Introduction
Venous malformation is one of the most common types
of congenital vascular dysplasia, mainly caused by venous system
stagnation at different stages during embryo development (1). The oropharynx represents the common
site for venous malformation in children, where not only the
appearance and function would be influenced, but also the
psychological development (due to the affected appearance and organ
dysfunction). Due to the complex and specific anatomical structure
and physiological function of the oropharynx, the inappropriate
choice or dosage of sclerosing agent may lead to tissue necrosis
and dysfunction, dysphagia, sleep apnea and respiratory obstruction
caused by lesion swelling. Therefore, the oropharyngeal venous
malformation is difficult to treat.
At present, interventional sclerotherapy is the
treatment recommended by the International Association of Veins
(2,3). Interventional sclerotherapy is
characterized by easy surgical procedures, limited trauma and
satisfactory curative effects (4).
In traditional sclerotherapies, sclerosing agents are usually
injected under digital subtraction angiography (DSA) guidance,
which can lead to complications such as local tissue necrosis,
ulceration and ectopic embolism (5).
The most commonly used interventional sclerosing agents include
pingyangmycin, bleomycin, polyglycerol, polidocanol and ethanol
(3). Sclerosing agents may cause
local vascular endothelium damage, which may be followed by
thrombosis, endothelial exfoliation, collagen fiber shrinkage and
blood vessel occlusion. However, there is still disagreement on the
application of sclerosing agents for venous malformation (6-8).
Clinicians generally opt for different sclerosing
agents with varying dosages based on experience. The efficacy and
adverse reactions to the sclerosing agents still need to be fully
elucidated (7-10).
This is of importance for the improvement of treatment efficacy for
oropharyngeal venous malformation in children, by reducing
postoperative adverse reactions and improving quality of life. In
the present study, the efficacy and safety of DSA-guided
polidocanol foam and the combination of bleomycin A5 and
dexamethasone in the treatment of oropharyngeal low-flow venous
malformation in children were investigated and analyzed. Both
pingyangmycin and polidocanol have effects, with different
efficacies and complications. The present study aimed to determine
an effective treatment plan with lesser side effects for
oropharyngeal venous malformation in children.
Materials and methods
Study subjects
A total of 27 children with oropharyngeal low-flow
venous malformation who were admitted to Qilu Children's Hospital
of Shandong University from January 2016 to June 2017 were included
in this study. The subjects comprised 11 males and 16 females, with
an average age of 2.5±0.7 years, and the age of onset ranged
between 4 months and 7 years old. The diagnostic criteria were
consistent with the treatment guidelines for Oral and Maxillofacial
Hemangioma and Vascular Malformations (11). Inclusion criteria for the study were
as follows: i) Children with complete data and follow-up records;
ii) children receiving no previous interventional sclerotherapy;
iii) according to clinical history, physical examination and
imaging data, cases diagnosed by direct puncturing diagnosis under
DSA fluoroscopy, with angiographic results indicating low-flow
venous malformation, with a slender reflux vein and slow reflux
speed, in which there was still obvious contrast agent residue in
the tumor after 5 mins of angiography (12); and iv) cases having normal liver and
kidney function, without sepsis, coagulopathy or cardiopulmonary
insufficiency, nor a history of allergies for iodine angiography
and anhydrous ethanol. The exclusion criteria were as follows: i)
Cases with incomplete data; ii) cases with lesions that had been
previously treated with sclerotherapy; iii) cases with high-flow
venous malformations; and iv) cases with other vascular diseases
such as arteriovenous malformations and lymphatic malformations.
The present study was approved by the ethics committee of Qilu
Hospital of Shandong University. The guardians of all parents
provided written informed consents and were informed of the
possible risks and complications of interventional sclerotherapy
for oropharyngeal venous malformation. All 27 children underwent an
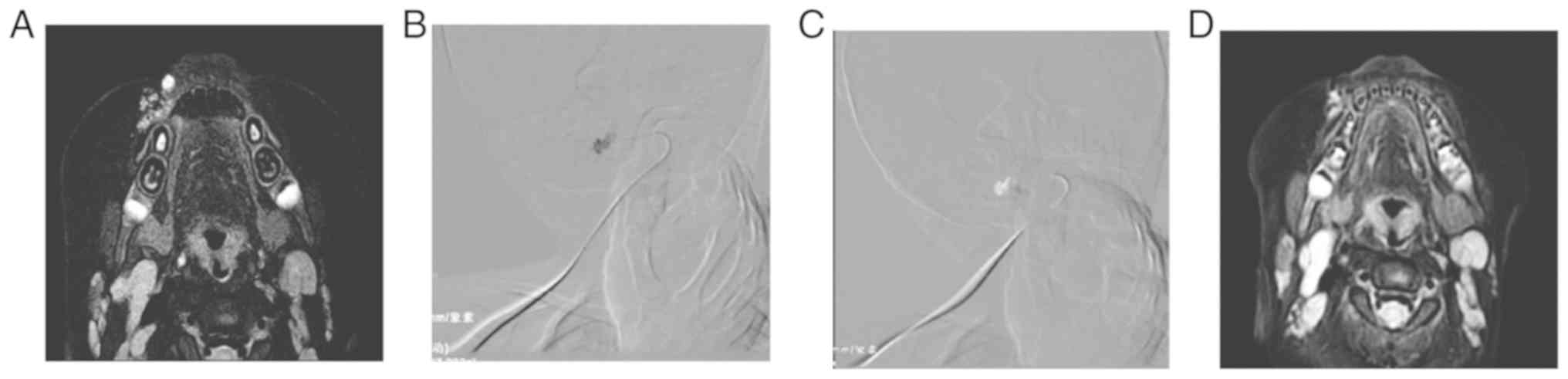
MRI examination, which showed round or irregular long T1 and T2
signals, with clear boundaries. Enhanced scanning showed varying
degrees of enhancement. The ultrasound examination showed uneven
echoes within the lesions, with a tubular echo signal inside. Study
subjects were randomly divided into the following groups: Group A,
which included 13 cases, with 16 lesions, subjected to treatment
with polidocanol foam sclerosing agent; and Group B, which included
14 cases with 19 lesions, subjected to the combination treatment of
pingyangmycin + dexamethasone (Table
I). Among the patients, the smallest lesion size was
0.5x0.5x1.2 cm, while the largest lesion size was 3.1x2.7x3.8 cm.
All subjects were unaware of the grouping process.
 | Table IClinical data of included cases. |
Table I
Clinical data of included cases.
| Characteristics | Group A (n=13) | Group B (n=14) |
|---|
| Sex, n | | |
|
Males | 5 | 6 |
|
Females | 8 | 8 |
| Age at initiation of
treatment, years | 2.7±1.2 | 2.1±5.7 |
| Location of IH,
n | | |
|
Lip | 4 | 3 |
|
Pharynx | 2 | 2 |
|
Tongue | 5 | 6 |
|
Multiple | 2 | 3 |
| Average no. of
treatments, frequency | 2.45±0.6 | 2.07±0.4 |
Preparation of sclerosing agents
Polidocanol foam sclerosing agent was prepared
according to the Tessari method (13). Two 2.5-ml screw syringes were briefly
connected with a three-way valve. A total of 0.5 ml polidocanol
(3%; Chemische Fabrik Kreussler & Co. GmbH) in one syringe was
mixed with 2 ml CO2 in another syringe by pumping the
syringes 20 times. The valve was switched down as much as possible,
and the syringes were rapidly pumped another 10 times to obtain the
foam agent. A volume of <10 ml of 3% polidocanol was injected
each time. For the preparation of pingyangmycin + dexamethasone, 8
mg pingyangmycin (Jilin Aodong Pharmaceutical Group Co., Ltd.) was
dissolved in 4 ml contrast agent (Iodixanol injection; Beijing
Beilu Pharmaceutical Co., Ltd.). The dosage was determined
according to the body surface area (10 mg/m2), which was
subsequently mixed with 1-2 mg dexamethasone.
Treatment methods
Sclerotherapy was performed using DSA equipment
(Artis zee; Siemens Healthineers). Prior to surgery, the lesions
were marked on the surface according to the results of the MRI
examination. Following general anesthesia, the child was placed in
a relaxed position. The skin of the lesion area was routinely
disinfected. A 4.5-scalp needle was used to puncture the lesion,
and the injection depth was determined based on the MRI imaging. A
venous malformation that could be observed under radiography was
defined as a successful puncture. Local angiography was performed
under DSA fluoroscopy. The shape, extent, and venous drainage of
the tumor nests were determined. For Group A, 3% polidocanol foam
was injected under the path diagram mode. The sclerosing agent was
shown as a negative shadow, until the reflux vein was filled with
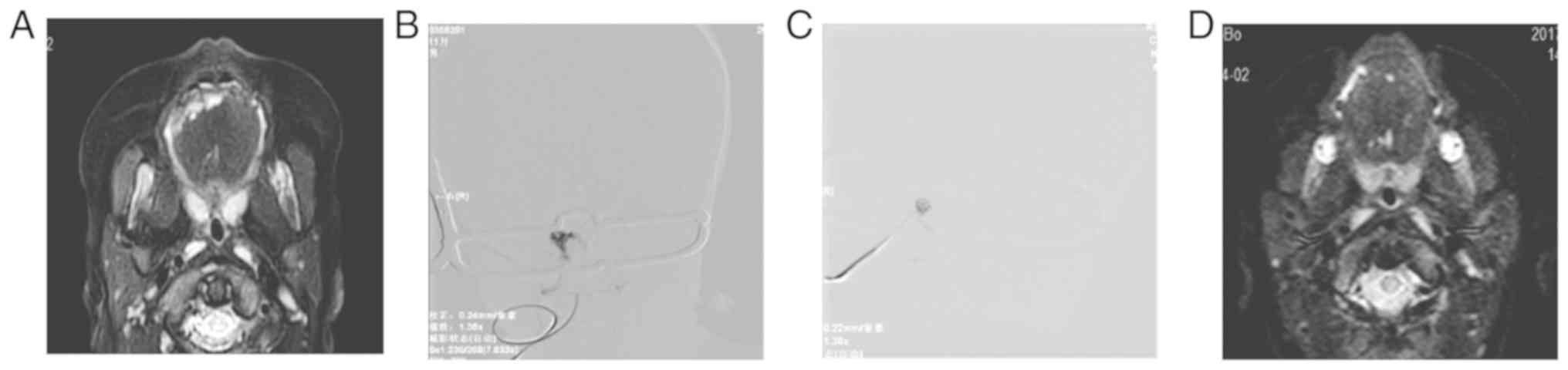
the sclerosing agent. For Group B, DSA fluoroscopy indicated that
the pingyangmycin dilution was directly injected into the venous
malformation vascular mass. The original contrast in the tumor nest
was displaced, and the injection was stopped when the tumor nest
was filled or the venous drainage was observed. Thereafter,
multi-point and multi-angle puncture and angiography were performed
in the lesion area to determine whether there were any residual or
new lesions. Subsequently, the treatment was continued according to
the above protocol. Following treatment, routine DSA fluoroscopy
was performed to confirm the sclerosing agent within the lesion
without subcutaneous exudation or abnormal reflux. The patients
were then carefully observed. For cases with disappearing or
partial remission of clinical symptoms, but with the MRI
examination showing a residual lesion >10%, or those suffering
from recurrence following stabilized symptoms, the interventional
therapy was repeated (with time intervals of >1 month).
Treatment endpoints were as follows: i) When clinical symptoms
disappeared, and the imaging examination showed residual lesions of
<10%; ii) cases reporting no symptom relief or even aggravation,
after three sequential treatments; and iii) parents who ended
treatment.
Evaluation of drug efficacy and
adverse reaction to drugs
Post-operative adverse reactions including fever,
skin swelling, ulceration, digestive tract reaction, hemorrhage,
and abnormal function of surrounding tissues and organs were
observed. Follow-up observation was performed at 1, 3, 6 and 12
months after surgery. Therapeutic efficacy was evaluated based on
clinical symptoms and MRI examination. The efficacy criteria were
as follows (14): i) Cured, the
symptoms completely disappeared after interventional treatment,
with normal surface color and without recurrence; ii) basic
remission, the lesion generally disappeared (reduced by >80%),
with no dysfunction, mild skin pigmentation, and further treatment
needed; iii) effective (improved situation), the tumor was
significantly reduced (to <80%), and further treatment would be
needed and iv) invalidation, the tumor was not reduced, remained
unchanged or continued to develop. The therapeutic efficiency was
calculated according to the following formula: Efficiency=(cured
cases + basic remission cases + effective cases)/total cases x100.
Minor complications mainly included self-limiting symptoms
requiring no clinical intervention, and permanent residual
functional damage after clinical treatment such as fever, local
swelling, skin ulceration and pain. Major complications included
permanent nerve damage, extensive tissue necrosis, cerebral
embolism and death.
Statistical analysis
Statistical analysis was performed using SPSS 18.0
software (SPSS, Inc.). The count data was compared using the
χ2 test, while the measurement data were analyzed
with a Student's t-test. Data are presented as the mean ± SD.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Analysis of number of injections of
sclerosing agents
The number of injections of sclerosing agents were
analyzed and compared between Groups A and B. The results showed
that Group A subjects (polydoxan foam sclerotherapy), who underwent
treatment 2-3 times, had an average number of treatments of
2.45±0.6. Group B subjects (pingyangmycin and dexamethasone
combination), underwent treatment 1-3 times, with an average number
of treatments of 2.07±0.4. No significant difference was observed
in the number of injections between these two groups (P>0.05;
Table II).
 | Table IIAnalysis of number of treatments
between Groups A and B. |
Table II
Analysis of number of treatments
between Groups A and B.
| Characteristics | Group A (n=16) | Group B (n=19) | t | P-value |
|---|
| Number of treatment
times (range) | 2-3 | 1-3 | 10.985 | 0.072 |
| Average number of
treatment times, X±SD | 2.45±0.6 | 2.07±0.4 | | |
Analysis of therapeutic efficacy
The therapeutic efficacies of the drugs were
analyzed and compared between Groups A and B. The results showed
that the efficacy rate of Group A was 87.50%, which was not
significantly declined in Group B (84.21%; Table III; Figs. 1 and 2). The results suggested that both methods
resulted in the satisfactory treatment of children with
oropharyngeal low-flow venous malformation.
 | Table IIIAnalysis of therapeutic efficacy
between Groups A and B. |
Table III
Analysis of therapeutic efficacy
between Groups A and B.
| Therapeutic
efficacy | Group A (n=16)
(%) | Group B (n=19)
(%) | χ2 | P-value |
|---|
| Cured | 7 (43.75) | 9 (47.37) | 2.132 | 0.242a |
| Basic remission | 5 (31.25) | 4 (21.05) | | |
| Effective | 2 (12.50) | 2 (10.52) | | |
| Invalidation | 2 (12.50) | 3 (15.79) | | |
| Effective rate | 87.50 | 84.21 | | |
Analysis of postoperative adverse
reactions
Postoperative adverse reactions were analyzed and
compared between Groups A and B. The results showed that one fever
case, three cases of swelling in the lesion area and one case of
necrosis with rupture were found in Group A subjects, with an
adverse reaction incidence of 38.46%. One case of fever and one
case of vomiting was found in Group B subjects, with an adverse
reaction incidence of 14.29%. A significant difference was measured
in the adverse reaction incidence between these groups (P<0.05;
Table IV). These results suggested
that the combination of pingyangmycin and dexamethasone is safer
for the treatment of children with oropharyngeal low-flow venous
malformation.
 | Table IVAnalysis of postoperative adverse
reactions between Groups A and B. |
Table IV
Analysis of postoperative adverse
reactions between Groups A and B.
| Complication | Group A (n=16)
(%) | Group B (n=19)
(%) | χ2 | P-value |
|---|
| Fever | 1 (7.69) | 1 (7.14) | 5.286 |
<0.001a |
| Pain | 0 (0) | 0 (0) | | |
| Digestive tract
reaction | 0 (0) | 1 (7.14) | | |
| Local swelling | 3 (23.08) | 0 (0) | | |
|
Necrosis/ulceration | 1 (7.69) | 0 (0) | | |
| Adverse reaction
rate | 5 (38.46) | 2 (14.29) | | |
Discussion
Venous malformation mainly results from abnormal
development of the embryonic vascular plexus, rather than abnormal
proliferation of vascular endothelial cells (15). Direct puncture venography represents
the gold standard for the diagnosis of venous malformation, which
could contribute to the evaluation of morphological and blood flow
characteristics (16). According to
puncture angiography, venous malformation can be divided into low-
and high-flow types, based on the thickness, number and flow speed
of the reflux veins (16-18).
At present, sclerotherapy has become the first choice for the
treatment of venous malformation, especially under DSA guidance
(19). Sclerotherapy can clearly
define the size, extent and drainage of the lesions, and reduce the
extravasation of sclerosing agents, reducing the number of
treatments required as well as the ensuing complications (20). Polidocanol is widely used in Europe
and the United States, although its clinical application is still
in its infancy in China (7).
Polidocanol has certain anesthetic effects, causing less pain and
better pain tolerance (21). The
main side effects of polidoclanol treatment include liver and
kidney function damage, ulcer, necrosis, fever, dizziness, chest
tightness, nausea and visual impairment (21). Cabrera et al (22) used 0.5-3% polidocanol foam sclerosing
agent under ultrasound guidance to treat venous malformations, with
an efficacy rate of 92%, and no serious complications were
observed. Foam sclerosing agents have been gradually developed for
the sclerotherapy for venous malformations (10). The effects of pingyangmycin in the
treatment of venous malformation are based on the fact that it may
damage and destroy the vascular endothelial cells (by inducing cell
degeneration and atrophy), leading to the regression of venous
malformation (23). Since
pingyangmycin injection treatment does not cause damage to
peripheral nerves, blood vessels or other tissue structures, it is
suitable for the treatment of oropharyngeal venous malformations,
especially for children (24). The
main adverse reactions to pingyangmycin treatment include fever,
gastrointestinal reactions, pulmonary fibrosis, local pain and
stomatitis (25). Dexamethasone has
an inhibitory effect on the angiogenesis of vascular endothelial
cells and anti-inflammatory effects, which can reduce adverse
reactions such as fever (2). The
combination of dexamethasone and pingyangmycin has been shown to
not only improve the curative effect and shorten treatment
duration, but also significantly reduce local swelling and fever
and prevent the release of excessive heat in the body, caused by
pingyangmycin and related allergic reactions (26).
The results of the present study showed that there
was no significant difference in the number of treatments and
therapeutic efficiency between polidocanol treatment and the
combination treatment of dexamethasone and pingyangmycin. The
efficacy rate for polidocanol foam sclerosing agent was at 87.50%,
with an average number of treatments of 2.45±0.6. Moreover, 3%
polidocanol foam sclerosing agent was used to treat low-flow venous
malformation. The foam agent demonstrates unique adhesiveness and
compactness and is injected into the vein to form a mass. This
prevents blood from diluting the drug, enlarges the contact area
with the vascular endothelium, prolongs contact time and improves
hardening efficiency (27).
Moreover, the curative effect was clear, with reduced treatment
numbers, thereby effectively reducing the risk of adverse reactions
(28-30).
In the present study, 19 lesions were treated with a pingyangmycin
and dexamethasone combination. The results showed that the efficacy
rate for the combination treatment was 84.21%, with an average
number of treatments of 2.07±0.4, indicating satisfactory
therapeutic efficiency, consistent with previous findings (31). The main adverse reactions in these
two groups included fever, digestive tract reaction, local soft
tissue swelling and ulceration. No patients had life-threatening
complications such as cardiopulmonary and cerebrovascular
accidents. The adverse reaction incidences for Groups A and B were
38.46% and 14.29%, respectively, suggesting that the therapeutic
efficacy of pingyangmycin and dexamethasone combination was better
compared with polidocanol treatment. The postoperative adverse
reaction incidence for polidocanol foam sclerosing agent was
38.46%, including fever, local soft tissue swelling and ulceration.
In three cases, swollen lesions were observed, which generally
disappeared 2-5 days after surgery. After injection with
polidocanol foam sclerosing agent, the drug may accumulate to
induce local necrotic ulcers. In the present study, one case
reported local tissue ulceration after polidocanol foam sclerosing
agent injection, which was cured by local anti-infection treatment.
In addition, among the 14 patients subjected to the combination
treatment of pingyangmycin and dexamethasone, two of the cases
displayed adverse reactions (fever and digestive tract reactions),
with an incidence rate of 14.29%, which returned to normal after
treatment. Compared with the treatment of polidocanol foam
sclerosing agent, the adverse reactions to the combination
treatment were significantly reduced, making the combination
treatment a safe and reliable treatment method, with few adverse
reactions, as well as satisfactory appearance and functional
recovery.
The current study had several limitations. Firstly,
the sample size of children with low-flow oropharyngeal venous
malformation was limited. The sample size should be increased in
future research, which will improve the accuracy and reliability of
the data obtained. Secondly, the long-term efficacy of the
treatments remains to be further observed.
In conclusion, polidocanol foam sclerosing agent, as
well as the combination of pingyangmycin and dexamethasone,
represent effective treatment methods for children with
oropharyngeal low-flow venous malformations. Compared with the
polidocanol foam sclerosing agent, the combination of pingyangmycin
and dexamethasone was safer with fewer complications, and is worthy
of clinical promotion. Collectively, DSA-guided therapy is a
visualization technique that monitors the treatment process and
increases treatment safety.
Acknowledgements
Not applicable.
Funding
This work was supported by the Science and
Technology Plan Project of Jinan Health and Family Planning
Commission (grant no. 2016-1-16).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
DS, LG, HS, JL, LW, CWa, CWu, YN and QZ designed the
current study, performed the experiments, collected and analyzed
the data, and prepared the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Qilu Hospital of Shandong University. The guardians of
all patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rabe E and Pannier F: Sclerotherapy in
venous malformation. Phlebology. 28 (Suppl 1):S188–S191.
2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bai N, Chen YZ, Fu YJ, Wu P and Zhang WN:
A clinical study of pingyangmycin sclerotherapy for venous
malformation: An evaluation of 281 consecutive patients. J Clin
Pharm Ther. 39:521–526. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lee BB, Baumgartner I, Berlien P,
Bianchini G, Burrows P, Gloviczki P, Huang Y, Laredo J, Loose DA,
Markovic J, et al: Diagnosis and treatment of venous malformations.
Consensus document of the international union of phlebology (IUP):
Updated 2013. Int Angiol. 34:97–149. 2015.PubMed/NCBI
|
|
4
|
Greene AK and Alomari AI: Management of
venous malformations. Clin Plast Surg. 38:83–93. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Berenguer B, Burrows PE, Zurakowski D and
Mulliken JB: Sclerotherapy of craniofacial venous malformations:
Complications and results. Plast Reconstr Surg. 104:1–11,
Discussion 12-15. 1999.PubMed/NCBI
|
|
6
|
Van der Vleuten CJ, Kater A, Wijnen MH,
Schultze Kool LJ and Rovers MM: Effectiveness of sclerotherapy,
surgery, and laser therapy in patients with venous malformations: A
systematic review. Cardiovasc Intervent Radiol. 37:977–989.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jing Z, Haibo L and Shaoyi Z: Comparative
study of sclerotherapy of venous malformation in children using
absolute ethanol and pingyangmycin. Chin J Radiol. 46:350–353.
2012.
|
|
8
|
Xue-Guo LI, Ren-Rong L, Feng X, et al:
Meta analysis of treatment of superficial venous malformation with
Bleomycin injection treatment. Chin J Aesth Plast Surg, 2016.
|
|
9
|
Ribeiro MC, de Mattos Camargo Grossmann S,
do Amaral MBF, de Castro WH, Navarro TP, Procopio RJ, da Silva TA,
de Nazaré Alvesde, Oliveira Kato C and Mesquita RA: Effectiveness
and safety of foam sclerotherapy with 5% ethanolamine oleate in the
treatment of low-flow venous malformations in the head and neck
region: A case series. Int J Oral Maxillofac Surg. 47:900–907.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kumar S, Bhavana K, Kumar S and Kumar P:
Ultrasound-guided polidocanol foam sclerotherapy for treating
venous malformations. J Clin Ultrasound. 46:23–31. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Department of Vascular Diseases OaMS,
Chinese Academy of Stomatology: Guide to the treatment of oral and
maxillofacial hemangioma and vascular malformations. Nat Med J
China 88: 3102.3107, 2008.
|
|
12
|
McCafferty I: Management of low-flow
vascular malformations: Clinical presentation, classification,
patient selection, imaging and treatment. Cardiovasc Intervent
Radiol. 38:1082–1104. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tessari L, Cavezzi A and Frullini A:
Preliminary experience with a new sclerosing foam in the treatment
of varicose veins. Dermatol Surg. 27:58–60. 2001.PubMed/NCBI
|
|
14
|
Murakami T, Ogata D, Miyano K and Tsuchida
T: An enlarged intramuscular venous malformation in the femoral
region successfully treated with complete resection. Int J Surg
Case Rep. 21:83–86. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lee BB, Laredo J, Kim YW and Neville R:
Congenital vascular malformations: General treatment principles.
Phlebology. 22:258–263. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Puig S, Aref H, Chigot V, Bonin B and
Brunelle F: Classification of venous malformations in children and
implications for sclerotherapy. Pediatr Radiol. 33:99–103.
2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhenyin L, Haibo L, Shaoyi Z, Kaunshan C,
Chuanqiang N, Tao Z and Jing Z: Clinical study on sclerotherapy
using absolute ethanol combined with polidocanol injectable foam in
treatment of venous malformation in maxillofacial region of
children. Chin J Intervent. 5:235–240. 2017.
|
|
18
|
Aboelatta YA, Nagy E, Shaker M and Massoud
KS: Venous malformations of the head and neck: A diagnostic
approach and a proposed management approach based on clinical,
radiological, and histopathology findings. Head Neck. 36:1052–1057.
2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen AW, Liu YR, Li K, Zhang K, Wang T and
Liu S: Efficacy of sclerotherapy with radio-opaque foam guided by
digital subtraction angiography for the treatment of complex venous
malformations of the head and neck. Br J Oral Maxillofac Surg.
53:809–813. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ouvry P, Allaert FA, Desnos P and
Hamel-Desnos C: Efficacy of polidocanol foam ver-sus 1iquid in
sclerotherapy of the great saphenous vein: A multicenter randomized
controlled trial with a 2 year follow-up. Eur J Vasc Endovasc Surg.
36:366–370. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Blaise S, Charavin-Cocuzza M, Riom H, Brix
M, Seinturier C, Diamand JM, Gachet G and Carpentier PH: Treatment
of low-flow vascular malformations by ultrasound-guided
sclerotherapy with polidocanol foam: 24 cases and literature
review. Eur J Vasc Endovasc Surg. 41:412–417. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cabrera J, Cabrera J Jr, Garcia-Olmedo MA
and Redondo P: Treatment of venous malformations with sclerosant in
microfoam form. Arch Dermatol. 139:1409–1416. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhai J and Zhai XD: Combined injection of
pingyangmycin & dexamethasone for the treatment of
maxillofacial and cervical venous malformations. Zhonghua Zheng
Xing Wai Ke Za Zhi. 28:168–171. 2012.PubMed/NCBI(In Chinese).
|
|
24
|
Spence J, Krings T, TerBrugge KG and Agid
R: Percutaneous treatment of facial venous: Malformations: A
matched comparison of alcohol and Bleomycin sclerotherapy. Head
Neck. 33:125–130. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gao Y, Chen H, Jin Y, et al: Retrospective
analysis of neuropathy following sclerotherapy for treating venous
malformations. J Tissue Eng Reconstruct Surgery, 2016.
|
|
26
|
Zheng JW, Yang XJ, Wang YA, He Y, Ye WM
and Zhang ZY: Intralesional injection of Pingyangmycin for vascular
malformations in oral and maxillofacial regions: An evaluation of
297 consecutive patients. Oral Oncol. 45:872–876. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hsu TS and Weiss RA: Foam sclerotherapy: A
new era. Arch Dermatol. 139:1494–1496. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wenxian Z, Fangfang L, Yifan Y, Huiling Y
and Xue L: Clinical efficacy of combination therapy with ethanol
+foam sclerotherapy +bleomycin A5 +medical elastic sleeve for
venous malformation in children. Chin J Intervent Radiol
(Electronic Edition). 4:227–230. 2017.
|
|
29
|
Steiner F, FitzJohn T and Tan ST: Ethanol
sclerotherapy for venous malformation. ANZ J Surg. 86:790–795.
2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Weitz-Tuoretmaa A, Keski-Nisula L, Rautio
R and Laranne J: Quality of life after endovascular sclerotherapy
of low-flow venous malformations: The efficacy of polidocanol
compared with ethanol. Acta Radiol. 59:946–952. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jingwei WU: Pingyangmycin in combination
with dexamethasone injection in the treatment of oral and
maxillofacial and neck venous malformations of clinical effect
assessment. China Continuing Medical Education, 2016.
|