Introduction
Acute liver failure (ALF) is a devastating clinical
syndrome that is associated with a high mortality rate if not
treated promptly (1). Originally
termed fulminant hepatic failure, ALF is defined as severe liver
injury that is potentially reversible in nature with the onset of
hepatic encephalopathy (HE) occurring within 8 weeks of the first
symptoms in the absence of any pre-existing liver diseases
(2). Patients ALF with HE present
with various neuropsychiatric symptoms, including cognitive
deficiency, motor function impairment and alterations in
personality and consciousness (3,4).
Although the most effective therapy for ALF is liver
transplantation, due to the side effects associated with
immunosuppressant therapy and the shortage of donor organs, this
procedure is limited at present (5,6).
Therefore, further investigation into novel approaches for the
treatment of ALF is urgently sorted.
Hydrogen sulfide (H2S) was previously
known only for its unpleasant odor; however, an increasing number
of studies have revealed that endogenously produced H2S
serves a protective role in a number of physiological functions
(7). Numerous enzymes, including
cystathionine-γ-lyase (CSE), cystathionine-β-synthase and
3-mercaptopyruvate sulfurtransferase are responsible for
H2S synthesis (8). In the
liver, endogenously produced H2S participates in the
regulation of liver glucose metabolism, lipoprotein synthesis,
hepatic circulation, liver bioenergetics and oxidative stress
(8). A number of studies have
previously revealed the protective effects of H2S in
multiple models of hepatic diseases, including liver cirrhosis and
fibrosis (9), portal hypertension
(10) and hepatic
ischemia-reperfusion (I/R) injury (11). In addition, H2S has also
been demonstrated to be a novel signaling molecule and
neuromodulator in the central nervous system (12). Previous evidence has indicated that
H2S protects neurons from oxidative stress and
impairments of learning and memory in models of Alzheimer's disease
(13,14). Therefore, it could be hypothesized
that H2S may also exert protective role son mouse models
of ALF, with combined effects on both the brain and liver.
In the present study, ALF was induced in mice by
thioacetamide (TAA) treatment, where sodium hydrosulfide (NaHS)
served as the H2S donor. The results of the present
study revealed that H2S treatment alleviated cognitive
deficiency and preserved spatial orientation learning ability as
assessed by novel object recognition (NOR) and Y-maze tests,
respectively. In addition, H2S treatment reduced serum
aspartate transaminase (AST), alanine transaminase (ALT) and
ammonia levels and prevented weight loss following ALF induction.
These results suggested a protective effect of H2S in
ALF-model mice, indicating that H2S may serve as a
potential therapeutic agent for ALF.
Materials and methods
Animals
A total of 100 female Institute of Cancer Research
mice (age, 8 weeks; weight, 30±2 g) were provided by the Hunan SJA
Laboratory Animals Co., Ltd. and were housed individually in a
well-ventilated and temperature-controlled room (temperature, 25˚C;
humidity, 50%) under a 12-h light/dark cycle, with free access to
food and water. The mice had 7 days to habituate to their new
environment before they were subjected to experiments. All
experiments were conducted in accordance with the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals (15) and were approved by
the Animal Use and Protection Committee of the University of South
China (Hengyang, China). All efforts were made to minimize the
number of animals used and their suffering.
Drugs and treatments
NaHS, the H2S donor and TAA were
purchased from Sigma-Aldrich; Merck KGaA. NaHS and TAA were
dissolved in phosphate buffered saline (PBS) and sterile normal
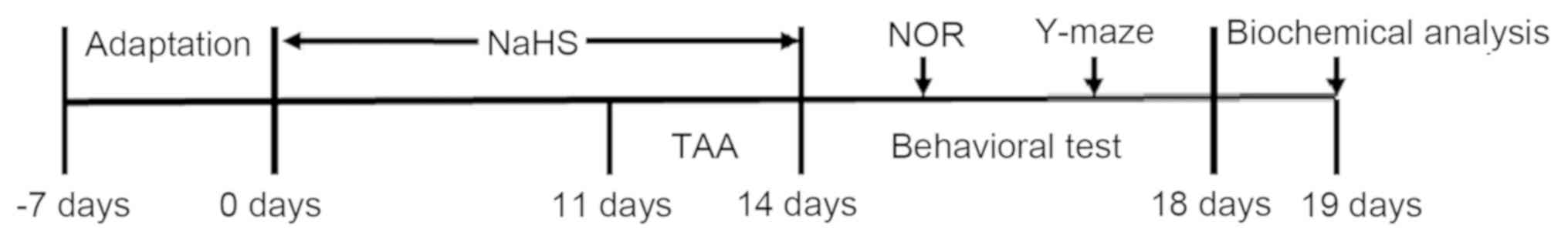
saline solutions, respectively. Following 7 days of adaptation, the
mice were randomly divided into five treatment groups (n=20 per
group; Fig. 1): i) The control
group, in which mice were injected intraperitoneally with PBS for
14 days; ii) the TAA-alone-treatment group, in which mice were
injected intraperitoneally with 150 mg/kg/day TAA for 3 days; iii)
the co-treatment with TAA and 30 µmol/kg/day NaHS group, in which
mice were pre-treated with 30 µmol/kg/day NaHS for 11 days and
subsequently co-treated with 150 mg/kg/day TAA for 3 days; iv) the
co-treatment with TAA and 100 µmol/kg/day NaHS group, in which the
mice were pre-treated with 100 µmol/kg/day NaHS for 11 days and
subsequently co-treated with 150 mg/kg/day TAA for 3 days; and v)
the NaHS-alone-treated group, in which mice were injected
intraperitoneally with 100 μmol/kg/day NaHS for 14 days. A
well-established model of TAA-induced ALF was used (16-18).
Following TAA injection for 24 h, all animals, including those in
the control group, were subcutaneously injected with 0.5 ml
solution containing 0.45% NaCl, 5% dextrose and 0.2% KCl to prevent
the development of hypovolemia, hypoglycemia and hypokalemia,
respectively. Hypothermia was prevented by intermittent exposure to
infrared light in a procedure described previously (19,20).
Survival analysis after TAA
challenge
Survival rates in each group were recorded on the
mornings of days 15-18. Housing was maintained at a temperature of
25˚C and relative humidity of 50% to reduce animal suffering.
Baseline body weight was measured at 8:00 am on the first day of
TAA injection and body weight was subsequently recorded three times
per day (at the beginning of the day and every 6 h between 8:00 am
and 8:00 pm). Animals were euthanized by sodium pentobarbital
overdose (100 mg/kg, intravenous injection) when a reduction of
>20% baseline body weight was observed. All mice were euthanized
when all the measurements were completed.
NOR test
Mice cognitive function was assessed by using a
novel objection recognition analysis system (BW-NOD405; Shanghai
Biowill Co., Ltd.). NOR test was performed once per day after the
induction of ALF. The NOR test included three trials. In the
habituation phase, all mice were habituated in a 38x38x38 cm test
box for 2 days. All individuals were allowed to explore the empty
arena for 5 min once per day. On day 3 of the NOR test, the mice
were allowed to explore two identical objects that were placed at
opposite corners of the box for 5 min, in a process known as the
familiarization phase. Following a 1-h retention interval, the mice
underwent a test session in the box with one familiar and one novel
object. Each animal was allowed to explore the objects for 5 min,
where the exploration time spent for each object was recorded.
Ethanol (75% v/v) was used to wipe the objects and the test
apparatus prior to each test to avoid olfactory cue formation.
Sniffing and touching of the objects (distance within 1 cm) were
considered exploratory behaviors, whilst climbing on the objects or
chewing were not. Discrimination index, which was calculated as the
time difference between new and familiar object exploration/total
time, was adopted to evaluate animal cognitive function (21,22).
Y-maze test
Spatial orientation learning ability was assessed by
using a Y-maze analysis system (BW-MYM103; Shanghai Biowill Co.,
Ltd.). The Y-maze test was performed 4 days following the induction
of ALF. The Y-maze was divided into three arms (A, B and C) with an
angle of 120˚ between them. The size of each arm was 50x18x35 cm.
Each mouse was habituated in the Y-maze for 10 min prior to the
test. Subsequently, the mice were placed into the first arm and
allowed to move freely for 5 min. Arm entry was recorded when all
four limbs of the mouse completely entered the arm. The appropriate
alternating sequence was defined as three consecutive entries made
into the different arms, whilst animals that entered the same arm
three times consecutively were considered to have performed an
incorrect alternating sequence. Ethanol (75% v/v) was used to wipe
the objects and the test apparatus prior to each test in order to
remove olfactory cues. A spontaneous alternation score was
estimated to measure spatial orientation learning ability (23).
Serum ammonia and liver enzymes
Blood samples (500 µl) were obtained from each mouse
via the orbital vein after Y maze test under anesthesia with an
intraperitoneal injection of sodium pentobarbital (50 mg/kg). After
blood extraction, all mice were euthanized by sodium pentobarbital
overdose (100 mg/kg, intravenous injection). ALT, AST and ammonia
levels were analyzed in glass tubes, following the Y-maze test.
Serum samples were centrifuged (speed, 1006.2 x g; temperature,
37˚C) for 5 min and analyzed on the day of sampling using a Hitachi
7600 series Automatic Analyzer (Hitachi, Ltd.). All serum samples
were processed in the same laboratory using the same methods and
reference values.
Statistical analysis
Statistical analysis was performed using the SPSS
18.0 software (SPSS, Inc.). The data are presented as the mean ±
SEM. One-way ANOVA with the least-significant difference multiple
comparison test (where there were 3 groups) or Tukey's post hoc
test (where there were >4 groups) was performed. Kaplan-Meier
survival analysis was performed using the Cox proportional hazards
regression and the Log-rank test. Weight data was processed using
mixed-design ANOVA and one-way ANOVA, following which Bonferroni's
test was used as the post hoc test for simple main effects.
P<0.05 was considered to indicate a statistically significant
difference.
Results
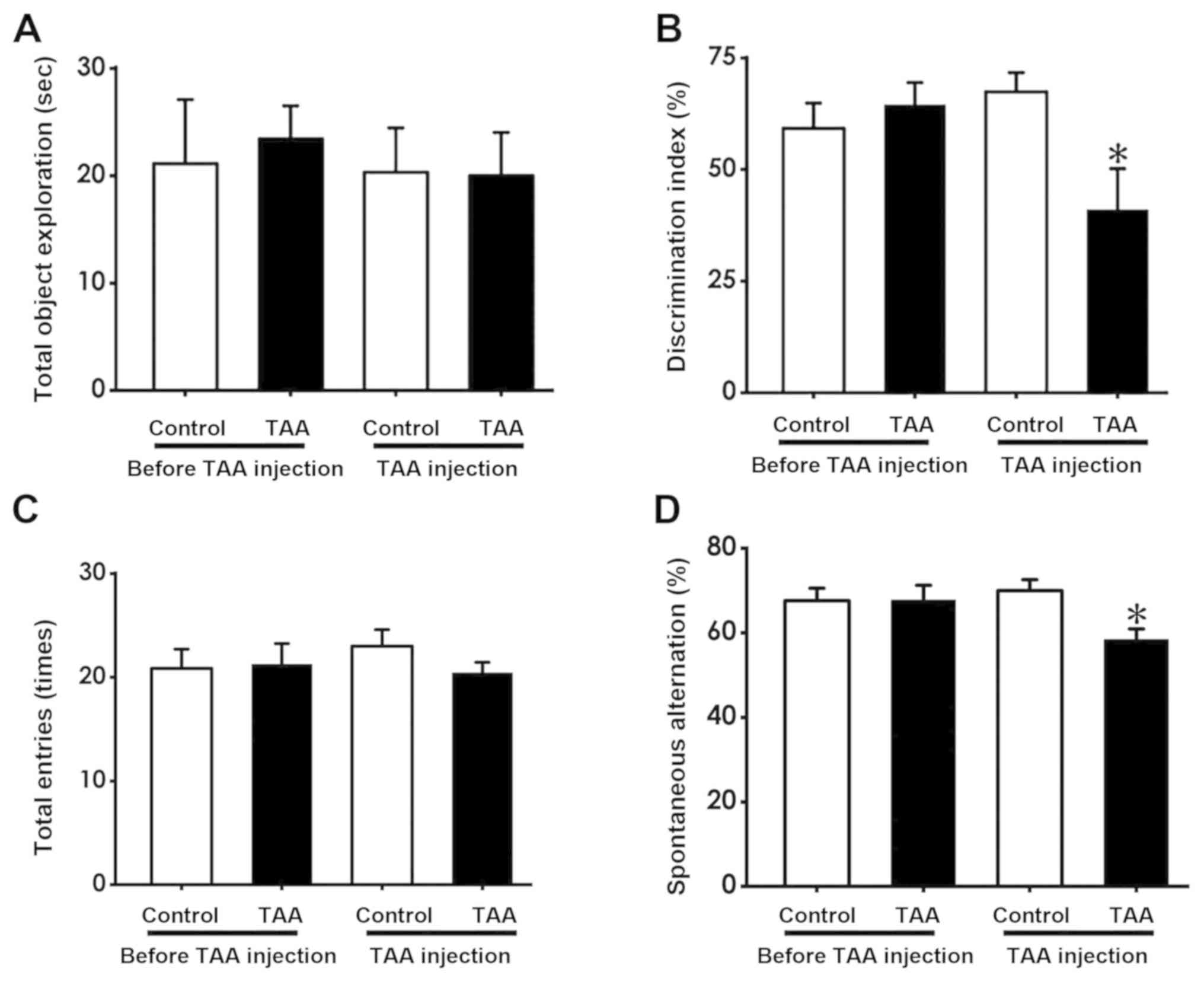
TAA induces cognitive deficiency as
demonstrated by the NOR and Y-maze tests
NOR and Y-maze tests were performed to evaluate
mouse cognitive function following and prior to TAA treatment (150
mg/kg, intraperitoneally). The NOR test revealed that the
discrimination index in TAA-treated mice was significantly reduced
following TAA injection compared with that in the TAA group prior
to injection (Fig. 2B). However, no
significant difference was observed between the total time spent
exploring the novel object prior to and following TAA injection
(Fig. 2A). In the Y-maze test, the
number of spontaneous alternations made by mice were found to be
significantly decreased following TAA treatment compared with those
prior to TAA treatment (Fig. 2D),
whilst no significant differences were observed in the number of
total entries made by the mice prior to and following TAA injection
(Fig. 2C). These results suggest
that TAA treatment resulted in cognitive deficiency.
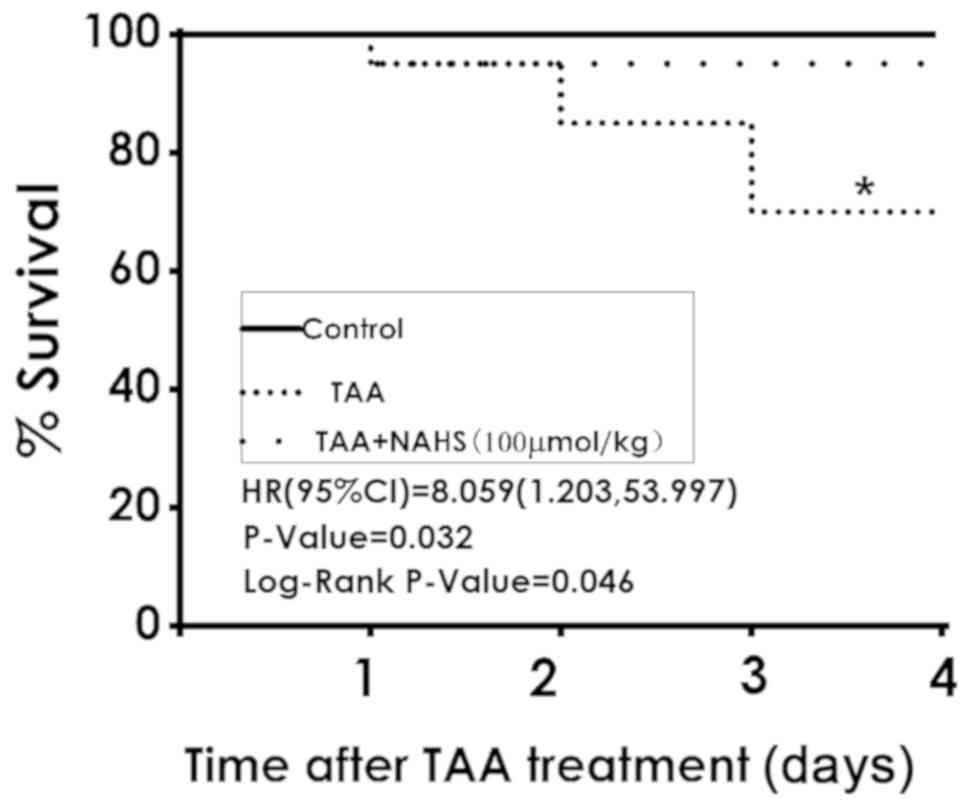
H2S improves the survival
rate of TAA-treated mice
The results of the present study revealed that 6 of
the 20 mice did not survive to day 18 following TAA administration
(150 mg/kg, intraperitoneally), whilst all mice in the control
group were viable. NaHS treatment (100 µmol/kg/day,
intraperitoneally) significantly reduced the mortality rate in the
co-treatment group, where 1 of the 20 mice did not survive
(P=0.046; Fig. 3). These results
demonstrated that H2S treatment improved the survival
rates of ALF mice.
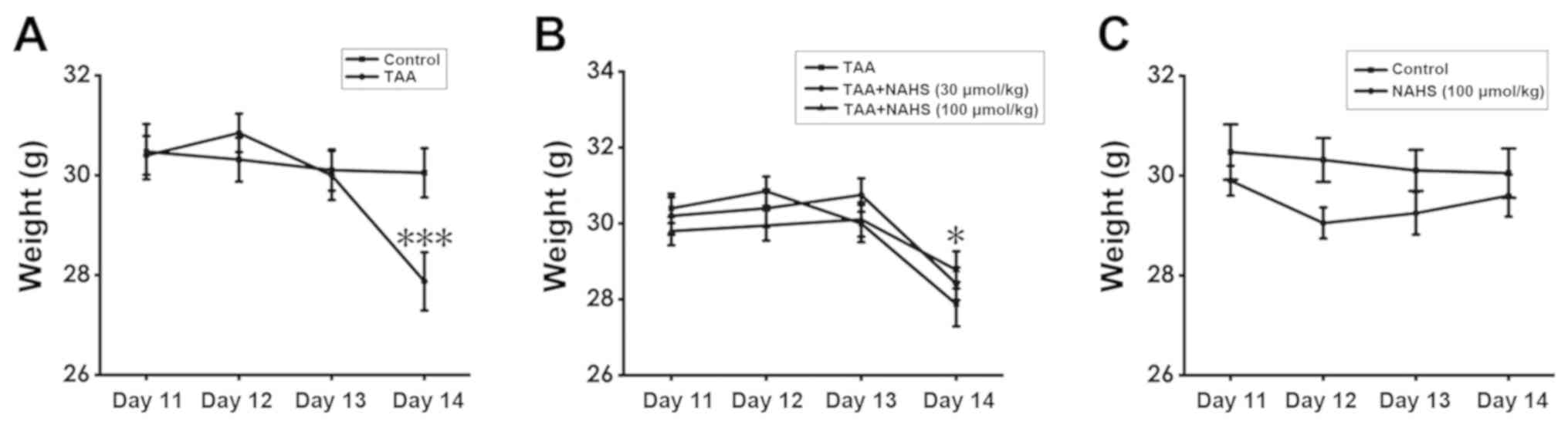
H2S prevents weight loss in
TAA-treated mice
To assess weight changes in the mice among the
treatment groups over time, a mixed ANOVA was conducted. The
results showed that the main effect of treatment regimen was not
significantly different (F=0.549; P=0.76) and the subsequent
post-hoc test for the main effects of treatment regimen also did
not reveal any significant differences. However, the main effect of
time (F=34.609; P<0.001) and the interaction between time and
treatment regimen (F=7.987; P<0.001) were found to be
significantly different. For the interaction between time and
treatment regimen, one-way ANOVA measuring the independent effects
of treatment regimen at a specific time point was performed. There
was no significant difference among the treatment regimens on days
11-13. However, on day 14, TAA treatment (150 mg/kg,
intraperitoneally) significantly reduced the body weight of the
animals compared with that of control animals (Fig. 4A), whilst NaHS treatment (100
µmol/kg/day, intraperitoneally) prevented weight loss in
TAA-treated mice compared with mice in the TAA-treatment alone
group (P=0.017; Fig. 4B). In
addition, no significant differences were noted in the body weight
between the mice in the control and those in the
H2S-alone-treated groups (Fig. 4C). These results suggest that
H2S pre-treatment prevented weight loss in ALF mice.
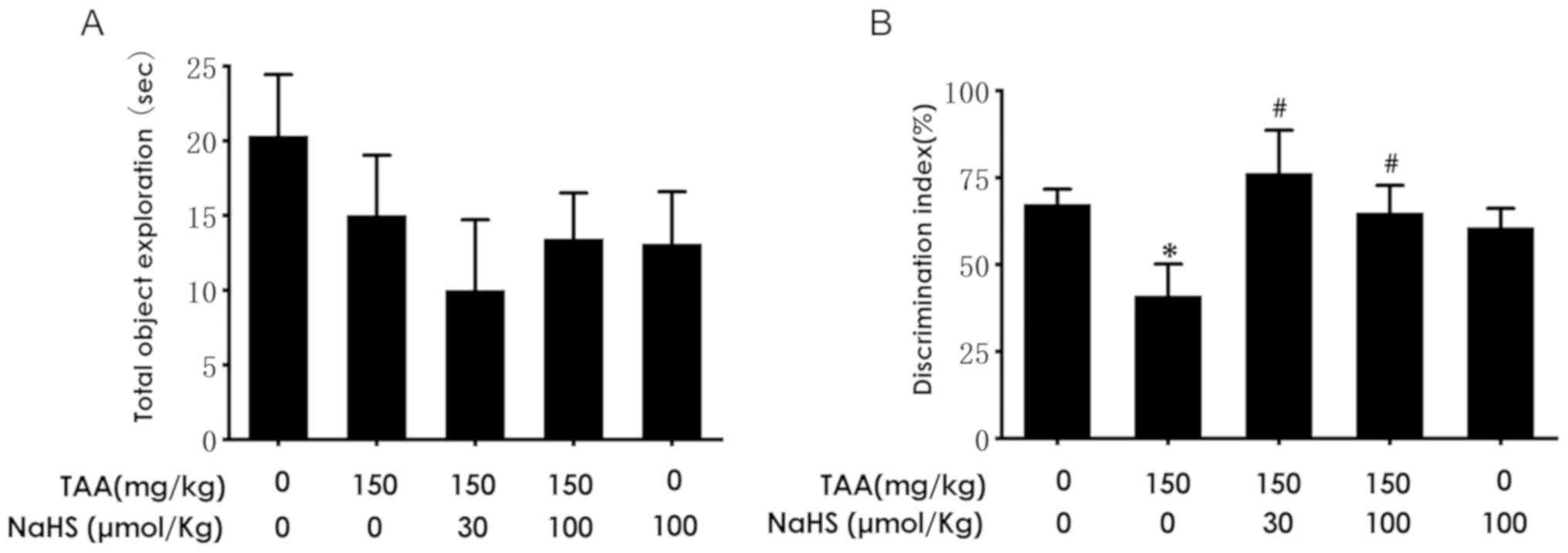
H2S attenuates cognitive
deficiency as determined by the NOR test
In the present study, the effect of H2S
treatment on TAA-induced cognitive dysfunction was subsequently
investigated. The mice were pre-treated with NaHS for 11 days and
co-treated with TAA for an additional 3 days, following which
cognitive function was investigated using the NOR test. No
significant differences were noted in the time of total object
exploration among the five groups of mice (Fig. 5A). The discrimination index in the
TAA-treated mice were found to be significantly decreased compared
with that noted in the control group (Fig. 5B). However, NaHS treatment (30 or 100
µmol/kg/day, intraperitoneal administration) significantly
increased the discrimination index compared with that in the TAA
treatment alone group (Fig. 5B).
These results suggested that H2S ameliorated cognitive
deficiency triggered by the TAA injection.
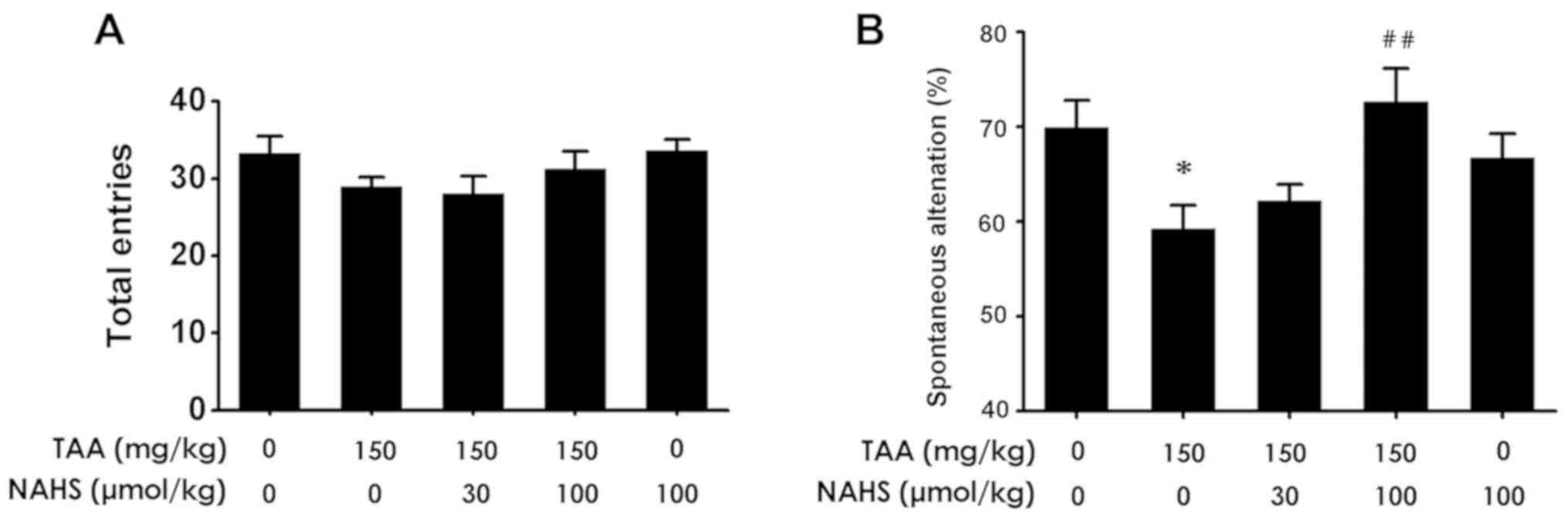
H2S improves spatial
orientation learning ability in the Y-maze test
To investigate further whether H2S
treatment improved the spatial orientation learning ability of mice
with ALF, the animals were subjected to the Y-maze test. No
significant differences were observed in the number of total
entries among the 5 groups of mice (Fig.
6A). Spontaneous alternations in the TAA-treated mice were
revealed to be significantly decreased compared with those noted in
the control group (Fig. 6B).
However, NaHS administration (100 µmol/kg/day, intraperitoneally)
increased the spontaneous alternations in the TAA-treated mice
compared with those treated with TAA alone. The results suggested
that H2S treatment protected mice from spatial
orientation learning ability impairment triggered by TAA
administration.
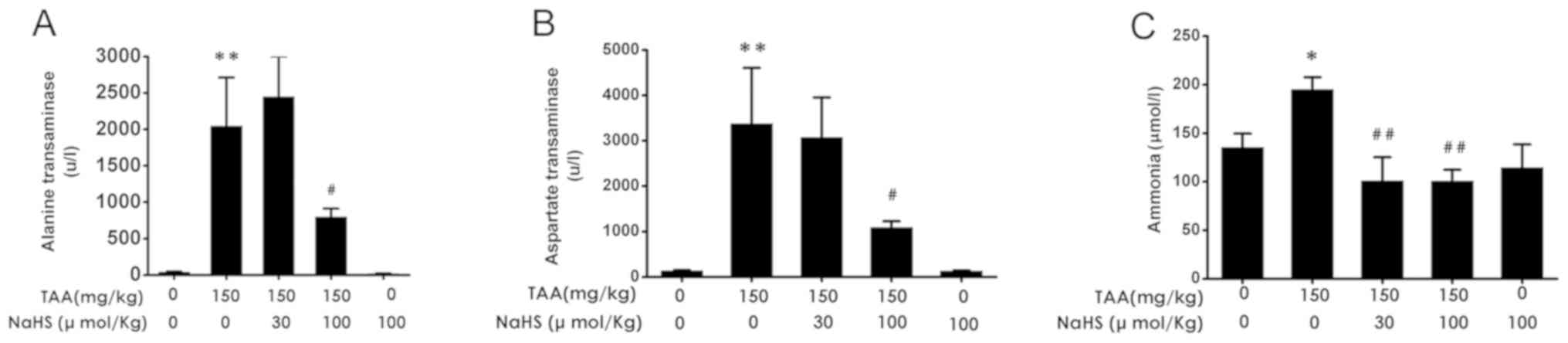
H2S decreased serum levels
of ALT, AST and ammonia after TAA treatment
AST, ALT and ammonia serum levels were next measured
among the five groups. TAA administration (150 mg/kg,
intraperitoneally) resulted in significant increases in serum ALT,
AST and ammonia levels in comparison with control treatment
(Fig. 7A-C). TAA co-administration
in the presence of NaHS (100 µmol/kg/day, intraperitoneally) caused
significant reductions in the serum levels of ALT, AST and ammonia
compared with TAA alone (Fig. 7A-C).
These results suggested that H2S treatment reduced liver
dysfunction induced by TAA administration.
Discussion
TAA administration induces ALF, leading to cognitive
deficiency and hepatic dysfunction in mice (17,24).
Previous studies have provided evidence that endogenous
H2S exerts a series of protective effects on the brain
and liver (25-27).
The aim of the present study was to investigate the role of
H2S treatment in TAA-induced mouse models of ALF where
the potential therapeutic value of H2S in this mouse
model was also evaluated. The main findings were as follows: i)
H2S treatment appeared to alleviate cognitive deficiency
and preserve spatial orientation learning ability in mice treated
with TAA according to NOR and Y-maze test data, respectively; and
ii) H2S treatment appeared to reduce serum levels of
ALT, AST and ammonia, in addition to preventing weight loss in
TAA-treated mice. Therefore, H2S treatment is suggested
to exhibit protective effects on both brain and liver function in
ALF mice.
TAA-induced ALF is a well-established rodent ALF
model (28). The Y-maze and NOR
tests are widely used as robust assays for assessing the cognitive
function in mice (28). In the NOR
test, NaHS administration led to marked increases in the
discrimination index in the TAA-treated mice compared with mice
treated with TAA alone, suggesting a protective effect of
H2S on cognition. The protective action of
H2S on cognition was confirmed further according to data
obtained using the Y maze test. Compared with the control
treatment, TAA treatment resulted in a reduction in spontaneous
alternation, an effect that was reversed by H2S
treatment. These findings suggest that H2S alleviated
cognitive deficiency induced by TAA. A previous study conducted by
our group (23) indicated that
H2S application ameliorated cognitive dysfunction in a
chronic restrain stress (CRS)-induced rat model, where
H2S reversed the production of malondialdehyde and the
reductions in superoxide dismutase activity and glutathione levels,
demonstrating that it could protect against CRS-induced oxidative
stress in the brain. To uncover the underlying molecular mechanisms
of this protective action of H2S further, additional
biochemical experiments are required in future studies.
The serum levels of ALT and AST are sensitive
markers for measuring liver injury (10,29). To
investigate the effect of H2S on liver function, the
serum levels of ALT, AST and ammonia were measured. H2S
significantly reduced the serum levels of ALT and AST, suggesting
that H2S exerted a hepato-protective effect. However, in
a previous study, NaHS administration (0.15 mmol/kg,
intraperitoneally) in rats augmented ALT and AST serum levels in
the TAA-treatment group, suggesting that H2S treatment
aggravated liver injury and manifested hepatotoxic effects
(30). Different NaHS concentrations
may be the cause of the aforementioned discrepancies. A previous
study reported that NaHS administration in mice resulted in
increased liver myeloperoxidase (MPO) activity, which is a marker
of tissue neutrophil infiltration and tumor necrosis factor-α
levels in the plasma (31). These
results indicated a pro-inflammatory effect of H2S. By
contrast, administration of the CSE inhibitor DL-propargylglycine
(50 mg/kg; intraperitoneally) exhibited marked anti-inflammatory
activity by reducing liver MPO activity and tissue damage (31). In addition, H2S has been
reported to induce a biphasic concentration-dependent effect
(32). In the present study, it was
observed that NaHS treatment (30 or 100 µmol/kg/day,
intraperitoneal administration) manifested neuroprotective effects
on TAA-treated mice, consistent with the previous study (33). Therefore, low and increased levels of
H2S exhibited cytoprotective and cytotoxic effects,
respectively (34). HE is a common
complication in patients with ALF, which is associated with poor
prognosis (35). Hyperammonemia is
strongly implicated in the pathogenesis of hepatic encephalopathy
that can cause brain edema, oxidative stress and inflammation
(36). Hyperammonemia can also
affect hepatocyte function (37).
The present study indicated that H2S treatment reversed
the increased serum ammonia levels in TAA-treated mice, suggesting
that H2S treatment inhibited the development of hepatic
encephalopathy by preserving liver function. Histological analysis
in an ALF model will be performed in future research, to verify the
aforementioned conclusions.
The present study did not directly measure
H2S levels, which is considered a limitation and should
be assessed in a future study. Additionally, H2S
pretreatment for 11 days can be considered excessive, where
H2S concentration in the body may have been compensated
for before TAA injection. Follow-up studies should therefore assess
H2S concentrations in the blood and liver tissues or
cerebrospinal fluid, in addition to adjusting the H2S
pretreatment time. In the present study, NaHS was applied as the
donor of H2S. Interestingly, Shirozu et al
(6) reported that sodium
thiosulfate, another donor of H2S, also attenuated liver
injury in mouse models of acute liver failure. Therefore, sodium
thiosulfate could have been adopted as an alternative donor of
H2S in a further study.
In conclusion, the present study demonstrated that
H2S treatment alleviated cognitive deficiency and
hepatic impairment in an ALF mouse model. The current findings
indicate that H2S treatment exerts combined beneficial
effects on the brain and liver in ALF mice, which may serve as a
protective molecule against ALF. However, the precise mechanisms
underlying the protective effect of H2S in ALF remain
elusive. Future studies should examine the molecular mechanisms
underlying the aforementioned physiological processes. In addition,
clinical studies are required to determine the potential clinical
application of H2S in the therapy of ALF.
Acknowledgements
Not applicable.
Funding
The presrnt study was supported by a Project of
Research-based Learning and Innovative Experiments for
Undergraduate Students grant (grant no. 2018XJXZ145).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding authors on reasonable
request.
Authors' contributions
DSY, YQH, YJF, JX, YLH, SSZ, and PYS performed the
experiments and analyzed the data. XQT was accountable for all the
aspects of the work and responsible for final approval of the
version to be published. DSY, YQH, YJF and XQT were responsible for
manuscript writing and revision and experimental design. All
authors approved the final version of the manuscript and
figures.
Ethics approval and consent to
participate
All experiments were conducted in accordance with
the National Institutes of Health Guide for the Care and Use of
Laboratory Animals and were approved by the Animal Use and
Protection Committee of the University of South China (approval no.
1807022; Hengyang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stravitz RT, Kramer AH, Davern T, Shaikh
AO, Caldwell SH, Mehta RL, Blei AT, Fontana RJ, McGuire BM, Rossaro
L, et al: Acute Liver Failure Study Group: Intensive care of
patients with acute liver failure: Recommendations of the U.S.
Acute Liver Failure Study Group. Crit Care Med. 35:2498–2508.
2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Trey C and Davidson CS: The management of
fulminant hepatic failure. Prog Liver Dis. 3:282–298.
1970.PubMed/NCBI
|
|
3
|
Felipo V: Hepatic encephalopathy: Effects
of liver failure on brain function. Nat Rev Neurosci. 14:851–858.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Poordad FF: Presentation and complications
associated with cirrhosis of the liver. Curr Med Res Opin.
31:925–937. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lee WM: Acute liver failure. N Engl J Med.
329:1862–1872. 1993.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shirozu K, Tokuda K, Marutani E, Lefer D,
Wang R and Ichinose F: Cystathionine γ-lyase deficiency protects
mice from galactosamine/lipopolysaccharide-induced acute liver
failure. Antioxid Redox Signal. 20:204–216. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen WL, Xie B, Zhang C, Xu KL, Niu YY,
Tang XQ, Zhang P, Zou W, Hu B and Tian Y: Antidepressant-like and
anxiolytic-like effects of hydrogen sulfide in behavioral models of
depression and anxiety. Behav Pharmacol. 24:590–597.
2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mani S, Cao W, Wu L and Wang R: Hydrogen
sulfide and the liver. Nitric Oxide. 41:62–71. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fiorucci S, Antonelli E, Mencarelli A,
Orlandi S, Renga B, Rizzo G, Distrutti E, Shah V and Morelli A: The
third gas: H2S regulates perfusion pressure in both the
isolated and perfused normal rat liver and in cirrhosis.
Hepatology. 42:539–548. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tan G, Pan S, Li J, Dong X, Kang K, Zhao
M, Jiang X, Kanwar JR, Qiao H, Jiang H and Sun X: Hydrogen sulfide
attenuates carbon tetrachloride-induced hepatotoxicity, liver
cirrhosis and portal hypertension in rats. PLoS One.
6(e25943)2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kang K, Zhao M, Jiang H, Tan G, Pan S and
Sun X: Role of hydrogen sulfide in hepatic
ischemia-reperfusion-induced injury in rats. Liver Transpl.
15:1306–1314. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Tan BH, Wong PT and Bian JS: Hydrogen
sulfide: A novel signaling molecule in the central nervous system.
Neurochem Int. 56:3–10. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Giuliani D, Ottani A, Zaffe D, Galantucci
M, Strinati F, Lodi R and Guarini S: Hydrogen sulfide slows down
progression of experimental Alzheimer's disease by targeting
multiple pathophysiological mechanisms. Neurobiol Learn Mem.
104:82–91. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xuan A, Long D, Li J, Ji W, Zhang M, Hong
L and Liu J: Hydrogen sulfide attenuates spatial memory impairment
and hippocampal neuroinflammation in beta-amyloid rat model of
Alzheimer's disease. J Neuroinflammation. 9(202)2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals, 8th
edition. National Academies Press (US), Washington, DC, 2011.
|
|
16
|
Zimmermann C, Ferenci P, Pifl C, Yurdaydin
C, Ebner J, Lassmann H, Roth E and Hörtnagl H: Hepatic
encephalopathy in thioacetamide-induced acute liver failure in
rats: Characterization of an improved model and study of amino
acid-ergic neurotransmission. Hepatology. 9:594–601.
1989.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Avraham Y, Grigoriadis N, Poutahidis T,
Vorobiev L, Magen I, Ilan Y, Mechoulam R and Berry E: Cannabidiol
improves brain and liver function in a fulminant hepatic
failure-induced model of hepatic encephalopathy in mice. Br J
Pharmacol. 162:1650–1658. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Avraham Y, Grigoriadis NC, Magen I,
Poutahidis T, Vorobiav L, Zolotarev O, Ilan Y, Mechoulam R and
Berry E: Capsaicin affects brain function in a model of hepatic
encephalopathy associated with fulminant hepatic failure in mice.
Br J Pharmacol. 158:896–906. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Panikashvili D, Simeonidou C, Ben-Shabat
S, Hanus L, Breuer A, Mechoulam R and Shohami E: An endogenous
cannabinoid (2-AG) is neuroprotective after brain injury. Nature.
413:527–531. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Avraham Y, Israeli E, Gabbay E, Okun A,
Zolotarev O, Silberman I, Ganzburg V, Dagon Y, Magen I, Vorobia L,
et al: Endocannabinoids affect neurological and cognitive function
in thioacetamide-induced hepatic encephalopathy in mice. Neurobiol
Dis. 21:237–245. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li M, Zhang P, Wei HJ, Li MH, Zou W, Li X,
Gu HF and Tang XQ: Hydrogen sulfide ameliorates
homocysteine-induced cognitive dysfunction by inhibition of
reactive aldehydes involving upregulation of ALDH2. Int J
Neuropsychopharmacol. 20:305–315. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ye T, Meng X, Wang R, Zhang C, He S, Sun G
and Sun X: Gastrodin alleviates cognitive dysfunction and
depressive-like behaviors by inhibiting ER stress and NLRP3
inflammasome activation in db/db mice. Int J Mol Sci.
19(E3977)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li XN, Chen L, Luo B, Li X, Wang CY, Zou
W, Zhang P, You Y and Tang XQ: Hydrogen sulfide attenuates chronic
restrain stress-induced cognitive impairment by upreglulation of
Sirt1 in hippocampus. Oncotarget. 8:100396–100410. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Avraham Y, Zolotarev O, Grigoriadis NC,
Poutahidis T, Magen I, Vorobiav L, Zimmer A, Ilan Y, Mechoulam R
and Berry EM: Cannabinoids and capsaicin improve liver function
following thioacetamide-induced acute injury in mice. Am J
Gastroenterol. 103:3047–3056. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wu DD, Wang DY, Li HM, Guo JC, Duan SF and
Ji XY: Hydrogen Sulfide as a Novel Regulatory Factor in Liver
Health and Disease. Oxid Med Cell Longev.
2019(3831713)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chen WL, Niu YY, Jiang WZ, Tang HL, Zhang
C, Xia QM and Tang XQ: Neuroprotective effects of hydrogen sulfide
and the underlying signaling pathways. Rev Neurosci. 26:129–142.
2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Panthi S, Chung HJ, Jung J and Jeong NY:
Physiological Importance of Hydrogen Sulfide: Emerging Potent
Neuroprotector and Neuromodulator. Oxid Med Cell Longev.
2016(9049782)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen SM, Yi YL, Zeng D, Tang YY, Kang X,
Zhang P, Zou W and Tang XQ: Hydrogen Sulfide Attenuates
β2-Microglobulin-Induced Cognitive Dysfunction: Involving Recovery
of Hippocampal Autophagic Flux. Front Behav Neurosci.
13(244)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bae J, Min YS, Nam Y, Lee HS and Sohn UD:
Humulus japonicus Extracts Protect Against
Lipopolysaccharide/d-Galactosamine-Induced Acute Liver Injury in
Rats. J Med Food. 21:1009–1015. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang X, Wang B, Huang Q, Zhang B and Hua
Z: Regulation of hydrogen sulfide on transporter protein Bsep and
Mdr2 in acute liver failure. Zhonghua Yi Xue Za Zhi. 95:3176–3179.
2015.PubMed/NCBI(In Chinese).
|
|
31
|
Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath
RD, Wang ZJ, Anuar FB, Whiteman M, Salto-Tellez M and Moore PK:
Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced
inflammation in the mouse. FASEB J. 19:1196–1198. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wedmann R, Bertlein S, Macinkovic I, Boltz
S, Miljkovic J, Munoz LE, Herrmann M and Filipovic MR: Working with
‘H2S’: facts and apparent artifacts. Nitric Oxide.
41:85–96. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yakovlev AV, Kurmasheva ED, Giniatullin R,
Khalilov I and Sitdikova GF: Hydrogen sulfide inhibits giant
depolarizing potentials and abolishes epileptiform activity of
neonatal rat hippocampal slices. Neuroscience. 340:153–165.
2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Brown KG and Strickland JA: Utilizing data
from multiple studies (meta-analysis) to determine effective
dose-duration levels. Example: Rats and mice exposed to hydrogen
sulfide. Regul Toxicol Pharmacol. 37:305–317. 2003.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Schwendimann RN and Minagar A: Liver
Disease and Neurology. Continuum (Minneap Minn). 23 (3, Neurology
of Systemic Disease):762–777. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Shawcross D and Jalan R: The
pathophysiologic basis of hepatic encephalopathy: Central role for
ammonia and inflammation. Cell Mol Life Sci. 62:2295–2304.
2005.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang Q, Wang Y, Yu Z, Li D, Jia B, Li J,
Guan K, Zhou Y, Chen Y and Kan Q: Ammonia-induced energy disorders
interfere with bilirubin metabolism in hepatocytes. Arch Biochem
Biophys. 555-556:16–22. 2014.PubMed/NCBI View Article : Google Scholar
|