Introduction
Diabetes is one of the most common causes of
erectile dysfunction (ED), such that the incidence of ED in
patients with diabetes is four times higher compared with that in
non-diabetic patients (1). During
the early stages of diabetes, 50-75% of diabetic patients
experience ED (2). Although
effective pharmacological interventions for treating ED are
available, there remains a lack of an effective treatment strategy
for ED associated with diabetes (DMED).
Corpus cavernosum smooth muscle function involves a
plethora of complex intracellular events and extracellular signals
(3,4). Since smooth muscle cell (SMC) function
depends heavily on mitochondrial activity a strong association
exists between mitochondrial dysfunction and dysfunction in the
corpus cavernosum smooth muscle (5).
In particular, long-term hyperglycemia has been reported to reduce
mitochondrial biogenesis, increase the ADP/ATP ratio, induce
ultra-structural changes and markedly increase the production of
reactive oxygen species (ROS) (6-8).
These changes severely impair the physiological function of the
corpus cavernosum smooth muscle.
Mitochondrial ATP synthase is the key enzyme of the
mitochondrial energy machinery that is responsible for the
synthesis of ATP in most eukaryotic cells. ATP synthase consists of
the catalytic F1 domain and the F0 domain
that is embedded in the inner mitochondrial membrane (9). It has been reported that low levels of
ATP synthase in patient fibroblasts can significantly limit
mitochondrial ATP production whilst increasing ROS production
through the mitochondrial electron transport chain (10). Increases in ROS can cause damage to
the diastolic function of penile cavernosum smooth muscle,
resulting in penile vasculopathy (11).
The relationship between ATP synthase and DMED
remains unclear. In the present study, F1-ATP synthase
expression in cavernosum smooth muscle under high glucose
conditions was measured; subsequently, the effects of
F1-ATP synthase overexpression on cavernosum smooth
muscle function under hyperglycemia were also investigated.
Materials and methods
Animal models and groups
A total of 20 male Sprague-Dawley (SD) rats (weight
range, 200-250 g), ~8 weeks old, were provided by Guangdong Medical
Experimental Animal Centre (Guangdong, China). The animal
procedures were approved by the Wenzhou Medical University Animal
Policy and Welfare Committee (Zhejiang, China). All rats were
housed with a 12 h light/dark cycle at 24±1˚C, and provided with
food and water ad libitum for a week before study.
Apomorphine (APO; 100 µg/kg) was injected into the loose neck skin
of the rats to verify normal erectile function previously (12). The rats were then randomly divided
into the control (n=4) and diabetic groups (n=5). A diabetic rat
model was generated by a single intraperitoneal injection of
streptozotocin (STZ; Sigma Aldrich; Merck KGaA; 55 mg/kg) after an
overnight fast (13). Plasma glucose
levels in blood samples obtained from the tail vein were measured
using a glucometer (Roche Diagnostics, Indianapolis, IN). A
randomized blood glucose concentration ≥16.67 mmol/l was considered
to indicate the successful establishment of the experimental
diabetic rat model (14). Rats in
the control group were injected with saline. During the experiment,
all rats were fed with a normal diet.
Evaluation of erectile function
According to a method established by He et al
(13), rats in each group were
placed in an observation cage after feeding. A period of 10 weeks
after feeding, APO was then injected subcutaneously into the loose
neck skin of the rat and each rat was videoed immediately with a
camera for 30 min after injection, and the number of penile
erections was observed and recorded. Each rat was examined at 8 am
and 8 pm for three consecutive days.
Sample acquisition
After successful modelling, rats continued to be fed
for 10 weeks. When APO experiment finished, rat corpus cavernosum
tissues were cut into three sections and each section was ~2 mm.
The middle section was fixed using 4% paraformaldehyde at 4˚C
overnight, whilst the remaining two sections were directly placed
in cryovials and stored at -80˚C.
Isolation of primary rat corpus
cavernosum smooth muscle cells (CCSMCs)
The corpus cavernosum tissues of normal SD rats were
cut into pieces of ~0.5x1x1 mm and placed at 0.5 cm intervals into
25 ml sterile culture flasks containing 2 ml DMEM (Hyclone; GE
Healthcare Life Sciences) with 20% FBS (Hyclone; GE Healthcare Life
Sciences). Under light microscopy (magnification, x100), a small
number of CCSMCs were observed to migrate out of the tissue
following 3 days of incubation.
Cell culture
CCSMCs after passage number three were cultured in
DMEM containing 10% heat-inactivated FBS (Hyclone; GE Healthcare
Life Sciences) and 1% penicillin/streptomycin (Beijing Solarbio
Science & Technology Co., Ltd.) and maintained in a humidified
incubator at 37˚C with 5% CO2. Cells were seeded in
six-well plates for immunocytochemical analysis, 12-well plates for
western blotting and reverse transcription-PCR (RT-PCR), and
96-well plates for high-throughput flow cytometry and ELISA
analysis.
Molecular cloning of Atp5b and cell
transfection
Briefly, the Atp5b gene (Shanghai Shenggong
Biology Engineering Technology Service, Ltd.) was cloned into the
pcDNA3.1(+) carrier (Taihe Biotechnology Co., Ltd.) within BamH I
(Thermo Fisher Scientific, Inc.) and Xho I (Thermo Fisher
Scientific, Inc.) restriction enzyme to create a pcDNA3.1 plasmid
encoding F1-ATP synthase (pcDNA3.1-F1-ATP).
Following sequencing to verify integration, 2 µg
pcDNA3.1-F1-ATP or blank vector pcDNA3.1 was transfected
into CCSMCs with Lipofectamine® 2000 (Thermo Fisher
Scientific, Waltham, USA) according to the manufacturer's protocol
The medium was replaced with complete medium at 6 h following
transfection. Transfected cells were collected for flow cytometry,
western blotting or RT-PCR at 48 h after transfection. Finally, the
cells were divided into the following groups: i) Control group,
where CCSMC cultures were incubated in normal glucose medium; ii)
high glucose group, where CCSMC cultures were incubated in medium
containing high glucose (30 mmol/l); iii) high glucose +
F1-ATP synthase overexpression (OE), where CCSMCs
transfected with the pcDNA3.1-F1-ATP were cultured in
medium containing high glucose; and iv) high glucose + blank vector
groups, where the CCSMCs transfected with blank pcDNA3.1 vector
were cultured in medium containing high glucose.
Analysis of apoptosis by TUNEL and
flow cytometry
TUNEL staining was performed using a Tissue TUNEL
apoptosis detection kit according to the manufacturer's protocol
(Enjing Biotechnology). The corpus cavernosum tissues sections were
fixed in 4% PFA overnight at 4˚C. Slides were first immersed in 3%
H2O2 for 10 min at room temperature.
Equilibration buffer (100 µl) was then added and slides were
incubated for 20 min at 37˚C, followed by incubation with a TdT
reaction mix (50 µl) for 60 min at 37˚C. The cells were then
stained with hematoxylin for 3 min at room temperature. Neutral
balsam was used to mount the slides. Brown staining was considered
as positive, and three fields of each slide were randomly selected
under light microscopy (magnification, x400). The number of cells
were counted and the percentage of positive cells was calculated
under a light microscope (magnification, x400).
For flow cytometry experiment, cells were first
digested with 0.25% trypsin without EDTA (15) for 2 min and then collected in
centrifuges. Cells were centrifuged (1,000 x g) for 5 min at room
temperature and resuspended in 500 µl binding buffer. Subsequently,
cells were incubated with 5 µl Annexin V-FITC and 5 ul propidium
iodide (Nanjing KeyGen Biotech Co., Ltd.) for 10 min at 37˚C in
darkness. Finally, cells were tested by flow cytometry within 2 h.
Flow cytometry was performed using FACS Calibur (BD Biosciences),
and FACS data were analyzed using CellQuest software (version 5.1;
BD Biosciences)
Oxidative stress testing by
ELISA.
In vivo and in vitro samples were
added to the wells, and biotin-labeled anti-endothelial nitric
oxide synthase (eNOS; Bioswamp Wuhan Beiyinlai Biotechnology Co.,
Ltd; cat. no. RA20010) antibodies or biotin-labeled anti-cyclic
guanosine monophosphate (cGMP; Bioswamp Wuhan Beiyinlai
Biotechnology Co., Ltd; cat. no. RA20105) antibodies were added and
cultured at room temperature, for 1 h, and then 50 µl
enzyme-labeled reagent was also added with the exception of the
blank well. After incubation for 30 min at 37˚C,
Tetramethylbenzidine was performed in the darkness for 15 min at
37˚C. Finally, stop solution (50 µl) was added to each well to
terminate the reaction. Absorbance (optical density at 450 nm) was
subsequently measured using a microplate reader.
Western blotting
Cells were collected in lysis buffer (Beyotime
Institute of Biotechnology) containing 1 mM phenylmethylsulfonyl
fluoride (PMSF) (Beyotime Institute of Biotechnology) for lysis at
4˚C for 20 min. Next, the samples were centrifuged (12,000 x g) for
10 min at 4˚C and the supernatant was used to detect the protein
concentration using a Bicinchoninic Acid protein assay kit
(Beyotime Institute of Biotechnology). Equal amounts (40 µg) of
protein samples were separated by 10% SDS-PAGE and transferred to a
PVDF membrane (EMD Millipore). After blocking with 5% non-fat milk
in TBST at room temperature for 1 h, the membranes were incubated
with primary anti-ATP synthase (1:1,000; cat. no. NBP1-91573;
Bio-Techne) or anti-GAPDH (1:10,000; cat. no. KC5G5; Aksomics)
primary antibodies at 4˚C overnight. The next day, the membranes
were incubated with horseradish peroxidase (HRP)-conjugated Goat
anti-Rabbit (1:10,000; cat. no. AP307P; Merck KGaA) antibody at
room temperature for 2 h. The bands were visualized using enhanced
chemiluminescence (Pierce; Thermo Scientific, Inc.) and quantified
by measuring the intensity of the signals using ImageJ software
(1.8.0; National Institutes of Health).
Fibrotic detection in vivo
To measure rat cavernosum collagen/smooth muscle
ratio, tissue sections were stained with Masson's trichrome stain
(16), followed by light microscopy
(magnification, x100) examination. The area ratio of the collagen
fibres to muscle was analyzed using Image-Pro Plus software (6.0,
Media Cybernetics, Inc.).
Immunocytochemistry
In aseptic conditions, dry glass slides were placed
in a six-well plate, onto which the 2x106 cells were
subsequently seeded and cultured overnight. The cells were then
fixed using 4% paraformaldehyde for 30 min at room temperature
followed by permeabilization with 0.2% Triton X-100 for another 5
min at room temperature. After blocking with 10% goat serum
(Beijing Solarbio Science & Technology Co., Ltd.) in PBS for 1
h at room temperature, the cells were incubated with primary α-SMA
antibody (1:200; cat. no. 55135-1-AP; Proteintech Group, Inc.)
overnight at 4˚C. This was followed by incubating with
HRP-conjugated secondary antibody (1:400; cat. no. AP307P; Merck
KGaA) for 1 h at room temperature, and then 3,3'-diaminobenzidine
(Beijing Solarbio Science & Technology Co., Ltd.) was incubated
for another 5 min at room temperature subsequently. Finally,
hematoxylin (Beijing Solarbio Science & Technology Co., Ltd.)
was used to stain the nuclei and neutral for 2 min at room
temperature and gum was used to seal the samples. Images of the
cells were captured under light microscopy (magnification, x100)
and the extent of immunostaining was analyzed using Image-Pro Plus
software (6.0, Media Cybernetics, Inc.).
RT-PCR
Total RNA was isolated from CCSMCs using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) and
reversely transcribed to cDNA using a GenestarScriptRT cDNA
Synthesis kit (Shanghai Jixing Biotechnology Co., Ltd.), the
thermocycling conditions for reverse transcription were as follows:
37˚C for 15 min and 85˚C for 5 sec. RT-PCR analysis was performed
to measure the mRNA levels using SYBR Green Clon Master mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.) in an ABI 7500
Rapid Thermal Cycler. The sequences of the primers used were as
follows: a-smooth muscle actin (α-SMA) forward,
5'-ATCTGGCACCACTCCTTCTA-3' and reverse, 5'-GTCCAGCACAATACCAGTTG-3';
smooth muscle myosin heavy chain (SMMHC) forward,
5'-CCGCTGCCTATGACAAACT-3' and reverse, 5'-CGCATACTTGGAGGAGATGT-3';
calponin forward, 5'-GGAACATCATTGGCCTACAG-3' and reverse,
GCGTGTCACAGTGTTCCAT-3'; osteopontin (OPN) forward,
5'-GCTATCAAGGTCATCCCAGTT-3' and reverse, 5'-GTTTCCACGCTTGGTTCAT-3';
β-actin forward, 5'-AGGGAAATCGTGCGTGACAT-3' and reverse,
5'-GAACCGCTCATTGCCGATAG-3'. The thermocycling conditions for RT-PCR
were as follows: 1 cycle of 95 ˚C for 5 min, 40 cycles of 50˚C for
2 min, 95˚C for 2 min, and 60˚C for 32 sec. The thermocycling
conditions for melting curves were: 95 ˚C for 15 sec, 60˚C for 1
min and 95˚C for 15 sec. Final values were computed using the
2-ΔΔCq method (17).
Statistical analysis
All statistical data are expressed as the mean ±
standard deviation. The differences among groups were analysed
using one-way ANOVA followed by Bonferroni correction. P<0.05
was considered to indicate a statistically significant difference.
GraphPad Prism software (version 5.0; GraphPad Software, Inc.) was
used to perform the statistical analyses.
Results
General characteristics of the
diabetic rats
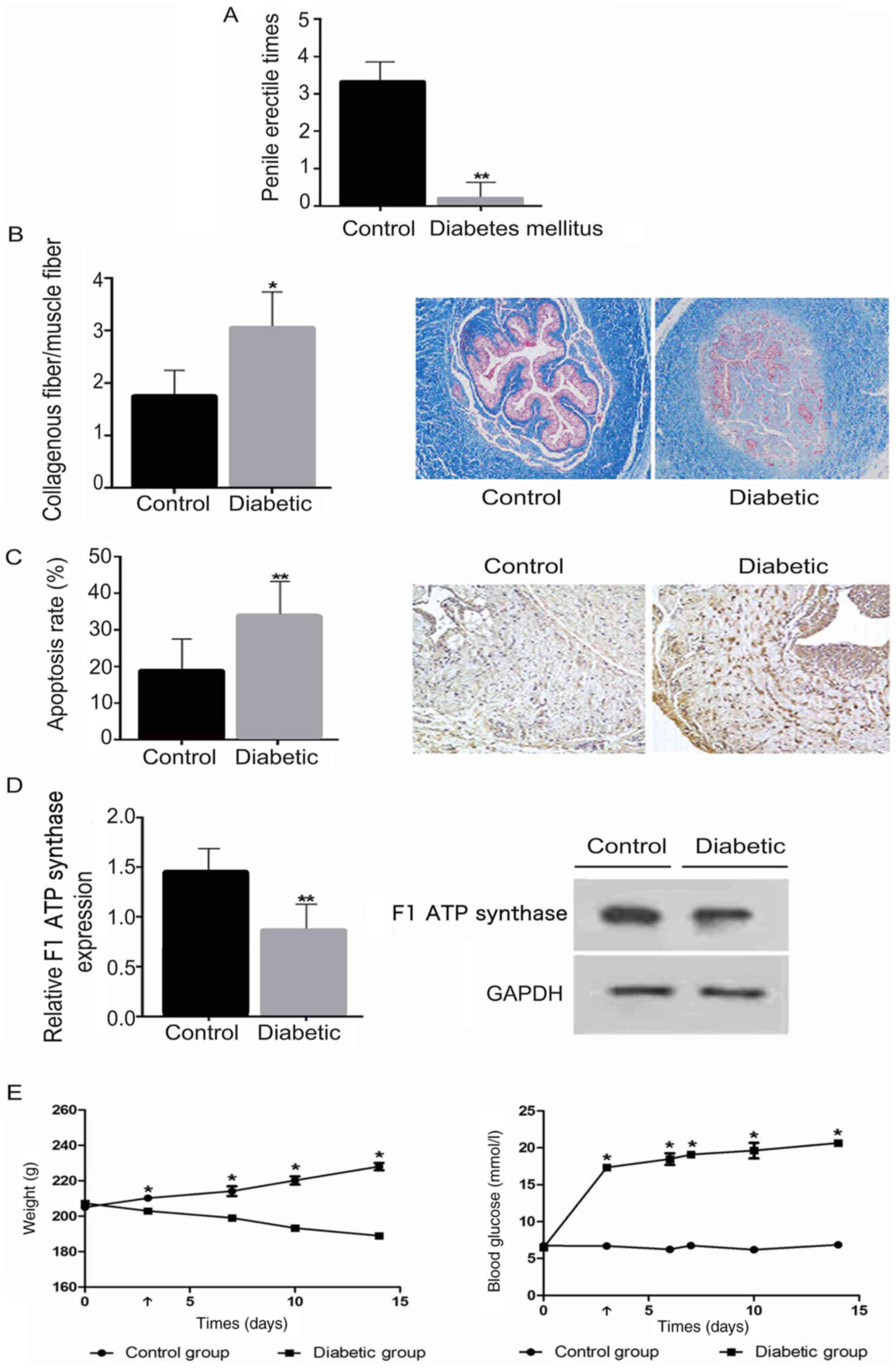
The characteristics of diabetic and healthy control
rats are compared in Fig. 1.
Diabetic rats exhibited significantly elevated blood glucose levels
and lower body weights compared with normal control rats
(P<0.05; Fig. 1E). Following
injection with APO, the erectile response in diabetic rats was
significantly lower compared with that in the control group
(P<0.01; Fig. 1A).
High glucose increases the
collagen/smooth muscle ratio and induces SMC apoptosis in diabetic
rats
The collagen/smooth muscle ratio was observed to be
significantly increased in diabetic rats compared with the control
group (P<0.05; Fig. 1B), whilst
the number of TUNEL-positive cells in the diabetic group was
significantly higher compared with that in the control group
(P<0.01; Fig. 1C).
F1-ATP synthase expression
is reduced in diabetic rats
To evaluate the activity of F1-ATP
synthase in cavernosum SMCs, western blot analysis was performed to
determine the expression of F1-ATP synthase protein in
the two groups. The expression of F1-ATP synthase was
found to be significantly lower in the diabetic group compared with
the control group (P<0.01; Fig.
1D).
F1-ATP synthase
overexpression promotes the expression of ATP synthase under high
glucose conditions in vitro
CCSMCs were isolated and cultured prior to α-SMA
immunocytochemical staining. Positive α-SMA cellular staining was
observed, suggesting that the isolation of CCSMCs was successful
(Fig. 2A). Consistent with the
findings of the in vivo experiment, F1-ATP
synthase expression in the CCSMCs incubated with high glucose was
markedly reduced compared with that in the control CCSMCs (Fig. 2B), and the high glucose-induced
effect was reversed by transfection with the vector encoding
F1-ATP synthase (Fig.
2B). No notable difference was observed in the expression of
F1-ATP synthase between the high glucose and high
glucose + blank vector groups.
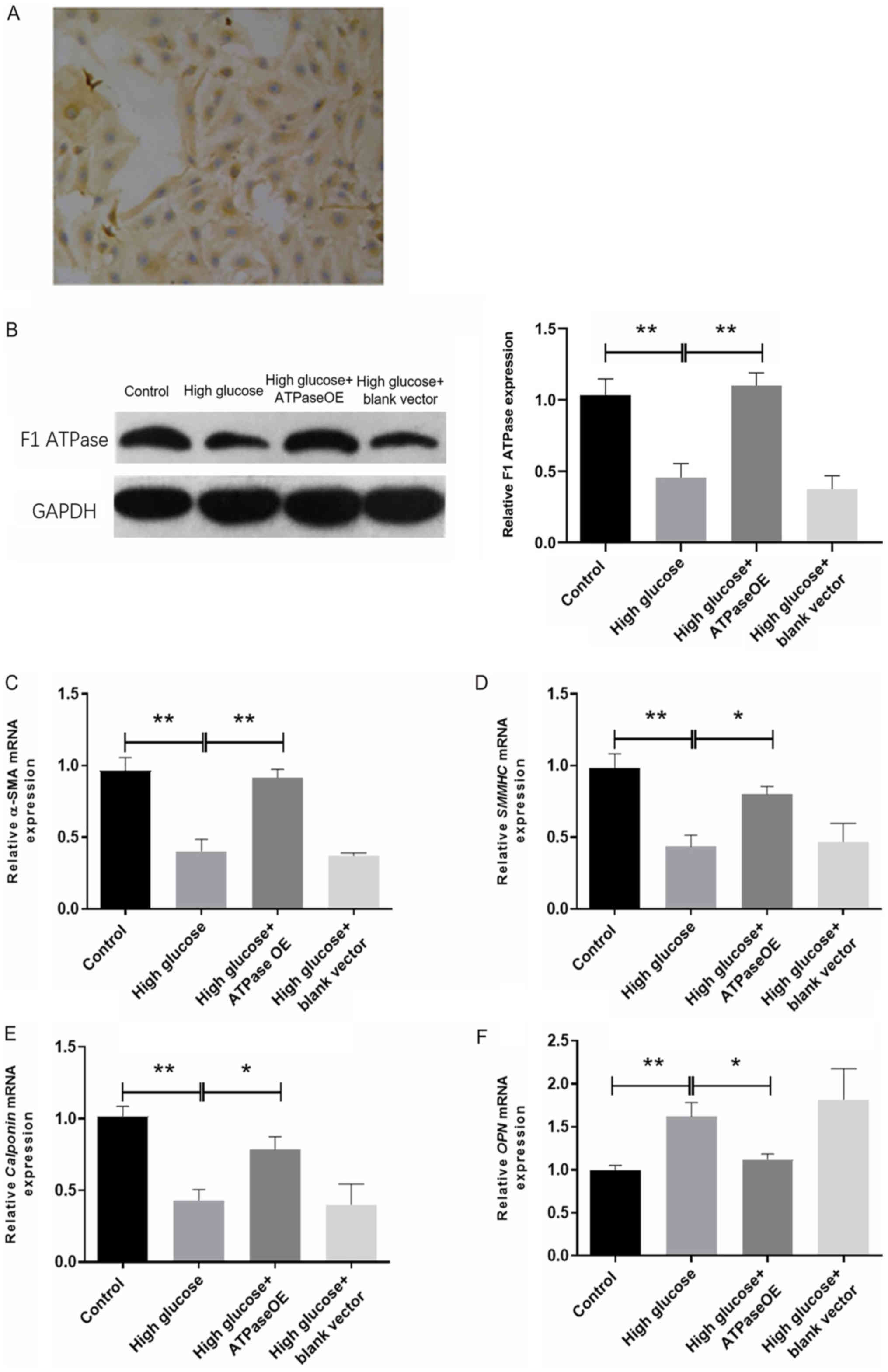 | Figure 2Effects of high glucose and ATP
synthase on smooth muscle markers in cultured rat CCSMCs. (A) The
α-SMA immunocytochemical staining of cultured rat CCSMCs.
Magnification, x100. (B) Western blot analysis of F1-ATP
synthase expression in cultured rat CCSMCs from the Control, high
glucose, high glucose + ATP synthase OE and high glucose + blank
vector groups. (C-F) mRNA expression of smooth muscle markers in
CCSMCs from control, high glucose, high glucose + ATP synthase and
high glucose + blank vector groups as measured using reverse
transcription-quantitative PCR. (C) α-SMA expression, (D) SMMHC
expression, (E) calponin expression and (F) OPN expression.
*P<0.05 and **P<0.01 as
indicated. CCSMCs, corpus cavernosum smooth muscle cells; OE,
overexpression; OPN, osteopontin; α-SMA, α-smooth muscle actin;
SMMHC, smooth muscle myosin heavy chain. |
Overexpression of F1-ATP
synthase promotes CCSMC phenotypic transformation under high
glucose
The mRNA expression levels of α-SMA, SMMHC and
calponin were significantly lower in the high glucose group
compared with the control (P<0.01; Fig. 2C-E). By contrast, OPN mRNA expression
in the high glucose group was significantly higher compared with
that in the control group (P<0.01; Fig. 2F). All of the aforementioned changes
were significantly reversed following transfection with the vector
encoding F1-ATP synthase (P<0.05; Fig. 2C-F), and no significant differences
in α-SMA, SMMHC, calponin and OPN mRNA expression were observed
between the high glucose and high glucose + blank vector
groups.
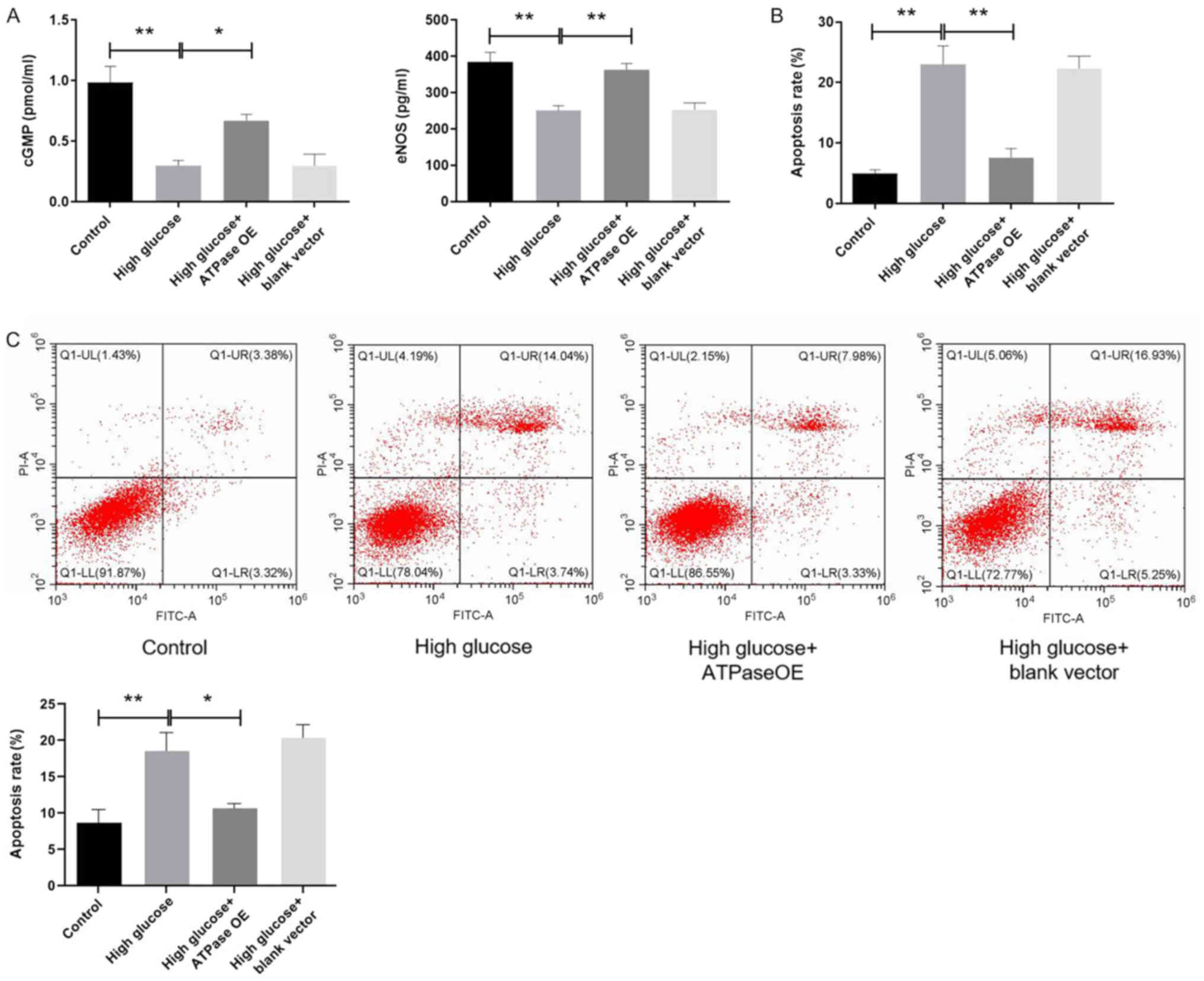
Overexpression of F1-ATP
synthase increases cGMP levels and eNOS expression in CCSMCs under
high glucose
In cultured rat CCSMCs, the levels of eNOS and cGMP
were measured using ELISA. Compared with the control group, the
eNOS and cGMP levels were significantly lower in the high glucose
group (P<0.01; Fig. 3A). Although
no significant differences were observed in the levels of eNOS
expression and cGMP between the high glucose and high glucose +
blank vector groups, eNOS expression and cGMP levels in the high
glucose group transfected with the F1-ATP synthase
vector were found to be significantly higher compared with those in
the high glucose group (P<0.05; Fig.
3A).
Overexpression of F1-ATP
synthase reduces the apoptosis of rat CCSMCs under high
glucose
A significant increase in the number of
TUNEL-positive cells was observed in the high glucose group
compared with the control group (P<0.01; Fig. 3B), and this increase was
significantly reversed by F1-ATP synthase overexpression
(P<0.01; Fig. 3B). No significant
differences were observed in the number of TUNEL-positive cells
between the high glucose + F1-ATP synthase
overexpression and control groups (Fig.
3B). According to the annexin V/PI assay, the apoptotic rate
was significantly higher in the high glucose group compared with
the control group, and the increase was markedly reversed by
F1-ATP synthase overexpression (Fig. 3B and C). No notable changes were observed in the
number of TUNEL-positive or apoptotic cells between the high
glucose and high glucose + blank vector groups (Fig. 3B and C).
Discussion
To the best of our knowledge, the present study is
the first to observe significantly reduced ATP synthase expression
in CCSMCs from the penile tissues of rats with DMED, and to
demonstrate that the upregulation of F1-ATP synthase
expression can increase eNOS expression and cGMP levels whilst
suppressing the apoptosis of rat CCSMCs under high glucose
conditions.
ATP synthase is the terminal enzyme in the oxidative
phosphorylation pathway and ATP formation in the mitochondria.
Previous studies have demonstrated that ATP synthase exists on the
outer surface of the plasma membrane of endothelial cells and tumor
cells (18,19). ATP synthase dysfunction can reduce
mitochondrial ATP production and lead to severe consequences for
all energy-dependent cellular activities in the human body.
However, the role of ATP synthase in human diseases remains
elusive. Previous studies have shown that the myocardial ATP
synthase levels in patients with ischemic cardiomyopathy are
significantly decreased along with the exhaustion of myocardial
contractile function, and the artificial restoration of ATP
synthase levels can restore the contractile function of
cardiomyocytes (20,21). It has also been reported that
downregulation of ATP synthase activity was detected in the
skeletal muscle of diabetic patients following the cessation of
insulin treatment (22). These
previous observations suggest that ATP synthase may be a promising
target for the regulation of muscle contractility.
In the penis, SMCs account for 40-52% of cells in
the cavernosum and serve to maintain penile contractility; in
particular, maintenance of the contractile phenotype in SMCs is
essential for cavernous space relaxation and penile erection
(12). Chronic hyperglycaemia
results in dysfunction of the vascular and nervous systems in the
corpus cavernosum, in addition to inducing CCSMC apoptosis and
changes in fiber and muscle content (23,24).
Under harmful external stimulation, including hypoxia and
hyperglycemia, CCSMCs display a tendency towards transforming from
a contractile to proliferative phenotype (25,26).
SMMHC, α-SMA and calponin are considered as molecular markers for
contractile CCSMCs, whereas OPN is considered as a molecular marker
for synthetic or proliferative CCSMCs (13,27). In
the present study, the expression of α-SMA, SMMHC and calponin in
CCSMCs incubated with high glucose group was significantly reduced,
whilst the expression of the OPN was significantly increased
compared with that in control CCSMCs. However, under high glucose
conditions, the changes in the expression levels of α-SMA, SMMHC,
calponin and OPN were reversed following F1-ATP synthase
transfection. These results suggest that under high glucose
treatment, rat CMSCs underwent phenotypic transformation from the
contractile to the proliferative phenotype, in a manner that could
be reversed by F1-ATP synthase overexpression. ATP
serves an important role in maintaining smooth muscle tone in the
corpus cavernosum. In a previous study, hyperglycemia has been
demonstrated to impair mitochondrial function and inhibit ATP
synthase activity, leading to reduced levels of ATP production
(28). Liu et al (5) showed that high glucose resulted in
abnormal lipid metabolism, in turn leading to a decrease in ATP
synthase expression through the Akt phosphorylation pathway in
CCSMCs, suggesting that ATP synthase is essential for CCSMC
function in hyperglycemia. In the present study, continuous
exposure to high glucose reduced the expression of the
F1-ATP synthase protein in both rat CCSMCs and smooth
muscle tissues from the corpus cavernosum in diabetic rats.
Additionally, the present study also confirmed that the
overexpression of F1-ATP synthase could switch the
cavernosum smooth muscle from a proliferative to a contractile
phenotype under high glucose conditions.
Impaired ATP synthase function as a result of
hyperglycemia can induce the production of excessive mitochondrial
ROS (29). Increased ROS levels may
inhibit the use of L-arginine, which is a substrate for NOS
(30). The present study showed that
the overexpression of F1-ATP synthase elevated eNOS
levels under hyperglycemia. NOS activates the NO-cGMP pathway,
which induces penile smooth muscle relaxation and the subsequent
initiation and maintenance of penile erection (31). This observation suggests that ATP
synthase can serve as a novel target for DMED treatment.
However, some limitations remain attached to the
present study. Additional studies are required to understand the
regulatory mechanism of ATP synthase activity in this DMED animal
model. Since chronic hyperglycemia not only impairs CCSMC function
but also leads to vascular sclerosis and neurological dysfunction
(32,33), further studies are required to
elucidate the relationship between ATP synthase activity and the
pathophysiological vascular and neurological features of DMED.
In conclusion, ATP synthase activity is closely
associated with CCSMC function under hyperglycemia. Further
understanding of ATP synthase may provide new therapeutic options
for treating DMED.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Health
and Family Planning Commission of Zhejiang Province (grant no.
2015KYB240), and the Science and Technology Department of Wenzhou
(grant no. Y20160340)
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZGW and LZ conceived and supervised the study; JC
and ZGW designed experiments; ZQX, JHC and SWC performed the
experiments; YBX and QQW developed new software and performed the
simulations; JHC and ZQX analyzed the data and wrote the
manuscript. All authors reviewed the results and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The animal procedures were approved by the Wenzhou
Medical University Animal Policy and Welfare Committee (Zhejiang,
China). All applicable international, national, and/or
institutional guidelines for the care and use of animals were
followed.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang X, Yang B, Li N and Li H: Prevalence
and risk factors for erectile dysfunction in Chinese adult males. J
Sex Med. 14:1201–1208. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hatzimouratidis K and Hatzichristou D: How
to treat erectile dysfunction in men with diabetes: From
pathophysiology to treatment. Curr Diab Rep. 14(545)2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sáenz de Tejada I: Molecular mechanisms
for the regulation of penile smooth muscle contractility. Int J
Impot Res. 14(Suppl 1):S6–S10. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Doyle C, Sergeant GP, Hollywood MA, Mchale
NG and Thornbury KD: ATP evokes inward currents in corpus
cavernosum myocytes. J Sex Med. 11:64–74. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu ZH, Yu LP, Xu T, Zhang XW, Yuan YQ,
Xiao YB, Li J, Hao YC, Zhao YP and Wang XF: Abnormal lipid
metabolism down-regulates adenosine triphosphate synthase β-subunit
protein expression in corpus cavernosum smooth muscle in vitro and
in vivo. Andrologia. 46:487–494. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Blake R and Trounce IA: Mitochondrial
dysfunction and complications associated with diabetes. Biochim
Biophys Acta. 1840:1404–1412. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Teodoro JS, Gomes AP, Varela AT, Duarte
FV, Rolo AP and Palmeira CM: Uncovering the beginning of diabetes:
The cellular redox status and oxidative stress as starting players
in hyperglycemic damage. Mol Cell Biochem. 376:103–110.
2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rolo AP and Palmeira CM: Diabetes and
mitochondrial function: Role of hyperglycemia and oxidative stress.
Toxicol Appl Pharmacol. 212:167–178. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jonckheere AI, Smeitink JA and Rodenburg
RJ: Mitochondrial ATP synthase: Architecture, function and
pathology. J Inherit Metab Dis. 35:211–225. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mrácek T, Pecina P, Vojtísková A, Kalous
M, Sebesta O and Houstek J: Two components in pathogenic mechanism
of mitochondrial ATP synthase deficiency: Energy deprivation and
ROS production. Exp Gerontol. 41:683–687. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Musicki B, Liu T, Sezen SF and Burnett AL:
Targeting NADPH oxidase decreases oxidative stress in the
transgenic sickle cell mouse penis. J Sex Med. 9:1980–1987.
2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wei AY, He SH, Zhao JF, Liu Y, Liu Y, Hu
YW, Zhang T and Wu ZY: Characterization of corpus cavernosum smooth
muscle cell phenotype in diabetic rats with erectile dysfunction.
Int J Impot Res. 24:196–201. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
He S, Zhang T, Liu Y, Liu L, Zhang H, Chen
F and Wei A: Myocardin restores erectile function in diabetic rats:
Phenotypic modulation of corpus cavernosum smooth muscle cells.
Andrologia. 47:303–309. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Araiza-Saldaña CI, Pedraza-Priego EF,
Torres-López JE, Rocha-González HI, Castañeda-Corral G, Hong-Chong
E and Granados-Soto V: Fosinopril prevents the development of
tactile allodynia in a streptozotocin-induced diabetic rat model.
Drug Dev Res. 76:442–449. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li T, Ni L, Liu X, Wang Z and Liu C: High
glucose induces the expression of osteopontin in blood vessels in
vitro and in vivo. Biochem Biophys Res Commun. 480:201–207.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bai WW, Tang ZY, Shan TC, Jing XJ, Li P,
Qin WD, Song P, Wang B, Xu J, Liu Z, et al: Up-regulation of
paired-related homeobox 2 promotes cardiac fibrosis in mice
following myocardial infarction by targeting of Wnt5a. J Cell Mol
Med. 27:2019.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
17
|
Bachman J: Reverse-transcription PCR
(RT-PCR). Methods Enzymol. 530:67–74. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Champagne E, Martinez LO, Collet X and
Barbaras R: Ecto-F1Fo ATP synthase/F1 ATP synthase: Metabolic and
immunological functions. Curr Opin Lipidol. 17:279–284.
2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Deshpande M, Notari L, Subramanian P,
Notario V and Becerra SP: Inhibition of tumor cell surface ATP
synthesis by pigment epithelium-derived factor: Implications for
antitumor activity. Int J Oncol. 41:219–227. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lou Q, Fedorov VV, Glukhov AV, Moazami N,
Fast VG and Efimov IR: Transmural heterogeneity and remodeling of
ventricular excitation-contraction coupling in human heart failure.
Circulation. 123:1881–1890. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang ZH, Cai XL, Wu L, Yu Z, Liu JL, Zhou
ZN, Liu J and Yang HT: Mitochondrial energy metabolism plays a
critical role in the cardioprotection afforded by intermittent
hypobaric hypoxia. Exp Physiol. 97:1105–1118. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ahmad F, Azevedo JL, Cortright R, Dohm GL
and Goldstein BJ: Alterations in skeletal muscle protein-tyrosine
phosphatase activity and expression in insulin-resistant human
obesity and diabetes. J Clin Invest. 100:449–458. 1997.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Malavige LS and Levy JC: Erectile
dysfunction in diabetes mellitus. J Sex Med. 6:1232–1247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lue TF: Erectile dysfunction. N Engl J
Med. 342:1802–1813. 2000.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lv B, Zhao J, Yang F, Huang X, Chen G,
Yang K, Liu S, Fan C, Fu H and Chen Z: Phenotypic transition of
corpus cavernosum smooth muscle cells subjected to hypoxia. Cell
Tissue Res. 357:823–833. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gur S, Kadowitz PJ and Hellstrom WJ: A
protein tyrosine kinase inhibitor, imatinib mesylate (Gleevec),
improves erectile and vascular function secondary to a reduction of
hyperglycemia in diabetic rats. J Sex Med. 7:3341–3350.
2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang HB, Wang ZQ, Chen FZ, Ding W, Liu
WB, Chen ZR, He SH and Wei AY: Maintenance of the contractile
phenotype in corpus cavernosum smooth muscle cells by Myocardin
gene therapy ameliorates erectile dysfunction in bilateral
cavernous nerve injury rats. Andrology. 5:798–806. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yan S, Du F, Wu L, Zhang Z, Zhong C, Yu Q,
Wang Y, Lue LF, Walker DG, Douglas JT and Yan SS: F1F0 ATP
synthase-cyclophilin D interaction contributes to diabetes-induced
synaptic dysfunction and cognitive decline. Diabetes. 65:3482–3494.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Matsuhashi T, Sato T, Kanno SI, Suzuki T,
Matsuo A, Oba Y, Kikusato M, Ogasawara E, Kudo T, Suzuki K, et al:
Mitochonic acid 5 (MA-5) facilitates ATP synthase oligomerization
and cell survival in various mitochondrial diseases. EBioMedicine.
20:27–38. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sifuentes-Franco S, Pacheco-Moisés FP,
Rodríguez-Carrizalez AD and Miranda-Díaz AG: The role of oxidative
stress, mitochondrial function, and autophagy in diabetic
polyneuropathy. J Diabetes Res. 2017(1673081)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rocha JT, Hipólito UV, Callera GE, Yogi A,
Neto Filho Mdos A, Bendhack LM, Touyz RM and Tirapelli CR: Ethanol
induces vascular relaxation via redox-sensitive and nitric
oxide-dependent pathways. Vascul Pharmacol. 56:74–83.
2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Dang VT, Zhong LH, Huang A, Deng A and
Werstuck GH: Glycosphingolipids promote pro-atherogenic pathways in
the pathogenesis of hyperglycemia-induced accelerated
atherosclerosis. Metabolomics. 14(92)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gasparova Z, Janega P, Weismann P, El
Falougy H, Tyukos Kaprinay B, Liptak B, Michalikova D and Sotnikova
R: Effect of metabolic syndrome on neural plasticity and morphology
of the hippocampus: Correlations of neurological deficits with
physiological status of the rat. Gen Physiol Biophys. 37:619–632.
2018.PubMed/NCBI View Article : Google Scholar
|