Introduction
The liver, which is the first organ to deliver
nutrients and toxins from the intestines through the portal vein
after consumption, is responsible for various biological process
including metabolizing nutrients and toxic compounds such as
alcohol and drugs (1). Since the
liver plays a crucial role in eliminating toxic compounds, it is
susceptible to damage by toxins such as alcohol, bacteria, and
viruses (1). The insults from the
toxins can induce acute liver damage via increased oxidative stress
and inflammation, and this can then progress to hepatitis, hepatic
cancer, and liver cirrhosis (2).
Accordingly, acute liver damage needs to be treated at an early
stage and it is important to explore effective therapeutic
strategies to recover from or protect against acute liver
damage.
Hepatic damage is induced by various chemicals
including alcohol, bacteria, and viruses. Several chemicals
including carbon tetrachloride (CCl4), D-galactose, and
lipopolysaccharide have been used to induce acute hepatic damage in
experimental animal models (1,3,4). Among these chemicals, D-galactosamine
derived from D-galactose works as a hepatotoxic compound to induce
hepatocyte necrosis and inflammation. D-galactosamine decreases the
cellular uridine-5'-triphosphate (UTP) concentration and reduces
the synthesis of hepatocyte RNA to block transcription in the liver
(5). D-galactosamine injection
results in a condition that resembles human viral hepatitis.
D-galactosamine is generally accepted for production of animal
models for acute liver damage because of its good reproducibility
and easy dosage control (3).
Mulberry (Morus alba L.) fruits contain
anthocyanins, which have anti-oxidant and anti-inflammation
properties. Mulberry fruit extracts have been reported to protect
against liver damage caused by tetrachloride, lipopolysaccharide,
and oxidative stress (2,6,7). In
addition, mulberry extracts improve alcohol-induced steatosis by
alleviating gut microbiota (4). The
amelioration of liver damage is mainly associated with reduction of
oxidative stress and inflammation via changes in the gut microbiota
(4,6). Silk amino acids have been shown to have
anti-inflammatory activities and to accelerate the removal of
toxins such as alcohol and acetaldehyde (8). Thus, both mulberry extracts and silk
amino acids protect against acute liver damage caused by toxins and
they may alleviate acute liver damage additively or
synergistically. In the present study, we hypothesized that a
mixture of mulberry water extracts and silk amino acids may protect
against acute liver damage in rats. This hypothesis was tested in
rats treated with intraperitoneal injection of D-galactosamine and
the action mechanism was explored. We found that D-galactosamine
acutely induced liver injury with decreased glycogen deposition and
increased triglyceride accumulation in the liver. The mixture of
mulberry and silk amino acids ameliorated liver damage by
potentiating anti-oxidant enzymes and reducing proinflammatory
cytokines.
Materials and methods
Effects of HepG2 cell line on cell
damage
Human hepatocellular carcinoma HepG2 cells were
acquired from American Type Culture Collection (HB-8065) were grown
and maintained with high glucose Dulbecco's modified Eagle's medium
(DMEM) containing 10% fetal bovine serum (FBS) (9). Cells were then transferred into 96-well
plates at 4x103 cells/well in high-glucose DMEM
containing 0.3% bovine serum albumin (BSA) and allowed to grow to
70% confluence. The cells were then treated with vehicle, 10 or 50
µg/ml mulberry extracts (MB), silk amino acids (SA) or their
mixtures (MS1:2, MS1:3, MS1:4, and MS1:5). After 1 h of treatment,
50 mM D-galactosamine was added to the cells and they were
incubated for another 8 h. The cell viability was then measured in
HepG2 cells by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl
tetrazolium bromide (MTT) assay using an Aureon plate reader
(Aureon Biosystems).
HepG2 cells were grown in 24-well plates at
6x104 cells/well until the cells were 70% confluent,
then treated with vehicle, 10 or 50 µg ml-1 mulberry
extracts (MB), silk amino acids (SA) or their mixtures (MS1:2,
MS1:3, MS1:4, and MS1:5) for 1 h. The cells were subsequently
treated with 50 mmol/l D-galactosamine and incubated for 8 h. Next,
cells were harvested with lysis buffer at 4˚C and centrifuged at
10,000 x g for 20 min at 4˚C, which was followed by collection of
the supernatants. Lipid peroxide levels as malondialdehyde (MDA)
contents in the liver were then measured using a thiobarbituric
acid reactive substance (TBARS) assay kit (Cayman Chemical).
Relative mRNA levels of tumor necrosis factor-α (TNF-α) were
measured by real-time polymerase chain reaction (PCR) in samples
treated with vehicle, 50 µg ml-1 mulberry extracts (MB),
silk amino acids (SA) or their mixtures (MS1:2, MS1:3, MS1:4, and
MS1:5).
Animal care and experimental
design
Male Sprague Dawley rats that weighed an average of
235±12 g were purchased from Daehan Bio, Inc. and acclimated in the
animal facility. All rats were raised in individual stainless steel
cages in a controlled environment (23˚C, 12-h light/dark cycle) and
had ad libitum access to food and water. All study procedures were
adopted based on the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health (NIH) and were
approved by the Institutional Animal Care and Use Committee of
Hoseo University (HSIACUC-17-068).
After 1-week of acclimation in the animal facility,
50 male rats received a single intraperitoneal injection of
D-galactosamine saline solution (800 mg/kg bw) to induce acute
liver damage. The rats were then randomly divided into five groups:
1) low dosage (200 mg/kg body weight) of mulberry extracts and silk
amino acid (1:3, MS1:3-L, or 1:5, MS1:5-L), 2) high dosage (600
mg/kg body weight) of mulberry extracts and silk amino acid (1:3,
MS1:3-H, or 1:5, MS1:5-H) and 3) cellulose (control). Ten rats
received a single intraperitoneal injection of saline solution.
Animals were given free access to water and 40 energy percent diet
containing mulberry extracts and silk amino acid during the 1-week
experimental period.
At the end of the experiment, epididymal and
retroperitoneal fat mass and uteruses were removed and weighed
after rats were sacrificed after anesthesia by subcutaneous
injection of a mixture of ketamine and xylazine (100 and 10 mg/kg
body weight, respectively). After blood collection, human insulin
(5 U/kg body weight) was injected through the inferior vena cava to
determine insulin signaling in the liver. Serum samples were then
stored at -70˚C for biochemical analysis. Serum was separated after
centrifugation of the blood and the serum levels of alanine
aminotransferase (ALT), aspartate aminotransferase (AST) and
γ-glutamyl transpeptidase (γ-GPT), which are markers for liver
damage, were measured by colorimetric methods using kits obtained
from Asan Pharmaceutical company (Seoul, Korea). Livers were
collected and stored at -70˚C for further study.
The livers were homogenized with 1.5N perchloric
acid and the lysates were treated with α-amyloglucosidase to
hydrolyze glycogen. The hydrolysate was then neutralized with NaOH
to pH 7.4 and centrifuged at 3000 rpm for 10 min, after which the
glucose concentration was measured using a glucose oxidase kit
(Young Dong Pharm.) and liver glycogen was calculated from the
glucose concentrations. Triacylglycerol was extracted from the
livers and brains with chloroform-methanol (2:1, vol/vol) and
resuspended in pure chloroform. After evaporating chloroform, the
residues were suspended with PBS containing 0.1% triton X-100 and
then sonicated and boiled for 5 min. Finally, the triacylglycerol
contents of the suspensions were assayed using a Trinder kit (Young
Dong Pharm.) (10).
Oxidative stress status
Lipid peroxide levels as MDA in the liver were
measured using a TBARS assay kit (Cayman Chemical). Triglyceride
(TG) contents were also measured in the liver and brain using a TG
kit (Asan Pharmaceutical). TNF-α levels in the liver lysate were
measured using ELISA kits (R & D Systems, Minneapolis, MN and
Amersham Biosciences, Piscataway, NJ, USA). The activities of
antioxidant enzymes such as Cu/Zn superoxide dismutase (SOD1) and
glutathione (GSH)-peroxidase were determined from the lysates of
liver tissues using colorimetry kits (Cayman Chemical and
Biovision), respectively (11). One
unit of each enzyme activity was defined as 50% inhibition of each
enzyme reaction and the enzyme activity was normalized by mg
protein in the lysate. Additionally, GSH levels in the liver were
determined using a GSH assay kit (Sigma-Aldrich; Merck KGaA).
Isolation of liver total RNA and
real-time PCR
Livers were powdered with a cold steel mortar and
pestle, then mixed with a monophasic solution of phenol and
guanidine isothiocyanate (TRIzol reagent; Gibco; Thermo Fisher
Scientific, Inc.) for total RNA extraction, which was conducted
according to the manufacturer's instructions. cDNA was synthesized
from equal amounts of total RNA using superscript III reverse
transcriptase, and polymerase chain reaction (PCR) was performed
with high-fidelity Taq DNA polymerase. Equal amounts of cDNA were
added to SYBR Green mix (Bio-Rad Laboratories, Inc.) and amplified
using a real-time PCR instrument (Bio-Rad Laboratories, Inc.). The
expression levels of the genes of interest were normalized to those
of the housekeeping gene β-actin. To assess changes in the
expression of genes related to fatty acid synthesis and oxidation
in the liver, the following primers were used: tumor necrosis
factor (TNF)-α (forward 5'-CCCTGGTACTAACTCCCAGAAA-3', reverse
5'-TGTATGAGAGGGACGGAACC-3'), interleukin (IL)-1β (forward
5'-CACCTCTCAAGCAGAGCACAG-3', reverse 5'-GGGTTCCATGGTGAAGTCAAC-3'),
Cu/Zn superoxide dismutase (SOD; forward 5'-CACTCTAAGAAACATGGCG-3',
reverse 5'-CTGAGAGTGAGATCACACG-3'), GSH-Px (forward
5'-GCGGGCCCTGGCATTG-3', reverse 5'-GGACCAGCGCCCATCTG-3') and
β-actin (forward 5'-AGCGTGGCTACAGCTTCACC-3', reverse
5'-AAGTCTAGGGCAACATAGCACAGC-3'). The relative gene expression was
quantified using the comparative cycle of threshold (CT) method
(2-ΔΔCq method) as previously described by Livak and
Schmittgen (12).
Histological analysis
At the end of the experimental period, the liver
samples were fixed in 10% buffered neutral formaldehyde and
embedded in paraffin wax. Histological sections that were 6 µm
thick were stained with hematoxylin and eosin (H&E) and used to
score the liver damage. The liver damage and glycogen contents were
determined in two sections with a random selection from six
consecutive sections at x200 magnification. The liver damage was
scored by summing each item such as the nucleus size and shape,
cell size and arrangement and the number of macrophages in the
histological scoring system. Each item was scored as 0 (no change),
1 (mid), 2 (moderate) and 3 (severe) (4). Higher scores indicated more hepatic
cell damage. In addition, glycogen contents were determined by the
red color intensity in periodic acid-Schiff (PAS) staining of the
stomach tissues. Lower scores indicated higher glycogen contents
(4).
Statistical analysis
Statistical analysis was performed with SAS software
version 7 (SAS Institute, Inc.) and the results are expressed as
the means ± standard deviation. The variables with results from
different time points were analyzed with one-way ANOVA to assess
the metabolic effects of the mixture of mulberry extracts and silk
amino acids in male rats fed a high-fat diet. Multiple comparisons
between groups were identified by Tukey's test at P<0.05.
Results
Cell viability of HepG2 cell line
damaged by D-galactosamine
As shown in Fig. 1A,
D-galactosamine reduced cell viability in HepG2 cells and high
dosage pretreatment of SA and MB protected against the cell damage
caused by D-galactosamine. The mixture of MS1:4 and MS1:5 improved
the cell viability in D-galactosamine intoxicated HepG2 cells.
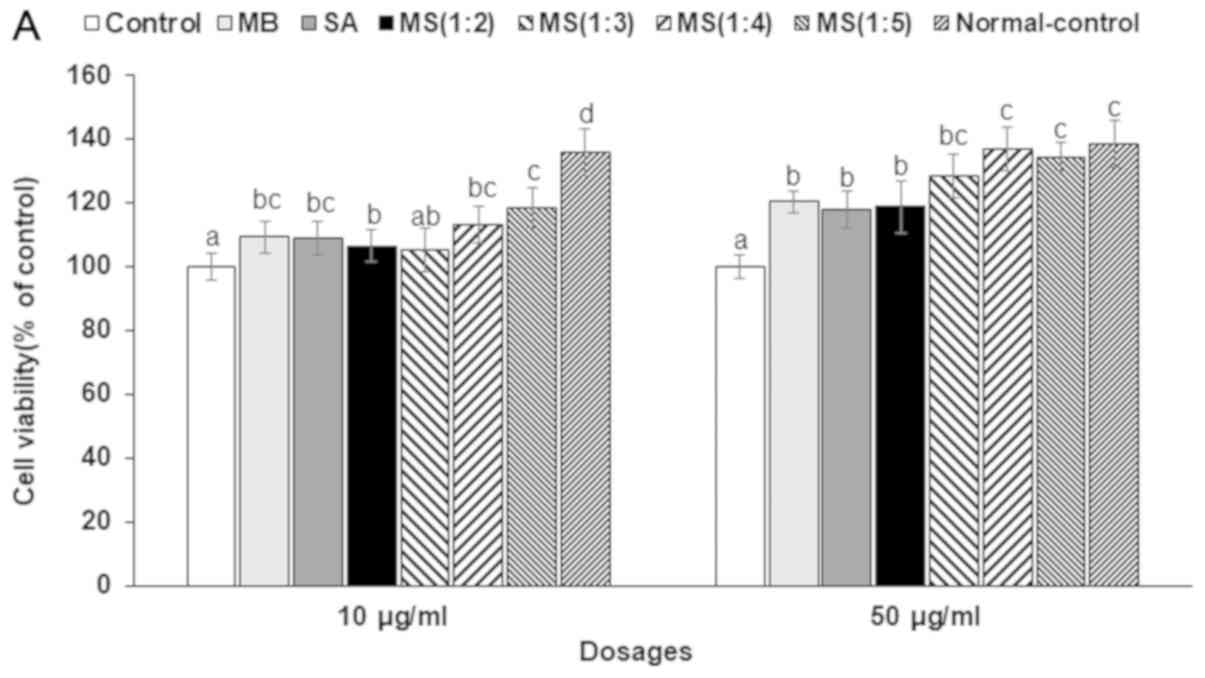 | Figure 1The effects of mulberry water extracts
and silk amino acids on HepG2 cells damaged by D-galactosamine.
HepG2 cells were treated with 0 (vehicle), 10 or 50 µg/ml MB, SA or
their mixtures (MS1:2, MS1:3, MS1:4, and MS1:5). After 1 h of
treatment, 50 mM D-galactosamine was added to the cells and they
were incubated for an additional 24 h. (A) Cell viability measured
by MTT assays. (B) Lipid peroxide levels measured as MDA. (C) Fold
changes of SOD1 and GSH-Px mRNA expression. (D) Fold changes of
IL-1β and TNF-α mRNA expression. *P<0.05,
**P<0.01 and
***P<0.001 vs. Control. MB,
mulberry extracts; SA, silk amino acids; MDA, malondialdehyde;
SOD1, superoxide dismutase 1; GSH-Px, glutathione peroxidase;
IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α. b P<0.05,
c P<0.01 and dP<0.001 vs. Control. |
Oxidative stress and inflammation in
HepG2 cell line
The cell damage was associated with increased
oxidative stress. Specifically, the MDA contents in HepG2 cells
were 2-fold higher in the HepG2 cells damaged with D-galactosamine
(control) than in the normal-controls (Fig. 1B). However, SA and MB reduced the
contents of MDA and lipid peroxide index induced by
D-galactosamine, with the high dosage mixture of SA and MB leading
to greater decreases than either compound alone.
Decreased MDA was related to increased anti-oxidant
enzyme expression. The SOD1 and GSH-Px expression were 2- and
1.7-fold lower, respectively, in the control group than the
normal-control group (Fig. 1C).
However, MB and SA (50 µg/ml) increased SOD1 and GSH-Px expression,
with MB leading to greater expression of both enzymes than SA. The
mixture of MS1:2, MS1:3, MS1:4 and MS1:5 elevated their expression
to levels similar to MB.
However, the expression of both proinflammatory
cytokines (IL-1β and TNF-α) was 1.7-fold higher in the control than
the normal-control. SA protected against the increase of IL-1β and
TNF-α expression better than MB and the mixture of MB and SA
reduced their expression (Fig. 1D).
MS1:5 decreased the expression of IL-1β and TNF-α as much as the
normal-control.
Energy metabolism in rats
Treatment with D-galactosamine for one week did not
change body weight. In addition, although MS treatments tended to
lower body weight, no significant differences were observed among
groups (Table I). Additionally,
there were no significant differences in energy intake between
control and normal-control groups and MS treatment did not alter
energy intake, indicating that D-galactosamine and MS treatments
did not alter food intake (Table I).
MB and SA intake were dependent on the food intake and the ratio
and dosage of MB and SA in the foods. Although body weight was not
significantly different between control and normal-control groups,
liver weight based on the body weight was higher in the control
than the normal-control group (Table
I). MS1:3-H suppressed the increase of liver weight in rats
injected with D-galactosamine.
 | Table IEnergy metabolism and mulberry and
silk amino acid intake. |
Table I
Energy metabolism and mulberry and
silk amino acid intake.
| Variables | Control (n=10) | MS1:3-L (n=10) | MS1:3-H (n=10) | MS1:5-L (n=10) | MS1:5-H (n=10) | Normal-control
(n=10) | P-valuea |
|---|
| Body weight at 1 week
(g) | 262±18 | 256±17 | 257±18 | 248±20 | 250±19 | 260±1 | 0.28 |
| Caloric intake
(kcal/day) | 70.9±7.4 | 67.1±6.8 | 68.7±6.2 | 65.7±6.6 | 68.4±7.1 | 68.1±4.8 | 0.17 |
| MB intake
(mg/day) | 0±0 |
16.5±1.7c |
50.9±5.1d |
9.5±1.1b |
30.7±2.5d | 0±0 | <0.001 |
| SA intake
(mg/day) | 0±0 |
33.0±3.5d | 102±10d |
40.3±4.2d | 122±13d | 0±0 | <0.001 |
| Liver weight
(weight %) | 4.3±0.1 | 4.1±0.2 |
3.8±0.2b | 4.2±0.2 | 4.1±0.2 |
3.7±0.1b | 0.03 |
Liver damage in rats
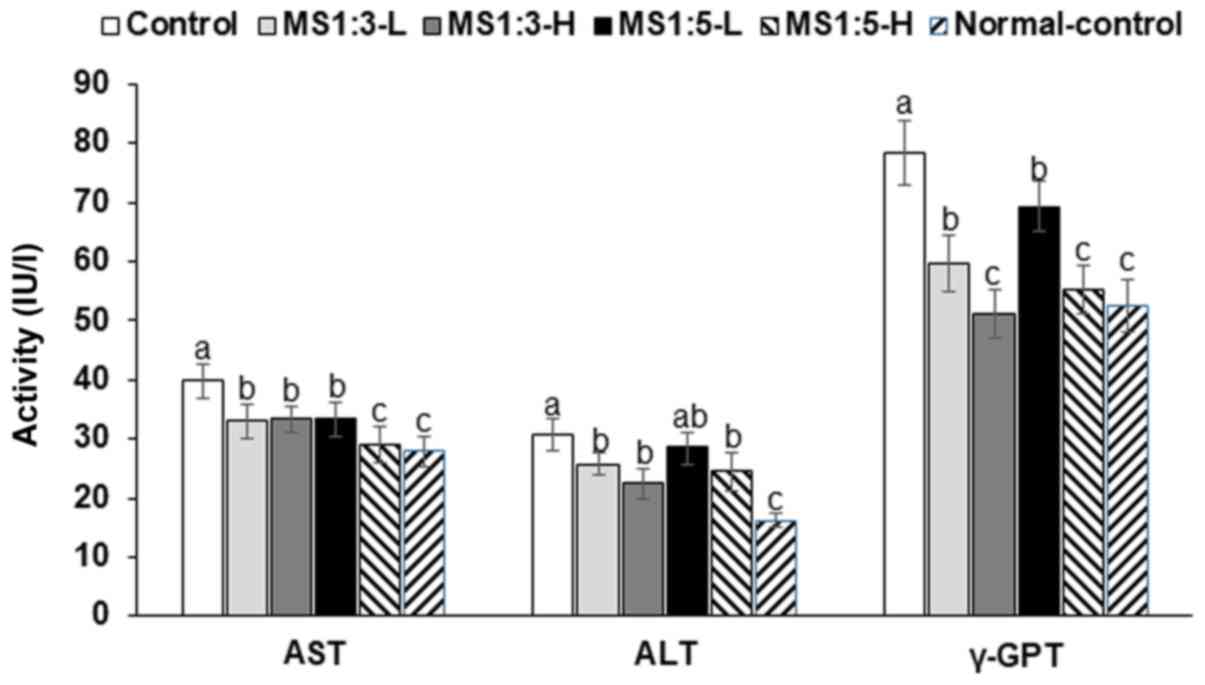
Serum AST and ALT levels, which are indexes of liver
damage, were higher in the control group than the normal-control
group (Fig. 2). However, MS1:3 and
MS1:5 suppressed these increases in a dose-dependent fashion. MS1:3
suppressed the decrease in serum ALT and AST levels more than
MS1:5. In addition, serum γ-GPT levels showed the same patterns as
serum AST and ALT levels. Additionally, serum TNF-α levels were
about 2-fold higher in the control than the normal-control group
(Table II), but MS1:3 and MS1:5
inhibited this increase. Taken together, these findings indicate
MS1:3 and MS1:5 reduced the liver damage induced by D-galactosamine
dose-dependently.
 | Table IILipid profiles and glucose metabolism
in the circulation. |
Table II
Lipid profiles and glucose metabolism
in the circulation.
| Variables | Control (n=10) | MS1:3-L (n=10) | MS1:3-H (n=10) | MS1:5-L (n=10) | MS1:5-H (n=10) | Normal-control
(n=10) |
P-valuea |
|---|
| Serum TNF-α
(pg/ml) | 57.6±4.7 |
44.6±4.9b |
38.2±4.1c |
36.2±4.3c |
31.2±3.4d |
23.1±2.9d | 0.004 |
| Serum total
cholesterol (mg/dl) | 108±9.8 | 99.7±8.2 | 97.3±6.8 | 99.3±9.7 | 101±8.6 | 96.9±7.3 | 0.07 |
| Serum HDL-C
(mg/dl) | 18.7±1.7 | 19.7±1.8 | 19.0±1.5 | 18.5±1.6 | 19.7±1.8 | 18.5±1.5 | 0.09 |
| Serum LDL-C
(mg/dl) | 63.8±5.9 | 58.0±5.5 | 58.7±5.6 | 64.9±6.7 | 65.2±6.2 | 61.2±5.8 | 0.08 |
| Serum triglyceride
(mg/dl) | 127±11.4 |
110±10.7b |
97.8±9.4b |
79.1±8.4c |
80.7±7.6c |
83.2±7.1c | 0.01 |
| Serum glucose
(mg/dl) | 139.5±8.3 |
127.6±8.1b |
126.9±7.6b |
115.7±7.2b |
118.9±6.8b |
130.7±7.9b | 0.02 |
| Serum insulin
(pg/ml) | 1.97±0.16 |
1.69±0.17b |
1.74±0.15b |
1.61±0.14b |
1.56±0.17b |
1.83±0.15b | 0.03 |
| HOMA-IR | 11.0±1.0 |
8.6±0.8b |
8.8±0.7b |
7.5±0.6c |
7.4±0.6c |
9.6±0.9b | 0.01 |
The liver plays a crucial role in lipid metabolism
and liver damage alters the metabolism. Serum total, high-density
lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol
levels were not significantly different following treatment with
D-galactosamine and MS (Table II).
However, serum triglyceride levels were elevated by D-galactosamine
injection and reduced by MS treatment. Treatment with MS1:5
decreased serum triglyceride levels compared to MS1:3 (Table II). In addition, triglyceride
deposition in the liver was elevated in the control group compared
to the normal-control group and MS1:3 and MS1:5 inhibited this
increase (Table III). In contrast,
glycogen deposition in the liver was lower in the control than the
normal-control and MS1:3 and MS1:5 prevented the decrease induced
by D-galactosamine injection (Table
III). Thus, D-galactosamine injection damaged the liver
tissues, which resulted in disturbance of triglyceride metabolism
but not cholesterol metabolism.
 | Table IIIHepatic lipid peroxides, antioxidant
enzyme activities, and triglyceride and glycogen deposition. |
Table III
Hepatic lipid peroxides, antioxidant
enzyme activities, and triglyceride and glycogen deposition.
| Variables | Control (n=10) | MS1:3-L (n=10) | MS1:3-H (n=10) | MS1:5-L (n=10) | MS1:5-H (n=10) | Normal-control
(n=10) |
P-valuea |
|---|
| Liver TG (mg/g
tissue) | 88.6±5.9 |
82.8±5.3b |
81.2±4.6b | 86.2±4.6 |
82.3±6.2b |
79.3±5.8b | 0.04 |
| Liver glycogen
(mg/g tissue) | 22.2±3.6 |
33.7±5.1c |
31.1±4.8c |
34.5±4.5c |
32.1±5.1c |
38.1±4.0d | 0.001 |
| Hepatic MDA
(nmol/mg protein) | 0.96±0.09 |
0.75±0.08b |
0.68±0.07c |
0.85±0.08b |
0.74±0.08b |
0.61±0.08c | 0.01 |
| Hepatic SOD (U/mg
protein) | 25.6±3.2 |
31.9±3.9b |
39.3±4.1c | 27.8±3.4 |
31.4±3.5b |
36.6±3.8b | 0.01 |
| Hepatic GSH
peroxide (U/mg protein) | 66.8±6.5 | 72.6±7.1 |
79.6±8.3b | 68.9±7.1 |
75.1±6.7b |
84.5±8.8b | 0.02 |
| Hepatic GSH (umol/g
protein) | 21.1±2.3 |
26.4±2.4b |
29.3±2.5b | 24.1±2.7 |
26.2±2.3b |
29.4±2.5b | 0.02 |
| Hepatic TNF-α (pg/g
tissue) | 9.5±0.9 | 8.7±0.9 |
7.6±0.7b |
8.0±0.9b |
6.8±0.7b |
4.1±0.5c | 0.004 |
Liver histology in rats
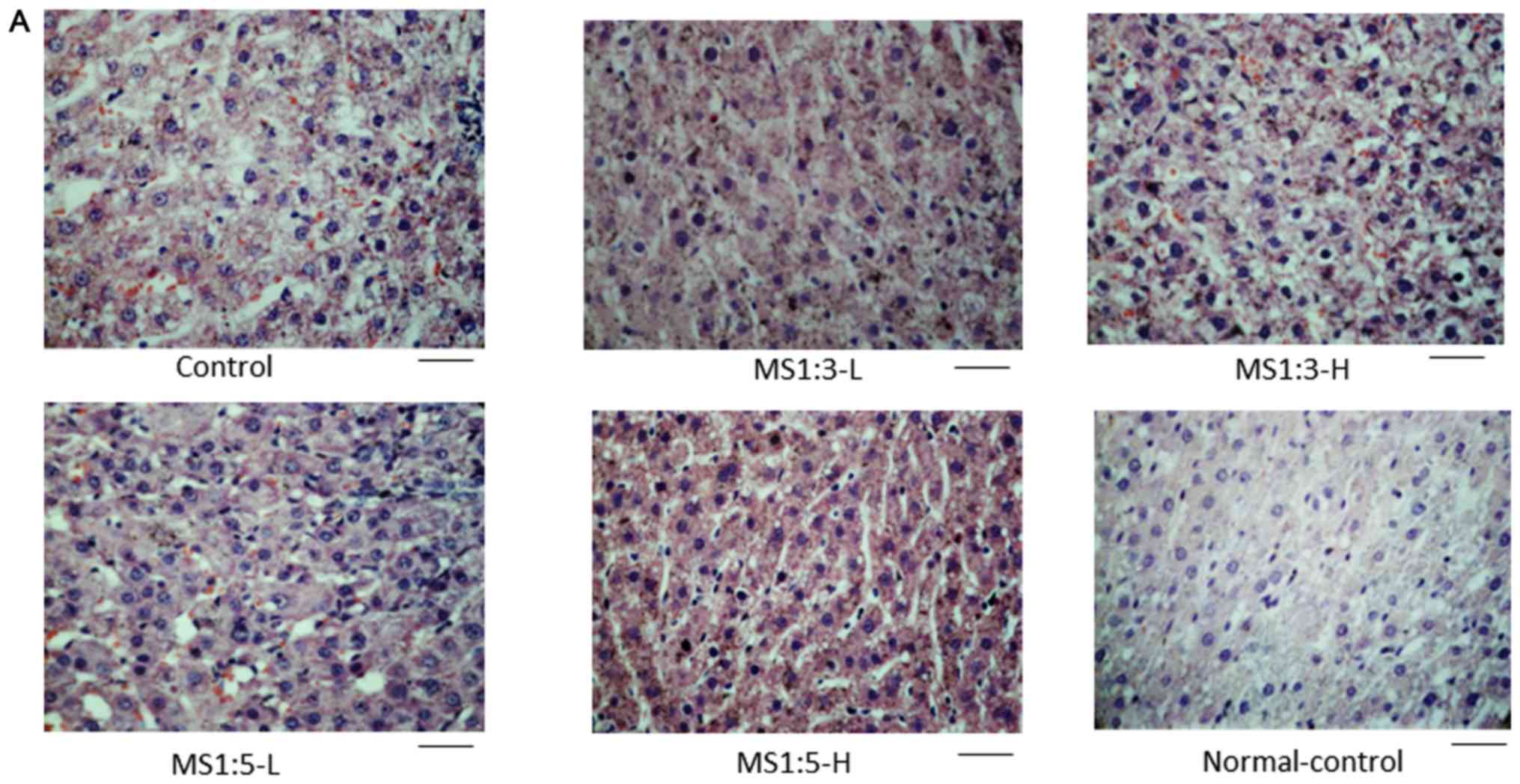
H&E staining showed that D-galactosamine
disrupted the hepatocytes (Fig. 3A).
The nuclei of the hepatocytes were enlarged, the cellular
arrangement was disrupted and the macrophage infiltration was
elevated compared to the control (Fig.
3A and B). The results indicated
that D-galactose induced hepatocyte damage. MS1:3 and MS1:5
protected against hepatocyte damage (Fig. 3B). Macrophage infiltration was also
reduced by MS1:3 and MS1:5 (Fig.
3B).
D-galactosamine depleted glycogen deposition in the
control group in comparison to the normal-control (Fig. 3B, C).
MS1:3 and MS1:5 increased the glycogen deposition and MS1:5-H
increased glycogen deposition more than the normal-control
(Fig. 3B and C).
Oxidative stress in the liver of the
rats
The amount of MDA, an index of lipid peroxide, was
higher in the control group than the normal-control group (Table III). The activity of SOD, which is
an enzyme that removes superoxide, was lower in the control group
than the normal-control group. MS1:3 dose-dependently increased the
SOD activity as did MS1:5. In parallel with SOD, GSH-Px activity
was also lower in the control than the normal-control and MA1:5
suppressed this decrease (Table
III). Evaluation of the hepatic level of GSH revealed that the
substrate of GSH-Px was lower in the control than the
normal-control, indicating that lower levels of reducing agents
increased lipid peroxides in the control than the normal-control
(Table III). MS1:3 better
inhibited the decrease of hepatic GSH activities than MS1:5.
Hepatic levels of TNF-α were more than 2-fold higher in the control
than the normal-control and they decreased more in response to
MS1:5 than MS1:3 (Table III).
In addition to the activity and contents of
anti-oxidant enzymes and proinflammatory cytokines, their mRNA
expression was influenced by D-galactosamine and MS mixtures
modified the mRNA expression (Fig.
4). The SOD1 and GSH-Px mRNA expression were 1.5-fold lower in
the control than the normal-control, while MS1:3 increased their
expression (Fig. 4). The mRNA
expression of IL-1β and TNF-α, proinflammatory cytokines was higher
in the control than the normal-control, while MS1:3 and MS1:5
lowered their expression (Fig. 4).
MS1:5-H decreased their mRNA expression similar to the
normal-control.
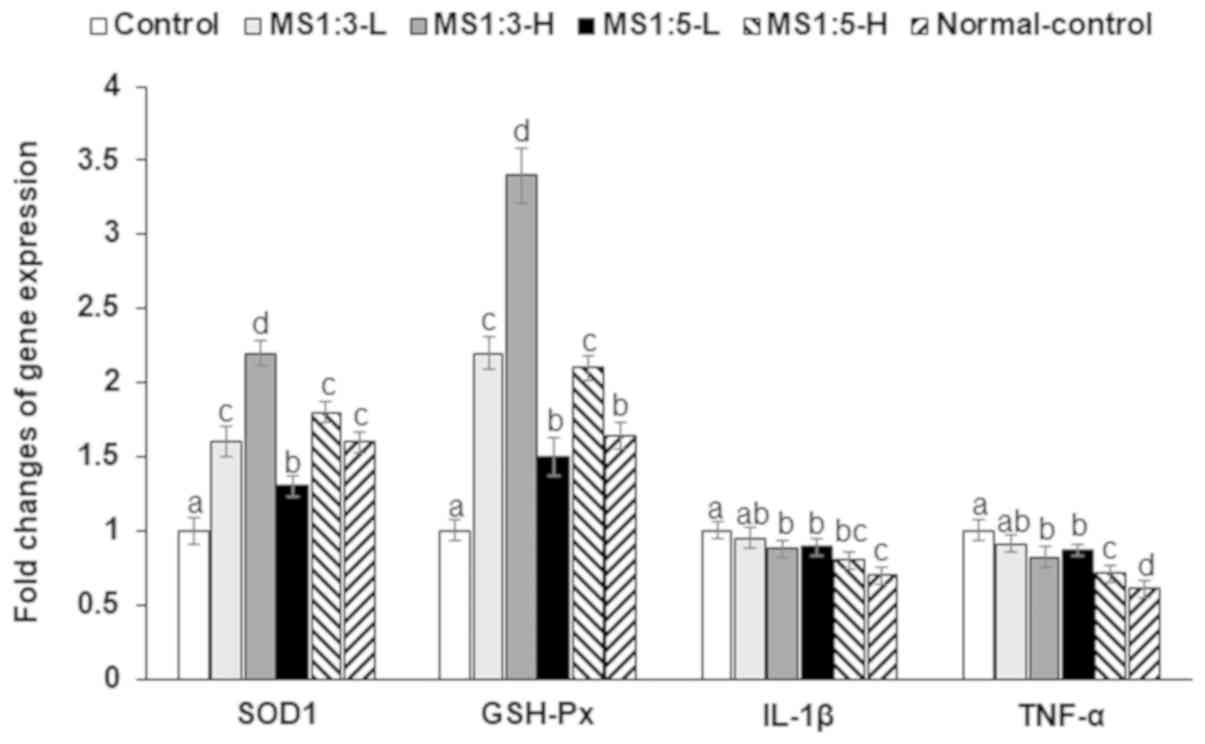 | Figure 4mRNA expression of SOD1, GSH-Px, and
TNF-α. Values are the means ± standard deviation. Rats received an
intraperitoneal injection of D-galactosamine and were provided with
200 and 600 mg/kg body weight of mulberry extracts and silk amino
acid (1:3, w/w; MS1:3-L and MS1:3-H), the same amounts of MS with
silk amino acid at different ratios (1:5, w/w; MS1:5-L and
MS1:5-H), or 600 mg/kg bw cellulose (control) for 1 week. The
normal-control rats were injected with saline instead of
D-galactosamine and they had the same diet as the control group.
*P<0.05, **P<0.01 and
***P<0.001 vs. Control. SOD1,
superoxide dismutase-1; GSH-Px, glutathione peroxidase; TNF-α,
tumor-necrosis factor-α. b P<0.05, c P<0.01 and dP<0.001
vs. Control. |
Discussion
The liver plays a crucial role in removal of toxins
and utilization of nutrients to regulate glucose and lipid
metabolism. Toxins such as CCL4 and D-galactosamine
induce acute liver damage and disturb lipid and glucose metabolism
(2,5,13).
D-galactosamine is known to induce a condition similar to human
viral hepatitis and is therefore widely used to generate
experimental animal models of the acute hepatic disease. The
etiology of D-galactosamine to induce acute liver damage is
associated with depleting UTP levels in the liver (5), stimulating proinflammatory cytokines
including IL-1β and TNF-α (14) and
elevating oxidative stress by reducing antioxidants (15). Depletion of UTP levels may be
involved in glycogen synthesis in the liver; thus, like other
toxins such as alcohol, D-galactosamine induces acute hepatocyte
necrosis toxicity and changes the glucose and lipid metabolism. We
investigated whether mixtures of mulberry water extracts and silk
amino acids could protect against acute liver damage in rats
induced by intraperitoneal injection of D-galactosamine and the
mechanism of the identified protective action. The results showed
that D-galactosamine induced acute liver damage was attenuated by a
mixture of silk amino acid and mulberry water extracts by reducing
oxidative stress and inflammation.
Silk amino acids are derived from the hydrolysis of
cocoons from the silkworm Bombyx mori. Silk amino acids
contain 75% fibroin and 25% sericin, and intake of silk amino
acids, including sericin hydrolysate, has been shown to exert
protection against alcohol-induced liver damage by accelerating
alcohol elimination through urine directly, which enhanced the
oxidation rate in the liver (8,16). Thus,
silk amino acid intake reduces ethanol concentration in the liver
and in circulation and may therefore reduce liver damage; however,
it may not ameliorate liver damage induced by other toxins besides
ethanol. In the present study, mulberry water extracts were
combined with silk amino acid to protect against D-galactosamine
induced liver damage in rats. The various ratios of mulberry water
extracts and silk amino acids were then examined to improve cell
survival in HepG2 cells treated with D-galactosamine.
Mulberry water extracts have been reported to
contain anthocyanins such as cyanidin-3-glucoside as well as
flavonoids such as rutin, quercetin, and luteolin (8); therefore, they can act as antioxidants.
Mulberry water extracts have been demonstrated to protect against
ethanol-induced liver disease by reducing inflammation and
inhibiting lipogenesis in the liver (8,17). In
addition, mulberry water extracts have been shown to ameliorate
obesity or CCL4-induced liver damage by decreasing lipid
peroxidation and inhibiting proinflammatory gene expression
(2,13). The results of the present study
showed that high dosages of MA and SA themselves increased cell
survival when liver damage was induced by D-galactosamine. However,
the mixture of MA and SA elevated cell survival more than either
extract alone. The mixture of MS reduced the MDA contents with
increased SOD1 and GSH-Px expression in the HepG2 cells treated
with D-galactosamine in HepG2 cells. Additionally, the levels of
TNF-α, which indicate inflammation, were lower samples treated with
high doses of MA and SA; however, the mixture of MS1:5 led to
greater decreases than either individual extract. Thus, the MS
mixture, especially MS1:5, may ameliorate acute liver damage better
than individual MB and SA.
In the present study, rats fed MS1:3 and MS 1:5
protected against acute liver injury by D-galactosamine. As shown
in other studies, D-galactosamine injection induced liver damage by
increasing oxidative stress and decreasing the activity of
antioxidants including SOD1 and GSH-Px and their mRNA expression
and inflammation by increasing proinflammatory cytokines such as
TNF-α. The MS1:3 and MS1:5 decreased oxidative stress and
inflammation. MS1:3 decreased lipid peroxide levels in the liver by
elevating the activities and mRNA expression of antioxidant enzymes
including SOD1 and GSH-Px more than MS1:5. However, MS1:5 reduced
proinflammatory cytokines such as TNF-α better than MS1:3. As a
result, both MS1:3 and MS1:5 improved the hepatic damage based on
histology and reduced liver damage indexes such as serum AST and
ALT. Serum AST and ALT activities were much lower in the present
study, which measured them 7 days after D-galactosamine injection
as opposed to 18-24 h in the previous studies (18-20).
The previous studies have showed substantial variations in their
activities (18-20).
In the present study, serum AST and ALT activities in the control
were much higher than the normal-control and MS1:3 and MS1:5
treatments decreased the activities compared to the control. The
decrease in AST and ALT was largely a time dependent improvement in
which the D-galactosamine-induced liver damage was naturally
alleviated in all groups including the control, the improvement was
accelerated by MS1:3 and MS1:5.
Mulberry extract may act as an antioxidant to reduce
oxidative stress and improve inflammation as shown in other studies
(6-8).
Silk amino acids have been reported to improve glucose metabolism
by increasing insulin secretion and pancreatic β-cell mass, even in
type 1 diabetes (21,22), suggesting that they have the
potential to improve the recovery of damaged cells. Thus, silk
amino acids may improve the recovery of damaged hepatocytes by
D-galactosamine.
No previous studies has reported that glycogen
deposition was reduced and TG accumulation was elevated in the
livers of the rats with D-galactosamine induced acute liver damage.
This was associated with the ability of D-galactosamine to reduce
UTP levels in the liver since UTP is associated with the synthesis
of RNA in the hepatocytes and glycogen synthesis (5). Cell damage by D-galactosamine may be
associated with the inhibition of protein production and glycogen
synthesis in the liver. Although glycogen storage disease type 1a
caused by the deficiency of glucose-6 phosphatase is associated
with decreasing glycogen utilization to excessively accumulate not
only glycogen storage but also triglycerides in the liver, it has
progressed to steatohepatitis, cirrhosis and hepatic adenomas and
carcinomas (23). However, depletion
of glycogen has been reported to be related to hepatocyte damage
with insulin resistance by environmental toxins (24,25).
Moreover, decreases in glycogen deposition have been shown to
contribute to increasing synthesis of fat from glucose and to
exacerbate glucose metabolism to increase insulin secretion to
normalize serum glucose levels, but not to decrease serum glucose
levels and increase insulin resistance (26). The present study showed that rats in
the control had increased HOMA-IR and insulin resistance and
developed glucose intolerance. In addition, serum triglyceride
levels increased in the control group relative to the MS1:3 and
MS1:5 treatments, even though the serum total, HDL and LDL
cholesterol levels did not differ among groups. D-galactosamine may
not influence cholesterol metabolism in the liver and MS1:3 and
MS1:5 did not alter the cholesterol metabolism. Glucose
intolerance, insulin resistance and serum triglyceride levels
induced by D-galactosamine were alleviated by both MS1:3 and MS1:5
and they were improved better in MS1:5 than MS1:3. These results
suggested that MS1:5 recovered hepatic function, especially glucose
and lipid metabolism, better than MS1:3 although both MS1:3 and
MS1:5 led to similar improvements in oxidative stress and
inflammation. Therefore, MS1:5 may be used as a therapeutic agent
for acute liver damage induced by D-galactosamine.
In conclusion, D-galactosamine injection induced
acute hepatic damage by increasing oxidative stress and
inflammation as well as the accumulation of decreased glycogen and
increased triglyceride levels. However, both MS1:3 and MS1:5
reduced serum ALT, AST and γ-GPT levels to ameliorate liver damage,
with MS1:5 having better effects on glycogen and triglyceride
accumulation. Therefore, MS1:5 may be a good therapeutic agent for
acute liver damage. Low and high doses of MS1:3 and MS1:5 of 1.5
and 4.5 g/day, respectively, were applied as human equivalents.
Acknowledgements
Not applicable.
Funding
This study was financially supported by the Ministry
of Trade, Industry, and Energy (MOTIE), Korea, under the ‘Regional
Specialized Industry Development Program’ (grant no. R0006422)
supervised by the Korea Institute for Advancement of Technology
(KIAT) and the Traditional Culture Convergence Research Program
through the National Research Foundation of Korea (NRF) funded by
the Ministry of Science and ICT (grant no.
NRF-2016M3C1B5907049).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SP designed the study and wrote the manuscript. TZ
and XW conducted biochemical experiments. TZ, JYQ and XW conducted
the animal study and biochemical assays. All authors reviewed the
manuscript and approved the submission.
Ethics approval and consent to
participate
The current study was approved by the Institutional
Animal Care and Use Committee of Hoseo University (approval no.
HSIACUC-17-068).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lim DW, Kim H, Park JY, Kim JE, Moon JY,
Park SD and Park WH: Amomum cardamomum L. ethyl acetate fraction
protects against carbon tetrachloride-induced liver injury via an
antioxidant mechanism in rats. BMC Complement Altern Med.
16(155)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hsu LS, Ho HH, Lin MC, Chyau CC, Peng JS
and Wang CJ: Mulberry water extracts (MWEs) ameliorated carbon
tetrachloride-induced liver damages in rat. Food Chem Toxicol.
50:3086–3093. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Radhiga T, Sundaresan A, Viswanathan P and
Pugalendi KV: Effect of protocatechuic acid on lipid profile and
DNA damage in D-galactosamine-induced hepatotoxic rats. J Basic
Clin Physiol Pharmacol. 27:505–514. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Park S, Kim DS, Wu X and Q JY: Mulberry
and dandelion water extracts prevent alcohol-induced steatosis with
alleviating gut microbiome dysbiosis. Exp Biol Med (Maywood).
243:882–894. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Decker K and Keppler D: Galactosamine
hepatitis: Key role of the nucleotide deficiency period in the
pathogenesis of cell injury and cell death. Rev Physiol Biochem
Pharmacol. 77–106. 1974.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ou TT, Kuo CY, Chyau CC, Lee HJ, Peng JS
and Wang CJ: Improvement of lipopolysaccharide-induced hepatic
injuries and inflammation with mulberry extracts. J Sci Food Agric.
93:1880–1886. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yan F, Chen Y, Azat R and Zheng X:
Mulberry anthocyanin extract ameliorates oxidative damage in HepG2
cells and prolongs the lifespan of Caenorhabditis elegans through
MAPK and Nrf2 pathways. Oxid Med Cell Longev.
2017(7956158)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yang HJ, Kim MJ, Kang ES, Kim DS and Park
S: Red mulberry fruit aqueous extract and silk proteins accelerate
acute ethanol metabolism and promote the antioxidant enzyme systems
in rats. Mol Med Rep. 18:1197–1205. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lopez-Terrada D, Cheung SW, Finegold MJ
and Knowles BB: Hep G2 is a hepatoblastoma-derived cell line. Hum
Pathol. 40:1512–1515. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jeong SY, Kang S, Hua CS, Ting Z and Park
S: Synbiotic effects of beta-glucans from cauliflower mushroom and
Lactobacillus fermentum on metabolic changes and gut microbiome in
estrogen-deficient rats. Genes Nutr. 12(31)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Moon NR, Kang S and Park S: Consumption of
ellagic acid and dihydromyricetin synergistically protects against
UV-B induced photoaging, possibly by activating both TGF-β1 and wnt
signaling pathways. J Photochem Photobiol B. 178:92–100.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lim HH, Lee SO, Kim SY, Yang SJ and Lim Y:
Anti-inflammatory and antiobesity effects of mulberry leaf and
fruit extract on high fat diet-induced obesity. Exp Biol Med
(Maywood). 238:1160–1169. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ganai AA, Khan AA, Malik ZA and Farooqi H:
Genistein modulates the expression of NF-κB and MAPK (P-38 and
ERK1/2), thereby attenuating d-Galactosamine induced fulminant
hepatic failure in Wistar rats. Toxicol Appl Pharmacol.
283:139–146. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sakaguchi S and Yokota K: Role of Ca2+ on
endotoxin-sensitivity by galactosamine challenge: Lipid peroxide
formation and hepatotoxicity in zymosan-primed mice. Pharmacol
Toxicol. 77:81–86. 1995.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li YG, Ji DF, Chen S and Hu GY: Protective
effects of sericin protein on alcohol-mediated liver damage in
mice. Alcohol Alcohol. 43:246–253. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tang CC, Huang HP, Lee YJ, Tang YH and
Wang CJ: Hepatoprotective effect of mulberry water extracts on
ethanol-induced liver injury via anti-inflammation and inhibition
of lipogenesis in C57BL/6J mice. Food Chem Toxicol. 62:786–796.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Saracyn M, Zdanowski R, Brytan M, Kade G,
Nowak Z, Patera J, Dyrla P, Gil J and Wankowicz Z: D-galactosamine
intoxication in experimental animals: Is it only an experimental
model of acute liver failure? Med Sci Monit. 21:1469–1477.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ganai AA and Husain M: Genistein
attenuates D-GalN induced liver fibrosis/chronic liver damage in
rats by blocking the TGF-β/Smad signaling pathways. Chem Biol
Interact. 261:80–85. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Banu S, Bhaskar B and Balasekar P:
Hepatoprotective and antioxidant activity of Leucas aspera against
D-galactosamine induced liver damage in rats. Pharm Biol.
50:1592–1595. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Do SG, Park JH, Nam H, Kim JB, Lee JY, Oh
YS and Suh JG: Silk fibroin hydrolysate exerts an anti-diabetic
effect by increasing pancreatic β cell mass in C57BL/KsJ-db/db
mice. J Vet Sci. 13:339–344. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Song Z, Zhang M, Xue R, Cao G and Gong C:
Reducing blood glucose levels in TIDM mice with an orally
administered extract of sericin from hIGF-I-transgenic silkworm
cocoons. Food Chem Toxicol. 67:249–254. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Farah BL, Sinha RA, Wu Y, Singh BK, Lim A,
Hirayama M, Landau DJ, Bay BH, Koeberl DD and Yen PM: Hepatic
mitochondrial dysfunction is a feature of Glycogen Storage Disease
Type Ia (GSDIa). Sci Rep. 7(44408)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Henrique da Silva G, Barros PP, Silva
Goncalves GM and Landi MA: Hepatoprotective effect of Lycopodium
clavatum 30CH on experimental model of paracetamol-induced liver
damage in rats. Homeopathy. 104:29–35. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Irimia JM, Meyer CM, Segvich DM, Surendran
S, DePaoli-Roach AA, Morral N and Roach PJ: Lack of liver glycogen
causes hepatic insulin resistance and steatosis in mice. J Biol
Chem. 292:10455–10464. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Crescenzo R, Bianco F, Mazzoli A, Giacco
A, Liverini G and Iossa S: A possible link between hepatic
mitochondrial dysfunction and diet-induced insulin resistance. Eur
J Nutr. 55:1–6. 2016.PubMed/NCBI View Article : Google Scholar
|