Introduction
Osteoporosis (OP) is a systemic bone disease
manifested as low bone mass, destruction of bone microstructure,
increased bone fragility and fracture risk (1). The prevalence of OP is on the rise due
to the aging of population. It has been reported that in 2010 in
China, the prevalence of OP in females >70 years of age was
40.0-59.3%, and in males was 14.2-18.9% (2). OP-induced pain and fracture severely
affect life quality and pose economic burden on the elderly
(3). It is of significance to focus
on the prevention and treatment of OP.
The prevention and treatment strategies of OP are
comprehensive, including lifestyle adjustment, calcium and vitamin
D supplementation, and application of anti-OP drugs. Estrogen
replacement therapy (ERT) can prevent bone loss caused by estrogen
deficiency; however, ERT increases the risks of uterine and breast
cancer (4). Selective estrogen
receptor modulators (SERMs) have been developed as tissue-specific
estrogen agonists that are applied for the treatment of
postmenopausal OP (5). Raloxifene
(RLF) is a second-generation SERM for treating postmenopausal OP
which does not have the adverse effects of ERT (6). As an estrogen agonist on bone and
several other tissues, RLF suppresses bone loss and reduces
fracture risks. In addition, RLF reduces the susceptibility to
uterine cancer as an estrogen antagonist (7). Currently, RLF has been utilized in the
clinical treatment of OP (8);
however, the specific pharmacological role of RLF remains to be
further explored.
Tumor necrosis factor-α (TNF-α) is a cytokine
produced by activated macrophages/monocytes that exerts a crucial
role in osteogenic differentiation of stem cells (9). A great number of studies have
demonstrated the involvement of TNF-α in mediating multiple
pathways related to osteogenic differentiation, such as Wnt, Smads
and NF-κB pathways (10-12).
Nevertheless, controversies exist regarding the factors that
determine the promotive or inhibitory role of TNF-α in osteogenic
differentiation (13). The aim of
the present study was to investigate the effect of RLF on
TNF-α-induced inhibition of osteogenic differentiation and the
potential mechanism, and the conclusions of the study may provide
new insights for the clinical treatment of OP.
Materials and methods
Experimental animals
Fifty-four female Sprague-Dawley (SD) rats, 12-weeks
old and weighing 238.4±14 g, were provided by the Shanghai SIPPR-Bk
Lab Animal Co., Ltd. Rats were randomly divided into the sham
group, ovariectomy (OVX) group and OVX+RLF group, with 18 rats in
each group. The rats in the OVX+RLF group were administered with
0.2 µM RLF. The body weight of each rat was recorded every week.
Rats were housed in a temperature controlled room (21±2˚C) on a
12:12-h light/dark cycle (lights on at 06:00), and all rats had
free access to water and food. The study was approved by the Animal
Ethics Committee of Beihang University Animal Center (Beijing,
China).
Preparation of OVX procedure
Rats were anesthetized by peritoneal administration
of 40 mg/kg pentobarbital sodium. After shaving and skin
disinfection, a 2-cm longitudinal incision at 1 cm near the spine
and 2 cm above posterior iliac crest was made. The abdominal cavity
was exposed to resect ovaries. The wound was sutured in layers.
Postoperative peritoneal administration of 1 mg/kg gentamicin was
applied. The rats in the sham group were subjected to abdominal
cavity exposure and removal of some fat tissues.
MicroCT
After rats were sacrificed, the femoral metaphysis
and attached surrounding soft tissues were removed, fixed in 4%
paraformaldehyde solution, and scanned with SCANCO medical microCT
(SCANCO Medical AG). Bone histomorphology indicators were
determined and analyzed using Image Processing Language (version X;
Adobe Systems, Inc.).
Biomechanical examinations
Three-point bending experiment was conducted to
record elastic/max radial degree and elastic/max load of rat
femora. Briefly, the right femora were placed on the Instron
Material Mechanics Testing Device (Instron). At the middle position
of femora, a persistent test velocity of 1 mm/min was loaded until
femoral fracture. Data were recorded and analyzed to obtain the max
load.
Isolation and culture of bone marrow
mesenchymal stem cells (BMSCs)
Rats were sacrificed by cervical dislocation after
being anesthetized with pentobarbital sodium via peritoneal
administration at a dose of 40 mg/kg. The rats were immersed in 75%
ethanol for 5 min and isolated for femora and humeri. Rat bones
were washed and the epiphysis of long bones was removed. Marrow
cavity was repeatedly washed by Dulbecco's modified Eagle's medium
(DMEM), and the fluids were inoculated in a 25-ml culture bottle.
After incubation for 48 h, the un-adherent cells were washed. Cell
passage was performed until 80-90% confluence. The fourth
generation BMSCs were harvested and divided into control group,
TNF-α group, RLF group and TNF-α+RLF group.
Osteogenic differentiation
Fourth generation BMSCs were cultured in a 25-ml
culture bottle at 1x107 cells/l. BMSCs were subjected to
osteogenic differentiation in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.), 10 nmol/l dexamethasone, 10 mmol/l
β-glycerophosphate, 50 µg/ml ascorbic acid, 1% L-glucose and 1%
penicillin-streptomycin. Osteogenic induction medium was replaced
every 2 days.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from BMSCs using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and the concentration of total RNA was measured using an
ultraviolet spectrophotometer (Hitachi, Ltd.). Total RNA was
reverse transcribed into cDNA at 50˚C for 45 min by using
PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd.),
according to the manufacturer's protocol. qPCR was subsequently
performed using the SYBR-Green Master kit (Roche Diagnostics). The
reaction system volume was 25 µl in total and the thermocycling
conditions were: pre-denaturation at 95˚C for 5 min, denaturation
at 95˚C for 30 sec, annealing at 60˚C for 45 sec, extension at 72˚C
for 3 min, with 35 cycles, and then extension at 72˚C for 5 min.
qPCR products were stored at 4˚C. The relative levels were
quantitatively analyzed using the 2-∆∆Cq method
(14). GAPDH was used as internal
reference. Primer sequences were as follows: osteocalcin (OCN)
forward, 5'-GCCCTGACTGCATTCTGC CTCT-3' and reverse,
5'-TCACCACCTTACTGCCCTCCTG-3'; Runx2 forward,
5'-GGACCGACACAGCCATATAAA-3' and reverse,
5'-GCCTCATTCCCTAACCTGAAA-3'; GAPDH forward,
5'-GCAAGGATACTGAGAGCAAGAG-3' and reverse,
5'-GGATGGAATTGTGAGGGAGATG-3'.
Determination of alkaline phosphatase
(ALP) activity
BMSCs were subjected to osteogenic differentiation
for 7 days. After cell lysis and centrifugation, the supernatant
was harvested for determine the absorbance at 520 nm. The relative
ALP activity was calculated based on the protocols of ALP
determination kit (Beyotime Institute of Biotechnology).
Alizarin red staining
BMSCs were subjected to osteogenic differentiation
for 21 days. Cells were washed with PBS twice, fixed in 4%
paraformaldehyde for 10 min at 37˚C and stained with 0.1% alizarin
red (pH 4.1) for 10 min at 37˚C. Calcification nodules were
observed and captured using a light inverted microscope
(magnification, x200; BX-42, Olympus Corporation).
Cell Counting Kit-8 (CCK-8)
BMSCs were seeded in a 96-well plate with
3x104 cells/well. The absorbance at 450 nm was recorded
at the appointed time-points using the CCK-8 kit (Dojindo Molecular
Technologies, Inc.) for depicting the viability curve.
Western blot analysis
Total protein was extracted from BMSCs using
radioimmunoprecipitation assay and was quantified by bicinchoninic
acid (both from Beyotime Institute of Biotechnology) method. A
total of 30 µg of protein were loaded per lane for electrophoresis.
The extracted proteins were separated using a 10% SDS-PAGE gel.
After transferred onto PVDF membranes (EMD Millipore) the proteins
were blocked with 5% skim milk at 20˚C for 2 h. The membranes were
incubated with primary antibodies at 4˚C overnight and secondary
antibodies at 20˚C for 2 h. Bands were exposed by enhanced
chemiluminescence (ECL) detection kit (Amersham; GE Healthcare) and
analyzed by ImageJ Software (version 1.38; National Institutes of
Health). Rabbit polyclonal NF-κB antibody (dilution: 1:500; cat.
no. ab8805), rabbit polyclonal GAPDH antibody (dilution: 1:500,
cat. no. ab37168) and secondary goat anti-rabbit (HRP) IgG antibody
(dilution: 1:2,000; cat. no. ab6721) were all purchased from
Abcam.
Statistical analysis
SPSS 20.0 statistical software (IBM Corp.) was used
for data analysis. Data were expressed as the mean ± standard
deviation. Comparisons between multiple groups were made using
one-way ANOVA followed by the Least Significant Difference post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
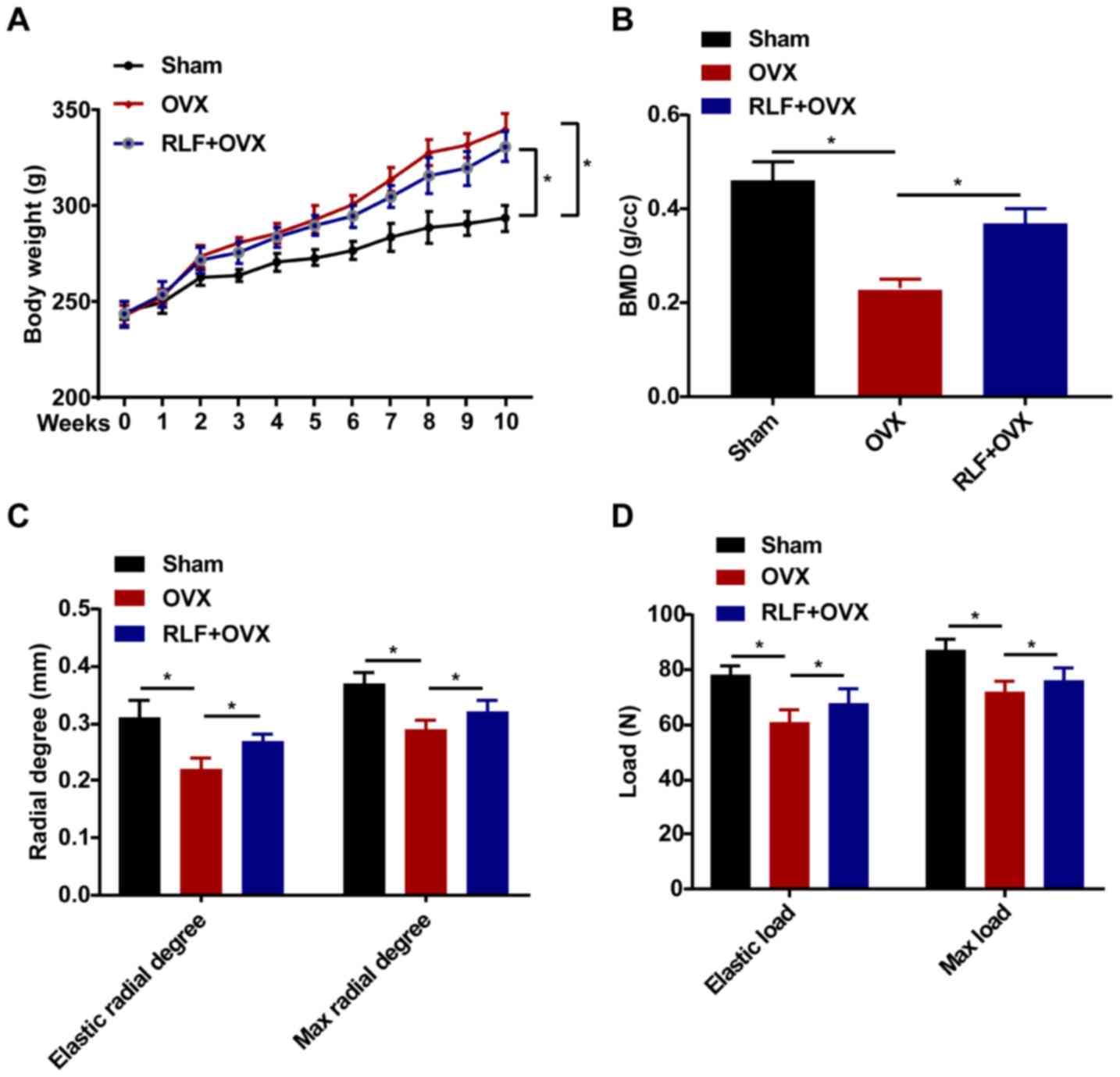
RLF influences body weight, bone
metabolism index (BMD) and biomechanical parameters
Rat body weight was weekly recorded during the whole
experiment. All rats in the three groups presented an increased
trend in the body weight. Compared with the sham group, the body
weight of the rats in the RLF+OVX and OVX groups was gradually
elevated in the 8th week. However, no significant difference was
observed in the body weight of rats between the RLF+OVX and OVX
group (Fig. 1A). In the RLF+OVX
group, BMD was higher compared with that in the OVX group and lower
than that of the sham group (Fig.
1B). The biomechanical parameters of femoral radial degree and
load were also examined. Compared with the sham group, the elastic
radial degree/load and max radial degree/load were reduced in the
OVX group. However, the RLF treatment markedly increased these
biomechanical parameters (Fig. 1C
and D).
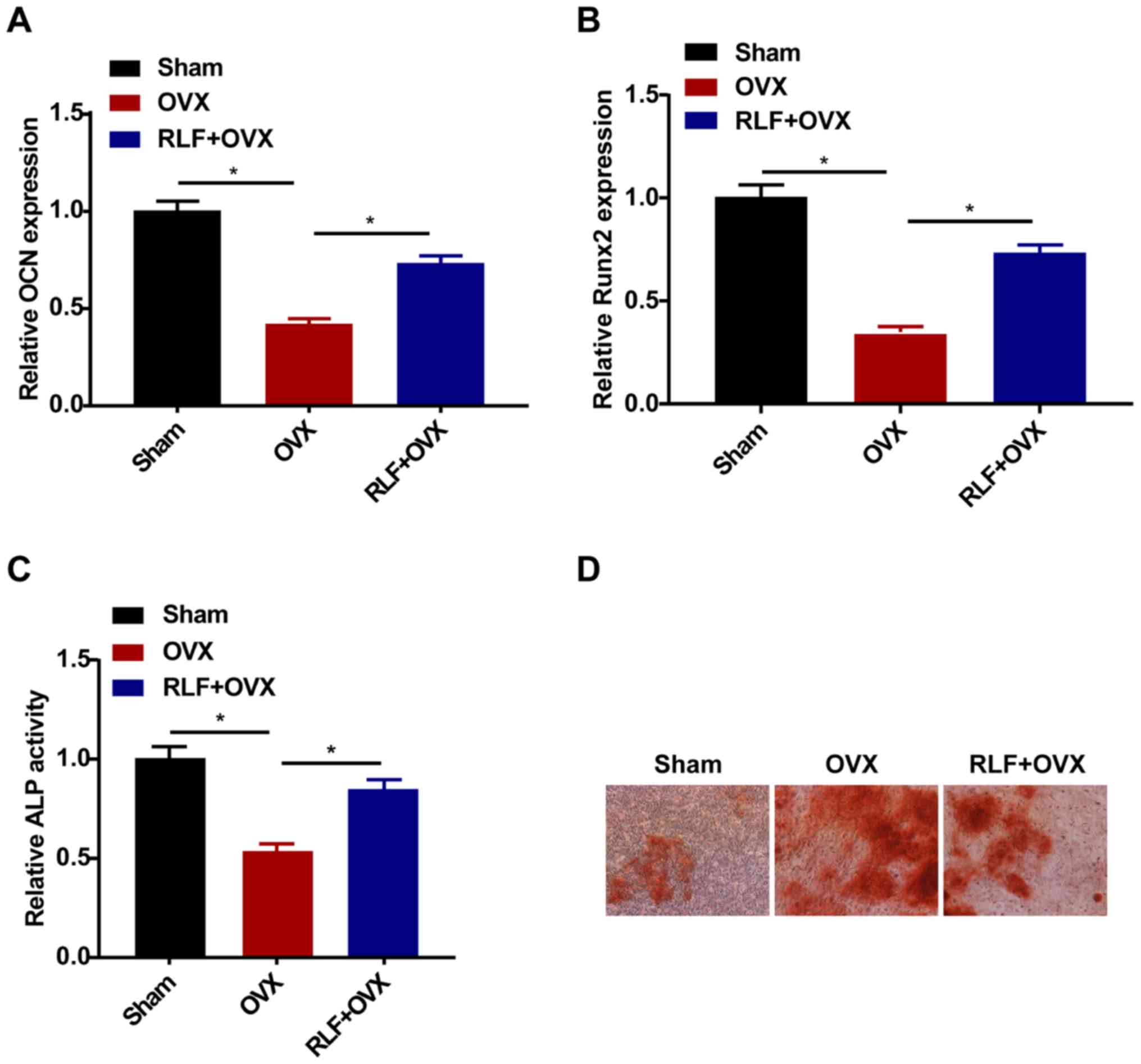
RLF enhances the osteogenic
differentiation ability of BMSCs
The relative levels of OCN and Runx2 were
downregulated in the OVX and RLF+OVX group, and especially in the
OVX group (Fig. 2A and B). Similarly, the ALP activity was lower in
rats undergoing OVX compared with that in rats of the sham group.
RLF treatment enhanced ALP activity in OVX rats; however, ALP
activity was still lower than that of the sham group (Fig. 2C). Alizarin red staining revealed a
pronounced increase in the calcification ability of the RLF+OVX
group, indicating the promotive effect of RLF on osteogenic
differentiation (Fig. 2D).
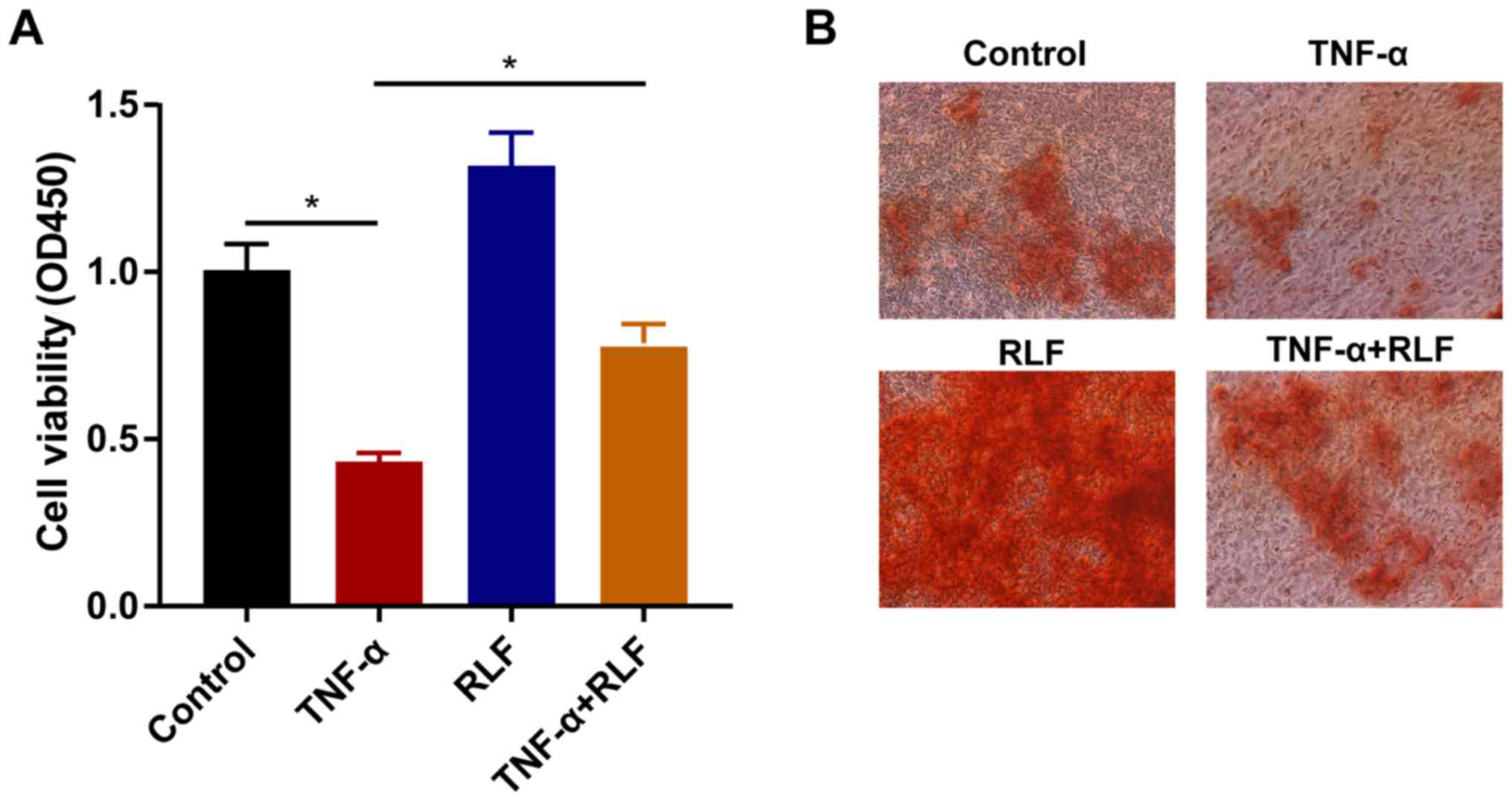
RLF improves viability and
calcification ability of BMSCs
To further uncover the in vitro effects of
RLF on osteogenic differentiation of BMSCs, the primary BMSCs were
divided into four groups based on different treatments. Cell
viability markedly decreased after TNF-α induction in BMSCs.
Conversely, RLF treatment could promote their viability. Notably,
RLF treatment accelerated the viability in TNF-α-induced BMSCs
(Fig. 3A). Furthermore, the RLF
treatment stimulated the calcification ability in BMSCs; however,
the calcification ability was markedly inhibited by TNF-α induction
(Fig. 3B).
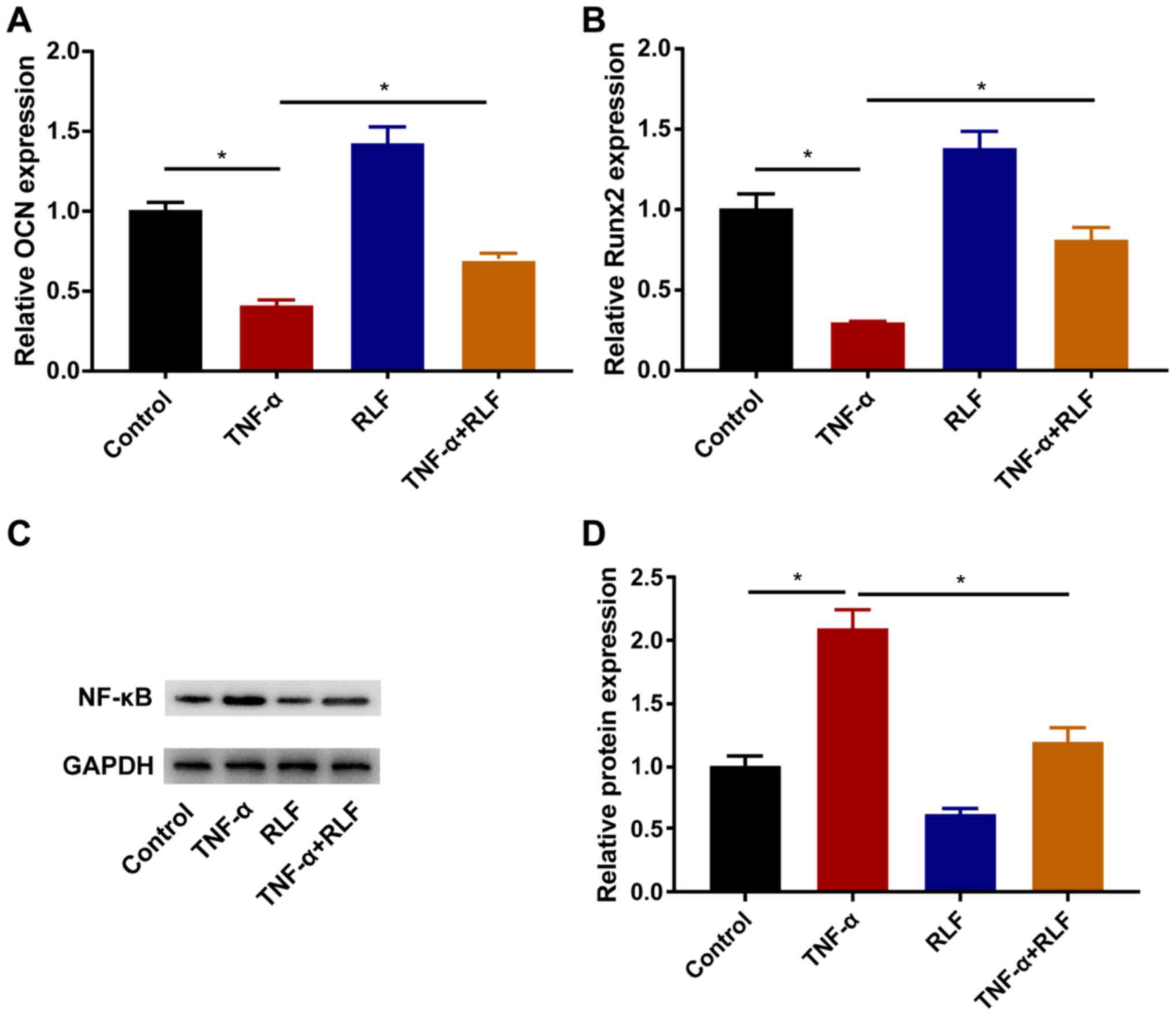
RLF regulates osteogenesis-related
genes and the NF-κB pathway
TNF-α induction downregulated OCN and Runx2 in
BMSCs, which effect was partially reversed after the RLF treatment
(Fig. 4A and B). The protein level of NF-κB was
determined in each group. After TNF-α induction, the protein level
of NF-κB was remarkably upregulated, which was suppressed by the
RLF treatment (Fig. 4C and D).
Discussion
Bones maintain structural stability through
sustained bone remodeling and resorption. Bone tissues include
osteoblasts, osteoclasts, and osteocytes. The osteogenic capacity
of osteoblasts and the bone resorption function of osteoclasts
maintain the balance of bone remodeling. Once the balance breaks,
it leads to bone diseases, such as OP (14). OP is common in the elderly and
enhances the fracture risks. It is estimated that 72% of females
and 62% of males >50 years of age will suffer from OP or
osteopenia in 2022(15). OP-induced
fractures pose a huge economic burden on the society (16).
ERT markedly alleviates bone mass reduction and
reduces the incidence of fracture. However, several large-scale
clinical trials have proposed that ERT increases the susceptibility
to invasive breast cancer, coronary disease, stroke and pulmonary
embolism (17). Selective estrogen
receptor modulators (SERMs) have attracted attention because of
their estrogen agonistic or antagonistic effects. RLF, the new
generation of SERMs, has been applied as an ideal drug for
postmenopausal OP (18).
Cytokines can stimulate the development of
osteoclasts in the presence of stromal cells or osteoblasts,
including IL-1β, IL-1, IL-6 and TNF-α. It has been suggested that
these cytokines may indirectly induce osteoclast formation through
autocrine or paracrine modes (19).
TNF is mainly synthesized by mononuclear macrophages, and can also
be secreted by T cells, natural killer cells, chondrocytes, and
osteoblasts (20). Roggia et
al (21) have pointed out the
rapid bone loss in OVX mice, whereas TNF-α deficiency mice do not
present such a change, indicating the crucial role of TNF-α in the
development of OP induced by ovarian function decline. It has been
reported that estrogens suppress the expression of TNF-α in
monocytes (22). Consistently, this
study demonstrated the inhibitory effect of TNF-α on osteogenic
differentiation, which was reversed by RLF treatment through the
NF-κB pathway.
In conclusion, TNF-α induction inhibits osteogenic
differentiation of BMSCs, which could be remarkably alleviated by
RLF. It is suggested that RLF contributes to the alleviation of OP
progression.
Acknowledgements
Not applicable.
Funding
The study was supported by the National Natural
Science Foundation of China (81503601), the Young Talents Training
Program of Dongzhimen Hospital of the Beijing University of Chinese
Medicine (DZMYS-201802), the Basic Scientific Research Service Fee
project of the Beijing University of Chinese Medicine
(2015-JYB-JSMS080, 2016-JYB-JSMS-045) and the 111 Project (no.
B13003).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
FY, JL and XN designed the study and performed the
experiments. FY, YJ and QS established the animal model. CZ and CL
acquired the data and were also involved in the conception of the
study. WW, LD and SK analyzed the data. FY, JL and XN prepared the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Animal Ethics
Committee of Beihang University Animal Center (Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hu BT and Chen WZ: MOTS-c improves
osteoporosis by promoting osteogenic differentiation of bone marrow
mesenchymal stem cells via TGF-β/Smad pathway. Eur Rev Med
Pharmacol Sci. 22:7156–7163. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhu H, Fang J, Luo X, Yu W, Zhao Y, Li X,
Du J and Lu Y: A survey of bone mineral density of healthy Han
adults in China. Osteoporos Int. 21:765–772. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hernlund E, Svedbom A, Ivergård M,
Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B and Kanis
JA: Osteoporosis in the European Union: Medical management,
epidemiology and economic burden. A report prepared in
collaboration with the International Osteoporosis Foundation (IOF)
and the European Federation of Pharmaceutical Industry Associations
(EFPIA). Arch Osteoporos. 8(136)2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chavassieux P, Portero-Muzy N, Roux JP,
Garnero P and Chapurlat R: Are biochemical markers of bone turnover
representative of bone histomorphometry in 370 postmenopausal
women? J Clin Endocrinol Metab. 100:4662–4668. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rodan GA and Martin TJ: Therapeutic
approaches to bone diseases. Science. 289:1508–1514.
2000.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Brennan TC, Rizzoli R and Ammann P:
Selective modification of bone quality by PTH, pamidronate, or
raloxifene. J Bone Miner Res. 24:800–808. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lamas AZ, Caliman IF, Dalpiaz PL, de Melo
AF Jr, Abreu GR, Lemos EM, Gouvea SA and Bissoli NS: Comparative
effects of estrogen, raloxifene and tamoxifen on endothelial
dysfunction, inflammatory markers and oxidative stress in
ovariectomized rats. Life Sci. 124:101–109. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ettinger B, Black DM, Mitlak BH,
Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas
PD, Zanchetta JR, Stakkestad J, et al: Multiple Outcomes of
Raloxifene Evaluation (MORE) Investigators: Reduction of vertebral
fracture risk in postmenopausal women with osteoporosis treated
with raloxifene: Results from a 3-year randomized clinical trial.
JAMA. 282:637–645. 1999.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ding J, Ghali O, Lencel P, Broux O,
Chauveau C, Devedjian JC, Hardouin P and Magne D: TNF-alpha and
IL-1beta inhibit RUNX2 and collagen expression but increase
alkaline phosphatase activity and mineralization in human
mesenchymal stem cells. Life Sci. 84:499–504. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Qiu W, Andersen TE, Bollerslev J, Mandrup
S, Abdallah BM and Kassem M: Patients with high bone mass phenotype
exhibit enhanced osteoblast differentiation and inhibition of
adipogenesis of human mesenchymal stem cells. J Bone Miner Res.
22:1720–1731. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mukai T, Otsuka F, Otani H, Yamashita M,
Takasugi K, Inagaki K, Yamamura M and Makino H: TNF-alpha inhibits
BMP-induced osteoblast differentiation through activating SAPK/JNK
signaling. Biochem Biophys Res Commun. 356:1004–1010.
2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chang J, Liu F, Lee M, Wu B, Ting K, Zara
JN, Soo C, Al Hezaimi K, Zou W, Chen X, et al: NF-κB inhibits
osteogenic differentiation of mesenchymal stem cells by promoting
β-catenin degradation. Proc Natl Acad Sci USA. 110:9469–9474.
2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gilbert L, He X, Farmer P, Rubin J, Drissi
H, van Wijnen AJ, Lian JB, Stein GS and Nanes MS: Expression of the
osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2alpha A)
is inhibited by tumor necrosis factor-alpha. J Biol Chem.
277:2695–2701. 2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kemmler W, Häberle L and von Stengel S:
Effects of exercise on fracture reduction in older adults: A
systematic review and meta-analysis. Osteoporos Int. 24:1937–1950.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Harvey N, Dennison E and Cooper C:
Osteoporosis: Impact on health and economics. Nat Rev Rheumatol.
6:99–105. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rossouw JE, Anderson GL, Prentice RL,
LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA,
Howard BV, Johnson KC, et al: Writing Group for the Women's Health
Initiative Investigators: Risks and benefits of estrogen plus
progestin in healthy postmenopausal women: Principal results From
the Women's Health Initiative randomized controlled trial. JAMA.
288:321–333. 2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Passeri G, Girasole G, Jilka RL and
Manolagas SC: Increased interleukin-6 production by murine bone
marrow and bone cells after estrogen withdrawal. Endocrinology.
133:822–828. 1993.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Vitale RF and Ribeiro FA: The role of
tumor necrosis factor-alpha (TNF-alpha) in bone resorption present
in middle ear cholesteatoma. Rev Bras Otorrinolaringol (Engl Ed).
73:117–121. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rayl JM, Wellehan JFX Jr, Bunick D and
Allender MC: Development of reverse-transcriptase quantitative PCR
assays for detection of the cytokines IL-1β, TNF-α, and IL-10 in
chelonians. Cytokine. 119:16–23. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Roggia C, Gao Y, Cenci S, Weitzmann MN,
Toraldo G, Isaia G and Pacifici R: Up-regulation of TNF-producing T
cells in the bone marrow: A key mechanism by which estrogen
deficiency induces bone loss in vivo. Proc Natl Acad Sci USA.
98:13960–13965. 2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
An J, Tzagarakis-Foster C, Scharschmidt
TC, Lomri N and Leitman DC: Estrogen receptor beta-selective
transcriptional activity and recruitment of coregulators by
phytoestrogens. J Biol Chem. 276:17808–17814. 2001.PubMed/NCBI View Article : Google Scholar
|