
J: The effect of oral contraceptives on aromatase and Cox-2
expression in the endometrium of patients with idiopathic
menorrhagia or adenomyosis. Int J Womens Health. 5:293–299.
2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Manna PR, Molehin D and Ahmed AU:
Dysregulation of aromatase in breast, endometrial, and ovarian
cancers: An overview of therapeutic strategies. Prog Mol Biol
Transl Sci. 144:487–537. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen YJ, Li HY, Huang CH, Twu NF, Yen MS,
Wang PH, Chou TY, Liu YN, Chao KC and Yang MH: Oestrogen-induced
epithelial-mesenchymal transition of endometrial epithelial cells
contributes to the development of adenomyosis. J Pathol.
222:261–270. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Khan KN, Kitajima M, Hiraki K, Fujishita
A, Nakashima M and Masuzaki H: Involvement of hepatocyte growth
factor-induced epithelial-mesenchymal transition in human
adenomyosis. Biol Reprod. 92(35)2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bilyk O, Coatham M, Jewer M and Postovit
LM: Epithelial-to-mesenchymal transition in the female reproductive
tract: From normal functioning to disease pathology. Front Oncol.
7(145)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tsui KH, Lee WL, Chen CY, Sheu BC, Yen MS,
Chang TC and Wang PH: Medical treatment for adenomyosis and/or
adenomyoma. Taiwan J Obstet Gynecol. 53:459–465. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chamoun-Emanuelli AM, Bryan LK, Cohen ND,
Tetrault TL, Szule JA, Barhoumi R and Whitfield-Cargile CM: NSAIDs
disrupt intestinal homeostasis by suppressing macroautophagy in
intestinal epithelial cells. Sci Rep. 9(14534)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lands LC and Stanojevic S: Oral
non-steroidal anti-inflammatory drug therapy for lung disease in
cystic fibrosis. Cochrane Database Syst Rev.
9(CD001505)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Vallée A, Lecarpentier Y and Vallée JN:
Targeting the canonical WNT/β-catenin pathway in cancer treatment
using non-steroidal anti-inflammatory drugs. Cells.
8(E726)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Rižner TL: The important roles of steroid
sulfatase and sulfotransferases in gynecological diseases. Front
Pharmacol. 7(30)2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Urabe M, Yamamoto T, Kitawaki J, Honjo H
and Okada H: Estrogen biosynthesis in human uterine adenomyosis.
Acta Endocrinol (Copenh). 121:259–264. 1989.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Benagiano G and Brosens I: The endometrium
in adenomyosis. Womens Health (Lond). 8:301–312. 2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kitawaki J, Noguchi T, Amatsu T, Maeda K,
Tsukamoto K, Yamamoto T, Fushiki S, Osawa Y and Honjo H: Expression
of aromatase cytochrome P450 protein and messenger ribonucleic acid
in human endometriotic and adenomyotic tissues but not in normal
endometrium. Biol Reprod. 57:514–519. 1997.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kitawaki J, Koshiba H, Ishihara H, Kusuki
I, Tsukamoto K and Honjo H: Progesterone induction of
17beta-hydroxysteroid dehydrogenase type 2 during the secretory
phase occurs in the endometrium of estrogen-dependent benign
diseases but not in normal endometrium. J Clin Endocrinol Metab.
85:3292–3296. 2000.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Pastushenko I and Blanpain C: EMT
transition states during tumor progression and metastasis. Trends
Cell Biol. 29:212–226. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Liu X, Shen M, Qi Q, Zhang H and Guo SW:
Corroborating evidence for platelet-induced epithelial-mesenchymal
transition and fibroblast-to-myofibroblast transdifferentiation in
the development of adenomyosis. Hum Reprod. 31:734–749.
2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Manta L, Suciu N, Toader O, Purcărea RM,
Constantin A and Popa F: The etiopathogenesis of uterine
fibromatosis. J Med Life. 9:39–45. 2016.PubMed/NCBI
|
|
50
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Hinz B: Formation and function of the
myofibroblast during tissue repair. J Invest Dermatol. 127:526–537.
2007.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Thannickal VJ, Zhou Y, Gaggar A and Duncan
SR: Fibrosis: Ultimate and proximate causes. J Clin Invest.
124:4673–4677. 2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Tai Y, Zhang LH, Gao JH, Zhao C, Tong H,
Ye C, Huang ZY, Liu R and Tang CW: Suppressing growth and invasion
of human hepatocellular carcinoma cells by celecoxib through
inhibition of cyclooxygenase-2. Cancer Manag Res. 11:2831–2848.
2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Saxena P, Sharma PK and Purohit P: A
journey of celecoxib from pain to cancer. Prostaglandins Other
Lipid Mediat. 147(106379)2019.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|