Introduction
Atherosclerosis is one of the leading cause of death
worldwide (1). Atherosclerotic
plaques are difficult to reverse once formed; however, statins are
regarded as the most effective drugs for the prevention of
atherosclerotic plaque formation (1). Numerous studies have reported that
statins can reduce the plaque volume following prolonged high-dose
statin administration (2-4).
However, these studies have also demonstrated that the reduction in
the plaque volume was limited despite long-term treatment with high
doses of statins (2-4),
making statins an unsatisfactory solution to the global demand for
clinical treatment.
A novel drug delivery system (DDS) has been
established using nanoparticle (NP)-mediated approaches. NPs are
biodegradable copolymers that change the pharmacokinetic properties
of drugs in vivo and increase the distribution of drugs in
organs (5). The NP-DDS can enhance
the efficacy and safety of therapeutic agents and overcome numerous
drawbacks of the agents such as toxicity, low water solubility,
poor bioavailability and low organ specificity (5). Previous studies have demonstrated that
pitavastatin inhibits pulmonary artery smooth muscle cell
proliferation more effectively compared with other statins,
including rosuvastatin, pravastatin, simvastatin, atorvastatin and
lovastatin (6), which might explain
that pitavastatin has advantages over other statins in preventing
atherosclerosis. Pitavastatin-NP (P-NP) has exhibited significant
advantages in experimental studies of angiogenesis in ischemic
tissue (7,8) and in the prevention and treatment of
pulmonary hypertension (6). These
effects may result primarily from the efficiency and durability of
the NP-DDS, as well as its ability to target the location of the
lesion. Additionally, pitavastatin-incorporated NP-eluting stents
were reported to attenuate in-stent stenosis without delayed
endothelial healing effects in a porcine coronary artery model
(9). Administration of P-NP is
frequently used in experimental studies and its theory and
technology are well understood (6-9).
Therefore, P-NP was used in the present study.
A limited number of reports have focused on the
effects of P-NP on atherosclerotic plaques. The present study aimed
to examine whether administration of P-NP may inhibit the
progression of atherosclerotic plaques and to elucidate its
mechanism of action.
Materials and methods
Preparation of nanoparticles
Lactide/glycolide copolymer (PLGA) with a copolymer
ratio of lactide to glycolide of 75:25 was used for the NP. PLGA
nanoparticles encapsulated with FITC (FITC-NP) and P-NP were
constructed using an emulsion solvent diffusion method as
previously described (6). The
FITC-loaded NP contained 4.06% (wt/vol) FITC, and the
pitavastatin-loaded NP contained 12% (wt/vol) pitavastatin.
Animal preparation and experimental
protocol
The study was approved by The Institutional Animal
Care and Use Committee, China Medical University (Shenyang, China).
C57BL/6J apolipoprotein E (ApoE)-knockout mice (n=60, male,
8-week-old, 20-30 g) were purchased from Beijing Vital River
Laboratory Animal Technology Co. Ltd. The mice were housed at a
constant temperature (20-22˚C) and humidity (40-50%) with a 12-h
light/dark cycle and received a high-fat diet and tap water to
establish the atherosclerosis model; the high-fat diet (0.2%
cholesterol, 21.2% fat, 13.7% saturated fatty acids, 7.3% total
unsaturated fatty acids; Harlan Teklad; cat. no. TD.88137) was
maintained throughout the experiment. The well-being of the mice
was monitored during the experimental period by assessing their
physical characteristics, activity, behavior, weight and reactions
to external stimuli. The humane endpoints were defined as 10-20%
weight loss, impaired ambulation preventing the animals from
reaching food or water, lack of physical or mental alertness,
abnormal breathing and prolonged inability to remain upright.
A total of 48 ApoE-knockout mice were randomly
divided into six groups (n=8 mice/group): i) Control group, in
which the mice were not treated; ii) PBS group, in which the mice
received intravenous injections of 0.1 ml PBS once a week via the
tail vein; iii) NP group, in which the mice received intravenous
injections of NP equivalent to the NP of 0.4 mg/kg P-NP once a week
via the tail vein [the P-NP contained 88% (wt/vol) NP]; iv) P-IV
group, in which the mice received an intravenous injection of 0.4
mg/kg pitavastatin once a week via the tail vein; v) P-NP group, in
which the mice received an intravenous injection of P-NP containing
0.4 mg/kg (7) pitavastatin at once a
week via the tail vein; and vi) P-Oral group, in which the mice
were orally administered 1 mg/kg pitavastatin once a day. The mice
were allowed free access to high-fat food and tap water throughout
the 12-week period. The cumulative effect of intravenous P-NP was
calculated using the following formula: P-NP group: Cumulative
dose=20,000 mg/animal x 0.4 mg/kg/day x 12 days=96,000 mg/animal;
P-Oral group: Cumulative dose=20,000 mg/animal x 1 mg/kg/day x 84
days=1,680,000 mg/animal); Cumulative effect=the cumulative dose of
the P-Oral group/the cumulative dose of the P-NP group.
The mice were then euthanized by an intraperitoneal
injection of 150 mg/kg sodium pentobarbital. Death was verified by
the absence of a corneal reflex, respiration and heartbeat.
Subsequently, the midline of the mouse abdomen was incised, and the
thorax was opened to expose the heart and aorta. The aorta was
dissected out between the aortic root and the abdominal aorta, and
the fat and connective tissues were removed with fine forceps.
Oil Red O staining of atherosclerotic
plaques
The mouse aortas were completely separated, and the
aorta strips were fixed with fine needles on a black rubber sheet.
The aorta strips were placed in PBS for 2 min at room temperature
and subsequently differentiated in a mixture of 60% isopropanol in
distilled water for 1 min at room temperature, incubated in Oil Red
O solution (cat. no. O8010; Beijing Solarbio Science and Technology
Co., Ltd) for 10 min at room temperature, differentiated in a
mixture of 60% isopropanol in distilled water for a further 2 min
at room temperature, and finally rinsed in PBS for 2 min at room
temperature. The stained aorta strips were imaged, and the Oil Red
O-positive area and total luminal surface area from the aortic root
to the abdominal aorta were measured using an SZX16 stereoscopic
microscope (Olympus Corporation; magnification x3).
Distribution and persistence of
FITC-NP
Following 12-week high-fat diet administration, six
randomly selected mice received 0.4 mg/kg FITC-NP and another six
randomly selected mice received 0.4 mg/kg FITC through an
intravenous injection into the tail vein. The mice were euthanized
1 or 7 days post-injection (four groups: FITC 1 day, FITC-NP 1 day,
FITC 7 days and FITC-NP 7 days; n=3 mice/group). The aortas were
dissected, frozen using optimal cutting temperature compound (cat.
no. 4583; Sakura Finetek USA, Inc.) and stored at -20˚C. Serial
cross-sections of 5 µm were made using a microtome at one side on
the aortic root. The nuclei were counterstained with DAPI (blue) at
room temperature for 3 min. Fluorescence distribution in
atherosclerotic plaques was observed under a fluorescence
microscope (Olympus Corporation; magnification, x200 and x400).
Histological and immunohistochemical
analysis
The aortic was removed, fixed with 4%
paraformaldehyde for 24 h and embedded in paraffin at room
temperature. Histological and immunohistochemical evaluation was
performed to evaluate the stability of atherosclerotic plaques in
5-µm paraffin-embedded sections from the dissected aortic roots
from the control, PBS, NP, P-IV, P-Oral and P-NP groups. The
cross-sections of aortic roots were stained with anti-mouse Mac-3
(Mac-3; 1:200; cat. no. 550292; BD Pharmingen; BD Biosciences) and
α-smooth muscle actin (α-actin; 1:50; cat. no. sc-32251; Santa Cruz
Biotechnology, Inc.) primary antibodies overnight at 4˚C, followed
by incubation with peroxidase-conjugated goat anti-rat
immunoglobulin G (IgG; 1:1,000; cat. no. zb2307; Zhongshan Jinqiao
Biotechnology Co., Ltd) or peroxidase-conjugated goat anti-mouse
IgG (1:1,000; cat. no. zb2305; Zhongshan Jinqiao Biotechnology Co.,
Ltd.) for another 30 min at room temperature. The nuclei were
counterstained with hematoxylin at room temperature for 2 min. The
images of five microscopic fields from three different sections
from each animal were obtained using a light microscope (Olympus
Corporation; magnification, x200). The positive staining integrated
optical density (IOD) value was measured by Image-Pro Plus 6.0
color microscopic image analysis software (Media Cybernetics, Inc.)
for semi-quantitative analysis.
Cell culture
The human monocyte cell line THP-1 was purchased
from The Shanghai institutes for Biological sciences. THP-1 cells
were cultured in RPMI-1640 medium (Thermo Fisher Scientific, Inc.)
containing 10% fetal bovine serum (FBS; GE Healthcare Life
Sciences), 100 U/ml penicillin and 100 U/ml streptomycin in a
humidified atmosphere with 5% CO2 at 37˚C. THP-1 cells
have been demonstrated to differentiate into macrophages following
stimulation with phorbol-12-myristate-13-acetate (PMA) (10). THP-1 cells were seeded in 6-well
culture plates at 1x106 cells/ml and stimulated with 100
ng/ml PMA (cat. no. P1585; Sigma-Aldrich; Merck KGaA) for 48 h to
obtain adherent macrophages. Differentiated cells exhibited
macrophage-like phenotypes, which were characterized by their
morphology and increased cell surface expression of CD11b by flow
cytometry analysis (Fig. S1) as
described in a previous study (10).
THP-1 macrophages were used in the subsequent experiments. THP-1
macrophages were incubated with 50 µg/ml oxidized low-density
lipoprotein (Beijing Xiesheng Biotechnology Co., Ltd.) for 48 h to
induce foam cell formation. FBS-free RPMI-1640 medium was
subsequently used.
A 1.0 ml suspension of 0.5 mg/ml pitavastatin, 0.5
mg/ml NP, P-NP containing 0.5 mg/ml pitavastatin or vehicle was
added to each well (n=6). After 1 h, the cells were washed three
times with PBS, incubated for another 7 days and collected for
future analysis.
Western blotting
The key enzymes of lipid metabolism regulation in
macrophages were evaluated by immunoblotting. The whole aortas of
the ApoE-knockout mice were isolated and collected. The frozen
samples were homogenized in RIPA lysis buffer (Beyotime Institute
of Biotechnology), and the protein concentration was measured using
the BCA Protein Assay Kit (Thermo Fisher Scientific, Inc.). A total
of 500-700 µg of protein was extracted from each aorta. The
proteins (20 µg/lane) were separated on 7.5% SDS-polyacrylamide
gels, blotted to polyvinylidene fluoride membranes, blocked with 5%
skim milk for 1 h at room temperature and incubated with scavenger
receptor-A1 (SR-A1; 1:500; cat. no. S3195; Sigma-Aldrich; Merck
KGaA) and cholesterol acyltransferase 1 (ACAT-1; 1:300; cat. no.
sc-161307; Santa Cruz Biotechnology, Inc.) antibodies overnight at
4˚C. The membranes were then washed with TBS-Tween-20 and incubated
with the appropriate horseradish peroxidase-linked secondary
antibody goat anti-rabbit IgG (1:1,000; cat. no. zb2301; Zhongshan
Jinqiao Biotechnology Co., Ltd.) or peroxidase-conjugated rabbit
anti-goat IgG (1:1,000; cat. no. zb2306; Zhongshan Jinqiao
Biotechnology Co., Ltd.), for 1 h at room temperature with gentle
agitation, and the immune complexes were detected by enhanced
chemiluminescence (ECL) using an ECL kit (Thermo Fisher Scientific,
Inc.). Densitometric analyses were performed using ImageJ 1.44
software (National Institutes of Health) and normalized to the
loading control protein β-actin (1:1,000; cat. no. TA-09; Zhongshan
Jinqiao Biotechnology Co., Ltd.).
ELISA
Blood samples were collected from the left
ventricles of the mice by heart puncture prior to euthanasia. The
samples were placed in heparin containing tubes and centrifuged at
4˚C at 2,000 x g for 10 min. The plasma was collected and stored at
-80˚C. The protein levels of total cholesterol (TC), triglyceride
(TG), low density lipoprotein cholesterol (LDL-C), high density
lipoprotein cholesterol (HDL-C), creatine kinase-MB (CK-MB) and
alanine transaminase (ALT) in the plasma of the mice were measured
by ELISA kits according to the manufacturer's instructions (cat.
nos.: TC, 201-02-0614; TG, 201-02-0376; LDL-C, 201-02-0333; HDL-C,
201-02-0332; CK-MB, 201-02-0308; ALT, 201-02-0495; Shanghai
Shanghong Biotechnology Co., Ltd.).
To measure the content of cholesterol ester (CE) in
THP-1-derived macrophages, CE levels were analyzed using commercial
reagents (cat. no. JL19339; Shanghai Future Industry Co., Ltd.).
The levels of the inflammatory parameters interleukin-6 (IL-6) and
tumor necrosis factor-α (TNF-α) were analyzed using ELISA kits
(cat. nos.: IL-6, 201-12-0091; TNF-α, 201-12-0083; Shanghai
Shanghong Biotechnology Co., Ltd.) according to the manufacturer's
instructions. Absorbance was detected with a microplate reader at
450 nm for all kits.
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was used to assess the impact of P-NP on the
expression of inflammatory factors in vivo. The aortas of
ApoE-knockout mice were isolated and collected. Total RNA was
extracted from the aortas using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). The RNA concentration
was measured by NanoDrop ND-1000 (NanoDrop Technologies; Thermo
Fisher Scientific, Inc.). cDNA was synthesized at 37˚C for 15 min
followed by 85˚C for 5 sec using the PrimeScript™ RT reagent
(Takara Bio, Inc.). PCR amplification with the SYBR PrimeScript
RT-PCR kit (Takara Bio, Inc.) was performed using an ABI PRISM 7500
Sequence Detection System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The thermocycling conditions were 30 sec at
95˚C, followed by 40 cycles of 5 sec at 95˚C and 34 sec at 60˚C.
TaqMan primer sequences (Takara Biotechnology Co, Ltd.) used for
RT-qPCR were as follows: Monocyte chemotactic protein 1 (MCP-1)
forward, 5'-AGCAGCAGGTGTCCCAAAGA-3' and reverse,
5'-GTGCTGAAGACCTTAGGGCAGA-3'; macrophage colony-stimulating factor
(M-CSF) forward, 5'-ACCACTACCCTCTCCTACCATCTTC-3' and reverse,
5'-CATCCTCCAGCCCTTTCTCTT-3'; and GAPDH forward,
5'-GGTTGTCTCCTGCGACTTCA-3' and reverse,
5'-TGGTCCAGGGTTTCTTACTCC-3'. GAPDH served as the internal reference
gene. Normalization and fold-changes were calculated using the
2-ΔΔCq method (11).
Statistical analysis
All data are presented as the mean ± standard
deviation values from at least four independent experiments.
Statistical analysis was performed by one-way ANOVA followed by
Tukey's post-hoc test. SPSS 20.0 (IBM Corp.) was used for
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
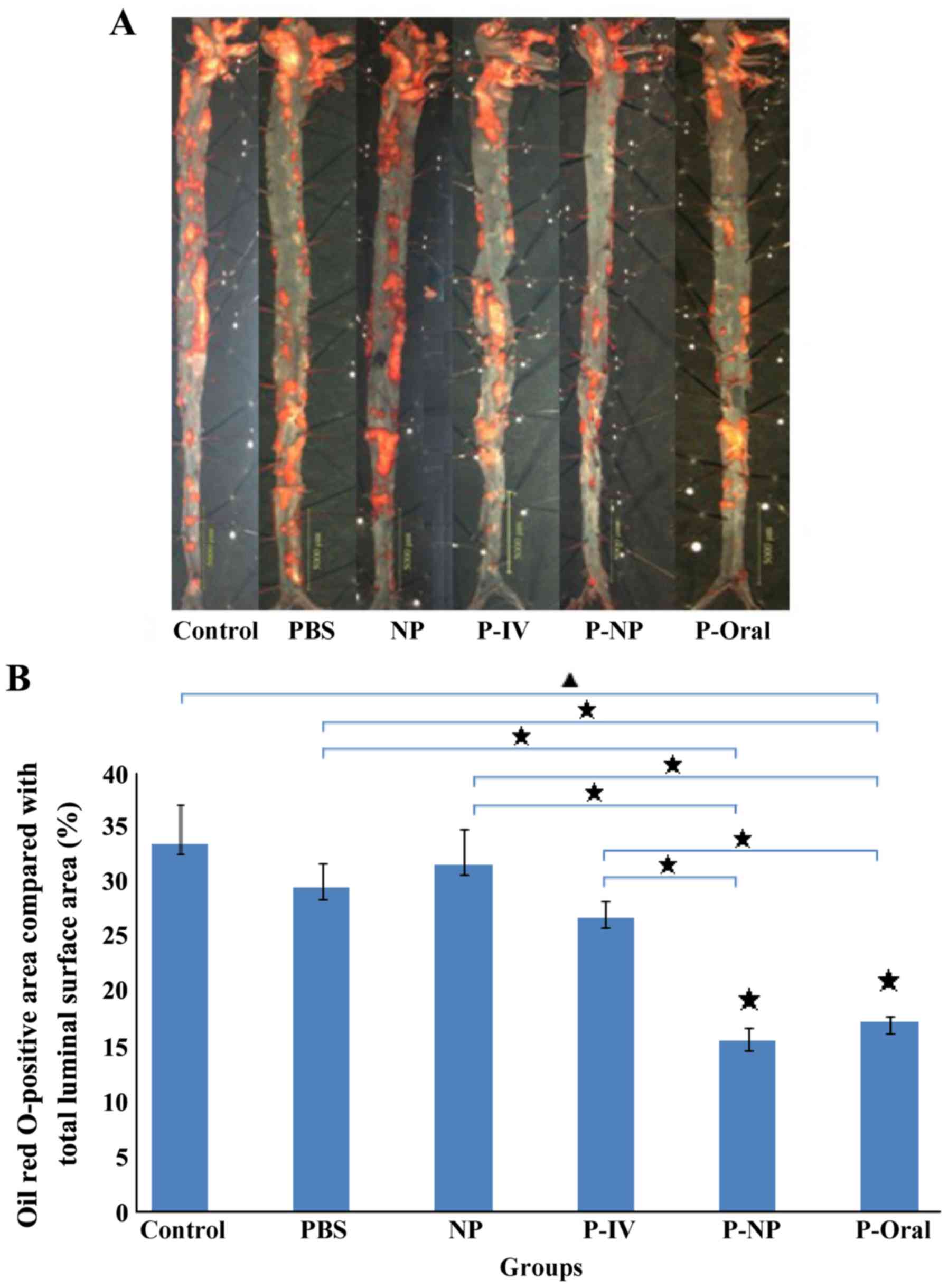
Effects of P-NP on aorta plaque
size
The Oil Red O-positive area compared with the total
luminal surface area in ApoE-knockout mice indicated the area
percentage of plaques (Fig. 1A). The
percentages of the Oil Red O-positive area as follows: Control,
33.6±3.57%; PBS, 29.5±2.26%; NP, 31.7±3.2%; P-IV, 26.9±1.55%; P-NP,
15.7±1.08% and P-Oral, 17.3±0.56% (n=4 mice/group). Significant
differences were observed in the percentages of the Oil Red
O-positive areas among the groups, and this percentage was
significantly lower in the P-Oral and P-NP groups compared with the
other groups (Fig. 1B).
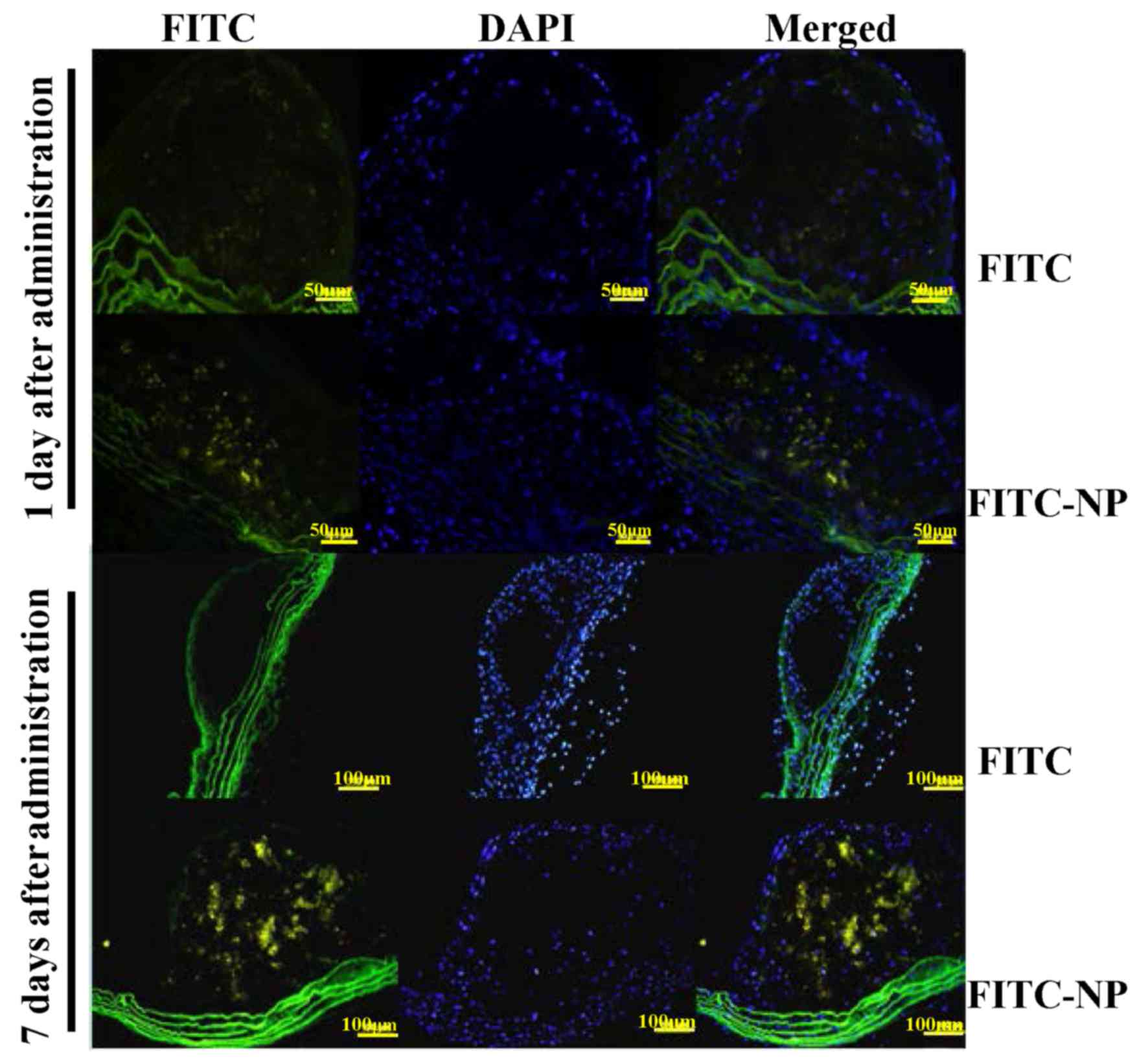
Localization of FITC-NP in the
atherosclerotic plaques
The localization of FITC was analyzed after a single
tail vein administration of FITC-NP in ApoE-knockout mice. On day 1
after injection, stronger FITC signals were detected in the plaques
of FITC-NP-injected mice compared with FITC-injected mice (Fig. 2). On day 7 post-injection, the
observations were similar, with strong FITC signals detected in
plaques formed in the FITC-NP-injected mice and very low FITC
signals observed in the FITC-injected mice (Fig. 2). These results indicated that FITC
signals were localized in the plaques of aortas for 7 days
post-injection with FITC-NP.
Effects of P-NP on lipid metabolism
regulation
The in vivo protein levels of SR-A1 and
ACAT-1 in the P-NP and P-Oral groups were significantly lower
compared with the other groups. No significant difference was
observed between the P-NP and P-Oral groups, but the levels of
SR-A1 and ACAT-1 were slightly lower in the P-NP compared with the
P-Oral group (Fig. 3A). The in
vitro protein levels of ACAT-1 and SR-A1 were lower in the P-NP
group compared with the control and NP groups. Additionally, the
ACAT-1 level was significantly lower in the P-NP group compared
with the P group (Fig. 3B).
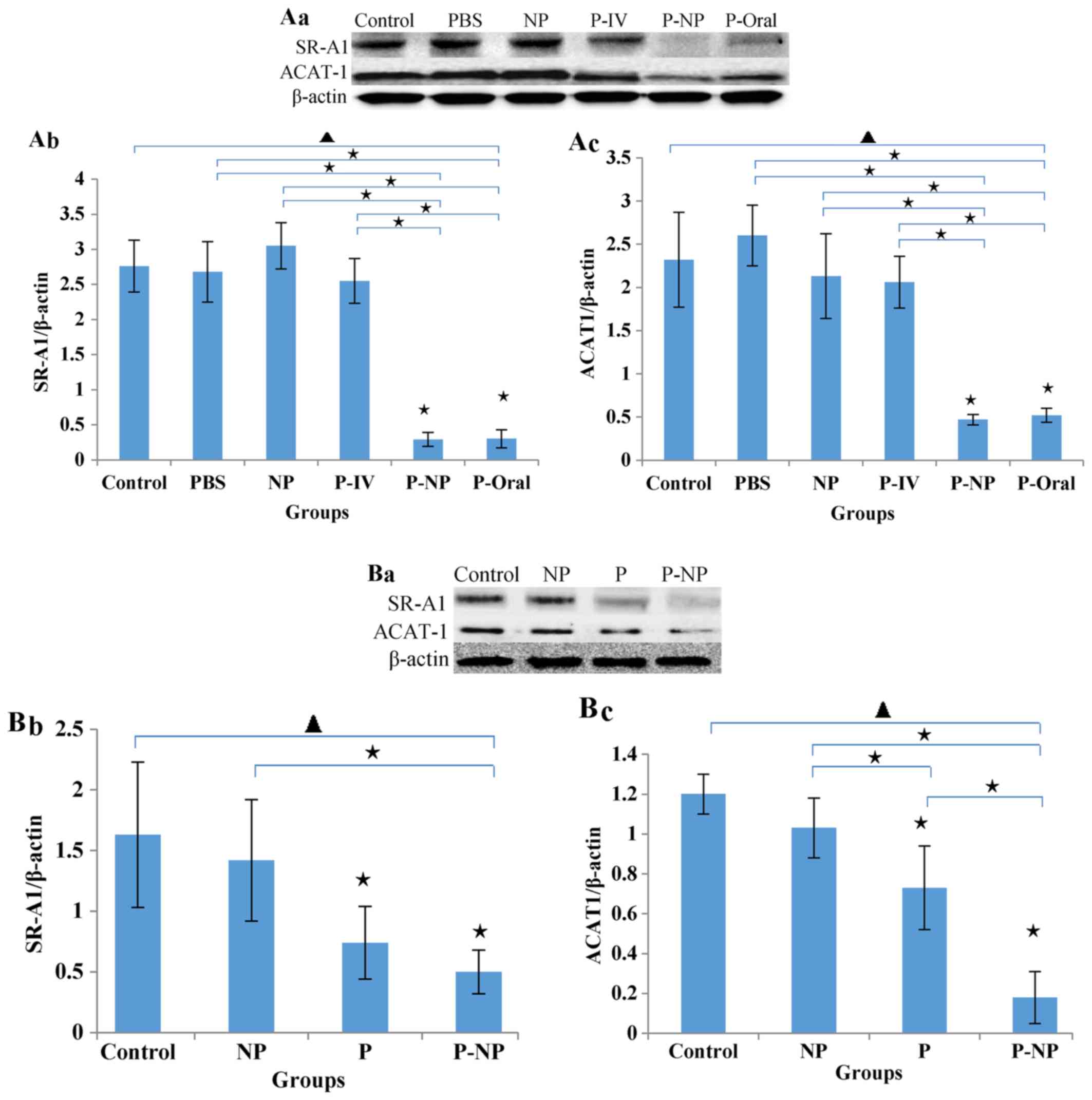 | Figure 3Effects of P-NP on regulation of lipid
metabolism. (A) Effects of P-NP on SR-A1 and ACAT-1 protein
secretion in ApoE-knockout mice. (A-a) SR-A1 and ACAT-1 protein
secretion in ApoE-knockout mice. (A-b) The SR-A1 level is presented
as a percentage of the β-actin level (n=4). (A-c) The ACAT-1 level
is presented as a percentage of the β-actin level (n=4). (B)
Effects of P-NP on SR-A1 and ACAT-1 protein levels in THP-1-derived
macrophages. (B-a) SR-A1 and ACAT-1 protein levels in THP-1-derived
macrophages. (B-b) The SR-A1 level is presented as a percentage of
the β-actin level (n=6). (B-c) The ACAT-1 level is presented as a
percentage of the β-actin level (n=6). Before the first antibody
staining, the three protein bands were excised according to their
molecular weight. The protein bands were incubated with different
antibodies and exposed to X-ray film; thus, although the blot
images each of these figures were from the same membrane, there was
variability among each set of images. ▲P<0.05 among
all groups; «P<0.05 vs. control or as indicated. NP,
nanoparticle; P-IV, pitavastatin intravenous; P-NP, pitavastatin
nanoparticle; P-Oral, pitavastatin oral; SR-A1, scavenger
receptor-A1; ACAT-1, cholesterol acyltransferase 1; ApoE,
apolipoprotein E. |
Effects of P-NP on the stability of
atherosclerotic plaques
Histological and immunohistochemical analysis
revealed significant differences in the α-actin expression in
atherosclerotic plaques among all groups (Fig. 4). The IOD proportion of the α-actin
positive area [IOD (sum)/area (sum)] was significantly higher in
the P-NP group compared with that in the control, PBS, NP and P-IV
groups (Fig. 4A and B). No significant differences were observed
in Mac-3 expression in atherosclerotic plaques among the groups;
however, the IOD proportion of the Mac-3-positive area was
significantly lower in the P-NP group compared with the control,
PBS and NP groups (Fig. 4C and
D). These results indicated that
P-NP significantly increased the stability of atherosclerotic
plaques by increasing the expression of α-actin and reducing the
expression of Mac-3.
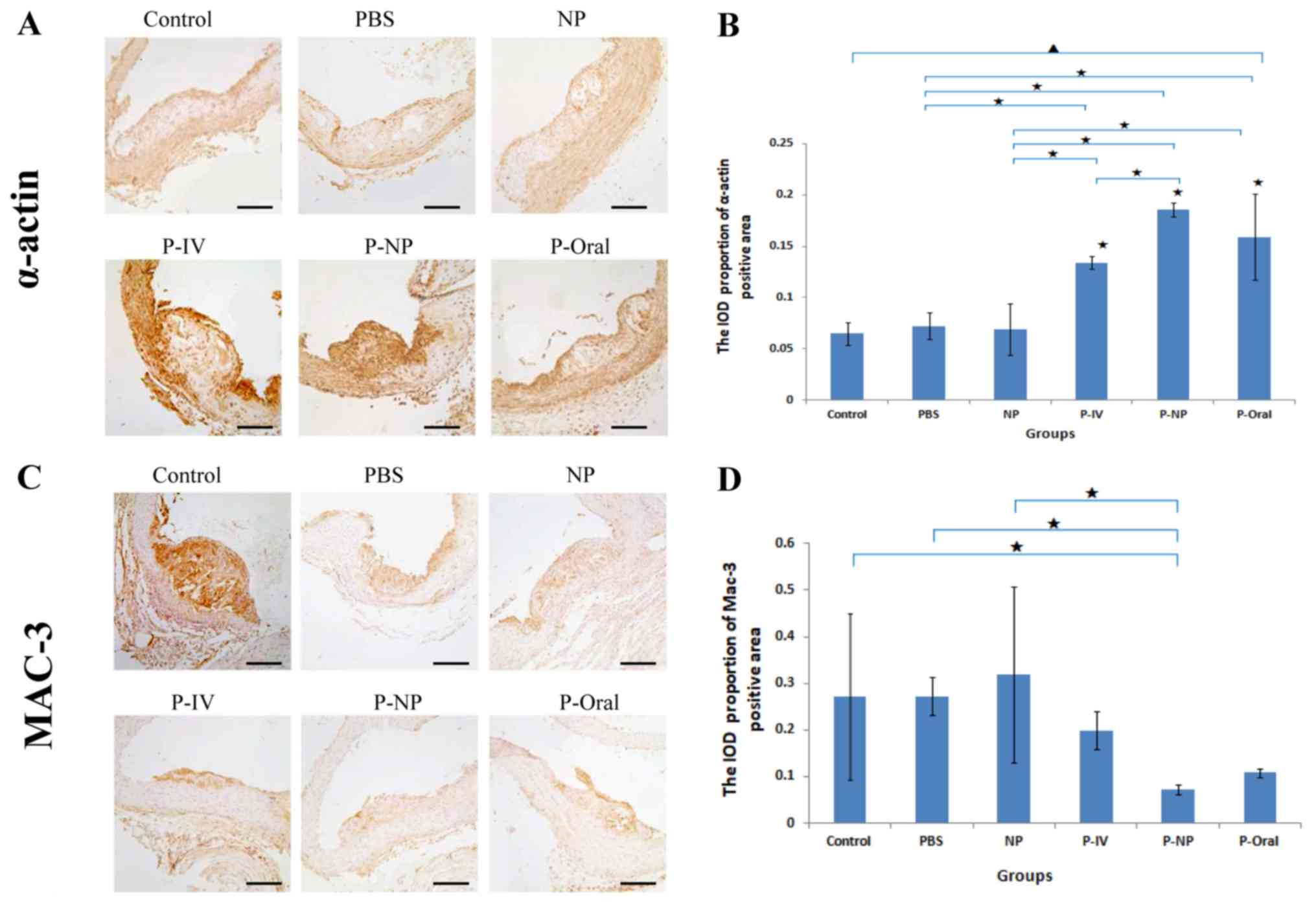 | Figure 4Effects of P-NP on the expression
levels of α-actin and Mac-3 in atherosclerotic plaques of aortas.
(A) Photomicrographs of cross sections of aortic roots
immunohistochemically stained with α-actin. (B) Effects of P-NP on
the α-actin-positive area (n=4). (C) Photomicrographs of
cross-sections of aortic roots immunohistochemically stained with
Mac-3. (D) Effects of P-NP on the Mac-3-positive area (n=4). Scale
bar, 100 µm. ▲P<0.05 among all groups;
«P<0.05 vs. control or as indicated. Mac-3,
Macrophage-3; PBS, phosphate-buffered saline; NP, nanoparticle;
P-IV, pitavastatin intravenous; P-NP, pitavastatin nanoparticle;
P-Oral, pitavastatin oral.; SR-A1, scavenger receptor-A1; ACAT-1,
cholesterol acyltransferase 1; IOD, integrated optical density. |
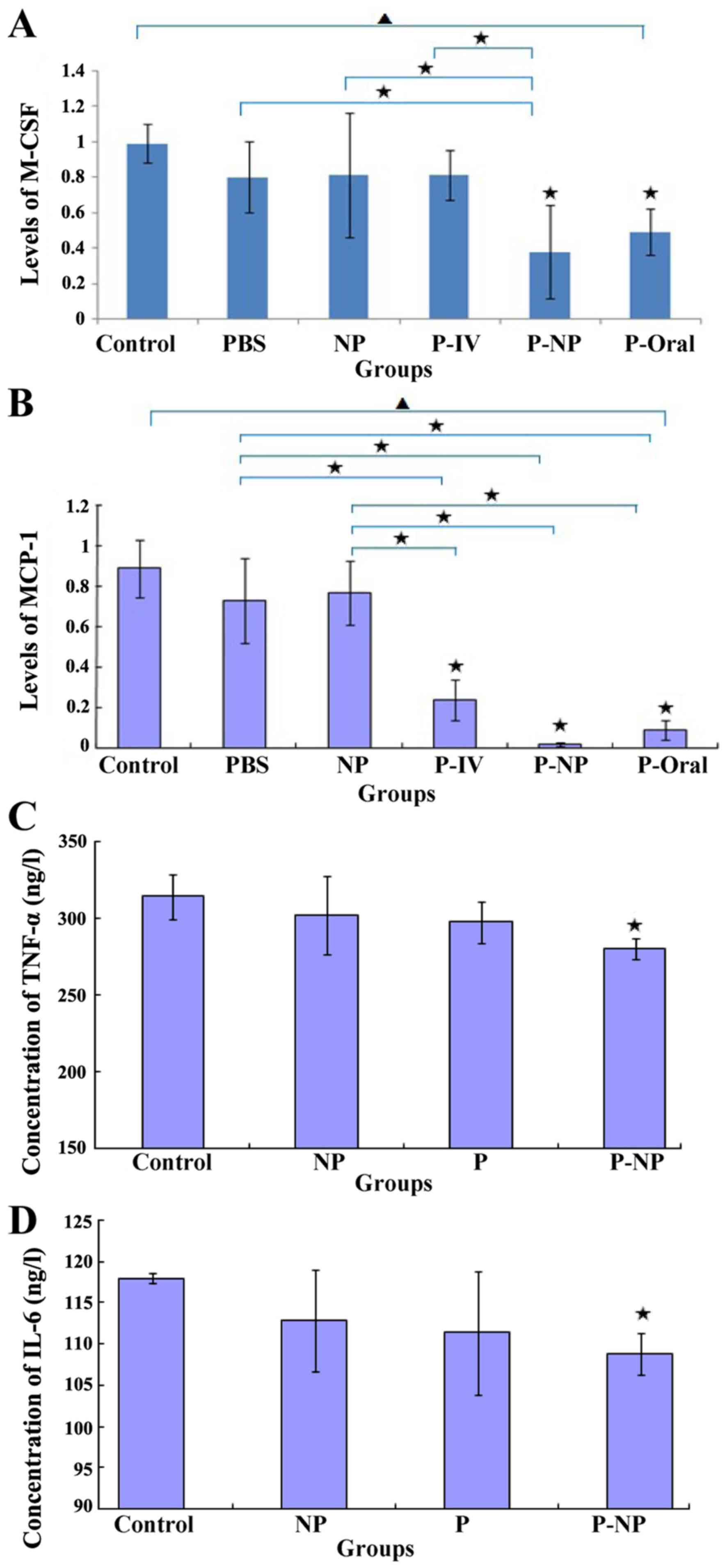
Effects of P-NP on ALT, CK-MB and
lipid levels in the ApoE-knockout mice and CE in THP-1-derived
macrophages
ELISA results demonstrated that the ALT level was
significantly lower in the P-NP group compared with that in the
P-oral group (Fig. 5A) and that the
CK-MB level was lower in the P-NP group compared with the P-Oral
group, although this was not statistically significant (Fig. 5B). Additionally, no significant
difference was observed in the TG, TC or HDL-C levels between the
P-NP and the other groups (Fig. 5C,
D and F). The LDL-C level was significantly lower
in the P-IV, P-NP and P-Oral groups compared with the control
group; however, there were no significant differences in the LDL-C
level among the P-IV, P-NP and P-Oral groups (Fig. 5E). In THP-1-derived macrophages, the
CE level was significantly lower in the P-NP group compared with
the control group (Fig. 5G). The
results suggested that P-NP could reduce the adverse drug reactions
and P-NP has no advantage in terms of regulating lipid levels.
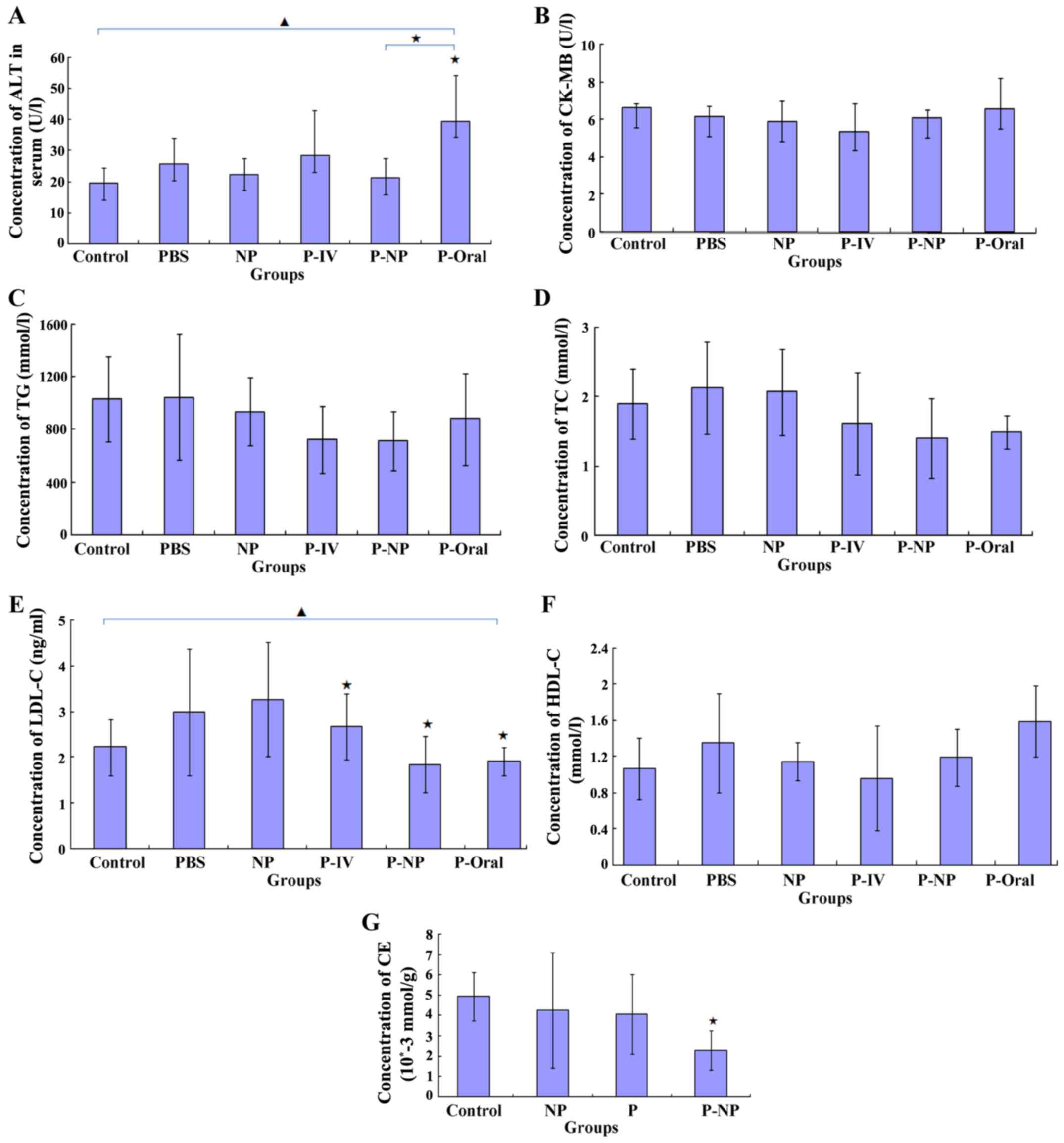 | Figure 5Effects of P-NP on biochemical
parameters. (A-F) Effects of P-NP on the levels of (A) ALT, (B)
CK-MB, (C) TG, (D) TC, (E) LDL-C, (F) HDL-C in the serum of the
ApoE-knockout mice (n=6). (G) The effects of P-NP on the levels of
intracellular CE in THP-1-derived macrophages (n=6).
▲P<0.05 among all groups; «P<0.05 vs.
control or as indicated. ALT, alanine transaminase; CK-MB, creatine
kinase-MB; TG, triglyceride; TC, total cholesterol; HDL-C, high
density lipoprotein cholesterol; LDL-C, low density lipoprotein
cholesterol; CE, cholesterol ester; PBS, phosphate-buffered saline;
NP, nanoparticle; P-IV, pitavastatin intravenous; P-NP,
pitavastatin nanoparticle; P-Oral, pitavastatin oral.; SR-A1,
scavenger receptor-A1; ACAT-1, cholesterol acyltransferase 1; ApoE,
apolipoprotein E. |
Effects of P-NP on the expression of
inflammatory factors
The in vivo mRNA expression levels of MCP-1
and M-CSF were significantly lower in the P-NP and P-oral groups
compared with those in the control, PBS and NP groups.
Additionally, the mRNA expression levels of M-CSF and MCP-1 were
lower in the P-NP group compared with the P-oral group (Fig. 6A and B). The in vitro protein expression
of TNF-α and IL-6 was significantly lower in the P-NP group
compared with the control group (Fig.
6C and D). P-NP significantly
reduced the expression of inflammatory factors in vivo and
in vitro.
Discussion
NP-DDS can be identified and swallowed by the
mononuclear phagocyte system (including monocytes, macrophage and
neutrophils), affecting the tissue/cell distribution and half-life
of drugs delivered by NP-DDS (12-14). The current
study demonstrated that NP carrier systems delivered pitavastatin
in a site-specific manner to atherosclerotic plaques and that a
fluorescent FITC signal was localized in atherosclerotic plaques
for up to 7 days after a single intravenous injection of FITC-NP
into ApoE-knockout mice. The results of the present study indicated
that intravenous injection of low doses of P-NP reduced the size of
atherosclerotic plaques and enhanced their stability, and reduced
the ALT and CK-MB levels in the blood. In addition, P-NP regulated
lipid metabolism and inhibited inflammatory reactions in THP-1
macrophages, exhibiting a significantly enhanced effect compared
with pitavastatin alone. These effects were determined to be equal
to or better than those resulting from a high dose of pitavastatin
administered orally in ApoE-knockout mice; additionally, P-NP was
demonstrated to be safer compared with large oral doses of
pitavastatin.
The effects of statins on arteriosclerosis are
mainly lipid-lowering and pleiotropic effects independent of lipid
lowering (15). A number of clinical
trials have demonstrated that long-term and high-dose application
of statins can decelerate the progression of atherosclerosis;
however, the way in which statins are used can increase the risk of
adverse effects and costs of medical treatment, thus reducing
compliance with this medication (16,17).
Therefore, in practical clinical applications, the prolonged use of
high doses of statins has limitations. In recent years, NP-DDSs
have been the focus of increasing attention and have been widely
applied in cardiovascular research. The use of NP-DDS in computed
tomography has been demonstrated to be helpful for detecting the
macrophage-rich arterial walls in animal models (18) and is being tested in clinical trials
(19). In addition, nanoscale
contrast enhancers for magnetic resonance imaging have been applied
to imaging of the formation process of atherosclerotic lesions and
myocardial infarction (20-22). A number of
studies have tested NP-DDS as an effective strategy for therapeutic
neovascularization in clinical limb ischemia (7,23),
pulmonary artery hypertension (6,24), vein
graft disease (25),
ischemia-reperfusion injury (26)
and coronary stents (9,27). These studies have confirmed that P-NP
at a 100 to 840-fold lower single dose appears to be as effective
as a cumulative systemic dose of pitavastatin (6,7). The
properties of NP-DDS may be the result of two major mechanisms: i)
Recognition and incorporation by the mononuclear phagocyte system
(monocytes, macrophages and neutrophils), which may affect the
blood circulation time and tissue/cell distribution of drugs
delivered by the NP-DDS; and ii) enhanced vascular permeability
contributing to enhanced drug delivery for atherosclerotic arterial
walls and macrophages (28). In the
present study, FITC-NP was still detected in the atherosclerotic
plaque 7 days after a single administration, leading to the
conclusion that FITC-NP may be recognized and incorporated by
macrophages. The NP-DDS is likely retained in the macrophage
cytoplasm for a prolonged period (≥7 days), with the encapsulated
drug being slowly released as the NPs are degraded in the
macrophages. This suggested that the persistence of NP-DDS in the
macrophages of plaques might serve an important role in the
treatment of atherosclerosis. In the present study, the mice in the
P-IV and P-NP groups received the same concentration of
pitavastatin by intravenous injection and P-NP; however, the
treatment effects were stronger in the P-NP group compared with
those in the P-IV group. This suggested that delivering
pitavastatin using an NP-DDS might increase its effectiveness.
Macrophages have been recognized as crucial
components of atherosclerotic initiation and progression (29). In addition, macrophage foam cell
formation reflects the disruption of the homeostatic mechanism that
controls the uptake, intracellular metabolism and efflux of
cholesterol within macrophages (30). SR-A1 and ACAT-1 are the key enzymes
of uptake and intracellular metabolism of cholesterol within
macrophages (30). Adjustment of
these enzymes may be used to inhibit macrophage foam cell formation
as a means of treating atherosclerosis. Studies have demonstrated
that statins attenuate macrophage foam cell formation as a result
of regulating cholesterol metabolism within macrophages (31). Inflammation is responsible for the
initiation and progression of atherosclerosis; MCP-1 recruits
monocytes to migrate to the subendothelial membrane, and M-CSF is
the main facilitator that induces monocyte differentiation into
macrophages. MCP-1 and M-CSF are proinflammatory factors that serve
important roles in the pathogenesis of atherosclerosis (30). Statins are considered to reduce
cardiovascular morbidity and mortality through anti-inflammatory
effects (32). An important finding
of the present study was that weekly intravenous administration of
P-NP in small doses significantly attenuated the development of
atherosclerotic plaques. The plaque area was the smallest in the
P-NP group compared with the other groups, which may have been
associated with the significantly lower levels of SR-A1, ACAT-1 and
inflammatory factors (MCP-1 and M-CSF). The in vitro results
further confirmed that P-NP significantly reduced the levels of
SR-A1, ACAT-1, IL-6 and TNF-α. These results suggested that the
advantage of anti-atherosclerosis treatment by P-NP may partly be
the result of the intensive adjustment of key cholesterol
metabolism enzymes and the anti-inflammatory action of P-NP. The
lipid levels (TG, TC, LDL-C and HDL-C) were not significantly lower
in the P-NP group compared with the other intervention groups
(including P-IV and P-Oral groups), suggesting that the advantages
of the P-NP treatment may be independent of its lipid-lowering
effect. The results of the present study suggested that NP-mediated
delivery of pitavastatin promoted plaque stability, which was
consistent with a previously published study (33); however, the research methods and
established mechanisms were not identical. Therefore, the
beneficial effects of P-NP on atherosclerosis may be attributable
to the pleiotropic effects of pitavastatin, independent of its
lipid-lowering effect, including the inhibition of the key enzymes
of cholesterol metabolism and inflammation.
The present study evaluated whether a small dose of
P-NP may be superior to a large oral dose of pitavastatin in
preventing the progression of atherosclerosis. The results
demonstrated that weekly administration of 0.4 mg/kg P-NP
intravenously for 12 weeks significantly decelerated the
progression of atherosclerosis and increased the stability of the
atheromatous plaques, the effect of small doses P-NP on
atherosclerosis is as large or larger than daily oral large dose of
pitavastatin (1 mg/kg for 84 days). The cumulative effect of
intravenous P-NP appeared to be as effective at a ≥17.5-fold in
oral pitavastatin. Additionally, the ALT level was significantly
lower in the P-NP group compared with the P-Oral group, indicating
that P-NP is safer compared with pitavastatin alone.
In conclusion, the results of the present study
demonstrated that the use of an NP-DDS for the delivery of
pitavastatin appeared to have advantages in attenuating the
progression of atherosclerosis and improving the stability of
plaques compared with pitavastatin alone. The present results may
be limited by species and research period. Despite certain
limitations, the present study indicated that NP-mediated delivery
of pitavastatin may exert favorable effects in patients with
atherosclerosis. In summary, NP-mediated delivery of pitavastatin
may provide a new targeting agent that is more feasible, effective
and safer for the treatment of atherosclerotic plaques. The present
study provided experimental evidence to support potential clinical
applications. However, further research is needed to confirm
whether P-NP may be safer and more effective in treating
arteriosclerosis compared with pitavastatin alone.
Supplementary Material
The surface receptor expression of
CD11b in the THP-1-derived macrophages differentiated with 100
ng/ml PMA. (A) THP-1 cells stained using anti-CD11b antibodies were
analyzed by flow cytometry. THP-1 cells were differentiated for two
days (green) in the presence of PMA (100 ng/ml), and the
undifferentiated THP-1 cells were used as the control group
(black). (B) Quantification of MFI for CD11b. n=3. *P<0.001.
PMA, phorbol-12-myristate-13-acetate; MFI, mean fluorescence
intensity.
Acknowledgements
The authors would like to thank Professor Kensuke
Egashira (Department of Cardiovascular Research, Development, and
Translational Research, Kyushu University, Fukuoka, Japan) for
providing the NP, FITC-NP and P-NP for our study.
Funding
This study was supported by Grants-in-Aid for The
LiaoNing Science and Technology Project (grant no. 2013020200-206),
The Key Laboratory of Myocardial Ischemia, Chinese Ministry of
Education (grant no. KF201306) and National Natural Science
Foundation of China (grant nos.: 81200083 and 81300038).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YJS performed most experiments and analyzed the
data, and was a major contributor in designing and writing the
manuscript. LC and SJZ performed the animal experiments. LYS
performed the cell experiments. HL collected and analyzed the data.
WT analyzed and reviewed the results. GXQ made substantial
contributions to conception and design of the study and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by The Institutional Animal
Care and Use Committee (IACUC issue no. 2019211), China Medical
University. The study followed internationally recognized
guidelines on animal welfare, as well as local and national
regulations.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Baigent C, Keech A, Kearney PM, Blackwell
L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, et
al: Efficacy and safety of cholesterol-lowering treatment:
Prospective meta-analysis of data from 90,056 participants in 14
randomised trials of statins. Lancet. 366:1267–1278.
2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kawashiri MA, Yamagishi M, Sakamoto T,
Takayama T, Hiro T, Daida H, Hirayama A, Saito S, Yamaguchi T,
Matsuzaki M and COSMOS Investigators: Impact of intensive lipid
lowering on lipid profiles over time and tolerability in stable
coronary artery disease: Insights from a subanalysis of the
coronary atherosclerosis study measuring effects of rosuvastatin
using intravascular ultrasound in Japanese subjects (COSMOS).
Cardiovasc Ther. 31:335–343. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wakabayashi K, Nozue T, Yamamoto S,
Tohyama S, Fukui K, Umezawa S, Onishi Y, Kunishima T, Sato A,
Miyake S, et al: Efficacy of statin therapy in inducing coronary
plaque regression in patients with low baseline cholesterol levels.
J Atheroscler Thromb. 23:1055–1066. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nissen SE, Nicholls SJ, Sipahi I, Libby P,
Raichlen JS, Ballantyne CM, Davignon J, Erbel R, Fruchart JC,
Tardif JC, et al: Effect of very high-intensity statin therapy on
regression of coronary atherosclerosis: The ASTEROID trial. JAMA.
295:1556–1565. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Matoba T and Egashira K:
Nanoparticle-mediated drug delivery system for cardiovascular
disease. Int Heart J. 55:281–286. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chen L, Nakano K, Kimura S, Matoba T,
Iwata E, Miyagawa M, Tsujimoto H, Nagaoka K, Kishimoto J, Sunagawa
K and Egashira K: Nanoparticle-mediated delivery of pitavastatin
into lungs ameliorates the development and induces regression of
monocrotaline-induced pulmonary artery hypertension. Hypertension.
57:343–350. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kubo M, Egashira K, Inoue T, Koga J, Oda
S, Chen L, Nakano K, Matoba T, Kawashima Y, Hara K, et al:
Therapeutic neovascularization by nanotechnology-mediated
cell-selective delivery of pitavastatin into the vascular
endothelium. Arterioscler Thromb Vasc Biol. 29:796–801.
2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Oda S, Nagahama R, Nakano K, Matoba T,
Kubo M, Sunagawa K, Tominaga R and Egashira K:
Nanoparticle-mediated endothelial cell-selective delivery of
pitavastatin induces functional collateral arteries (therapeutic
arteriogenesis) in a rabbit model of chronic hind limb ischemia. J
Vasc Surg. 52:412–420. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tsukie N, Nakano K, Matoba T, Masuda S,
Iwata E, Miyagawa M, Zhao G, Meng W, Kishimoto J, Sunagawa K and
Egashira K: Pitavastatin-incorporated nanoparticle-eluting stents
attenuate in-stent stenosis without delayed endothelial healing
effects in a porcine coronary artery model. J Atheroscler Thromb.
20:32–45. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Starr T, Bauler TJ, Malik-Kale P and
Steele-Mortimer O: The phorbol 12-myristate- 13-acetate
differentiation protocol is critical to the interaction of THP-1
macrophages with Salmonella Typhimurium. PLoS One.
13(e0193601)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Daley JM, Thomay AA, Connolly MD, Reichner
JS and Albina JE: Use of Ly6G-specific monoclonal antibody to
deplete neutrophils in mice. J Leukoc Biol. 83:64–70.
2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lee PY, Wang JX, Parisini E, Dascher CC
and Nigrovic PA: Ly6 family proteins in neutrophil biology. J
Leukoc Biol. 94:585–594. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Leuschner F, Dutta P, Gorbatov R,
Novobrantseva TI, Donahoe JS, Courties G, Lee KM, Kim JI, Markmann
JF, Marinelli B, et al: Therapeutic siRNA silencing in inflammatory
monocytes in mice. Nat Biotechnol. 29:1005–1010. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Banfi C, Baetta R, Gianazza E and Tremoli
E: Technological advances and proteomic applications in drug
discovery and target deconvolution: Identification of the
pleiotropic effects of statins. Drug Discov Today. 22:848–869.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Helin-Salmivaara A, Lavikainen PT,
Korhonen MJ, Halava H, Martikainen JE, Saastamoinen LK, Virta L,
Klaukka T and Huupponen R: Pattern of statin use among 10 cohorts
of new users from 1995 to 2004: A register-based nationwide study.
Am J Manag Care. 16:116–122. 2010.PubMed/NCBI
|
|
17
|
Jackevicius CA, Mamdani M and Tu JV:
Adherence with statin therapy in elderly patients with and without
acute coronary syndromes. JAMA. 288:462–467. 2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bhavane R, Badea C, Ghaghada KB, Clark D,
Vela D, Moturu A and Annapragada A, Johnson GA, Willerson JT and
Annapragada A: Dual-energy computed tomography imaging of
atherosclerotic plaques in a mouse model using a liposomal-iodine
nanoparticle contrast agent. Circ Cardiovasc Imaging. 6:285–294.
2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Weissleder R, Nahrendorf M and Pittet MJ:
Imaging macrophages with nanoparticles. Nat Mater. 13:125–138.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Mulder WJ and Fayad ZA: Nanomedicine
captures cardiovascular disease. Arterioscler Thromb Vasc Biol.
28:801–802. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Leuschner F and Nahrendorf M: Molecular
imaging of coronary atherosclerosis and myocardial infarction:
Considerations for the bench and perspectives for the clinic. Circ
Res. 108:593–606. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Michalska M, Machtoub L, Manthey HD, Bauer
E, Herold V, Krohne G, Lykowsky G, Hildenbrand M, Kampf T, Jakob P,
et al: Visualization of vascular inflammation in the
atherosclerotic mouse by ultrasmall superparamagnetic iron oxide
vascular cell adhesion molecule-1-specific nanoparticles.
Arterioscler Thromb Vasc Biol. 32:2350–2357. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nagahama R, Matoba T, Nakano K,
Kim-Mitsuyama S, Sunagawa K and Egashira K: Nanoparticle-mediated
delivery of pioglitazone enhances therapeutic neovascularization in
a murine model of hindlimb ischemia. Arterioscler Thromb Vasc Biol.
32:2427–2434. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kimura S, Egashira K, Chen L, Nakano K,
Iwata E, Miyagawa M, Tsujimoto H, Hara K, Morishita R, Sueishi K,
et al: Nanoparticle-mediated delivery of nuclear factor kappaB
decoy into lungs ameliorates monocrotaline-induced pulmonary
arterial hypertension. Hypertension. 53:877–883. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kimura S, Egashira K, Nakano K, Iwata E,
Miyagawa M, Tsujimoto H, Hara K, Kawashima Y, Tominaga R and
Sunagawa K: Local delivery of imatinib mesylate
(STI571)-incorporated nanoparticle ex vivo suppresses vein graft
neointima formation. Circulation. 118(14 Suppl):S65–S70.
2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Nagaoka K, Matoba T, Mao Y, Nakano Y,
Ikeda G, Egusa S, Tokutome M, Nagahama R, Nakano K, Sunagawa K and
Egashira K: A new therapeutic modality for acute myocardial
infarction: Nanoparticle-mediated delivery of pitavastatin induces
cardioprotection from ischemia-reperfusion injury via activation of
PI3K/Akt pathway and anti-inflammation in a rat model. PLoS One.
10(e0132451)2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nakano K, Egashira K, Masuda S, Funakoshi
K, Zhao G, Kimura S, Matoba T, Sueishi K, Endo Y, Kawashima Y, et
al: Formulation of nanoparticle-eluting stents by a cationic
electrodeposition coating technology: Efficient nano-drug delivery
via bioabsorbable polymeric nanoparticle-eluting stents in porcine
coronary arteries. JACC Cardiovasc Interv. 2:277–283.
2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Matoba T, Koga JI, Nakano K, Egashira K
and Tsutsui H: Nanoparticle-mediated drug delivery system for
atherosclerotic cardiovascular disease. J Cardiol. 70:206–211.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang W, Yancey PG, Su YR, Babaev VR,
Zhang Y, Fazio S and Linton MF: Inactivation of macrophage
scavenger receptor class B type I promotes atherosclerotic lesion
development in apolipoprotein E-deficient mice. Circulation.
108:2258–2263. 2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
McLaren JE, Michael DR, Ashlin TG and
Ramji DP: Cytokines, macrophage lipid metabolism and foam cells:
Implications for cardiovascular disease therapy. Prog Lipid Res.
50:331–347. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Leon C, Hill JS and Wasan KM: Potential
role of acyl-coenzyme A: Cholesterol transferase (ACAT) inhibitors
as hypolipidemic and antiatherosclerosis drugs. Pharm Res.
22:1578–1588. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Berman JP, Farkouh ME and Rosenson RS:
Emerging anti-inflammatory drugs for atherosclerosis. Expert Opin
Emerg Drugs. 18:193–205. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Katsuki S, Matoba T, Nakashiro S, Sato K,
Koga J, Nakano K, Nakano Y, Egusa S, Sunagawa K and Egashira K:
Nanoparticle-mediated delivery of pitavastatin inhibits
atherosclerotic plaque destabilization/rupture in mice by
regulating the recruitment of inflammatory monocytes. Circulation.
129:896–906. 2014.PubMed/NCBI View Article : Google Scholar
|