Introduction
Esophageal cancer is the sixth leading cause of
cancer-related mortality. It caused the mortality of 440,000 people
in 2013(1). It is the third
deadliest cancer in Chinese males and the fifth deadliest
malignance in Chinese females (2).
In China and other Asian countries, 95% of the esophageal cancer is
esophageal squamous cell carcinoma (ESCC) and the 5-year overall
survival rate is ~10-20% (3).
Therefore, it is important to identify the tumor-specific markers
in ESCC (4).
Histone deacetylases (HDACs) are expressed in
plants, animals, fungi, archaebacteria and eubacteria (5). HDACs are classified into Class I, II
and IV or Class III (coenzyme-nicotinamide adenine dinucleotide).
HDAC1, -2, -3 and -8 belong to Class I (6). HDACs together with histone acetylases
can regulate gene transcription and carcinogenesis by modulating
chromatin structure (7). Class I
HDACs have been shown to be widely expressed in all kinds of solid
cancers (8,9). HDAC1 expression in ESCC is higher
compared with normal esophageal mucosa. When cancer cells invade
the deep layer of the esophageal wall, the expression of HDAC 1 is
significantly decreased compared with normal control samples
(10). Nevertheless, the expression
and role of HDAC2 and 3 in ESCC remain to be elucidated.
The relationship between histone deacetylation and
cellular events, including cell proliferation, differentiation and
cell cycle regulation has been demonstrated (11-13).
For example, HDACs may inhibit target gene expression by binding to
the transcriptional cofactor PC3/Tis21, thereby inhibiting cell
proliferation (14). HDAC2
overexpression enhances the aggressiveness of gastric carcinoma
cells and HDAC2 inhibition attenuates the carcinogenic potential of
gastric carcinoma cells in xenotransplanted nude mice (13). In addition, several studies have
shown that HDAC3 is over-expressed in various solid tumors and is
closely associated with poor prognosis (14-16).
The present study investigated the expression levels
of HDAC1, -2 and -3 and their clinical significance in ESCC. The
association of HDAC1, -2 and -3 with clinicopathological
characteristics of ESCC was also analyzed.
Materials and methods
Patient information and tissue
specimens
Primary ESCC tissues and paired distal normal
tissues (>5 cm away from the margin of tumor tissue) were
collected from 88 patients (Kazak, n=40; Han, n=44). Esophagus
excision was performed between December 2009 and September 2015 at
the Xinjiang Medical University Affiliated Cancer Hospital. No
patient received preoperative chemotherapy or radiotherapy. ESCC
diagnosis was confirmed by pathology. Patient characteristics
including sex, age, lymph node (LN) metastasis, differentiation and
depth of invasion were recorded (Table
I). The present study research was approved by the Ethics
Committee of Xinjiang Medical University and all patients provided
written informed consent.
 | Table IClinicopathological characteristics
of esophageal squamous cell carcinoma patients. |
Table I
Clinicopathological characteristics
of esophageal squamous cell carcinoma patients.
| Basic data | Number |
|---|
| Sex |
|
Male | 62 |
|
Female | 26 |
| Ethnicity | 48 |
|
Han | 40 |
|
Kazakh | |
| Age |
|
<60
yrs | 46 |
|
≥60 yrs | 42 |
| LN Metastasis |
|
Positive | 46 |
|
Negative | 42 |
|
Differentiation |
|
Well and
Moderate | 69 |
|
Poor | 19 |
| Depth of
invasion |
|
T1 and
T2 | 13 |
|
T3 and
T4 | 75 |
Reverse transcription semi-quantified
PCR
Total RNA was extracted with TRIzol (Invitrogen,
Thermo Fisher Scientific, Inc.) from ESCC tissues and paired distal
normal tissues. Reverse transcription of RNA was performed with
avian myeloblastosis virus reverse transcriptase (Promega
Corporation). Reverse transcription was performed under the
following heat conditions: 55˚C for 90 min and 4˚C for 10 min. The
cDNA products were identified on a 2% agarose gel with ethidium
bromide. The PCR reaction system included 2 µl reverse-transcribed
cDNA, 10 µl 2XPCR master mix, 1 µl of each primer (10 µM) and 6 µl
ddH2O (Ready-to-Use PCR kit, Beijing Transgen Biotech
Co., Ltd.). Amplification was performed on an iCycler™
Thermal Cycler (Bio-Rad Laboratories, Inc.). GAPDH was an internal
control.
The PCR primers were: HDAC1 forward,
5'-AGTGCGGTGGTCTTACAGTG-3', HDAC1 reverse,
5'-TCTCCCTCCTCTTCAGAATCG-3', HDAC2 forward,
5'-GCTGGTCTTGAACTCCTT-3', HDAC2 reverse,
5'-TACAACCCATCTGGCATC-3', HDAC3 forward,
5'-GGGACATTATTGGCAGTG-3', HDAC3 reverse,
5'-GGATTCAGGTGTTAGGGAG-3', GAPDH forward,
5'-GCGGGCTCTCCAGAACATCAT-3' and GAPDH reverse,
5'-CCAGCCCCAGCGTCAAAGGTG-3'. The thermocyling conditions were as
follows: Initial denaturation at 95˚C for 5 min; 35 cycles of 95˚C
for 30 sec, 58˚C for 20 sec for HDAC1. 54˚C for 20 sec for
HDAC2, 58˚C for 30 sec for HDAC3 and 60˚C for 30 sec
for GAPDH, 72˚C for 30s; and a final extension at 72˚C for 7
min. The PCR products were identified on a 1.5% agarose gel with
ethidium bromide and analyzed with the Gel Doc XR System (Bio-Rad
Laboratories, Inc.). The DNA ladder maker was purchased from Sangon
Biotech Co., Ltd. The intensities of PCR product bands were
quantified using Quantity One software version 4.5.2 (Bio-Rad
Laboratories, Inc.). The quantity of each PCR product band was
standardized to that of GAPDH. The presence or absence of
bands was considered positive or negative. Grey scale ratio >2
was defined as high expression.
Statistical analysis
SPSS 17.0 software (SPSS Inc.) was used for data
analysis. Experiments were performed in triplicate. Measurement
data were presented as the mean ± SD. Count data were expressed as
the number (%). Correlations among HDAC1, -2 and -3 expression were
analyzed by the Pearson correlation test. Differences in gene
expression were analyzed using a paired t-test and variances in
clinicopathologic features were analyzed using χ2 test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
First, the basic information of the patients was
investigated (Table I). Of the 88
patients, 26 (29.5%) were women and 62 (70.5%) were men. Their mean
age was 58.2 years, 37-84 years old. The most common tumor location
was at the middle and lower esophagus (95.5%). There were 69 ESCC
cases with moderate to high differentiation and 19 with poor
differentiation. A total of 46 cases had LN metastasis and 42 did
not have LN metastasis. There were 13 cases of
T1+T2 and 75 cases of
T3+T4.
Expression levels of HDAC1, -2 and -3
in human ESCC and distal normal tissues
To compare the expression levels of HDAC1, -2
and -3 in tumor issues and distal normal tissues, reverse
transcription semi-quantified PCR analysis was performed. The
representative amplification results of HDAC1, -2 and
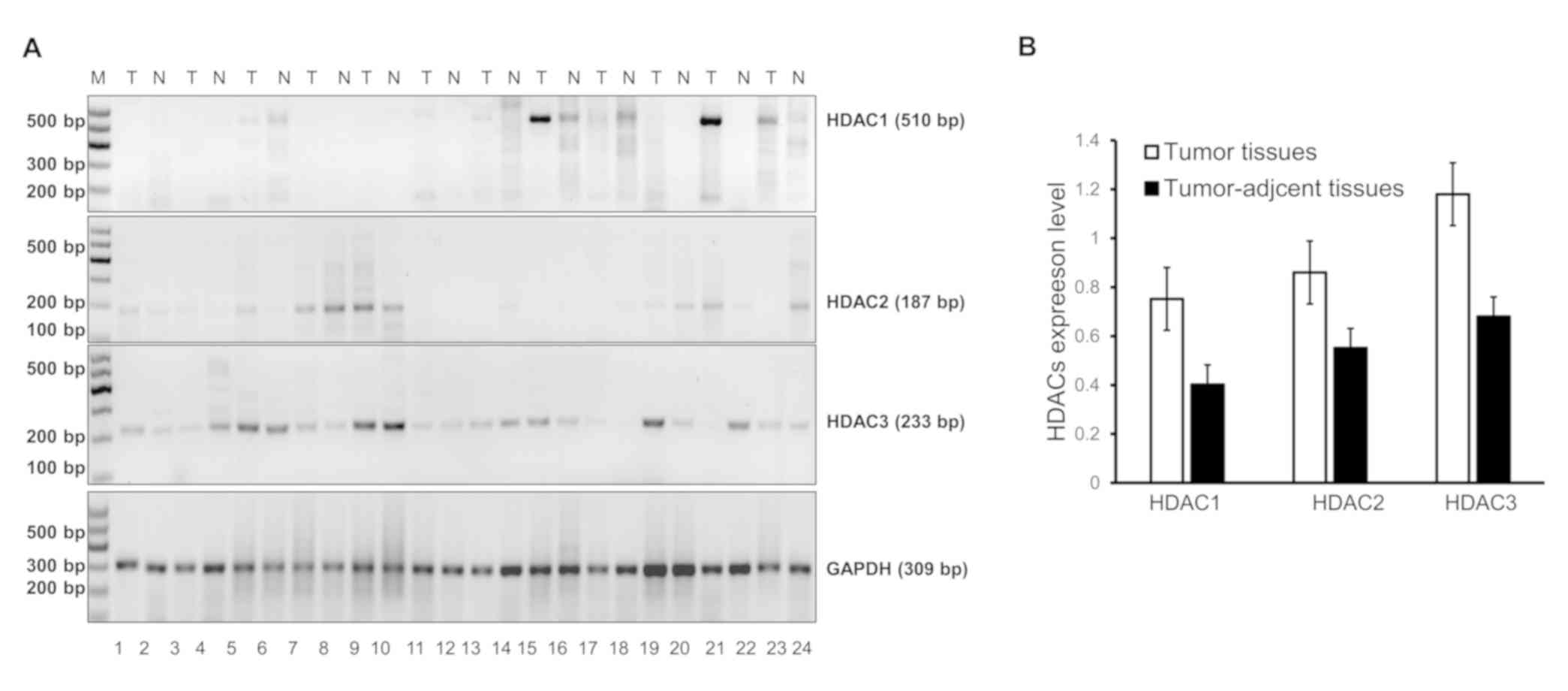
-3 are presented in Fig. 1A.
It demonstrated that HDAC1 was 510 bp in length,
HDAC2 was 187 bp, HDAC3 was 233 bp and GAPDH
was 309 bp (Fig. 1A). Following
quantification, expression of HDAC1, -2 and -3 mRNA
was elevated in tumor tissues compared with distal normal tissue,
although no significant difference was observed (Fig. 1B). The HDAC1 mRNAs were
positive in 48.9% (43/88) of the tumor tissues, HDAC2 mRNAs
were positive in 77.3 (68/88) of the tumor tissues and HDAC3
mRNAs were positive in 93.2% (82/88) of the tumor tissues. Their
positive rate in distal normal tissues was 31.8% (28/88), 85.2%
(75/88) and 87.5% (77/88), respectively. The expression of
HDAC1, but not HDAC2 or HDAC3, was higher in
ESCC samples than in distal normal samples (P<0.05; Table II). The expression of HDAC1,
-2 and -3 in patients of different ethnicities was also
analyzed. As shown in Table III,
the expression of HDAC1 in tumor tissue and normal tissue of
Kazak people was lower compared with Han people (P<0.05). The
expression of HDAC2 in normal tissue of Kazak people was
decreased compared with that in Han people. The expression of
HDAC3 in tumor tissue of Kazak people was decreased compared
with that in Han people (P<0.05).
 | Table IIExpression of HDAC1, -2
and -3 mRNA in tumor and normal tissue. |
Table II
Expression of HDAC1, -2
and -3 mRNA in tumor and normal tissue.
| Expression of
mRNA | Specimens | No | Positive (+) | Negative (-) | Positive rate
(%) |
X2 | P-value |
|---|
| HDAC1 | T | 88 | 43 | 45 | 48.9 (43/88) | 5.312 | 0.021a |
| N | 88 | 28 | 60 | 31.8 (28/88) | | |
| HDAC2 | T | 88 | 68 | 20 | 77.3 (68/88) | 1.828 | 0.176 |
| N | 88 | 75 | 13 | 85.2 (75/88) | | |
| HDAC3 | T | 88 | 82 | 6 | 93.2 (82/88) | 1.628 | 0.202 |
| N | 88 | 77 | 11 | 87.5 (77/88) | | |
 | Table IIIExpression of HDAC1,
HDAC2 and HDAC3 mRNA in tumor and normal tissue of
Han and Kazak people. |
Table III
Expression of HDAC1,
HDAC2 and HDAC3 mRNA in tumor and normal tissue of
Han and Kazak people.
| Expression of
mRNA | Specimens | Positive no.
(+) | Negative no.
(-) |
X2 | P-value |
|---|
| HDAC1 | T | 43 | Han | 31 | 45 | Han | 17 | 10.443 | 0.001a |
| | | Kazak | 12 | | Kazak | 28 | | |
| N | 28 | Han | 21 | 60 | Han | 27 | 6.930 | 0.008a |
| | | Kazak | 7 | | Kazak | 33 | | |
| HDAC2 | T | 68 | Han | 44 | 20 | Han | 4 | 12.458 | 0.176 |
| | | Kazak | 24 | | Kazak | 16 | | |
| N | 75 | Han | 46 | 13 | Han | 3 | 6.571 | 0.010a |
| | | Kazak | 29 | | Kazak | 10 | | |
| HDAC3 | T | 82 | Han | 48 | 6 | Han | 0 | 7.727 | 0.005a |
| | | Kazak | 34 | | Kazak | 6 | | |
| N | 77 | Han | 45 | 11 | Han | 3 | 3.771 | 0.052 |
| | | Kazak | 32 | | Kazak | 8 | | |
Relationship of HDAC1, -2 and -3
expression with clinicopathological characteristics
Next the relationship of HDAC1, -2 and -3 expression
with the clinicopathological characteristics of ESCC patients was
detected. No significant differences were found in correlation
analysis between HDAC1 expression and clinicopathological indexes
(Table IV). The expression of HDAC2
was significantly related with invasion depth (P<0.05; Table V). However, there were no significant
correlation between HDAC2 and age, sex, depth of invasion and tumor
differentiation. Moreover, no significant differences were found
between HDAC3 expression and clinicopathological indexes (Table VI). For the Kazak and Han
ethnicities, the expression of HDAC1 in male patients, patients
with well and moderate differentiated ESCC and T3 and T4 ESCC were
significantly related with ethnicity (P<0.01; Table VII). The expression of HDAC1 in
patients less than 60 years old was related with ethnicity
(P<0.05; Table VII). The
expression of HDAC2 in positive LN metastasis, well and moderate
differentiation and T3 and T4 stages were significantly related
with ethnicity (P<0.01; Table
VIII). The expression of HDAC2 in female patients and negative
LN metastasis were related with ethnicity (P<0.05; Table VIII). The expression of HDAC3 in
male, negative LN metastasis and well and moderate differentiation
was related with ethnicity (P<0.05; Table IX).
 | Table IVCorrelation of HDAC1
expression with clinicopathologic characteristics of esophageal
squamous cell carcinoma patients (n=88). |
Table IV
Correlation of HDAC1
expression with clinicopathologic characteristics of esophageal
squamous cell carcinoma patients (n=88).
| | Expression of
HDAC1 mRNA | | |
|---|
| Clinicopathological
characteristics | No. | Positive (+) | Negative (-) | Positive rate
(%) |
X2 | P-value |
|---|
| Sex |
|
Male | 62 | 31 | 31 | 50.0 (31/62) | 0.108 | 0.742 |
|
Female | 26 | 12 | 14 | 46.2 (12/26) | | |
| Age |
|
<60
yrs | 46 | 20 | 26 | 43.5 (20/46) | 1.119 | 0.290 |
|
≥60 yrs | 42 | 23 | 19 | 54.8 (23/42) | | |
| LN Metastasis |
|
Positive | 46 | 22 | 24 | 47.8 (22/46) | 0.042 | 0.839 |
|
Negative | 42 | 21 | 21 | 50.0 (21/42) | | |
|
Differentiation |
|
Well and
Moderate | 69 | 36 | 33 | 52.26 (36/69) | 1.401 | 0.236 |
|
Poor | 19 | 7 | 12 | 36.8 (7/19) | | |
| Depth of
invasion |
|
T1 and
T2 | 13 | 6 | 7 | 46.2 (6/13) | 0.045 | 0.832 |
|
T3 and
T4 | 75 | 37 | 38 | 49.3 (37/75) | | |
 | Table VCorrelation of HDAC2
expression with clinicopathologic characteristics of esophageal
squamous cell carcinoma patients (n=88). |
Table V
Correlation of HDAC2
expression with clinicopathologic characteristics of esophageal
squamous cell carcinoma patients (n=88).
| Clinicopathological
characteristics | No. | Expression of
HDAC2 mRNA |
X2 | P-value |
|---|
| Positive (+) | Negative (-) | Positive rate
(%) |
|---|
| Sex |
|
Male | 62 | 47 | 15 | 75.8 (47/62) | 0.257 | 0.612 |
|
Female | 26 | 21 | 5 | 80.8 (21/26) | | |
| Age |
|
<60
yrs | 46 | 37 | 9 | 80.4 (37/46) | 0.549 | 0.459 |
|
≥60 yrs | 42 | 31 | 11 | 73.8 (31/42) | | |
| LN Metastasis |
|
Positive | 46 | 39 | 7 | 84.8 (39/46) | 3.095 | 0.079 |
|
Negative | 42 | 29 | 13 | 69.0 (29/42) | | |
|
Differentiation |
|
Well and
Moderate | 69 | 52 | 17 | 75.4 (52/69) | 0.664 | 0.415 |
|
Poor | 19 | 16 | 3 | 84.2 (16/19) | | |
| Depth of
invasion |
|
T1 and
T2 | 13 | 6 | 7 | 13 | 8.411 | 0.004a |
|
T3 and
T4 | 75 | 62 | 13 | 75 | | |
 | Table VICorrelation of HDAC3
expression with clinicopathologic characteristics of esophageal
squamous cell carcinoma patients (n=88). |
Table VI
Correlation of HDAC3
expression with clinicopathologic characteristics of esophageal
squamous cell carcinoma patients (n=88).
| Clinicopathological
characteristics | No. | Expression of
HDAC3 mRNA |
X2 | P-value |
|---|
| Positive (+) | Negative (-) | Positive rate
(%) |
|---|
| Sex |
|
Male | 62 | 59 | 3 | 95.2 (59/62) | 1.294 | 0.255 |
|
Female | 26 | 23 | 3 | 88.5 (23/26) | | |
| Age |
|
<60
yrs | 46 | 45 | 1 | 97.8 (45/46) | 3.272 | 0.070 |
|
≥60 yrs | 42 | 37 | 5 | 88.1 (37/42) | | |
| LN Metastasis |
|
Positive | 46 | 43 | 3 | 93.5 (43/46) | 0.013 | 0.908 |
|
Negative | 42 | 39 | 3 | 92.9 (39/42) | | |
|
Differentiation |
|
Well and
Moderate | 69 | 64 | 5 | 92.8 (64/69) | 0.092 | 0.761 |
|
Poor | 19 | 18 | 1 | 94.7 (18/19) | | |
| Depth of
invasion |
|
T1 and
T2 | 13 | 13 | 0 | 100 (13/13) | 1.116 | 0.291 |
|
T3 and
T4 | 75 | 69 | 6 | 92.0 (69/75) | | |
 | Table VIICorrelation of HDAC1
expression with clinicopathologic characteristics of esophageal
squamous cell carcinoma in Han and Kazak patients (n=88). |
Table VII
Correlation of HDAC1
expression with clinicopathologic characteristics of esophageal
squamous cell carcinoma in Han and Kazak patients (n=88).
| Clinicopathological
characteristics | | Expression No. of
HDAC1 mRNA |
X2 | P-value |
|---|
| Positive No.
(+) | Negative No.
(-) |
|---|
| Sex | Male | 31 | Han | 25 | 31 | Han | 12 | 11.328 | 0.001a |
| | | Kazak | 6 | | Kazak | 19 | | |
| Female | 12 | Han | 6 | 14 | Han | 5 | 0.540 | 0.462 |
| | | Kazak | 6 | | Kazak | 9 | | |
| Age | <60 yrs | 20 | Han | 11 | 26 | Han | 6 | 4.945 | 0.026a |
| | | Kazak | 9 | | Kazak | 20 | | |
| ≥60 yrs | 23 | Han | 19 | 19 | Han | 12 | 2.036 | 0.154 |
| | | Kazak | 4 | | Kazak | 7 | | |
| LN Metastasis | Positive | 22 | Han | 13 | 24 | Han | 10 | 1.394 | 0.238 |
| | | Kazak | 9 | | Kazak | 14 | | |
| Negative | 21 | Han | 18 | 21 | Han | 7 | 2.059 | 0.151 |
| | | Kazak | 3 | | Kazak | 14 | | |
|
Differentiation | Well and
moderate | 36 | Han | 24 | 33 | Han | 11 | 7.654 | 0.006a |
| | | Kazak | 12 | | Kazak | 22 | | |
| Poor | 7 | Han | 6 | 12 | Han | 7 | 1.534 | 0.216 |
| | | Kazak | 1 | | Kazak | 5 | | |
| Depth of
invasion | T1 and T2 | 6 | Han | 3 | 7 | Han | 2 | 0.627 | 0.429 |
| | | Kazak | 3 | | Kazak | 5 | | |
| T3 and T4 | 37 | Han | 28 | 38 | Han | 15 | 10.044 | 0.002a |
| | | Kazak | 9 | | Kazak | 23 | | |
 | Table VIIICorrelation of HDAC2 expression with
clinicopathologic characteristics of esophageal squamous cell
carcinoma in Han and Kazaks patients (n=88). |
Table VIII
Correlation of HDAC2 expression with
clinicopathologic characteristics of esophageal squamous cell
carcinoma in Han and Kazaks patients (n=88).
| Clinicopathological
characteristics | | Expression No. of
HDAC1 mRNA |
X2 | P-value |
|---|
| Positive (+)
No. | Negative (-) |
|---|
| Sex | Male | 47 | Han | 31 | 15 | Han | 6 | 3.184 | 0.074 |
| | | Kazak | 16 | | Kazak | 9 | | |
| Female | 21 | Han | 11 | 5 | Han | 0 | 4.540 | 0.033a |
| | | Kazak | 10 | | Kazak | 5 | | |
| Age | <60 yrs | 37 | Han | 17 | 9 | Han | 0 | 6.559 | 0.010a |
| | | Kazak | 20 | | Kazak | 9 | | |
| ≥60 yrs | 31 | Han | 26 | 11 | Han | 5 | 6.198 | 0.013a |
| | | Kazak | 5 | | Kazak | 6 | | |
| LN Metastasis | Positive | 39 | Han | 23 | 7 | Han | 0 | 8.256 | 0.004a |
| | | Kazak | 16 | | Kazak | 7 | | |
| Negative | 29 | Han | 21 | 13 | Han | 4 | 6.461 | 0.011a |
| | | Kazak | 8 | | Kazak | 9 | | |
|
Differentiation | Well and
moderate | 52 | Han | 32 | 17 | Han | 3 | 9.874 | 0.002a |
| | | Kazak | 20 | | Kazak | 14 | | |
| Poor | 16 | Han | 12 | 3 | Han | 1 | 2.030 | 0.154 |
| | | Kazak | 4 | | Kazak | 2 | | |
| Depth of
invasion | T1 and T2 | 6 | Han | 4 | 7 | Han | 1 | 3.745 | 0.053 |
| | | Kazak | 2 | | Kazak | 6 | | |
| T3 and T4 | 62 | Han | 40 | 13 | Han | 3 | 7.544 | 0.006a |
| | | Kazak | 22 | | Kazak | 10 | | |
 | Table IXCorrelation of HDA3 expressions with
clinicopathologic characteristics of esophageal squamous cell
carcinoma in Han and Kazak patients (n=88). |
Table IX
Correlation of HDA3 expressions with
clinicopathologic characteristics of esophageal squamous cell
carcinoma in Han and Kazak patients (n=88).
| Clinicopathological
characteristics | | Expression No. of
HDAC3 mRNA |
X2 | P-value |
|---|
| Positive (+)
No. | Negative (-) |
|---|
| Sex | Male | 59 | Han | 37 | 3 | Han | 0 | 4.666 | 0.031a |
| | | Kazak | 22 | | Kazak | 3 | | |
| Female | 23 | Han | 11 | 3 | Han | 0 | 2.487 | 0.115 |
| | | Kazak | 12 | | Kazak | 3 | | |
| Age | <60 years | 45 | Han | 17 | 1 | Han | 0 | 0.599 | 0.439 |
| | | Kazak | 28 | | Kazak | 1 | | |
| ≥60 years | 37 | Han | 28 | 5 | Han | 3 | 0.560 | 0.454 |
| | | Kazak | 9 | | Kazak | 2 | | |
| LN metastasis | Positive | 43 | Han | 22 | 3 | Han | 1 | 0.357 | 0.550 |
| | | Kazak | 21 | | Kazak | 2 | | |
| Negative | 39 | Han | 25 | 3 | Han | 0 | 4.751 | 0.029a |
| | | Kazak | 14 | | Kazak | 3 | | |
|
Differentiation | Well and
moderate | 64 | Han | 35 | 5 | Han | 0 | 5.549 | 0.018a |
| | | Kazak | 29 | | Kazak | 5 | | |
| Poor | 18 | Han | 13 | 1 | Han | 0 | 2.287 | 0.130 |
| | | Kazak | 5 | | Kazak | 1 | | |
| Depth of
invasion | T1 and T2 | 13 | Han | 5 | 0 | Han | 0 | / | |
| | | Kazak | 8 | | Kazak | 0 | | |
| T3 and T4 | 69 | Han | 41 | 6 | Han | 2 | 1.536 | 0.215 |
| | | Kazak | 28 | | Kazak | 4 | | |
Correlation among HDAC1, -2 and -3
expression in human ESCC tissues
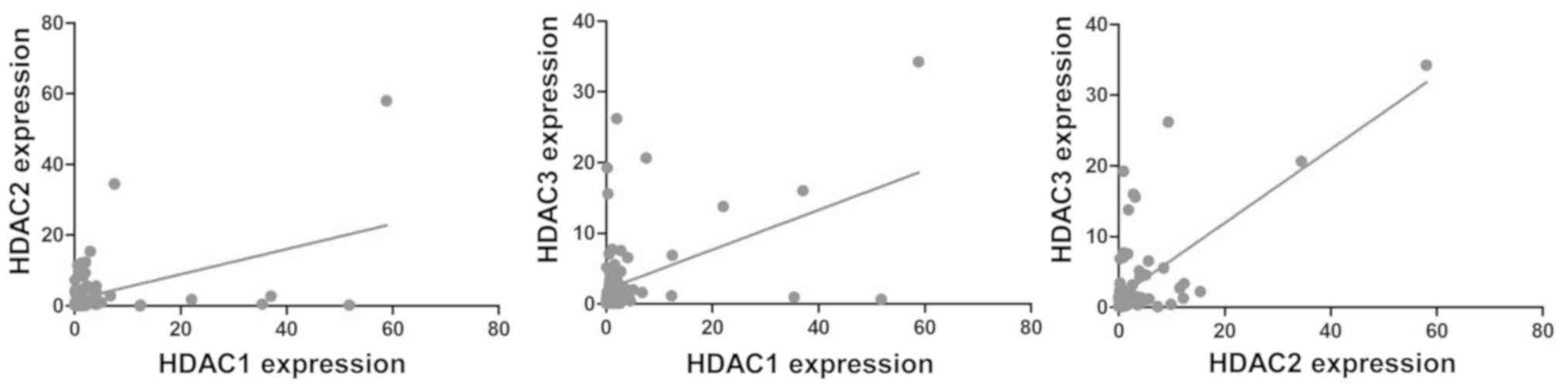
The correlation among HDAC1, -2 and -3 was obtained
by using Pearson's correlation test. The result showed that there
were no significant correlations between HDAC1 and HDAC2 (r=-0.042,
P=0.694), HDAC1 and HDAC3 (r=-0.084, P=0.430), or HDAC2 and HDAC3
(r=-0.176, P=0.099) in ESCC tissues (Fig. 2). These results indicated that there
was no correlation among the three HDACs.
Discussion
ESCC is a common malignant cancer, which can easily
invade adjacent areas and metastasize to lymph nodes and distant
tissues (15,16). It has a high degree of prevalence and
a low survival rate. Thus, it is urgent to develop molecular
markers that can facilitate the early detection of ESCC.
HDAC1 contains 482 amino acids, identified in 1996
by Taunton et al (17). HDAC1
regulates genes involved in cell differentiation and the cell
cycle, and participates in the development of various diseases such
as viral infectious diseases, degenerative diseases and cancer
(18-20).
HDAC1 can mediate site-specific DNA-binding transcriptional
repression and a high level of HDAC1 is observed in tumor invasion
and metastasis (21). Burdelski
et al (22) identified that a
high level of HDAC1 was associated with high Gleason grade,
advanced pathological tumor stage, positive LN metastasis, elevated
preoperative prostate specific antigen level and cell
proliferation; therefore, HDAC1 expression detection may have
clinical significance for the risk stratification of prostate
cancer. Huang et al (23),
demonstrated high expression of HDACs in cervical cancer and
cervical intraepithelial neoplasia tissues. Mutze et al
(24), identified high levels of
HDAC1 and HDAC2 in gastric carcinomas and they were not related to
the response to platinum/5-fluorouracil. High HDAC1 level is
related to lower overall survival (25). There is a significant association
between HDAC1 high expression and advanced age (26). High levels of HDAC1 expression are
associated with a poor histological differentiation and prognosis
in liver cell carcinoma (27). There
is higher expression of HDAC1 in gastrointestinal malignant tumor,
particularly in colorectal cancer and HDAC1 expression is closely
related to clinical characteristics of gastrointestinal cancer
(8). Zhong et al (28), noted that the high expression of
HDAC1 may serve as a potential therapeutic target for ESCC.
Miyashita et al (29),
reported that the expression of HDAC1 may be involved in
duodenoesophageal reflux-induced neoplastic transformation of the
esophageal mucosa into cancer cells with squamous and adeno
differentiation. By contrast, Langer et al (30) stated HDAC1 expression is not changed
based on pT, pN category or esophageal adenocarcinoma
differentiation level. Xu et al (31), noted that HERG1 contributes to poor
prognosis of ESCC and suggest that targeting HERG1 may have
potential diagnostic and therapeutic value for ESCC treatment. In
the present study, the positive rate of HDAC1 expression in ESCC
was increased compared with normal tissues. However, the expression
did not change according to sex, age, metastasis, differentiation
degree and invasion depth.
HDAC2 can promote tumorigenesis through several
mechanisms, including promoting the degradation of β-catenin and
decreasing epigenetic modification (15,32). In
addition, HDAC2 can inhibit the expression of tumor suppressor p53
and p21 while promoting expression of oncogene Myc (33,34).
HDAC2 participates in chronic obstructive pulmonary disease
(35,36). It is also over expressed in lung
cancer (37-39).
Huang et al (40),
demonstrated that HDAC2 affects chromatin remodeling following DNA
damage in ovarian cancer cells. It is also reported that high HDAC2
expression indicates high aggressive behavior in esophageal
adenocarcinoma (30). Wang et
al (41), noted that the level
of HDAC2 increases dramatically in ESCC compared with adjacent
non-tumor tissues. Li et al (42) reported that the HDAC2 protein level
in ESCC tissues was significantly increased and was closely
associated with the histological grade, invasion depth and lymph
node metastasis. Göder et al (43) noted that HDAC1 and HDAC2 regulate
checkpoint kinase phosphorylation through suppression of PR130.
HDACi, as well as an elimination of HDAC1/HDAC2, induces
PR130-dependent mechanisms that inhibit checkpoint kinase
phosphorylation. However, the present study failed to detect
notable changes in HDAC2 expression between ESCC and normal
tissues. However, the expression of HDAC2 was related to the
invasion depth.
HDAC3 is also an essential factor for cell survival
and proliferation in tumors and in maintaining the structure of
chromatin and the stability of genome (44). HDAC3 can form large corepressor
complexes with N-CoR and SMRT (45).
High HDAC3 expression has been found in colon or gastric tumor
cells and it has an antiapoptotic function (46,47).
High expression of HDAC3 is also noted in gastric cancer, where it
is associated with poor prognosis (14). HDAC3 stimulates cell migration of
ovarian carcinoma and high HDAC3 expression indicates poor
prognosis of lung adenocarcinoma patients (48,49).
Jiao et al (50) found that
increased HDAC3 level in the nucleus, but not in the cytoplasm, was
related with LN metastasis and advanced clinical staging of
pancreatic cancer. It has also been reported that HDAC3 is a risk
factor of ESCC and may be used to evaluate the grade of malignancy
and prognosis of ESCC (51). The
present study found no significant difference in the expression of
HDAC3 in ESCC tissue compared with normal tissue and identified
that the expression of HDAC3 was not associated with sex, age,
metastasis, degree of esophageal tissue differentiation or the
depth of invasion. It was hypothesized that perhaps regional
differences and ethnic specificity lead to the discrepancy between
the present results and a previous study (51). These results indicate that the
expression of HDAC3 has no relationship with the development of
ESCC.
Additionally, the present study found that the
expression of HDACs in tumor tissue from Kazak people was lower
compared with Han people. In ESCC, the positive rate of HDAC1
expression in ESCC tissues was increased compared with normal
tissue. The expression of HDAC2 varied according to the invasion
depth of ESCC patients. However, HDAC3 showed no significant
expression changes in ESCC and was not correlated with the
clinicopathological characteristics of ESCC patients. The
expression of HDACs in tumor tissue of Kazak people was lower
compared with Han people. Additionally, it was noted that the
expression of HDAC1, -2 and -3 had ethnic differences.
As for treatment, Ahrens et al (52) proposed that targeting epigenetic
modifiers in esophageal cancers may represent a potential future
therapeutic approach. Kano et al (53) found that CHAP31 sensitized SCC cells
to carbon-ion radiotherapy and this combination treatment may be a
potentially useful therapeutic strategy for ESCC. Hoshino et
al (54) suggested that
HDACi-FK228 has a potent ability to augment the effect of
adenovirus-mediated p53 gene therapy in ESCC. Thus, targeting HDACs
may be an effective approach for treatment of ESCC.
In summary, HDAC1 may be used as a risk factor for
ESCC and HDAC2 may be used to predict ESCC invasion. The different
clinical parameter expression is related to ethnic differences.
Future research should focus on the effect of HDAC inhibitors on
ESCC treatment in Xinjiang, China.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Nature
Science Foundation of China (grant nos. 81460419 and 81460359).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
LL designed the study. HuiwL, HuiL and YW performed
data collection. HuiwL and HuiL performed statistical analysis.
HuiwL provided data interpretation. YinL and YikL participated in
data collection of patient information. YC and LY provided help in
RT-qPCR assay. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Xinjiang Medical University and informed consent was obtained
from all patients.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Wang QL, Xie SH, Wahlin K and Lagergren J:
Global time trends in the incidence of esophageal squamous cell
carcinoma. Clin Epidemiol. 10:717–728. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Parkin DM, Bray FI and Devesa SS: Cancer
burden in the year 2000. The global picture. Eur J Cancer. 37(Suppl
8)(S4-S66)2001.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kohler BA, Ward E, McCarthy BJ, Schymura
MJ, Ries LA, Eheman C, Jemal A, Anderson RN, Ajani UA and Edwards
BK: Annual report to the nation on the status of cancer, 1975-2007,
featuring tumors of the brain and other nervous system. J Natl
Cancer Inst. 103:714–736. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Glozak MA and Seto E: Histone deacetylases
and cancer. Oncogene. 26:5420–5432. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hull EE, Montgomery MR and Leyva KJ: HDAC
inhibitors as epigenetic regulators of the immune system: Impacts
on cancer therapy and inflammatory diseases. Biomed Res Int.
2016(8797206)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yamagoe S, Kanno T, Kanno Y, Sasaki S,
Siegel RM, Lenardo MJ, Humphrey G, Wang Y, Nakatani Y, Howard BH
and Ozato K: Interaction of histone acetylases and deacetylases in
vivo. Mol Cell Biol. 23:1025–1033. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cao LL, Yue Z, Liu L, Pei L, Yin Y, Qin L,
Zhao J, Liu H, Wang H and Jia M: The expression of histone
deacetylase HDAC1 correlates with the progression and prognosis of
gastrointestinal malignancy. Oncotarget. 8:39241–39253.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nemati M, Ajami N, Estiar MA, Rezapour S,
Gavgani RR, Hashemzadeh S, Kafil HS and Sakhinia E: Deregulated
expression of HDAC3 in colorectal cancer and its clinical
significance. Adv Clin Exp Med. 27:305–311. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Toh Y, Yamamoto M, Endo K, Ikeda Y, Baba
H, Kohnoe S, Yonemasu H, Hachitanda Y, Okamura T and Sugimachi K:
Histone H4 acetylation and histone deacetylase 1 expression in
esophageal squamous cell carcinoma. Oncol Rep. 10:333–338.
2003.PubMed/NCBI
|
|
11
|
Micheli L, D'Andrea G, Leonardi L and
Tirone F: HDAC1, HDAC4, and HDAC9 bind to PC3/Tis21/Btg2 and are
required for its inhibition of cell cycle progression and cyclin D1
expression. J Cell Physiol. 232:1696–1707. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nakashima H, Nguyen T and Chiocca EA:
Combining HDAC inhibitors with oncolytic virotherapy for cancer
therapy. Oncolytic Virother. 4:183–191. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yoon S and Eom GH: HDAC and HDAC
inhibitor: From cancer to cardiovascular diseases. Chonnam Med J.
52:1–11. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Thangaraju M, Carswell KN, Prasad PD and
Ganapathy V: Colon cancer cells maintain low levels of pyruvate to
avoid cell mortality caused by inhibition of HDAC1/HDAC3. Biochem
J. 417:379–389. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu C, Liu L, Shan J, Shen J, Xu Y, Zhang
Q, Yang Z, Wu L, Xia F, Bie P, et al: Histone deacetylase 3
participates in self-renewal of liver cancer stem cells through
histone modification. Cancer Lett. 339:60–69. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xu G, Zhu H, Zhang M and Xu J: Histone
deacetylase 3 is associated with gastric cancer cell growth via the
miR-454-mediated targeting of CHD5. Int J Mol Med. 41:155–163.
2018. View Article : Google Scholar
|
|
17
|
Taunton J, Hassig CA and Schreiber SL: A
mammalian histone deacetylase related to the yeast transcriptional
regulator Rpd3p. Science. 272:408–411. 1996.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen L, Wang C, Luo J, Su W, Li M, Zhao N,
Lyu W, Attaran H, He Y, Ding H and He H: Histone deacetylase 1
plays an acetylation-independent role in influenza a virus
replication. Front Immunol. 8(1757)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Datta M, Staszewski O, Raschi E, Frosch M,
Hagemeyer N, Tay TL, Blank T, Kreutzfeldt M, Merkler D,
Ziegler-Waldkirch S, et al: Histone deacetylases 1 and 2 regulate
microglia function during development, homeostasis, and
neurodegeneration in a context-dependent manner. Immunity.
48:514–529. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Qiao W, Liu H, Liu R, Liu Q, Zhang T, Guo
W, Li P and Deng M: Prognostic and clinical significance of histone
deacetylase 1 expression in breast cancer: A meta-analysis. Clin
Chim Acta. 483:209–215. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sekhavat A, Sun JM and Davie JR:
Competitive inhibition of histone deacetylase activity by
trichostatin A and butyrate. Biochem Cell Biol. 85:751–758.
2007.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Burdelski C, Ruge OM, Melling N, Koop C,
Simon R, Steurer S, Sauter G, Kluth M, Hube-Magg C, Minner S, et
al: HDAC1 overexpression independently predicts biochemical
recurrence and is associated with rapid tumor cell proliferation
and genomic instability in prostate cancer. Exp Mol Pathol.
98:419–426. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Huang BH, Laban M, Leung CH, Lee L, Lee
CK, Salto-Tellez M, Raju GC and Hooi SC: Inhibition of histone
deacetylase 2 increases apoptosis and p21Cip1/WAF1 expression,
independent of histone deacetylase 1. Cell Death Differ.
12:395–404. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mutze K, Langer R, Becker K, Ott K,
Novotny A, Luber B, Hapfelmeier A, Gottlicher M, Hofler H and
Keller G: Histone deacetylase (HDAC) 1 and 2 expression and
chemotherapy in gastric cancer. Ann Surg Oncol. 17:3336–3343.
2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shinke G, Yamada D, Eguchi H, Iwagami Y,
Asaoka T, Noda T, Wada H, Kawamoto K, Gotoh K, Kobayashi S, et al:
Role of histone deacetylase 1 in distant metastasis of pancreatic
ductal cancer. Cancer Sci. 109:2520–2531. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang Z, Yamashita H, Toyama T, Sugiura H,
Ando Y, Mita K, Hamaguchi M, Hara Y, Kobayashi S and Iwase H:
Quantitation of HDAC1 mRNA expression in invasive carcinoma of the
breast. Breast Cancer Res Treat. 94:11–16. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang H, Kohashi K, Yoshizumi T, Okumura Y,
Tanaka Y, Shimokawa M, Iwasaki T, Aishima S, Maehara Y and Oda Y:
Co-expression of SALL4 with HDAC1 and/or HDAC2 is associated with
underexpression of PTEN and poor prognosis in patients with
hepatocellular carcinoma. Hum Pathol. 64:69–75. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhong L, Zhou S, Tong R, Shi J, Bai L, Zhu
Y, Duan X, Liu W, Bao J, Su L and Peng Q: Preclinical assessment of
histone deacetylase inhibitor quisinostat as a therapeutic agent
against esophageal squamous cell carcinoma. Invest New Drugs.
37:616–624. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Miyashita T, Tajima H, Munemoto M, Shah
FA, Harmon JW, Watanabe T, Shoji M, Okamoto K, Nakanuma S, Sakai S,
et al: Impact of histone deacetylase 1 and metastasis-associated
gene 1 expression in esophageal carcinogenesis. Oncol Lett.
8:758–764. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Langer R, Mutze K, Becker K, Feith M, Ott
K, Hofler H and Keller G: Expression of class I histone
deacetylases (HDAC1 and HDAC2) in oesophageal adenocarcinomas: An
immunohistochemical study. J Clin Pathol. 63:994–998.
2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Xu J, Lv H, Zhang B, Xu F, Zhu H, Chen B,
Zhu C and Shen J: miR-30b-5p acts as a tumor suppressor microRNA in
esophageal squamous cell carcinoma. J Thorac Dis. 11:3015–3029.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kiweler N, Brill B, Wirth M, Breuksch I,
Laguna T, Dietrich C, Strand S, Schneider G, Groner B, Butter F, et
al: The histone deacetylases HDAC1 and HDAC2 are required for the
growth and survival of renal carcinoma cells. Arch Toxicol.
92:2227–2243. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fritsche P, Seidler B, Schuler S, Schnieke
A, Gottlicher M, Schmid RM, Saur D and Schneider G: HDAC2 mediates
therapeutic resistance of pancreatic cancer cells via the BH3-only
protein NOXA. Gut. 58:1399–1409. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Witt O, Deubzer HE, Milde T and Oehme I:
HDAC family: What are the cancer relevant targets? Cancer Lett.
277:8–21. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Barnes PJ: Histone deacetylase-2 and
airway disease. Ther Adv Respir Dis. 3:235–243. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yao H and Rahman I: Role of histone
deacetylase 2 in epigenetics and cellular senescence: Implications
in lung inflammaging and COPD. Am J Physiol Lung Cell Mol Physiol.
303(L557-L566)2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Brodie SA, Li G, El-Kommos A, Kang H,
Ramalingam SS, Behera M, Gandhi K, Kowalski J, Sica GL, Khuri FR,
et al: Class I HDACs are mediators of smoke carcinogen-induced
stabilization of DNMT1 and serve as promising targets for
chemoprevention of lung cancer. Cancer Prev Res (Phila). 7:351–361.
2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Han Y, Zhang Y, Yang LH, Mi XY, Dai SD, Li
QC, Xu HT, Yu JH, Li G, Zhao J, et al: X-radiation inhibits histone
deacetylase 1 and 2, upregulates Axin expression and induces
apoptosis in non-small cell lung cancer. Radiat Oncol.
7(183)2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Jung KH, Noh JH, Kim JK, Eun JW, Bae HJ,
Xie HJ, Chang YG, Kim MG, Park H, Lee JY and Nam SW: HDAC2
overexpression confers oncogenic potential to human lung cancer
cells by deregulating expression of apoptosis and cell cycle
proteins. J Cell Biochem. 113:2167–2177. 2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Huang R, Langdon SP, Tse M, Mullen P, Um
IH, Faratian D and Harrison DJ: The role of HDAC2 in chromatin
remodelling and response to chemotherapy in ovarian cancer.
Oncotarget. 7:4695–4711. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang F, Qi Y, Li X, He W, Fan QX and Zong
H: HDAC inhibitor trichostatin A suppresses esophageal squamous
cell carcinoma metastasis through HADC2 reduced MMP-2/9. Clin
Invest Med. 36(E87-E94)2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Li S, Wang F, Qu Y, Chen X, Gao M, Yang J,
Zhang D, Zhang N, Li W and Liu H: HDAC2 regulates cell
proliferation, cell cycle progression and cell apoptosis in
esophageal squamous cell carcinoma EC9706 cells. Oncol Lett.
13:403–409. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Göder A, Emmerich C, Nikolova T, Kiweler
N, Schreiber M, Kühl T, Imhof D, Christmann M, Heinzel T, Schneider
G and Krämer OH: HDAC1 and HDAC2 integrate checkpoint kinase
phosphorylation and cell fate through the phosphatase-2A subunit
PR130. Nat Commun. 9(764)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bhaskara S, Knutson SK, Jiang G,
Chandrasekharan MB, Wilson AJ, Zheng S, Yenamandra A, Locke K, Yuan
JL, Bonine-Summers AR, et al: Hdac3 is essential for the
maintenance of chromatin structure and genome stability. Cancer
Cell. 18:436–447. 2010.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Li J, Jin W, Wang J, Nawaz Z, Liu JM, Qin
J and Wong J: Both corepressor proteins SMRT and N-CoR exist in
large protein complexes containing HDAC3. EMBO J. 19:4342–4350.
2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Weichert W, Röske A, Niesporek S, Noske A,
Buckendahl AC, Dietel M, Gekeler V, Boehm M, Beckers T and Denkert
C: Class I histone deacetylase expression has independent
prognostic impact in human colorectal cancer: Specific role of
class I histone deacetylases in vitro and in vivo. Clin Cancer Res.
14:1669–1677. 2008.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Weichert W, Röske A, Gekeler V, Beckers T,
Ebert MP, Pross M, Dietel M, Denkert C and Röcken C: Association of
patterns of class I histone deacetylase expression with patient
prognosis in gastric cancer: A retrospective analysis. Lancet
Oncol. 9:139–148. 2008.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hayashi A, Horiuchi A, Kikuchi N, Hayashi
T, Fuseya C, Suzuki A, Konishi I and Shiozawa T: Type-specific
roles of histone deacetylase (HDAC) overexpression in ovarian
carcinoma: HDAC1 enhances cell proliferation and HDAC3 stimulates
cell migration with downregulation of E-cadherin. Int J Cancer.
127:1332–1346. 2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Minamiya Y, Ono T, Saito H, Takahashi N,
Ito M, Motoyama S and Ogawa J: Strong expression of HDAC3
correlates with a poor prognosis in patients with adenocarcinoma of
the lung. Tumour Biol. 31:533–539. 2010.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Jiao F, Hu H, Han T, Zhuo M, Yuan C, Yang
H and Wang L and Wang L: Aberrant expression of nuclear HDAC3 and
cytoplasmic CDH1 predict a poor prognosis for patients with
pancreatic cancer. Oncotarget. 7:16505–16516. 2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yao Z, Xu M and Zhou Z: The role of
Histone deacetylase 3 in esophageal squamous cell carcinoma
pathogenesis. Chin J Dig. 32:760–762. 2012.(In Chinese). PubMed/NCBI View Article : Google Scholar
|
|
52
|
Ahrens TD, Timme S, Hoeppner J, Ostendorp
J, Hembach S, Follo M, Hopt UT, Werner M, Busch H, Boerries M and
Lassmann S: Selective inhibition of esophageal cancer cells by
combination of HDAC inhibitors and Azacytidine. Epigenetics.
10:431–445. 2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kano M, Yamada S, Hoshino I, Murakami K,
Akutsu Y, Sakata H, Nishimori T, Usui A, Miyazawa Y, Kamada T, et
al: Effects of carbon-ion radiotherapy combined with a novel
histone deacetylase inhibitor, cyclic hydroxamic-acid-containing
peptide 31 in human esophageal squamous cell carcinoma. Anticancer
Res. 29:4433–4438. 2009.PubMed/NCBI
|
|
54
|
Hoshino I, Matsubara H, Akutsu Y,
Nishimori T, Yoneyama Y, Murakami K, Sakata H, Matsushita K,
Komatsu A, Brooks R and Ochiai T: Role of histone deacetylase
inhibitor in adenovirus-mediated p53 gene therapy in esophageal
cancer. Anticancer Res. 28:665–671. 2008.PubMed/NCBI
|