Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic
fibrosing lung disease of unknown etiology (1). IPF is associated with an increased
fibroblast population, leading to excessive deposition of collagen
in the lung alveolar spaces (1,2). This
pathological change subsequently leads to decreased gas exchange
and respiratory failure. While disease progression is usually slow,
a number of patients experience acute deterioration of respiratory
function, which is termed as acute exacerbation (AE) of IPF
(AE-IPF) (2). In a retrospective
review of 461 patients with IPF in South Korea, the 1- and 3-year
incidences of AE-IPF were estimated to be 14.2 and 20.7%,
respectively (3). While the exact
etiology of AE-IPF remains elusive, the prognosis is poor, with a
systematic review estimating the mortality rate to be 60% at 1
month and 67% at 3 months after disease onset (4).
The pathophysiology of AE-IPF has been attributed to
pro-coagulant and antifibrinolytic activity in affected patients
(3). Imokawa et al (5) demonstrated the strong presence of
tissue factor, a coagulation initiator, in the lung tissues of
patients with IPF. Kotani et al (6) have reported higher concentrations of
plasminogen activator inhibitor (PAI)-1 and PAI-2 antigen levels in
bronchoalveolar lavage (BAL) supernatant fluids and cell lysates,
suggestive of an antifibrinolytic activity. While one study
corroborate this theory based on improved survival of patients
treated with low-molecular-weight heparin (7), another study indicated a lack of
benefit of anticoagulant therapy in such patients (8). High-dose corticosteroids and
immunosuppressive drugs, including cyclosporine A, have also been
used for managing AE-IPF, but the clinical efficacy of these drugs
is still under dispute (9,10).
Thrombomodulin, a transmembranous protein expressed
on the surface of vascular endothelial cells, is an essential
component in the regulation of intravascular coagulation (11). Due to its anticoagulant and
anti-inflammatory effects, thrombomodulin has been successfully
used in the management of disseminated intravascular coagulation
(DIC) (11). Recombinant human
soluble thrombomodulin (rhTM; Recomodulin; Asahi Kasei Pharma
Corp.), which comprises only the extracellular domain of
thrombomodulin, has also been explored in the management of various
respiratory, renal and cardiovascular diseases (12). Recently, several trials reported on
the use of rhTM in the management of AE-IPF (13-15).
While research is still at the nascent stage, there is a
requirement to summarize the results of these multiple studies to
evaluate the quality of evidence and provide a direction for
further research. Therefore, the purpose of the present systematic
review and meta-analysis was to evaluate the efficacy and safety of
rhTM when used in the management of AE-IPF.
Materials and methods
Guidelines
The guidelines of the Preferred Reporting Items for
Systematic Reviews and Meta-analyses (16) and the Cochrane Handbook for
Systematic Reviews of Intervention (17) were followed during the preparation of
this review.
Search strategy
An electronic search of titles and abstracts in the
PubMed (https://pubmed.ncbi.nlm.nih.gov), Biomed Central
(https://www.biomedcentral.com), Scopus
(https://www.scopus.com/home.uri) and
Embase (https://www.embase.com/login#search) databases was
performed using the following key words: ‘Recombinant human
thrombomodulin’, ‘thrombomodulin’, ‘pulmonary fibrosis’, ‘AE’,
‘IPF’ and ‘clinical outcomes’. Titles were searched by two
independent reviewers (BW and TL) with the last search performed on
31st August 2019. The reference lists of published studies and
review articles on the subject were also searched for the
identification of any further trials. After screening records by
their titles and abstracts, the full texts of selected articles
were retrieved. Each of the two reviewers assessed individual
studies based on the inclusion criteria. Disagreements, if any,
were resolved by discussion.
Study selection and outcomes
Using the common evidence medicine framework,
population, intervention, comparison, outcome (18), all types of studies performed on
patients with AE-IPF (Population) were included. Trials
comparing rhTM (Intervention) vs. control
(Comparison) for AE-IPF and assessing mortality and adverse
events (Outcomes) were included in the review. Duplicate
studies based on the same dataset, non-comparative studies, case
series, case reports, review articles and studies that were not
published in English language were excluded. In the case of studies
with overlapping datasets, the study encompassing the largest
dataset was selected.
Two independent reviewers retrieved data from
selected studies using a standardized data collection form. The
details sourced were the following: Authors, publication year,
country of origin, sample size, baseline characteristics of study
participants, treatment protocol, mortality and adverse events. The
primary outcome of interest was the incidence of 28- and 90- day
mortality. The secondary outcome was the incidence of adverse
events.
Risk of bias assessment
The Risk of Bias Assessment Tool for Non-randomized
Studies was used for quality assessment (19). Studies were rated as having low, high
or unclear risk of bias regarding the following points: Selection
of participants, confounding variables, measurement of exposure
blinding of outcome assessment, incomplete outcome data and
selective outcome reporting.
Statistical analysis
RevMan version 5.3 (Cochrane Collaboration) was used
for meta-analysis. Considering the methodological heterogeneity
amongst studies, a random-effects model was used to calculate the
pooled effect size. Data were summarised using the Mantel-Haenszel
odds ratio (OR) with 95% CI. Inter-study heterogeneity was
estimated using the I2 statistic. I2 values
of 25-50% were considered to indicate low heterogeneity, 50-75%
medium heterogeneity and >75% represented substantial
heterogeneity.
Results
Literature search and study
selection
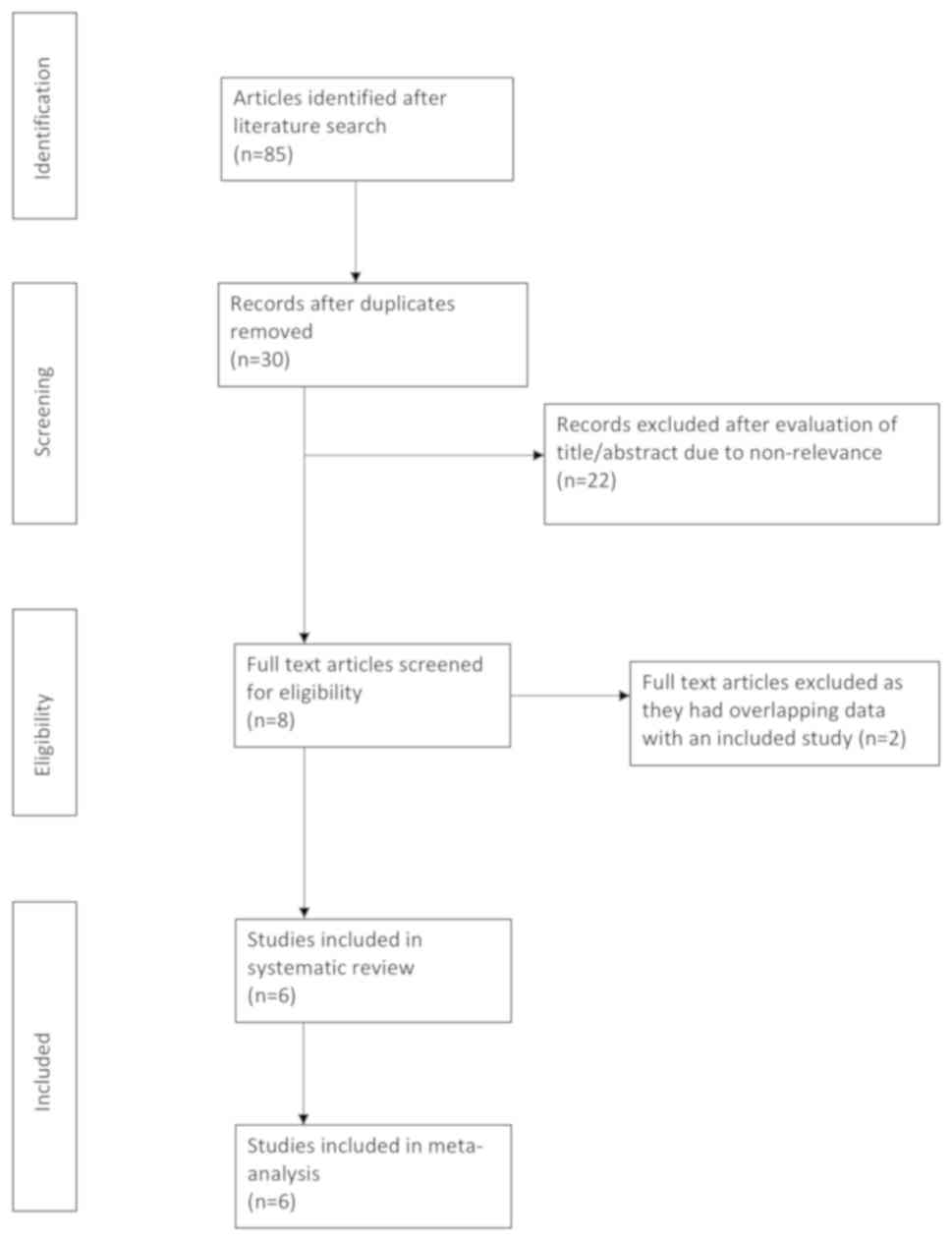
The literature search yielded a total of 85 records.
After the exclusion of duplicates, 30 unique articles were
identified. The titles and abstracts of these 30 articles were then
screened for possible inclusion in the present study. A total of 22
articles were excluded due to non-relevance and full texts of 8
articles were retrieved (Fig. 1). A
total of three studies (19-21)
had overlapping datasets, of which two (20,21) were
excluded. A total of six studies (13-15,
21-23)
were finally included in the present systematic review and
meta-analysis.
Features of the studies included
Details of the studies included are presented in
Table I. Two studies were
retrospective studies (13,22) and three were single-arm prospective
studies utilizing a historical control group (14,15,23),
while one was a non-randomized prospective trial (24). All trials were performed in Japan.
The sample size varied across the studies, with 6-61 participants
per group. The baseline parameters of the experimental and control
groups as reported by the studies are presented in Table II. The authors reported negligible
differences in the baseline parameters between the rhTM and control
groups. The dosage of rhTM was the same across the studies (0.06
mg/kg/day is equivalent to 380 U/kg/day). Methylprednisolone pulse
therapy was administered for three days in all studies in the
experimental and control groups.
 | Table ICharacteristics of included
studies. |
Table I
Characteristics of included
studies.
| | | Sample size | Age (years) | | |
|---|
| Authors (year) | Study type | rhTM | Control | rhTM | Control | Treatment
protocol | (Refs.) |
|---|
| Tsushima et
al (2014) | Prospective with
historical controls | 20 | 6 | 76.2±0.4 | 73.7±1.2 | rhTM group: MP
pulse therapy at 1 g/day for 3 days, followed by prednisolone 1
mg/kg/day. rhTM (0.06 mg/kg/day) for 6 days. Control group: Similar
treatment without rhTM. | (15) |
| Kataoka et
al (2015) | Retrospective | 20 | 20 | 73.5±NR | 71.0±NR | rhTM group: MP
pulse therapy at 1 g/day for 3 days with cyclosporine A 2.5
mg/kg/day followed by tapered dose of steroids. rhTM (0.06
mg/kg/day) for 6 days followed by continuous IV infusion of LMWH
750.000 IU/kg/day. LMWH discontinued if any adverse events were
observed. Control group: Similar treatment without rhTM | (13) |
| Abe et al
2015) | Prospective,
non-randomized | 11 | 11 | 68.9±7.3 | 73.1±11.3 | rhTM group: MP
pulse therapy at 1 g/day for 3 days with cyclophosphamide and/or
cyclosporine A and 0.06 mg/kg/day rhTM for 6 days. Control group:
Similar treatment without rhTM. | (24) |
| Hayakawa et
al (2016) | Prospective with
historical controls | 10 | 13 | 73.2±9.5 | 69.7±8.5 | rhTM group: MP
pulse therapy at 1 g/day for 3 days, then 80 mg/day for 7 days with
rhTM 380 U/kg/day for 30 min for 7 days. Concomitant administration
of anticoagulants and immunosuppressants prohibited. Control group:
Similar treatment without rhTM but no restriction of other
drugs. | (14) |
| Sakamoto et
al (2017) | Retrospective | 45 | 35 | 75.0±NR | 75.0±NR | rhTM group: MP
pulse therapy at 1 g/day for 3 days with cyclosporine A 2.5
mg/kg/day in 66 patients, followed by tapered dose of steroids and
0.06 mg/kg/day rhTM for 6 days. Control group: Similar treatment
without rhTM. Furthermore, 11 patients received LMWH 75 IU for 14
days. | (22) |
| Arai et al
(2019) | Prospective with
historical controls | 39 | 61 | 74.0±NR | 74.0±NR | rhTM group:
Generally treated with IV MP for 3 days. Polymyxin B-immobilized
fibre column therapy administered using toraymyxin. Anticoagulant
and antiplatelet drugs used as required. rhTM (380 U/kg/day) for 6
days. Control group:Similar treatment without rhTM. | (23) |
 | Table IIBaseline characteristics of
participants of the studies included. |
Table II
Baseline characteristics of
participants of the studies included.
| | Tsushima et
al (15) | Kataoka et
al (13) | Abe et al
(24) | Hayakawa et
al (14) | Sakamoto et
al (22) | Arai et al
(23) |
|---|
| Parameters | rhTM | Control | rhTM | Control | rhTM | Control | rhTM | Control | rhTM | Control | rhTM | Control |
|---|
|
PaO2/FiO2
(mmHg) | NR | NR | 224.0±NR | 238.0±NR | 198.0±62 | 234.0±126 | 168.0±56 | 183.0±47 | 256.0±NR | 260.0±NR | 161.0±NR | 194.6±NR |
| WBC count,
(mm3) | 14,000±462 | 15,820±291 | 10,350±NR | 10,350±NR | 14,590±6,031 | 9,518±2,835 | NR | NR | 10,500±NR | 10,200±NR | 10,200±NR | 9,700±NR |
| LDH (IU/l) | 782±41 | 477±13 | 300±NR | 313±NR | 430±148 | 419±150 | 378±118 | 444±173 | 353±NR | 351±NR | 349±NR | 347±NR |
| CRP (mg/dl) | 13.1±0.7 | 11.8±0.5 | 4.7±NR | 6.8±NR | 6.8±5.8 | 11.3±7.3 | 11.5±8.3 | 11±11.1 | 6.0±NR | 6.9±NR | 12.4±NR | 9.1±NR |
| KL-6 (U/l) | 1,812±111 | 1,956±218 | 1,278±NR | 1,255±NR | 2,018±780 | 1,489±1,385 | 1,512±583 | 2,060±1,520 | 1,299±NR | 1,038±NR | 1,192±NR | 1,516±NR |
| SP-D (ng/ml) | NR | NR | 202±NR | 203±NR | 759±638 | 338±338 | 482±527 | 676±711 | 288±NR | 317±NR | NR | NR |
| D-dimer
(mg/ml) | NR | NR | 2.3±NR | 2.4±NR | 4.4±3.7 | 3.3±4.9 | 5.3±7.9 | 6.9±8.9 | 4.2±NR | 4.8±NR | NR | NR |
| TAT (ng/ml) | 34.7±1.4 | 24.2±2.2 | NR | NR | 9.1±11.0 | 14.8±22.7 | 8.1±13.5 | NR | NR | NR | NR | NR |
| FDP
(μg/ml) | 99.0±11.0 | 39.6±7.4 | NR | NR | 8.6±4.7 | 8.4±5.6 | NR | NR | 8.8±NR | 9.4±NR | NR | NR |
| PIC (ng/ml) | NR | NR | NR | NR | 1.4±0.4 | 1.8±0.7 | 3.0±5.1 | NR | NR | NR | NR | NR |
Outcomes
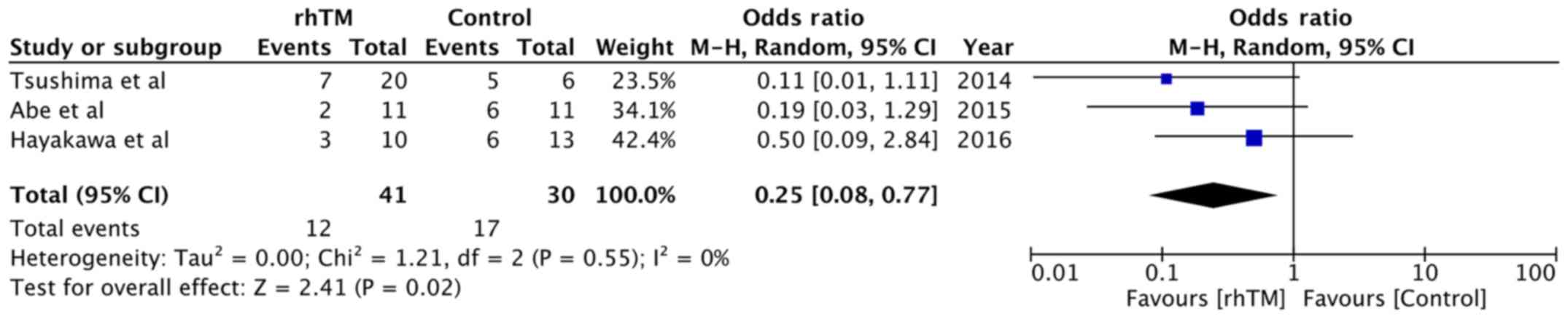
The 28-day mortality rates were reported by three
studies (14,15,24).
Within the rhTM group, 29.26% of patients died, while 56.66% of
patients died in the control group. The meta-analysis indicated
that treatment with rhTM was associated with a significant
reduction in 28-day mortality compared with that in the control
group (OR, 0.25; 95% CI, 0.08-0.77; P=0.02; I2=0%;
Fig. 2).
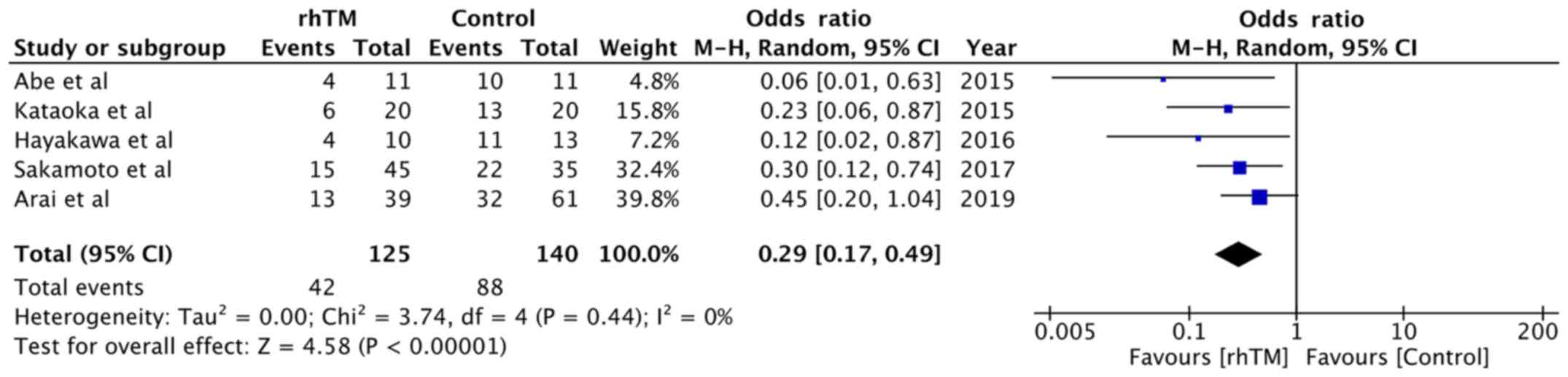
The 90-day mortality rates were reported by five
(13,14,21-23)
of the six studies included. A total of 125 patients received rhTM,
while 140 patients served as controls. The overall 90-day mortality
rate was 33.6% in the rhTM group and 62.85% in the control group.
Pooled analysis indicated that rhTM significantly reduced the
90-day mortality in patients with AE-IPF (OR, 0.29; 95% CI,
0.17-0.49; P<0.00001; I2=0%; Fig. 3).
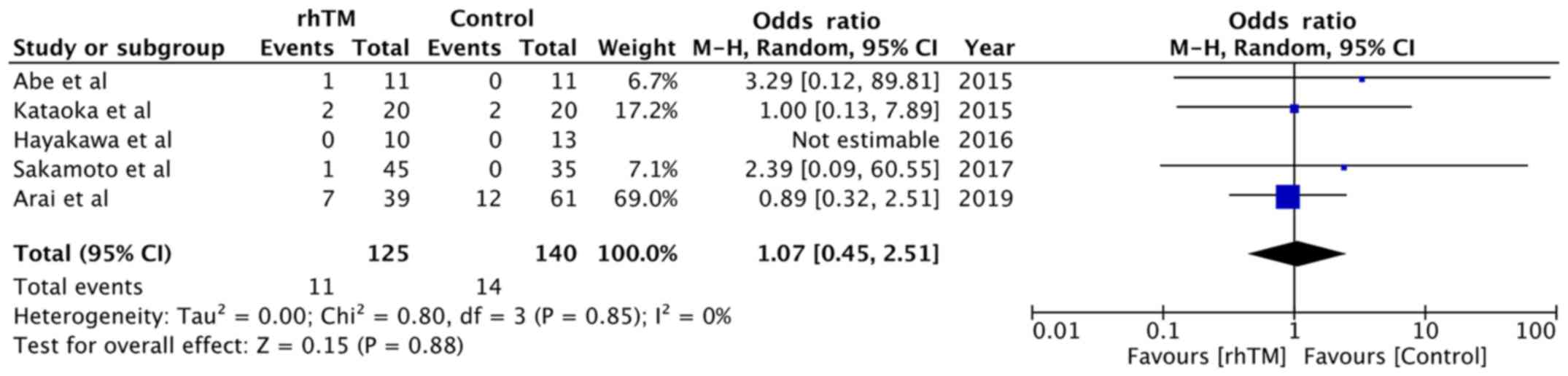
Data on adverse events were reported by five trials
(13,14,21-23).
Details of adverse events in the studies included are presented in
Table III. Hayakawa et al
(14) did not report any adverse
events, while Sakamoto et al (22) reported only minor adverse events in
the study group, which did not require discontinuation of
treatment. Abe et al (24)
reported one case of hepatic and renal failure in the study group.
The authors suspected the cause of death in this patient to be a
drug interaction between warfarin and cyclosporine, leading to
uncontrolled serum levels of cyclosporine and subsequent renal and
hepatic failure. Kataoka et al (13) reported hemorrhagic complications in
the study and control groups, which were managed by discontinuation
of rhTM and low-molecular-weight heparin, respectively. Arai et
al (23) reported two cases of
bleeding (1 case of gastrointestinal and 1 case of nasal) in the
control group but no hemorrhagic complication in the rhTM group.
All adverse events were pooled for a meta-analysis, which
demonstrated no significant difference between the rhTM and control
groups (OR, 1.07; 95% CI, 0.45-2.51; P=0.88; I2=0%;
Fig. 4).
 | Table IIIAdverse events reported by the
studies included. |
Table III
Adverse events reported by the
studies included.
| Study | rhTM | Control | (Ref.) |
|---|
| Tsushima et
al | NR | NR | (15) |
| Kataoka et
al | One case of
hemosputum on day 5. and one case of acute DVT on day 4. | One case of
bleeding from central venous catheter on day 3 and 1 case of
subcutaneous bleeding on day 8. | (13) |
| Abe et
al | One patient died of
hepatic and renal failure due to high concentration of cyclosporine
(suspected drug interaction with warfarin). | None. | (24) |
| Hayakawa et
al | None. | None. | (14) |
| Sakamoto et
al | Mild hemoptysis and
hematuria in one patient on day 1. No case of serious
bleeding. | None. | (22) |
| Arai et
al | One case of
gastrointestinal bleeding, one case of nasal bleeding, one case of
DVT, one case of cerebral infarction, one case of drug-induced
hepatitis, 1 case of an unspecified vascular event, one case of
fungal infection. | Two cases of
pneumomediastinum, one case of pneumothorax, three cases of DVT,
one case of hepatitis B, one case of hepatitis B PCR positive, one
case of sulfamethoxazole/trimethoprim, -associated
thrombocytopenia, one case of MRSA pneumonia, one case of fungal
infection. | (23) |
Risk of bias
The assessment of the risk of bias in the included
studies is presented in Table IV. A
high risk of bias was noted across all studies in the selection of
participants, which were recruited at different time intervals in
all trials.
 | Table IVRisk of bias summary. |
Table IV
Risk of bias summary.
| Study | Selection of
participants | Confounding
variables | Measurement of
exposure | Blinding of outcome
assessment | Incomplete outcome
data | Selective outcome
reporting | (Ref.) |
|---|
| Tsushima et
al | High risk | High risk | Low risk | Low risk | Low risk | Low risk | (15) |
| Kataoka et
al | High risk | Low risk | Low risk | Low risk | Low risk | Low risk | (13) |
| Abe et
al | High risk | Low risk | Low risk | Low risk | Low risk | Low risk | (24) |
| Hayakawa et
al | High risk | High risk | Low risk | Low risk | Low risk | Low risk | (14) |
| Sakamoto et
al | High risk | Low risk | Low risk | Low risk | Low risk | Low risk | (22) |
| Arai et
al | High risk | Low risk | Low risk | Low risk | Low risk | Low risk | (23) |
Discussion
Several different therapies have been studied to
improve outcomes in AE-IPF. These include corticosteroids,
anticoagulants, antacids, ambrisentan, imatinib, nintedanib,
phosphodiesterase-5 inhibitors, pirfenidone, dual endothelin
receptor antagonists (e.g. macitentan) and combination therapy of
prednisone, azathioprine and N-acetylcysteine (25). Despite several attempted treatment
strategies, no single management protocol has been accepted as the
standard to date. The updated American Thoracic Society/European
Respiratory Society/Japanese Respiratory Society/Latin American
Thoracic Society guidelines do not recommend the use of warfarin,
ambrisentan, imatinib or the combination therapy of prednisone,
azathioprine and N-acetylcysteine, while providing a conditional
recommendation for nintedanib, pirfenidone, macitentan and
phosphodiesterase-5 inhibitors (25).
The use of immunosuppressants, including
cyclo-phosphamides, cyclosporine or tacrolimus, has been examined,
but only in small retrospective studies providing inconclusive
evidence for the potential benefits of these drugs (9,26). In
routine practice, administration of corticosteroids in the form of
methylprednisolone pulse therapy is the most commonly employed
management technique, despite low evidence supporting its use
(27). Corticosteroid therapy was
utilized in all six of the included studies of the present review
but with disparity in the use of immunosuppressant drugs.
Cyclophosphamide and/or cyclosporine were administered in three
studies in the experimental and control groups, while in the
clinical trial by Hayakawa et al (14), only the control group received
immunosuppressants. The variance in the treatment strategies for
AE-IPF may be attributed to the poorly understood
pathophysiological mechanism of the disease.
In an attempt to elucidate the underlying pathology
of AE, Collard et al (28)
compared the plasma biomarker profiles between patients with
AE-IPF, acute lung injury and stable IPF. The results indicated a
significant increase in the levels of type II alveolar epithelial
cell injury/proliferation markers [Krebs von den Lungen-6 (KL-6)
and surfactant protein D], endothelial cell injury markers (von
Willebrand factor), inflammation markers (interleukin-6) and
coagulation markers (protein C, thrombomodulin and PAI-1) in
patients with AE-IPF. Kataoka et al (13) detected high levels of D-dimer and
thrombomodulin in BAL fluid from patients with AE-IPF. Song et
al (3) reported that increased
levels of C-reactive protein are a poor prognostic factor for
AE-IPF. These studies suggested that dysfunction of endothelial
cells, coagulation abnormalities and inflammation have an important
role in the pathophysiology of AE-IPF.
The dual anticoagulant and antiinflammatory action
of rhTM has been utilized in the management of AE-IPF (13). rhTM activates protein C by the
formation of reversible complexes with thrombin. These complexes
inactivate coagulation factors, thereby downregulating
intravascular coagulation (23).
Inflammation is also suppressed by inhibition of neutrophil
adhesion, improved endothelial barrier function, decreased
complement activation and expression of inflammatory cytokines,
inactivation of bradykinin C3a and C5a and increased high-mobility
group box-1 degradation (12,29).
Following a systematic literature search, six unique
studies evaluating the efficacy and safety of rhTM in AE-IPF were
identified. The dose of rhTM was the same across studies (0.06
mg/kg/day is equivalent to 380 U/kg/day) (30). All studies utilized a 6-day drug
protocol with the exception of one study (14), in which the drug was administered for
seven days. With the limited evidence, the difference between the
6- and 7-day rhTM protocol remains to be determined. After pooling
the data of 145 patients in the study group and 146 patients in the
control group, the present results indicated that administration of
rhTM decreased the 90-day mortality by 71% (CI, 51-83%) compared
with that in patients who were not treated with the drug.
Similarly, the 28-day mortality was reduced by 75% (CI, 23-92%)
with the administration of rhTM, albeit with a large CI, probably
due to the small number of studies with limited sample size
included in the present analysis. The results of the present study
are similar to a recently published meta-analysis of Kamiya et
al (31). In their analysis of
four studies, the authors noted a statistical significant
difference in 90 day mortality with the use of rhTM in AE-IPF.
However, due to fewer studies, no meta-analysis was conducted for
28 day mortality in their study.
The survival rate in the control arm of the present
analysis was similar to that in studies utilizing only
corticosteroid therapy for the management of AE-IPF (3,4). It is
important to note that to date, no randomized controlled trial
(RCT) has been performed and the present review is a pooled
analysis of non-randomized studies. The absence of randomization
potentially introduces bias due to the presence of confounding
factors. Mortality in AE-IPF may be dependent on several
confounding factors, including disease severity, oxygen partial
pressure/fraction of inspired oxygen ratio, D-dimer and KL-6
levels, high-resolution computed tomography (HRCT) pattern
(diffuse/non-diffuse) and use of concomitant therapies, including
anticoagulants and immunosuppressants (3,4). While
multivariate regression analysis performed in the clinical trials
by Arai et al (23) and
Sakamoto et al (22)
indicated that rhTM independently improved the 90-day survival in
patients with AE-IPF, the absence of robust RCTs diminishes the
overall level of evidence.
Data from the post-marketing surveillance of rhTM
therapy in patients with DIC demonstrated an incidence of all
adverse events of 7.1%, with bleeding and serious bleeding
complication rates of 5.5 and 6.8%, respectively (32). With similar rates of adverse events
in the study (8.8%) and control groups (10%), the present results
indicated rhTM to be safe in the management of AE-IPF. None of the
included studies reported any serious bleeding events during the
treatment with rhTM.
The following limitations should be considered when
interpreting the results of the present review. First, as mentioned
above, no RCTs were available for inclusion in the present review.
The majority of the included studies used a historical control
group for which the diagnosis and treatment protocols would have
differed. In spite of the authors reporting baseline similarity
amongst the study groups, certain confounding factors may have
introduced bias in the overall results. Furthermore, the inherent
limitations of included studies, e.g. methodological variation,
limited sample size and differences in the use of adjunctive
therapies, including anticoagulants, immunosuppressants,
antibiotics and high-flow oxygen, may have led to skewed results.
As another limitation, BAL and HRCT were not rigorously utilized
for diagnostic purposes in all studies included. In addition, the
difference in general supportive care amongst studies, as well as
between the experimental and control groups, may have influenced
the results. In addition, it was not possible to pool data other
than those of mortality and adverse events in the present
meta-analysis. The lack of evaluation of other clinical outcomes in
the included studies restricted the present meta-analysis to these
variables only. Finally, all studies were reported from a single
country. The influence of the region and ethnicity on clinical
outcomes requires to be considered.
In conclusion, bearing in mind the limitations our
systematic review and meta-analysis, our results indicated that
rhTM may improve the short-term mortality in patients with AE-IPF.
The drug appears to be safe without any enhanced risk of adverse
events. There is a requirement for high-quality RCTs with a large
sample size to provide robust evidence supporting its use.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BW conceived and designed the study. BW and TL
collected the data and performed the literature search. BW was
involved in the writing of the manuscript. All authors read and
approved the final manuscript.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Crystal RG, Bitterman PB, Rennard SI,
Hance AJ and Keogh BA: Interstitial lung diseases of unknown cause.
Disorders characterized by chronic inflammation of the lower
respiratory tract (first of two parts). N Engl J Med. 310:154–166.
1984.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kondoh Y, Taniguchi H, Kawabata Y, Yokoi
T, Suzuki K and Takagi K: Acute exacerbation in idiopathic
pulmonary fibrosis. Analysis of clinical and pathologic findings in
three cases. Chest. 103:1808–1812. 1993.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Song JW, Hong S-B, Lim C-M, Koh Y and Kim
DS: Acute exacerbation of idiopathic pulmonary fibrosis: Incidence,
risk factors and outcome. Eur Respir J. 37:356–363. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Agarwal R and Jindal SK: Acute
exacerbation of idiopathic pulmonary fibrosis: A systematic review.
Eur J Intern Med. 19:227–235. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Imokawa S, Sato A, Hayakawa H, Kotani M,
Urano T and Takada A: Tissue factor expression and fibrin
deposition in the lungs of patients with idiopathic pulmonary
fibrosis and systemic sclerosis. Am J Respir Crit Care Med.
156:631–636. 1997.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kotani I, Sato A, Hayakawa H, Urano T,
Takada Y and Takada A: Increased procoagulant and antifibrinolytic
activities in the lungs with idiopathic pulmonary fibrosis. Thromb
Res. 77:493–504. 1995.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kubo H, Nakayama K, Yanai M, Suzuki T,
Yamaya M, Watanabe M and Sasaki H: Anticoagulant therapy for
idiopathic pulmonary fibrosis. Chest. 128:1475–1482.
2005.PubMed/NCBI
|
|
8
|
Noth I, Anstrom KJ, Calvert SB, de Andrade
J, Flaherty KR, Glazer C, Kaner RJ and Olman MA: Idiopathic
Pulmonary Fibrosis Clinical Research Network (IPFnet): A
placebo-controlled randomized trial of warfarin in idiopathic
pulmonary fibrosis. Am J Respir Crit Care Med. 186:88–95.
2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sakamoto S, Homma S, Miyamoto A, Kurosaki
A, Fujii T and Yoshimura K: Cyclosporin A in the treatment of acute
exacerbation of idiopathic pulmonary fibrosis. Intern Med.
49:109–115. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ryerson CJ, Cottin V, Brown KK and Collard
HR: Acute exacerbation of idiopathic pulmonary fibrosis: Shifting
the paradigm. Eur Respir J. 46:512–520. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ogawa Y, Yamakawa K, Ogura H, Kiguchi T,
Mohri T, Nakamori Y, Kuwagata Y, Shimazu T, Hamasaki T and Fujimi
S: Recombinant human soluble thrombomodulin improves mortality and
respiratory dysfunction in patients with severe sepsis. J Trauma
Acute Care Surg. 72:1150–1157. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ito T, Thachil J, Asakura H, Levy JH and
Iba T: Thrombomodulin in disseminated intravascular coagulation and
other critical conditions-a multi-faceted anticoagulant protein
with therapeutic potential. Crit Care. 23(280)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kataoka K, Taniguchi H, Kondoh Y,
Nishiyama O, Kimura T, Matsuda T, Yokoyama T, Sakamoto K and Ando
M: Recombinant human thrombomodulin in acute exacerbation of
idiopathic pulmonary fibrosis. Chest. 148:436–443. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hayakawa S, Matsuzawa Y, Irie T, Rikitake
H, Okada N and Suzuki Y: Efficacy of recombinant human soluble
thrombomodulin for the treatment of acute exacerbation of
idiopathic pulmonary fibrosis: A single arm, non-randomized
prospective clinical trial. Multidiscip Respir Med.
11(38)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tsushima K, Yamaguchi K, Kono Y, Yokoyama
T, Kubo K, Matsumura T, Ichimura Y, Abe M, Terada J and Tatsumi K:
Thrombomodulin for acute exacerbations of idiopathic pulmonary
fibrosis: A proof of concept study. Pulm Pharmacol Ther.
29:233–240. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group: Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. PLoS Med.
6(e1000097)2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Higgins J, Thomas J, Chandler J, Cumpston
M, Li T, Page M and Welch V (eds): Cochrane handbook for systemic
reviews of interventions. 2nd edition. Cochrane, 2019. John Wiley
& Sons, Inc., Hoboken NJ. https://doi.org/10.1002/9781119536604.
|
|
18
|
Schardt C, Adams MB, Owens T, Keitz S and
Fontelo P: Utilization of the PICO framework to improve searching
PubMed for clinical questions. BMC Med Inform Decis Mak.
7(16)2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS,
Hahn S, Jang BH and Son HJ: Testing a tool for assessing the risk
of bias for nonrandomized studies showed moderate reliability and
promising validity. J Clin Epidemiol. 66:408–414. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Isshiki T, Sakamoto S, Kinoshita A, Sugino
K, Kurosaki A and Homma S: Recombinant human soluble thrombomodulin
treatment for acute exacerbation of idiopathic pulmonary fibrosis:
A retrospective study. Respiration. 89:201–207. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shimizu H, Sakamoto S, Isshiki T, Furuya
K, Kurosaki A and Homma S: Association of serum high-mobility group
box protein 1 level with outcomes of acute exacerbation of
idiopathic pulmonary fibrosis and fibrosing nonspecific
interstitial pneumonia. PLoS One. 13(e0196558)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sakamoto S, Shimizu H, Isshiki T, Sugino
K, Kurosaki A and Homma S: Recombinant human soluble thrombomodulin
for acute exacerbation of idiopathic pulmonary fibrosis: A
historically controlled study. Respir Investig. 56:136–143.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Arai T, Kida H, Ogata Y, Marumo S,
Matsuoka H, Gohma I, Yamamoto S, Mori M, Sugimoto C, Tachibana K,
et al: Osaka Acute Exacerbation of Interstitial Pneumonia Research
Group: Recombinant thrombomodulin for acute exacerbation in
idiopathic interstitial pneumonias. Respirology. 24:658–666.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Abe M, Tsushima K, Matsumura T, Ishiwata
T, Ichimura Y, Ikari J, Terada J, Tada Y, Sakao S, Tanabe N, et al:
Efficacy of thrombomodulin for acute exacerbation of idiopathic
pulmonary fibrosis and nonspecific interstitial pneumonia: A
nonrandomized prospective study. Drug Des Devel Ther. 9:5755–5762.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Raghu G, Rochwerg B, Zhang Y, Garcia CA,
Azuma A, Behr J, Brozek JL, Collard HR, Cunningham W, Homma S, et
al: American Thoracic Society; European Respiratory society;
Japanese Respiratory Society; Latin American Thoracic Association:
An official ATS/ERS/JRS/ALAT clinical practice guideline: Treatment
of idiopathic pulmonary fibrosis. An update of the 2011 clinical
practice guideline. Am J Respir Crit Care Med. 192:3–19.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Horita N, Akahane M, Okada Y, Kobayashi Y,
Arai T, Amano I, Takezawa T, To M and To Y: Tacrolimus and steroid
treatment for acute exacerbation of idiopathic pulmonary fibrosis.
Intern Med. 50:189–195. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cuerpo S, Moisés J, Hernández-González F,
Benegas M, Ramirez J, Sánchez M, Agustí À and Sellares J: Acute
exacerbations of idiopathic pulmonary fibrosis: Does clinical
stratification or steroid treatment matter? Chron Respir Dis.
16(1479973119869334)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Collard HR, Calfee CS, Wolters PJ, Song
JW, Hong SB, Brady S, Ishizaka A, Jones KD, King TE Jr, Matthay MA,
et al: Plasma biomarker profiles in acute exacerbation of
idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol.
299:3–7. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Okamoto T, Tanigami H, Suzuki K and
Shimaoka M: Thrombomodulin: A bifunctional modulator of
inflammation and coagulation in sepsis. Crit Care Res Pract.
2012(614545)2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hayakawa M, Kushimoto S, Watanabe E, Goto
K, Suzuki Y, Kotani T, Kiguchi T, Yatabe T, Tagawa J, Komatsu F, et
al: Pharmacokinetics of recombinant human soluble thrombomodulin in
disseminated intravascular coagulation patients with acute renal
dysfunction. Thromb Haemost. 117:851–859. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kamiya H and Panlaqui OM: The efficacy of
recombinant human soluble thrombomodulin (rhsTM) treatment for
acute exacerbation of idiopathic pulmonary fibrosis: A systematic
review and meta-analysis. BMC Pulm Med. 20(57)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Eguchi Y, Gando S, Ishikura H, Saitoh D,
Mimuro J, Takahashi H, Kitajima I, Tsuji H, Matsushita T, Tsujita
R, et al: Post-marketing surveillance data of thrombomodulin alfa:
Sub-analysis in patients with sepsis-induced disseminated
intravascular coagulation. J Intensive Care. 2(30)2014.PubMed/NCBI View Article : Google Scholar
|