Introduction
Abdominal purpura is inflammation of systematic
small vessels of unknown etiology (1) and common in individuals aged <20
years (2). It is difficult to
diagnose abdominal purpura if children only have abdominal pain
(3) because abdominal purpura is an
acute, complex, immune-mediated vasculitis characterized by
abdominal pain (4), purpuric skin
lesions (5), and bloody diarrhea
(6) only without thrombocytopenia
(7). Differences in the clinical
characteristics and pathogenesis for abdominal purpura among
Xining, Tibet, inner Mongolia, and other areas were previously
reported (8).
According to The American College of Rheumatology
(ACR) criteria, two out of four criteria (acute abdominal pain, age
≤20 years or less, bowel angina, and granulocytes in the walls of
small venules and arterioles in the biopsies) must be met for
diagnosis of abdominal purpura (6).
These criteria have different sensitivities and specificities.
Moreover, biopsies are often difficult to obtain and some patients
can have atypical presentations. In addition, the age criteria are
considered to be redundant (9).
Lastly, imaging modalities are not used routinely in the evaluation
of abdominal purpura (10).
X-rays may suggest the presence of fecal impaction,
an obstruction and duodenal atresia (11). Computed tomography (CT) scans of the
abdomen and pelvis can provide a high yield of information
(12). Ultrasound scans avoid
exposure to radiation (11). There
is a risk of overtreatment if children with abdominal pain are
treated with steroids because steroids can cause a rapid reduction
in abdominal pain but do not prevent recurrence (13). Steroids can also cause weight gain
issues (4). Therefore, there is a
need for non-invasive and accurate diagnostic modality for
abdominal purpura.
In clinical practice, it is known that abdominal
ultrasound is the best approach for the correct diagnosis of
abdominal complications. However, in some cases, CT can provide
more detailed information. Therefore, each diagnostic technique has
its specific role.
The objectives of the present study were to compare
diagnostic parameters of ultrasound, X-ray and CT for diagnosis of
abdominal purpura, and to find additional parameters to evaluate
and administer early treatment, considering ACR criteria as the
‘gold standard (reference index test)’ in children with acute
abdominal pain in China.
Patients and methods
Compliance with ethical standards
The protocol of the present study was approved by
the Guiyang Maternal and Child Health Hospital review board. The
present study was a retrospective analysis of prospectively
collected data. An informed consent form was signed by the parents
of all participating children regarding diagnosis, radiology,
treatment and anesthesia (if required) during hospitalization. The
present study adheres to the law of China, the Strengthening the
Reporting of Observational Studies in Epidemiology statement and
the Declaration of Helsinki (2008).
Inclusion criteria
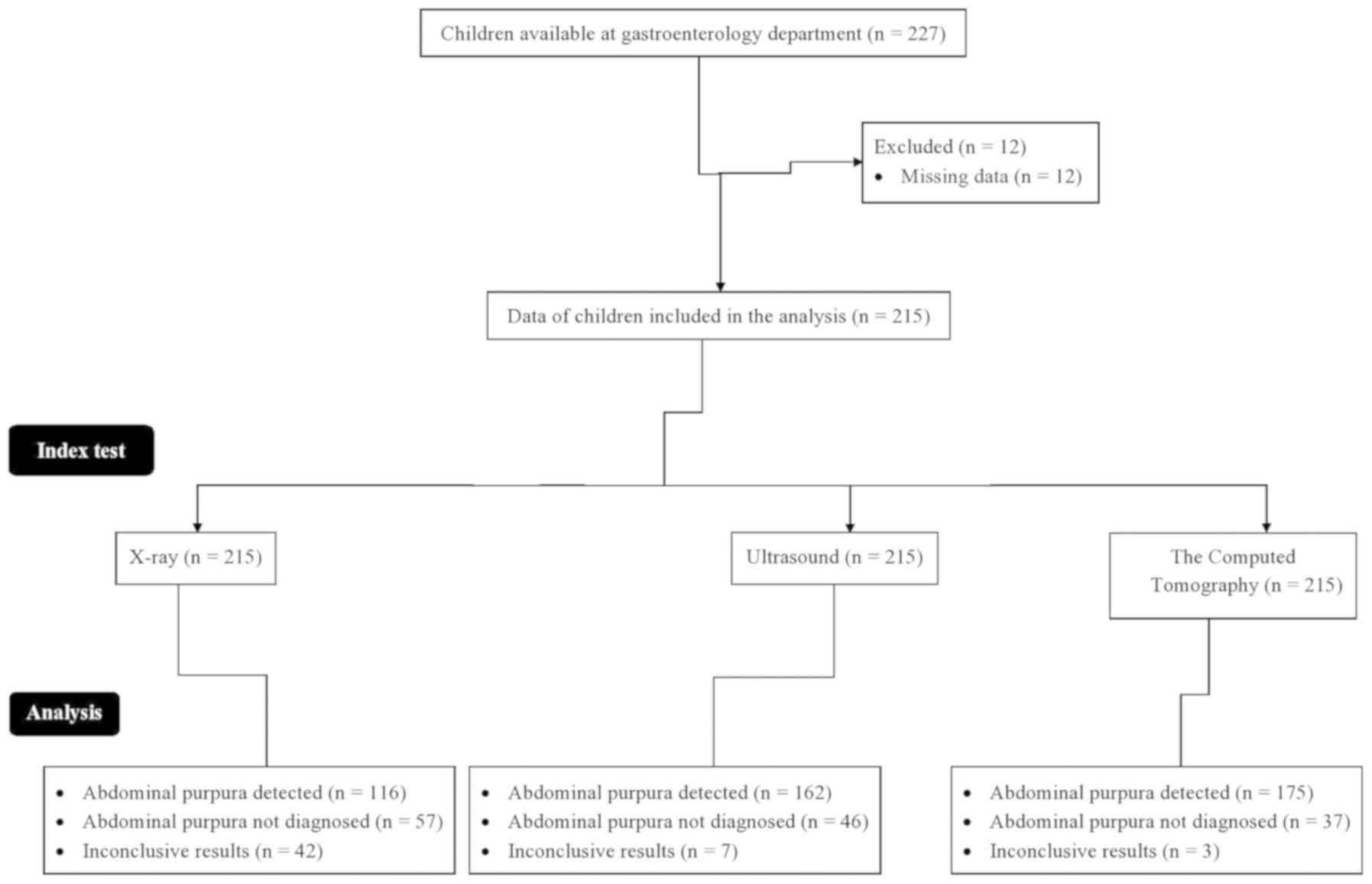
From January 1, 2013 to February 13, 2019, data from
227 children [109 (51%) boys and 106 (49%) girls] in the age range
from 3-16 years (mean: 11.25±2.11 years) were available at the
outpatient setting of Guiyang Maternal and Child Health Hospital.
The children experienced acute abdominal pain or abdominal
tenderness, and exhibited typical skin purpura.
Exclusion criteria
Among the available children, complete records of 12
children were not available. Therefore, they were excluded from the
present study. Medical records of 215 children were included in the
present analysis. A flow chart of the analysis is presented in
Fig. 1.
Data collection
Data regarding demographical parameters, laboratory
test results, X-ray, ultrasound findings, and CT images were
collected of enrolled children.
Image examination
Dilatation and motility of the intestinal segments,
intramural hemorrhage, and the wall of the small bowel were
investigated by image examinations. X-ray and CT scans were carried
out by radiologists with a minimum of 3 years of experience.
Ultrasound was performed by gastroenterologists with a minimum of 3
years of experience.
Abdominal X-ray
Supine anteroposterior and/or horizontal beam
(upright, decubitus or cross-Table lateral) views of abdominal
X-rays (Brivo XR115; GE Healthcare) were performed at 60-75 kVp,
100 mA and 200 kHz.
Abdominal ultrasound
Abdominal ultrasound was performed using the Philip
EPEQ5 5-13 MHz linear probe.
Abdominal CT
Abdominal CT was performed using the Revolution Apex
scanner (GE Healthcare).
Beneficial score analysis
Decision curve analysis was used for evaluation of
the beneficial score and working area for each diagnostic modality
as follows:
The working area is defined as the likelihood to
detect abdominal purpura. Sensitivity is the ratio of the value of
true positive abdominal purpura detected by imaging modality with
those detected by ACR criteria. Accuracy is the ratio of the value
of true negative abdominal purpura detected by imaging modality
with those detected by ACR criteria.
Statistical analysis
SPSS Statistics version 25 (IBM Corp.) was used for
statistical analysis. Categorical parameters are demonstrated as
frequency (percentage) and continuous parameters are demonstrated
as the mean ± SD. Categorical parameters were analyzed using the
χ2 test or the Fisher's exact test (14) and continuous parameters were analyzed
using the Wilcoxon sum rank test or the Mann-Whitney U test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinical features
In total, 99 (46%) children had abdominal tenderness
located in the periumbilical area. Nausea, vomiting and diarrhea
were frequent among the children. Bloody diarrhea was present in 8
(4%) children. Hematuria was observed in 18 (8%) children.
Laboratory tests
All children had an erythrocyte sedimentation rate
above the normal range, 33 (15%) children had a positive occult
blood stool test. All children were put on Deflazacort at 0.9 mg/kg
orally once a day for 6-8 days. Other laboratory test results are
presented in Table I.
 | Table ILaboratory test results. |
Table I
Laboratory test results.
| Test | Normal value | Test prediction | Test results |
|---|
| Serum amylase,
IU/l | 23-85 | Abnormal | 135±25 |
| Urine amylase,
IU/l | 20-400 | Abnormal | 1,215±85 |
| Hemoglobin, g/dl | 12-15 | Normal | 11.5±1.12 |
| Total leucocyte,
count/µl | 4,500-11,000 | Abnormal | 13,125±245 |
| Platelet,
count/µl | 150,000-450,000 | Normal | 353,561±7,245 |
| Erythrocytes
sedimentation rate, mm per the first hour | 12-20 | Abnormal | 28.12±3.45 |
| Serum immunoglobulin
A, mg/dl | 40-230 | Borderline | 210±35 |
| Protein urea,
mg/mmol | <100 | | |
|
Positive | | | 116(54) |
|
Negative | | | 99(46) |
| Granulocytes in the
walls of small venules and arterioles in the biopsies | N/A | Positive | 65(30) |
| | | Negative | 150(70) |
| Occult blood stool
test | N/A | Positive | 33(15) |
| | | Negative | 182(85) |
| Treatment | N/A | N/A | Deflazacort (0.9
mg/kg orally once a day for 6-8 days) |
| Onset of symptoms,
days | N/A | N/A | 7±2 |
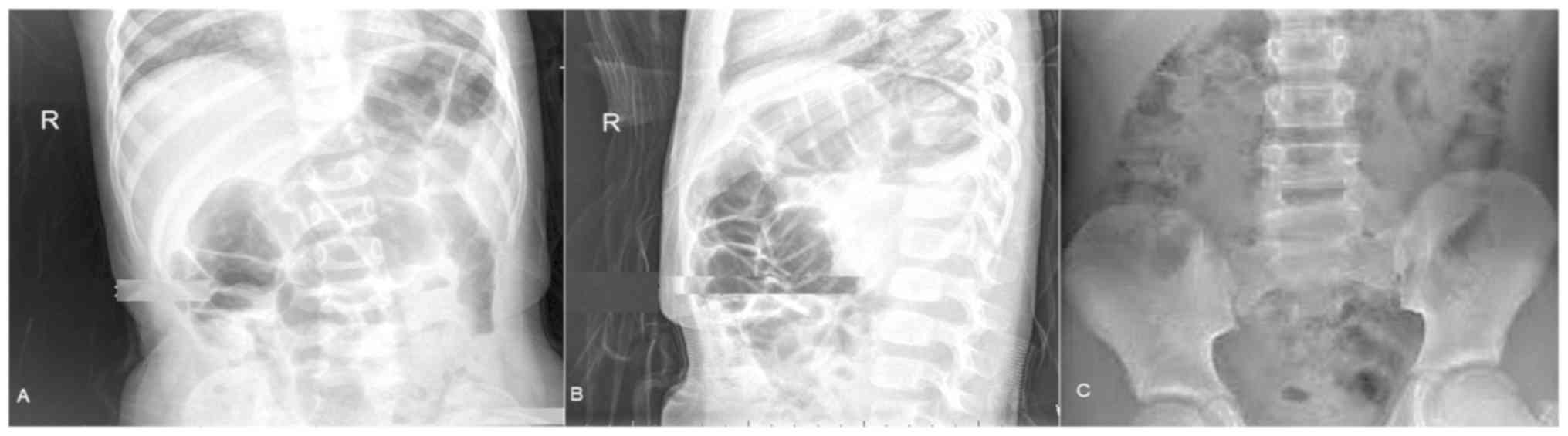
X-ray measurements
Interior posterior views (Fig. 2A) and lateral views (Fig. 2B) of the abdomen were successful in
the presentation of ileus and epididymitis (considering urinary
bladder as a reference limit) but X-ray did not detect abdominal
involvement where the occult blood stool test and biopsy results
were negative (Fig. 2C).
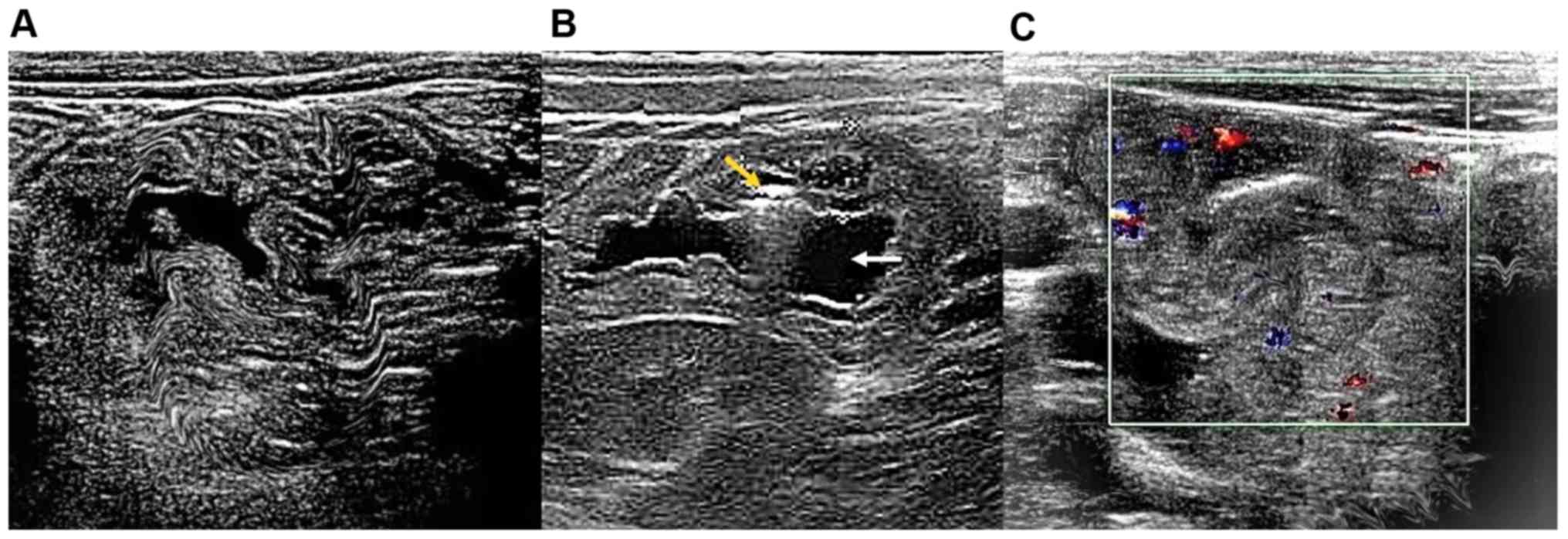
Ultrasound measurements
Ultrasound examinations identified thickening of the
bowel wall accompanied by reduced peristalsis and dilated bowel
loops. An outer hypoechoic ring from the edematous walls of the
intussuscipiens was identified around an echo-dense center of the
intussuceptum under ultrasound examination of empty ileum (n=85;
Fig. 3A). With confirmed ileocecal
intussusception, subdiaphragmatic free gas was not found on the
ultrasound scan of the duodenum (n=42; Fig. 3B). However, on the outer surface of
the colon, thick loops with extensive linear hemorrhages and bowel
perforation were identified (n=22; Fig.
3C).
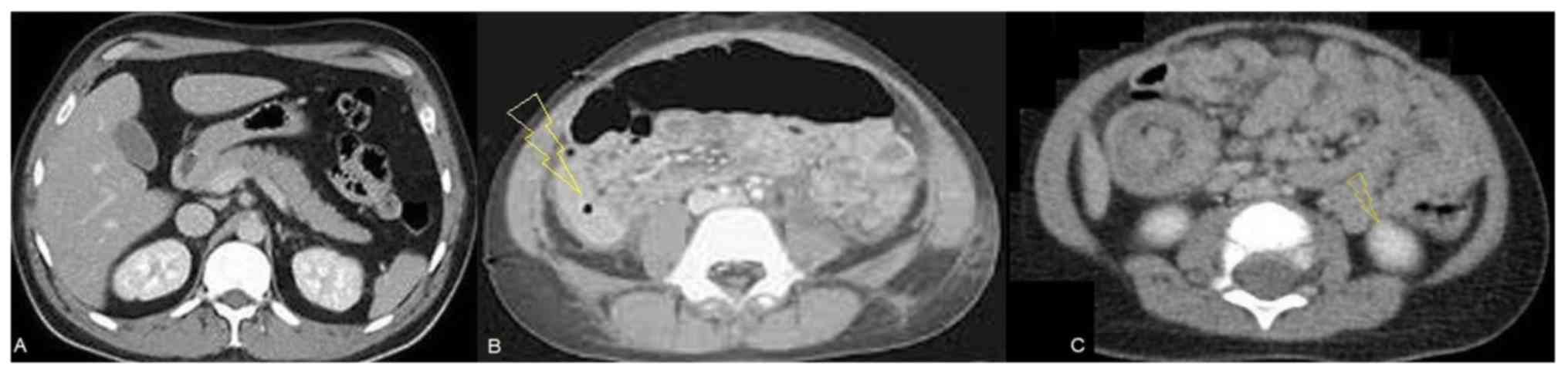
CT measurements
Thickening of the wall and focal dilated small loop
at the jejunum were detected in the abdominal CT scan (Fig. 4A). In addition, CT scans detected
bowel perforation (Fig. 4B) and
intussusception in abdomen (Fig.
4C).
Diagnostic parameters
CT had the highest sensitivity (0.939) but
ultrasound findings had the highest accuracy (0.861) among
diagnostic modalities for detection of abdominal purpura. The other
diagnostic parameters of imaging modalities are presented in
Table II.
 | Table IIDiagnostic parameters. |
Table II
Diagnostic parameters.
| | Diagnostic modalities
measurements |
|---|
| | X-ray | Ultrasound | Computed
tomography |
|---|
| Parameters | ACR
criteriaa | Result | P-valueb | Result | P-valueb | Result | P-valueb |
|---|
| True positive | 179(83) | 101(47) | <0.0001 | 154(72) | 0.006 | 168(78) | 0.223c |
| True negative | 36(17) | 24(11) | 0.126c | 31(14) | 0.595c | 25(12) | 0.167c |
| False positive | 0 (0) | 35(16) | <0.0001 | 8(4) | 0.013 | 7(3) | 0.022 |
| False negative | 0 (0) | 22(10) | <0.0001 | 15(7) | 0.0002 | 12(6) | 0.001 |
| Inconclusive
results | 0 (0) | 33(16) | <0.0001 | 7(3) | 0.022 | 3(1) | 0.247c |
| Sensitivity | 1 | 0.564 | <0.0001 | 0.86 | <0.0001 | 0.939 | <0.0001 |
| Accuracy | 1 | 0.667 | <0.0001 | 0.861 | <0.0001 | 0.694 | <0.0001 |
Beneficial score analysis
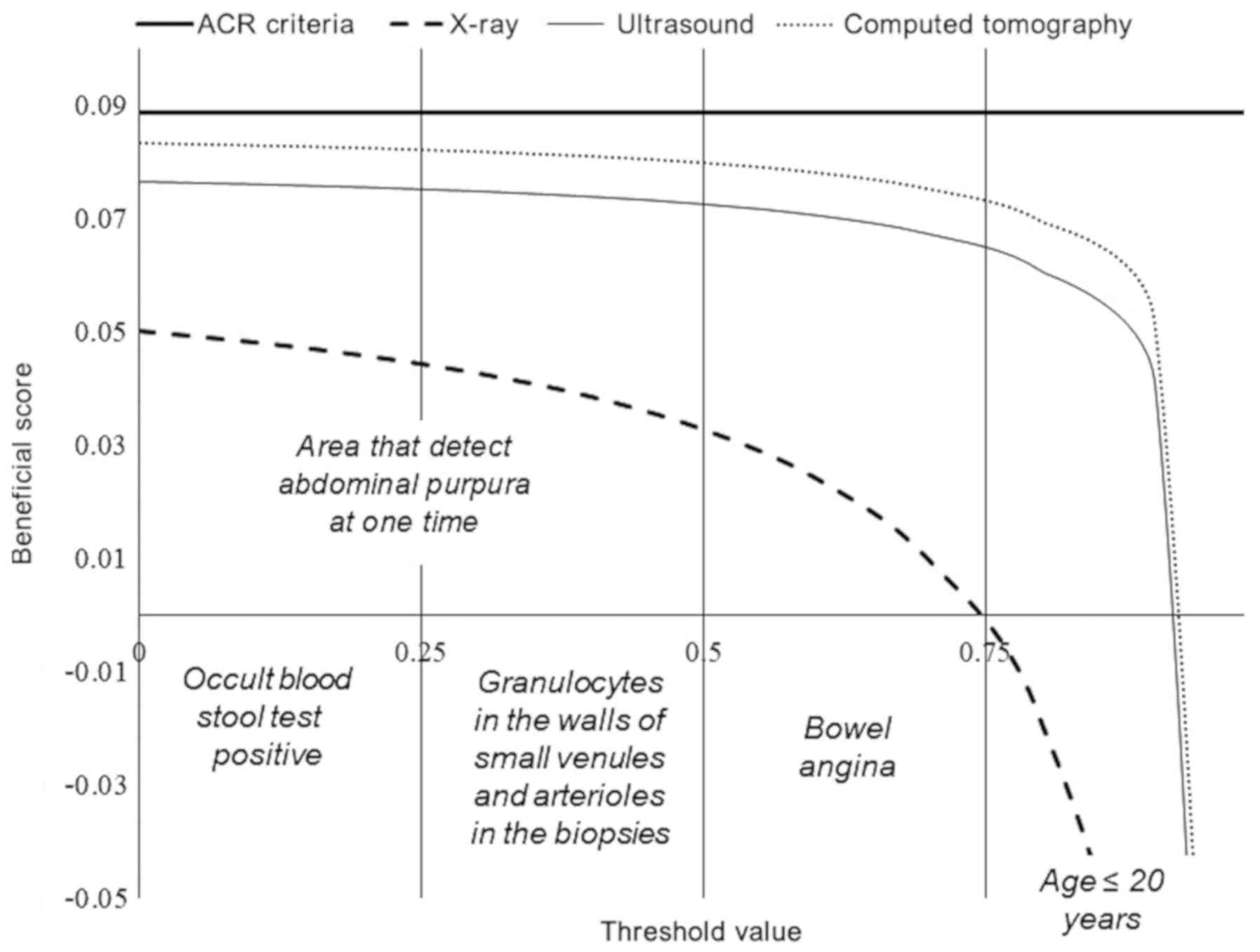
X-ray findings had 0-0.75 working area to detect
abdominal purpura at one time and >0.75 thresholds (only colic
pain with age <20 years, absent of the other laboratory tests)
there was a risk of over-diagnosis and overtreatment. Ultrasound
findings had 0-0.916 working area to detect abdominal purpura at
one time and >0.916 thresholds there was a risk of
over-diagnosis and over-treatment. CT had 0-0.922 working area to
detect abdominal purpura at one time and >0.922 thresholds there
was a risk of over-diagnosis and overtreatment. Ultrasound and CT
were successful at detecting abdominal purpura when children had
only colic pain and were aged <20 years; however; occult blood
stool test and biopsy results were absent (Fig. 5).
Discussion
Laboratory tests and clinical features provide
baseline information; however, do not rule out other etiologies of
colicky pain, except occult blood stool test and biopsy results.
However, biopsies are rarely preferred in the diagnosis of
abdominal pain by physicians (15).
Abdominal gastrointestinal symptoms are often observed prior to
clinical symptoms (4). Ultrasound,
CT and X-ray provide direct insight into visualization and
detection of complications and bowel involvement (for example,
bowel perforation and intussusception) (13) but imaging criteria are not used in
the diagnosis of purpura (9). Even
The European League Against Rheumatism, The Pediatric Rheumatology
International Trials Organization and The Pediatric Rheumatology
European Society 2010 criteria (15), which are the current gold standard,
do not include imaging criteria for the diagnosis of abdominal
purpura (4). However, imaging
modalities could play an important role in the diagnosis of
abdominal purpura in children.
In the present study, ultrasound, CT and X-ray were
successful at detecting abdominal purpura when children had colicky
pain, aged <16 years, and had bowel angina. Ultrasound and CT
had similar working areas but ultrasound had high accuracy among
all imaging modalities. X-ray was not effective in cases where
occult blood stool tests and biopsy results were negative. The
results of the present study were consistent with a previous
retrospective study (13) and a
previous case report (3). The As Low
As Reasonably Possible principle is required to maintain in the CT
of children to avoid the risk of cancer (12), which is difficult to manage in
children (16). Ultrasound has the
ability to assess hematomas, mural thickness, small bowel
intussusceptions and peritoneal fluid (3). Ultrasound provides an easy,
non-invasive and safe method for the detection of abdominal purpura
in children.
With respect to the ACR criteria, there were seven
and three inconclusive results for ultrasound and CT, respectively.
Abdominal fat may interfere with proper diagnosis in imaging
modalities. In addition, in non-obese Chinese children, CT
resolution was as good as ultrasound (17). Thus, abdominal CT is the best method
for diagnosis of abdominal purpura in Chinese children but has the
issue of radiation dose.
The use of an imaging method for the diagnosis of
abdominal involvement in purpura presents many limitations and is
only useful for some clinical presentations or complications. All
children were subjected to laboratory tests with imaging
modalities, which require exposure of high radiation doses. Indeed,
the treatment period and treatment dose were small, yet too high
for diagnosis methods. Demographical characteristics and seasons
also have an impact over the diverse etiology of abdominal purpura
in Chinese children (18,19); however, the present study did not
consider such parameters. Erosions, mural hematoma and ulcers can
be visualized by endoscopy (10).
Furthermore, endoscopic examinations can be more useful in view of
the pathophysiology of the disease, but endoscopy was not performed
at all. Additionally, serum immunoglobulin A was not used to detect
abdominal purpura in the children (9). The justification for that the level of
serum immunoglobulin A is varied with age and the other
demographical and clinical characters. Other limitations of the
present study include the retrospective nature of the analysis and
lack of a randomized trial.
In conclusion, laboratory tests and clinical
features do not rule out other etiologies of acute colicky pain.
Ultrasound and CT had the same findings in the diagnosis of
abdominal purpura; however, CT had an issue of radiation dose.
Abdominal ultrasound is an easy, non-invasive and safe method for
the detection of abdominal purpura.
Acknowledgements
Not applicable.
Funding
The present study was supported by a Provincial
Health and Family Planning Commission-Initiated Fund Project (grant
no. gzwjkj2015-1-067).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YG and SL contributed equally to the project
administration and conceptualization, data analysis using software,
formal analysis, validation, resources, data curation,
investigation, and literature review of the study. YG and SL
drafted, reviewed and edited the manuscript for intellectual
content. Both authors read and approved the final version of the
manuscript. Both authors agree to be accountable for all aspects of
work ensuring integrity and accuracy.
Ethics approval and consent to
participate
The protocol (approval no. GPH/CL/14/19; February
15, 2019) of the present study was approved by the Guiyang Maternal
and Child Health Hospital review board. An informed consent form
was signed by the parents of all participating children regarding
diagnosis, radiology, treatment and anesthesia (if required) during
hospitalization. The present study adheres to the law of China, the
Strengthening the Reporting of Observational Studies in
Epidemiology statement and the Declaration of Helsinki (2008).
Patient consent for publication
An informed consent form was signed by parents of
all participating children regarding publication of the present
study in all formats of the publication house, including personal
data and images, irrespective of time and language.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Başaran Ö, Cakar N, Uncu N, Celikel BA,
Kara A, Cayci FS, Taktak A and Gür G: Plasma exchange therapy for
severe gastrointestinal involvement of henoch schönlein purpura in
children. Clin Exp Rheumatol. 33 (Suppl 89):S176–S180.
2015.PubMed/NCBI
|
|
2
|
Srivali N, Ungprasert P, Ahmed S,
Cheungpasitporn W and Bischof EF: A case of childhood vasculitis
presenting in adulthood. Am J Emerg Med. 31:254–255.
2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lim CJ, Chen JH, Chen WL, Shen YS and
Huang CC: Jejunojejunum intussusception as the single initial
manifestation of Henoch-Schönlein purpura in a teenager. Am J Emerg
Med. 30(2085)2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
McPartland K and Wright G: Acute abdominal
pain: Henoch-Schönlein purpura case in a young adult, a rare but
important diagnosis. Clin Med (Lond). 19:77–79. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang Q, Guo Q, Gui M, Ren Z, Hu B, Lu L
and Deng F: Henoch-Schönlein purpura with acute pancreatitis:
Analysis of 13 cases. BMC Pediatr. 18(159)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yang YH, Yu HH and Chiang BL: The
diagnosis and classification of Henoch-Schönlein purpura: An
updated review. Autoimmun Rev. 13:355–358. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Keenswijk W, Van Renterghem K and Vande
Walle J: A case report of a child with purpura, severe abdominal
pain, and hematochezia. Gastroenterology. 153:e10–e11.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yang Z, Guo L, Xiong H, Gang Z, Li JX,
Deng YP, Dawa QZ, Pubu ZX and Li H: Clinical analysis of childhood
Henoch-Schonlein purpura on the Tibetan Plateau, China. Zhongguo
Dang Dai Er Ke Za Zhi. 16:1231–1235. 2014.PubMed/NCBI(In Chinese).
|
|
9
|
Batu ED and Ozen S: Vasculitis: Do we know
more to classify better? Pediatr Nephrol. 30:1425–1432.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Reamy BV, Williams PM and Lindsay TJ:
Henoch-Schönlein purpura. Am Fam Physician. 80:697–704.
2009.PubMed/NCBI
|
|
11
|
BMJ Best Practice. Evaluation of abdominal
pain in children. https://bestpractice.bmj.com/topics/en-us/787/diagnosis-approach.
Accessed December 1, 2018.
|
|
12
|
Brody AS, Frush DP, Huda W and Brent RL:
American academy of pediatrics section on radiology. Radiation risk
to children from computed tomography. Pediatrics. 120:677–682.
2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chang WL, Yang YH, Lin YT and Chiang BL:
Gastrointestinal manifestations in Henoch-Schönlein purpura: A
review of 261 patients. Acta Paediatr. 93:1427–1431.
2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Teng X, Gao C, Sun M and Wu J: Clinical
significance of fecal calprotectin for the early diagnosis of
abdominal type of Henoch-Schonlein purpura in children. Clin
Rheumatol. 37:1667–1673. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ozen S, Pistorio A, Iusan SM, Bakkaloglu
A, Herlin T, Brik R, Buoncompagni A, Lazar C, Bilge I, Uziel Y, et
al: Paediatric rheumatology international trials organisation
(PRINTO). EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura,
childhood polyarteritis nodosa, childhood wegener granulomatosis
and childhood takayasu arteritis: Ankara 2008. Part II: Final
classification criteria. Ann Rheum Dis. 69:798–806. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kirpalani H and Nahmias C: Letter:
Radiation risk to children from computed tomography. Pediatrics.
121:449–450. 2008.
|
|
17
|
Pelin M, Paquette B, Revel L, Landecy M,
Bouveresse S and Delabrousse E: Acute appendicitis: Factors
associated with inconclusive ultrasound study and the need for
additional computed tomography. Diagn Interv Imaging. 99:809–814.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen O, Zhu XB, Ren P, Wang YB, Sun RP and
Wei DE: Henoch schonlein purpura in children: Clinical analysis of
120 cases. Afr Health Sci. 13:94–99. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu LJ, Yu J and Li YN: Clinical
characteristics of Henoch-Schönlein purpura in children. Zhongguo
Dang Dai Er Ke Za Zhi. 17:1079–1083. 2015.PubMed/NCBI(In Chinese).
|