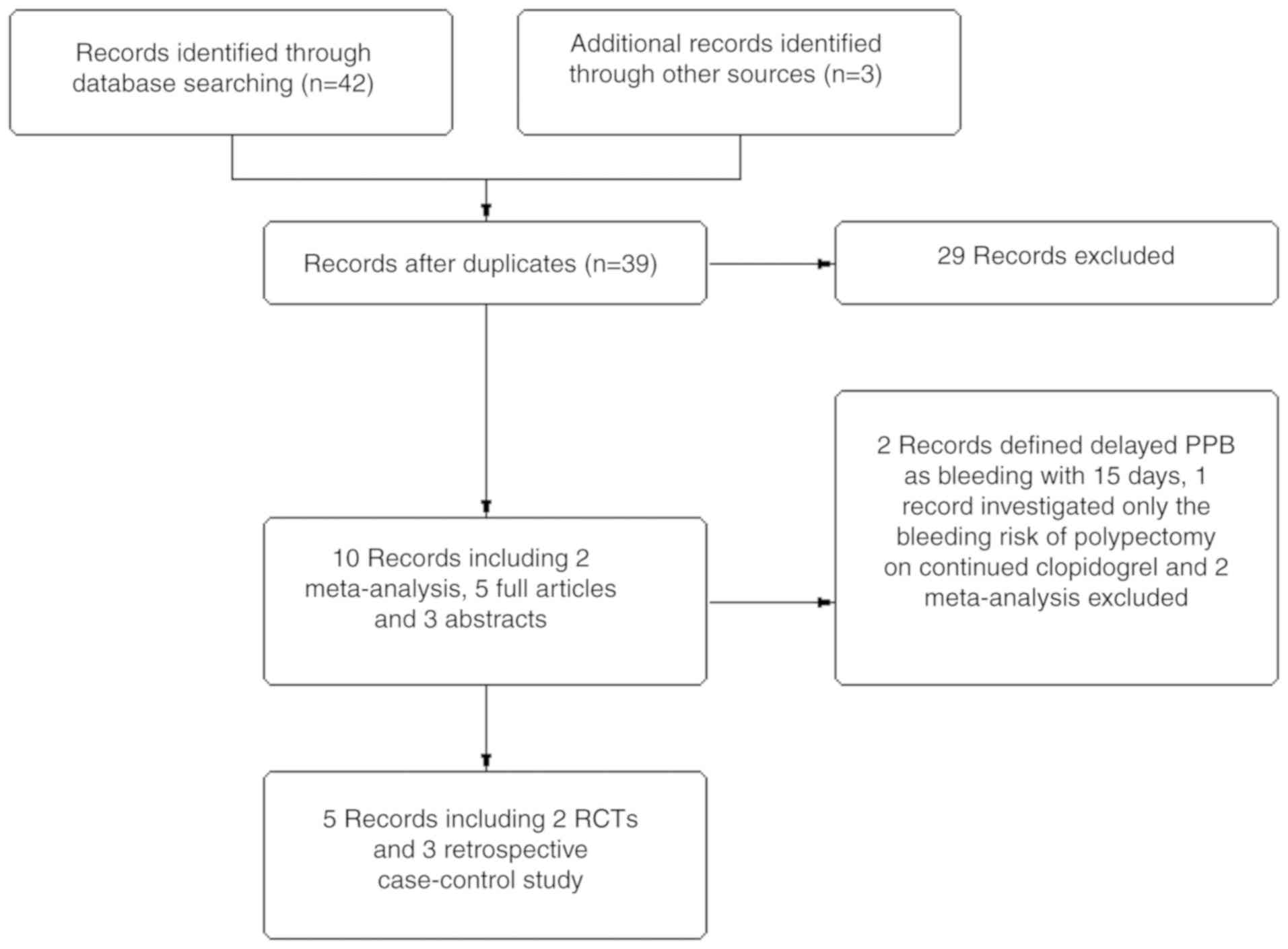
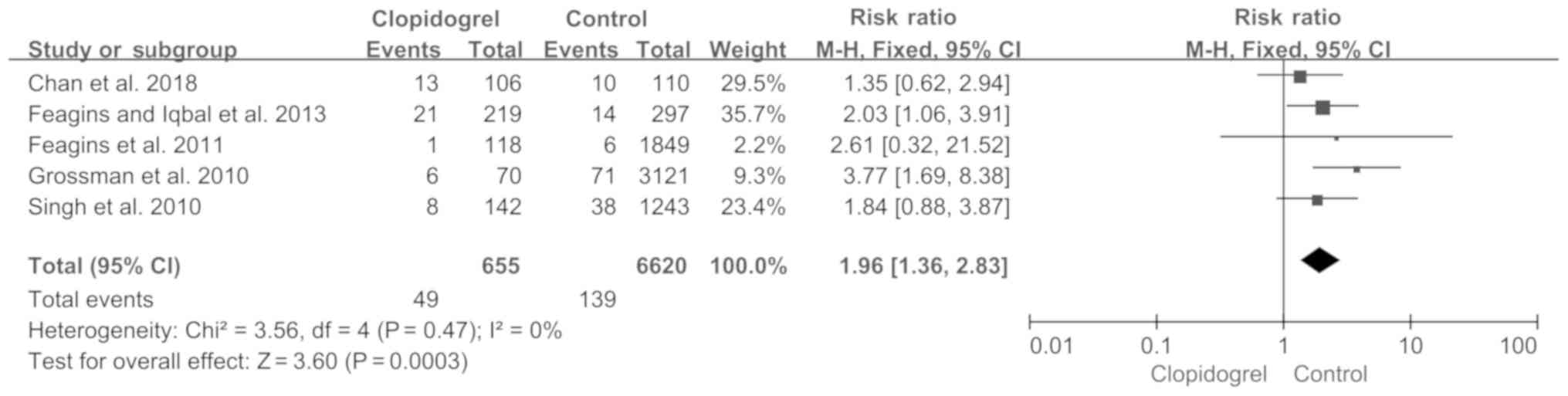
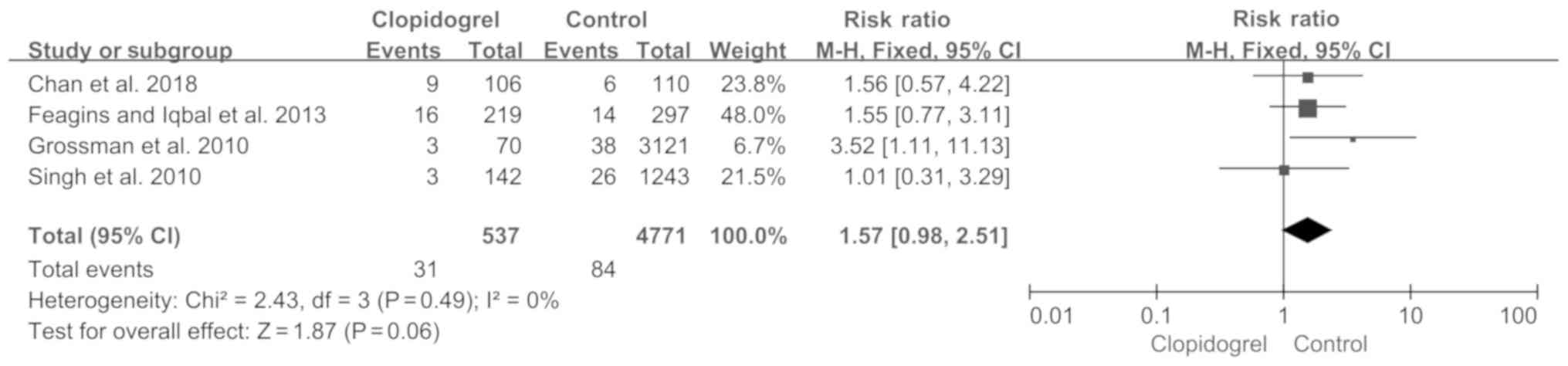
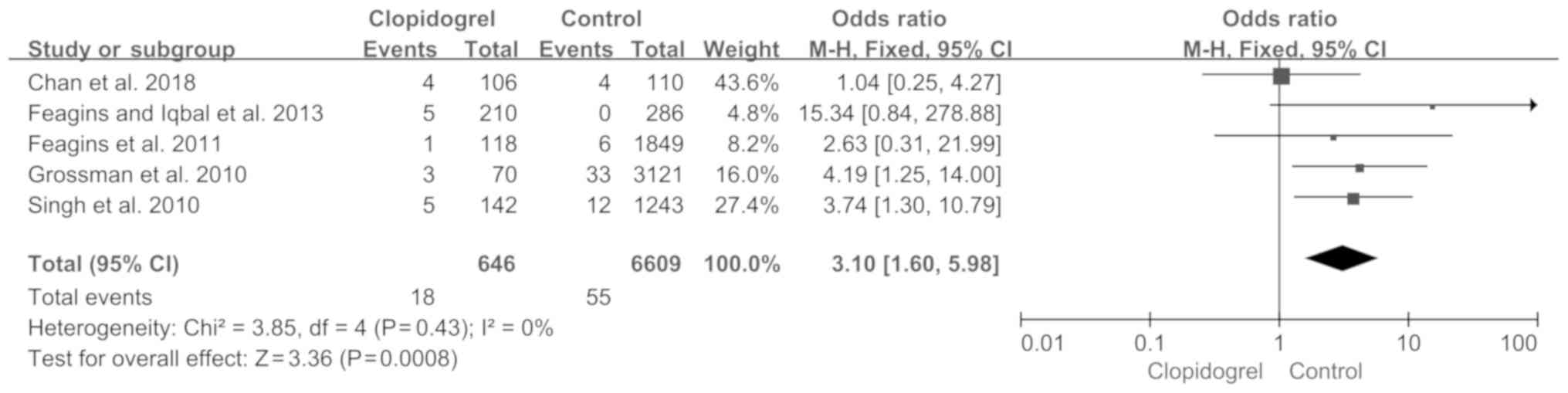
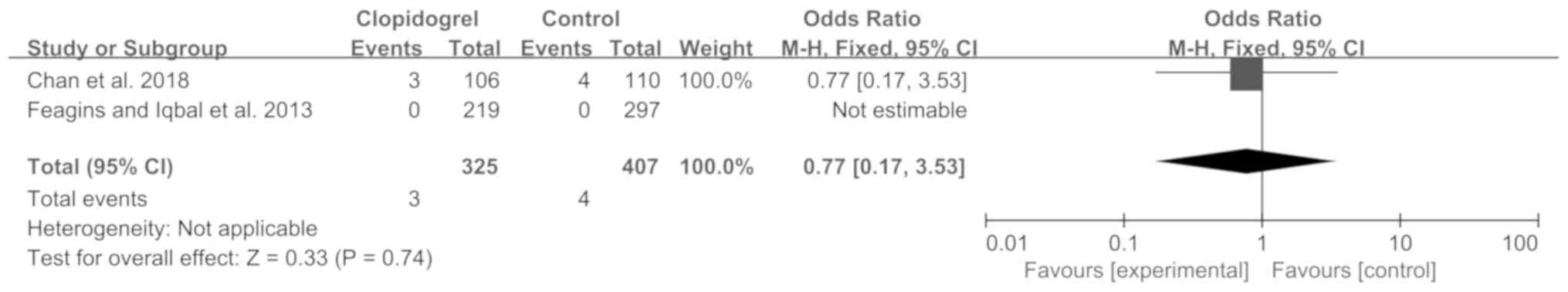
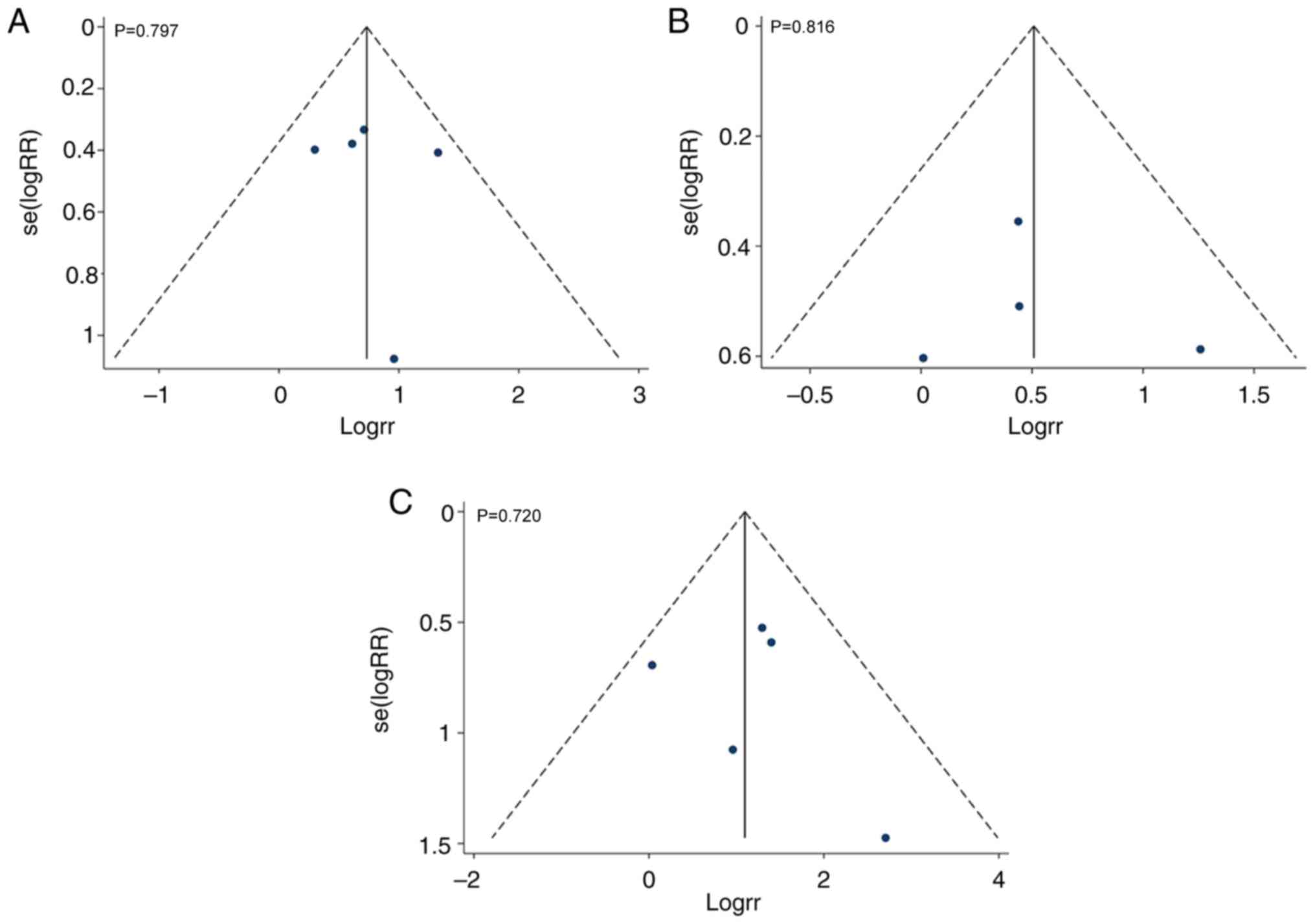
|
1
|
Kim AS and Johnston SC: Global variation
in the relative burden of stroke and ischemic heart disease.
Circulation. 124:314–323. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Writing Group Members. Mozaffarian D,
Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de
Ferranti S, Després JP, et al: Executive summary: Heart disease and
stroke statistics-2016 update: A report from the American heart
association. Circulation. 133:447–454. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rosen L, Bub DS, Reed JF III and Nastasee
SA: Hemorrhage following colonoscopic polypectomy. Dis Colon
Rectum. 36:1126–1131. 1993.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Reaume KT, Regal RE and Dorsch MP:
Indications for dual antiplatelet therapy with aspirin and
clopidogrel: Evidence-based recommendations for use. Ann
Pharmacother. 42:550–557. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Spertus JA, Kettelkamp R, Vance C, Decker
C, Jones PG, Rumsfeld JS, Messenger JC, Khanal S, Peterson ED, Bach
RG, et al: Prevalence, predictors, and outcomes of premature
discontinuation of thienopyridine therapy after drug-eluting stent
placement: Results from the PREMIER registry. Circulation.
113:2803–2809. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Singh M, Mehta N, Murthy UK, Kaul V, Arif
A and Newman N: Postpolypectomy bleeding in patients undergoing
colonoscopy on uninterrupted clopidogrel therapy. Gastrointest
Endosc. 71:998–1005. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Carter D, Beer-Gabel M, Eliakim R, Novis
B, Avidan B and Bardan E: Management of antithrombotic agents for
colonoscopic polypectomies in Israeli gastroenterologists relative
to published guidelines. Clin Res Hepatol Gastroenterol.
37:514–518. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Feagins LA, Uddin FS, Davila RE, Harford
WV and Spechler SJ: The rate of post-polypectomy bleeding for
patients on uninterrupted clopidogrel therapy during elective
colonoscopy is acceptably low. Dig Dis Sci. 56:2631–2638.
2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Feagins LA, Iqbal R, Harford WV, Halai A,
Cryer BL, Dunbar KB, Davila RE and Spechler SJ: Low rate of
postpolypectomy bleeding among patients who continue thienopyridine
therapy during colonoscopy. Clin Gastroenterol Hepatol.
11:1325–1332. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gandhi S, Narula N, Mosleh W, Marshall JK
and Farkouh M: Meta-analysis: Colonoscopic post-polypectomy
bleeding in patients on continued clopidogrel therapy. Aliment
Pharmacol Ther. 37:947–952. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shalman D and Gerson LB: Systematic review
with meta-analysis: The risk of gastrointestinal haemorrhage
post-polypectomy in patients receiving anti-platelet,
anti-coagulant and/or thienopyridine medications. Aliment Pharmacol
Ther. 42:949–956. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kato M, Uedo N, Hokimoto S, Ieko M,
Higuchi K, Murakami K and Fujimoto K: Guidelines for
gastroenterological endoscopy in patients undergoing antithrombotic
treatment: 2017 appendix on anticoagulants including direct oral
anticoagulants. Dig Endosc. 30:433–440. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chan FKL, Goh KL, Reddy N, Fujimoto K, Ho
KY, Hokimoto S, Jeong YH, Kitazono T, Lee HS, Mahachai V, et al:
Management of patients on antithrombotic agents undergoing
emergency and elective endoscopy: Joint Asian Pacific Association
of Gastroenterology (APAGE) and Asian Pacific Society for Digestive
Endoscopy (APSDE) practice guidelines. Gut. 67:405–417.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mehran R, Baber U, Steg PG, Ariti C, Weisz
G, Witzenbichler B, Henry TD, Kini AS, Stuckey T, Cohen DJ, et al:
Cessation of dual antiplatelet treatment and cardiac events after
percutaneous coronary intervention (PARIS): 2 year results from a
prospective observational study. Lancet. 382:1714–1722.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Eisenberg MJ, Richard PR, Libersan D and
Filion KB: Safety of short-term discontinuation of antiplatelet
therapy in patients with drug-eluting stents. Circulation.
119:1634–1642. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chan FKL, Kyaw MH, Hsiang JC, Suen BY, Kee
KM, Tse YK, Ching JYL, Cheong PK, Ng D, Lam K, et al: Risk of
postpolypectomy bleeding with uninterrupted clopidogrel therapy in
an industry-independent, double-blind, randomized trial.
Gastroenterology. 156:918–925. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Plumé Gimeno G, Bustamante-Balén M,
Satorres Paniagua C, Díaz Jaime FC and Cejalvo Andújar MJ:
Endoscopic resection of colorectal polyps in patients on
antiplatelet therapy: An evidence-based guidance for clinicians.
Rev Esp Enferm Dig. 109:49–59. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Abdel Samie A, Theilmann L and Labenz J:
ALGK : Endoscopic procedures in patients under clopidogrel or dual
antiplatelet therapy: A survey among German gastroenterologists and
current guidelines. Z Gastroenterol. 52:425–428. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liberati A, Altman DG, Tetzlaff J, Mulrow
C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J
and Moher D: The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate health care
interventions: Explanation and elaboration. Ann Intern Med.
151:W65–W94. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nelson DB, McQuaid KR, Bond JH, Lieberman
DA, Weiss DG and Johnston TK: Procedural success and complications
of large-scale screening colonoscopy. Gastrointest Endosc.
55:307–314. 2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Stroup DF, Berlin JA, Morton SC, Olkin I,
Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA and Thacker
SB: Meta-analysis of observational studies in epidemiology: A
proposal for reporting. Meta-analysis Of Observational Studies in
Epidemiology (MOOSE) group. JAMA. 283:2008–2012. 2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jadad AR, Moore RA, Carroll D, Jenkinson
C, Reynolds DJ, Gavaghan DJ and McQuay HJ: Assessing the quality of
reports of randomized clinical trials: Is blinding necessary?
Control Clin Trials. 17:1–12. 1996.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Grossman EB, Maranino AN, Zamora DC, et
al: Antiplatelet Medications Increase the Risk of Post-Polypectomy
Bleeding. GastrointestEndosc. 71(AB138)2010.
|
|
25
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhao D, Liu J, Wang M, Zhang X and Zhou M:
Epidemiology of cardiovascular disease in China: Current features
and implications. Nat Rev Cardiol. 16:203–212. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Iakovou I, Schmidt T, Bonizzoni E, Ge L,
Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M,
Carlino M, et al: Incidence, predictors, and outcome of thrombosis
after successful implantation of drug-eluting stents. JAMA.
293:2126–2130. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kim HS, Kim TI, Kim WH, Kim YH, Kim HJ,
Yang SK, Myung SJ, Byeon JS, Lee MS, Chung IK, et al: Risk factors
for immediate postpolypectomy bleeding of the colon: A multicenter
study. Am J Gastroenterol. 101:1333–1341. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sawhney MS, Salfiti N, Nelson DB, Lederle
FA and Bond JH: Risk factors for severe delayed postpolypectomy
bleeding. Endoscopy. 40:115–119. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Watabe H, Yamaji Y, Okamoto M, Kondo S,
Ohta M, Ikenoue T, Kato J, Togo G, Matsumura M, Yoshida H, et al:
Risk assessment for delayed hemorrhagic complication of colonic
polypectomy: Polyp-related factors and patient-related factors.
Gastrointest Endosc. 64:73–78. 2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rutter MD, Nickerson C, Rees CJ, Patnick J
and Blanks RG: Risk factors for adverse events related to
polypectomy in the English Bowel Cancer Screening Programme.
Endoscopy. 46:90–97. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Choung BS, Kim SH, Ahn DS, Kwon DH, Koh
KH, Sohn JY, Park WS, Kim IH, Lee SO, Lee ST and Kim SW: Incidence
and risk factors of delayed postpolypectomy bleeding: A
retrospective cohort study. J Clin Gastroenterol. 48:784–789.
2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Heldwein W, Dollhopf M, Rösch T, Meining
A, Schmidtsdorff G, Hasford J, Hermanek P, Burlefinger R, Birkner
B, Schmitt W, et al: The Munich Polypectomy Study (MUPS):
Prospective analysis of complications and risk factors in 4000
colonic snare polypectomies. Endoscopy. 37:1116–1122.
2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Rabeneck L, Paszat LF, Hilsden RJ, Saskin
R, Leddin D, Grunfeld E, Wai E, Goldwasser M, Sutradhar R and
Stukel TA: Bleeding and perforation after outpatient colonoscopy
and their risk factors in usual clinical practice.
Gastroenterology. 135:1899–1906.e1891. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Parikh ND, Zanocco K, Keswani RN and
Gawron AJ: A cost-efficacy decision analysis of prophylactic clip
placement after endoscopic removal of large polyps. Clin
Gastroenterol Hepatol. 11:1319–1324. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Matsumoto M, Kato M, Oba K, Abiko S, Tsuda
M, Miyamoto S, Mizushima T, Ono M, Omori S, Takahashi M, et al:
Multicenter randomized controlled study to assess the effect of
prophylactic clipping on post-polypectomy delayed bleeding. Dig
Endosc. 28:570–576. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Boumitri C, Mir FA, Ashraf I,
Matteson-Kome ML, Nguyen DL, Puli SR and Bechtold ML: Prophylactic
clipping and post-polypectomy bleeding: A meta-analysis and
systematic review. Ann Gastroenterol. 29:502–508. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Dokoshi T, Fujiya M, Tanaka K, Sakatani A,
Inaba Y, Ueno N, Kashima S, Goto T, Sasajima J, Tominaga M, et al:
A randomized study on the effectiveness of prophylactic clipping
during endoscopic resection of colon polyps for the prevention of
delayed bleeding. Biomed Res Int. 2015(490272)2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lin D, Soetikno RM, McQuaid K, Pham C,
Doan G, Mou S, Shergill AK, Somsouk M, Rouse RV and Kaltenbach T:
Risk factors for postpolypectomy bleeding in patients receiving
anticoagulation or antiplatelet medications. Gastrointest Endosc.
87:1106–1113. 2018.PubMed/NCBI View Article : Google Scholar
|