Introduction
Idiopathic inflammatory myopathies (IIMs) are a
group of inflammatory muscle diseases that share similar clinical
presentations, such as muscle weakness (1-5).
IIMs include dermatomyositis (DM), polymyositis (PM) and inclusion
body myositis (IBM). The pathogenesis of the individual conditions
may differ. However, inflammatory cell infiltration into skeletal
muscles is common across these disorders. In patients with IIMs,
helper T cells (CD4 T cells) and cytotoxic T cells (CD8 T cells)
have been shown to invade muscle fibers (6). Autoantibodies in the endomysium,
perimysium, perivascular areas and blood have also been reported
(7,8).
Despite years of research, the underlying mechanisms
of IIMs are not fully understood. As a result, there are no
targeted immunotherapies for these disorders. Currently, patients
with IIMs are treated with immune-suppressive corticosteroids, such
as short-term methylprednisolone (MP) or long-term prednisone.
However, treatment with corticosteroids is not ideal, since
long-term use of prednisone can result in severe loss of muscle
strength, which is contradictory to the treatment goal (1). Immunoglobulin infusion is another
treatment option, but it is costly and is typically reserved for
patients who are refractory to corticosteroids or have trouble
swallowing, and for patients for whom immunosuppressants are
contraindicated due to comorbid conditions. Thus, immunomodulatory
therapies become of interest, since they can improve the conditions
of the disease and decrease the adverse effects associated with
corticosteroids (1,9,10).
Rapamycin inhibits immune cell proliferation,
thereby suppressing the immune response, and has been shown to be
effective in treating various inflammatory conditions (11-17).
Rapamycin possibly suppresses the immune response by increasing the
frequency of regulatory T (Treg) cells, a subset of T cells that
modulates the immune system and has been postulated to prevent
autoimmune disorders. Depletion of Treg cells leads to excessive
proliferation of effecter T cells, which can lead to autoimmune
diseases such as polyendocrinopathy, enteropathy and X-linked
inheritance (18-20).
By contrast, increased numbers of Tregs and Treg activity can
protect individuals from autoimmune diseases (21).
In order to study the mechanisms underlying IIMs and
to test treatment efficacy, a murine model of experimental
autoimmune myositis (EAM) was developed. EAM shares disease
characteristics with PM, such as T cell infiltration of muscle
fibers (2,6). Allenbach et al (22) reported that the depletion of Treg
cells aggravated EAM, whereas polyclonal Treg injection improved
the condition. Furthermore, Prevel et al (23) found that rapamycin increased Treg
cell frequency and showed beneficial effects in treating EAM.
Therefore, rapamycin is a potential treatment option for myositis
because it suppresses the immune system by increasing Treg
frequency.
To date, no direct comparison between rapamycin and
steroids for treating myositis has been made, to the best of our
knowledge. In the present study a murine model of EAM was used to
compare the efficacy of rapamycin and MP, and to further
investigate the possible mechanism of action of rapamycin for
treating EAM.
Materials and methods
Animals
A total of 24 female BALB/c mice (weight 15-17 g;
age, 6-8 weeks) and 1 female guinea pig (weight, 480 g; age, 12
weeks) were purchased from the Experimental Animal Center of The
Fourth Military Medical University (Xi'an, China). All mice and
guinea pigs were housed in the specific pathogen-free facility of
the Experimental Animal Center at room temperature (22±1˚C) and
40-60% humidity with a 12/12 h light/dark cycle, and access to food
and water ad libitum. Mice were anesthetized via an
intraperitoneal injection of 10% chloral hydrate, at a dose of 3
ml/kg body weight (350 mg drug per kg body weight). Mice and guinea
pigs were euthanized using CO2 asphyxiation (the flow
rate of CO2 was about 10-30% of the chamber volume per
minute), followed by cervical dislocation. The animals did not
exhibit any evident signs of peritonitis following the
administration of 10% chloral hydrate.
EAM model
EAM was induced using a previously published
protocol (24). Briefly, skeletal
muscle from a guinea pig was dissected, weighed and frozen at
-70˚C. The frozen muscle tissue was subsequently thawed and minced.
The muscle tissue (10 g) was homogenized in 30 ml homogenizing
buffer (0.3 M KCl and 0.15 M sodium phosphate; pH 6.5) at 4˚C and
kept on ice for 1 h. The homogenate was subsequently centrifuged at
12,000 x g for 30 min at 4˚C, and the supernatants were collected
and filtered. The filtrates were diluted with cold Milli-Q-filtered
water (5X the volume of the filtrates), and the resultant solution
was then centrifuged at 7,000 x g at 4˚C for 30 min. The aggregated
myosin, the pellet obtained following centrifugation, was
resuspended in 0.5 M KCl and stored at -70˚C.
To induce EAM, mice were subcutaneously injected
with 100 µl of 50% complete Freund's adjuvant (Sigma-Aldrich; Merck
KGaA) containing 1.5 mg myosin and 5 mg/ml Mycobacterium
tuberculosis (BD Difco™ Adjuvants; BD Biosciences;
cat. no. DF3114-33-8) in the left hind limb. Booster shots were
administered at the tail base once a week for 2 weeks. Immediately
after each booster shot, the mice were intraperitoneally injected
with pertussis toxin (500 ng in 200 µl saline; Sigma-Aldrich; Merck
KGaA). The myosin solutions were freshly prepared or stored at
-70˚C for <1 month
A total of 10 days following the last injection of
myosin, rapamycin (1.5 mg/kg body weight) or MP (40 mg/kg body
weight) was administered intraperitoneally into the mice daily for
14 days. Mice in the placebo group received equal volumes of ~3%
DMSO (diluted in saline) via intraperitoneal injection. The mice
were assessed for muscle strength on day 15, following drug
treatment, and tissues were then collected for subsequent
analysis.
Inverted screen test
Muscle strength was assessed using the inverted
screen test, as previously described (21,24,25).
Briefly, the mice were placed at the center of a circular wire mesh
screen (50 cm2), composed of 1 mm diameter wire. The
screen was immediately rotated to the inverted horizontal position
for 3 sec, with the head of the mouse declining first. The screen
was then held steadily 20 cm above a padded surface. The time for
the mouse to fall from the mesh was recorded. Each mouse was
assessed five consecutive times.
Histological grading of
inflammation
Muscle tissue sections (10 µm thick) were randomly
selected from each block and stained with hematoxylin and eosin.
Briefly, samples were fixed with 10% neutral buffered formalin
overnight at room temperature and embedded in paraffin. The
paraffin sections, with a thickness of 10 µm, were deparaffinized
and rehydrated with xylene and ethanol in gradient concentrations
and stained with hematoxylin for 10 min at room temperature. After
destaining with 10% acid ethanol for 5-10 sec and washing with
water, the slides were stained with eosin for 30 sec at room
temperature and subjected to dehydration with ethanol and xylene.
The slides were mounted with Permount™ mounting medium
(Thermo Fisher Scientific, Inc.) and observed under a confocal
microscope (LeicaDRM; Leica Microsystems Inc.) with 10x, 20x and
40x magnification. Inflammation in six muscle tissue sections was
graded and expressed as a mean score (26-28),
which was defined as follows: Grade 1, fewer than five muscle
fibers involved; grade 2, a lesion involving 5-30 muscle fibers;
grade 3, a lesion involving a muscle fasciculus; and grade 4,
diffuse extensive lesions. When multiple lesions were found in one
section of muscle, 0.5 was added to the score. Five random fields
of view per sample were assessed for inflammation scoring in a
blinded manner by two independent trained pathologists.
Luminex assay
Plasma transforming growth factor-β (TGF-β) and
interleukin 10 (IL-10) levels were measured using the Luminex
assay. Briefly, blood samples from mice were collected via the
retro-orbital plexus under general anesthesia with 10% chloral
hydrate (350 mg/kg), as described above, placed at room temperature
for 30 min, and then plasma was sampled after centrifugation at
10,000 g/min at 4˚C for 10 min. The Luminex multiplex assays (cat.
no. TGFB-64K-01 for the detection of TGF-β and cat. no.
MPXMCYTO-70K for the detection of IL-10; EMD Millipore) were
performed according to the manufacturer's protocols. Samples were
assessed in duplicate with the Luminex 200 IS System (Luminex
Corporation). TGF-β and IL-10 were identified and classified with the
red laser, and their protein levels were quantified using the green
laser. Following laser excitation, digital images of the bead array
were captured and processed on a computer workstation. Standard
curves and reports of unknown samples were prepared using the
BeadView and MiraiBio software (Vigene Tech, Inc; version 3.1). The
limits of sensitivity for the assay were 9.8 pg/ml for TGF-β and
1.25 pg/ml for IL-10. All mice had detectable levels of these
cytokines.
Flow cytometry
The composition of splenocytes was analyzed using
flow cytometry. Fresh spleens were ground with the plunger of a 5
ml sterile syringe in PBS at 4˚C, and the homogenate was then
passed through a nylon mesh screen. The lymphocytes were separated
in EZ-Sep™ Mouse 1X lymphocyte separation medium (Dakewe Biotech
Co., Ltd.) and resuspended in flow cytometry staining buffer (PBS
supplemented with 2% FBS; Thermo Fisher Scientific, Inc.).
Splenocyte cell viability, as detected by trypan blue staining, was
>95%.
Cells were washed once in flow cytometry staining
buffer, and then resuspended to a density of 1x107
cells/ml. For surface staining, cells were incubated with
FITC-labeled anti-mouse CD4 (clone RM4-5; cat. no. 11-0042-82;
Thermo Fisher Scientific, Inc.) and allophycocyanin-labeled
anti-mouse CD25 (clone PC61.5; cat. no. 17-0251-82; Thermo Fisher
Scientific, Inc.) for ~30 min at 4˚C. Appropriate isotype control
antibodies (cat. no. 11-4321-80 and cat. no. 17-4301-82; Thermo
Fisher Scientific, Inc.) were used to exclude nonspecific binding
after washing, fixation and permeabilization of the cells. Cell
fixation and permeabilization were conducted at 4˚C for 2 h with
the eBioscience™ Mouse Regulatory T Cell Staining Kit #2
(cat. no. 88-8118-40; Thermo Fisher Scientific, Inc.) following the
manufacturer's instructions. Subsequently, the cells were stained
intracellularly with phycoerythrin-labeled anti-mouse/rat Foxp3
(FJK16-second; eBioscience; Thermo Fisher Scientific, Inc.),
following the manufacturer's instructions. The cells were then
applied to a FACScan cytometer equipped with the CellQuest software
(version 3.0, BD Biosciences) for analysis.
Statistical analysis
All data are presented as the mean ± SD (from at
least 3 repeats), unless otherwise noted. One-way analysis of
variance followed by the post hoc least significant difference test
was used to assess inter-group differences. P<0.05 was
considered to indicate a statistically significant difference. All
analyses were performed using SPSS 16.0. software (SPSS, Inc.).
Results
General body condition assessment
A murine model of EAM was first established, which
was induced by injections with myosin. All myosin-injected mice
developed dull and untidy fur, lost weight, and became less active.
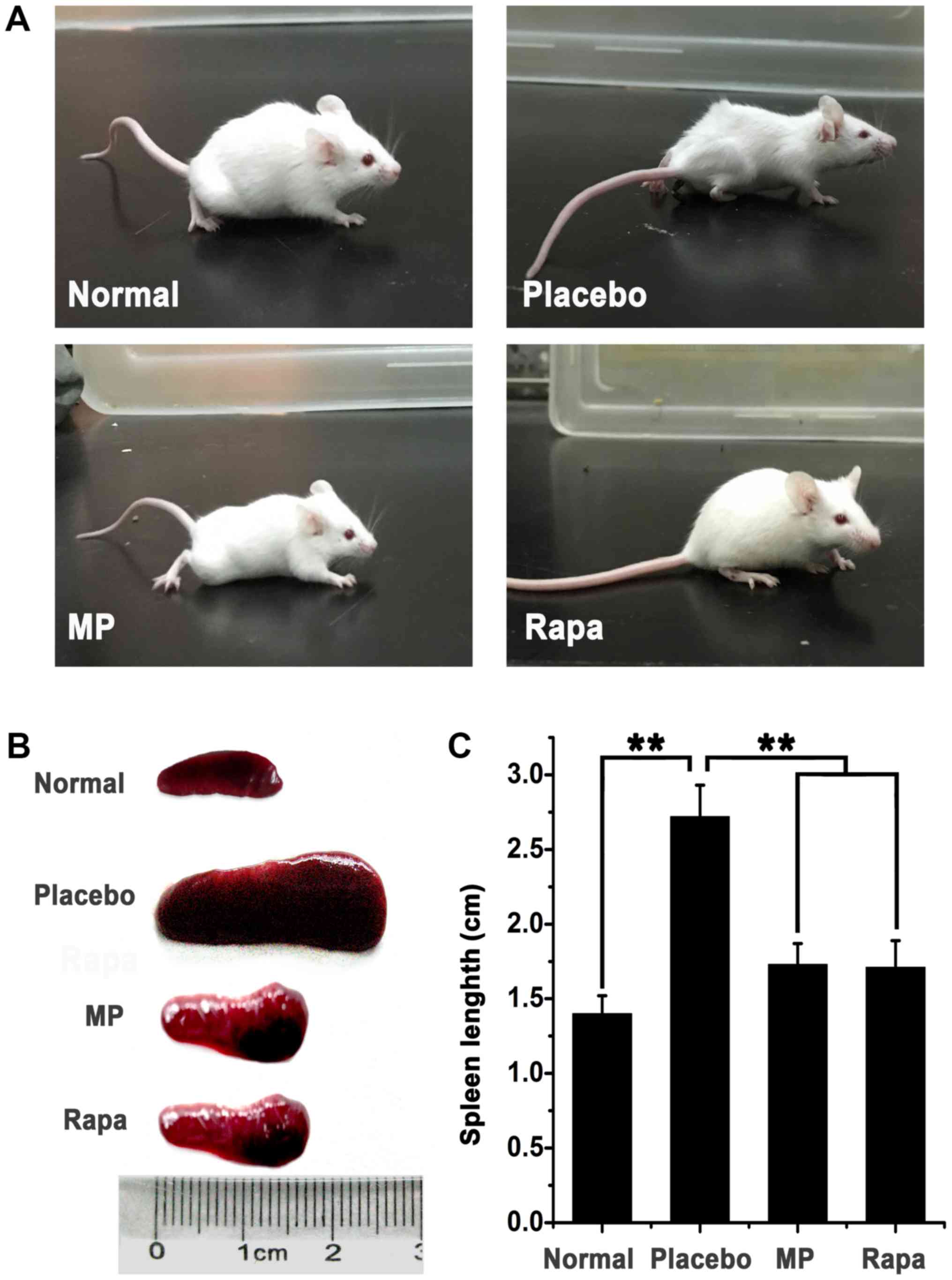
Moreover, skin ulcers were found at the injection sites (Fig. 1A). The spleens of all myosin-injected
animals were enlarged compared with the placebo animals (mean
spleen length, 1.40±0.12 cm), which indicated that inflammation was
induced upon myosin injection. Subsequent treatment with either MP
or rapamycin improved the general body condition of the animals and
resulted in less marked enlargement of the spleen compared with the
placebo group (Fig. 1B). The mean
spleen length was 2.72±0.21 cm in the placebo group compared with
1.73±0.14 cm in the MP-treated group and 1.71±0.18 in the
rapamycin-treated group. Spleens in the drug-treated groups were
significantly smaller compared with those in the placebo group
(P<0.01, treatment group vs. placebo group), suggesting that
drug treatments effectively ameliorated inflammation in mice.
Spleen sizes between the rapamycin-treated mice and MP-treated mice
did not differ significantly (P>0.05; Fig. 1C).
Histological grading of muscle
inflammation
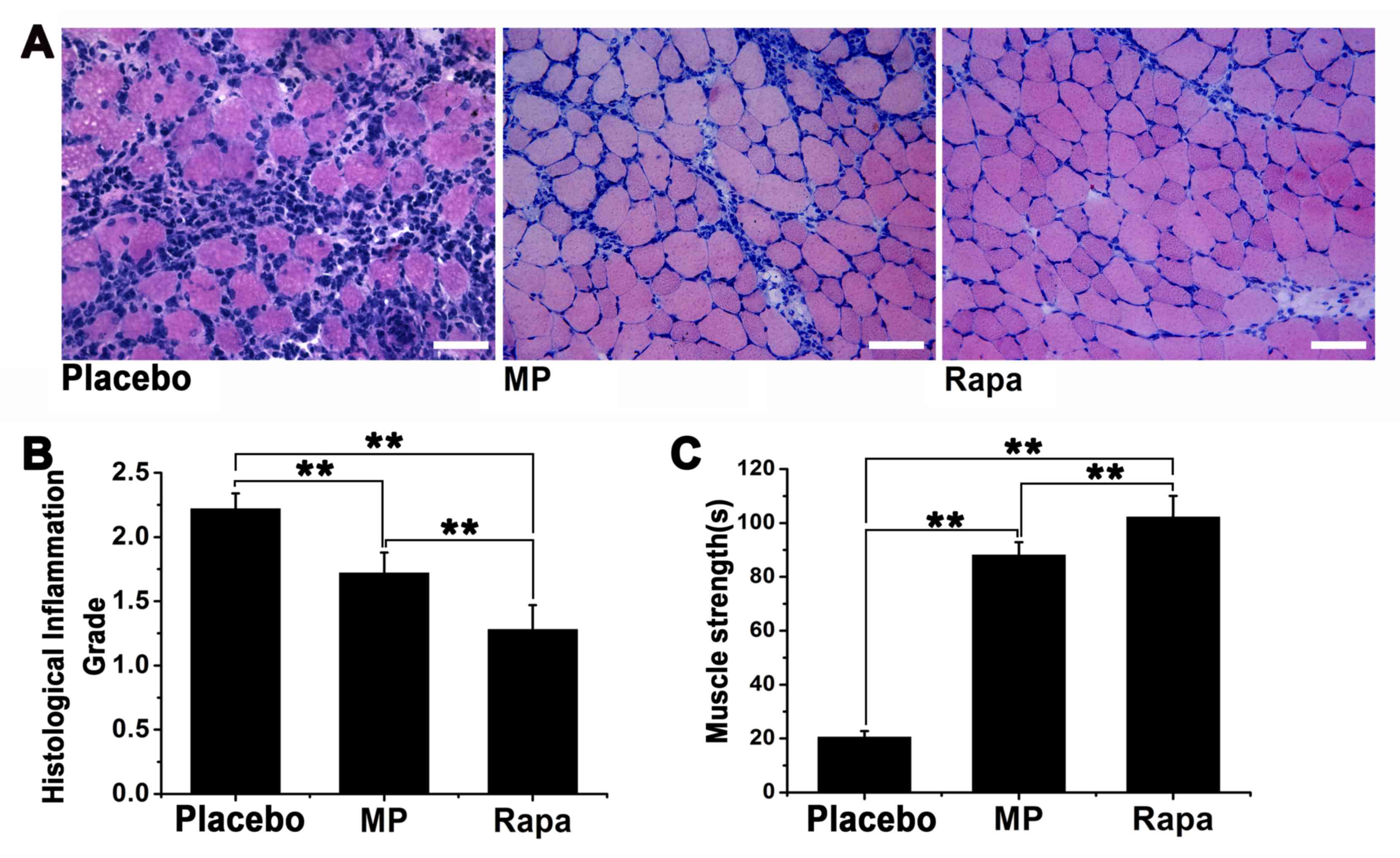
Muscle inflammation was scored using a 1-4 grading
system, with the score of 1 being the least severe (Fig. 2A-C). The inflammation score was
2.22±0.12 for the placebo group, 1.72±0.16 for the MP-treated group
(n=6; P<0.01 vs. placebo group) and 1.28±0.19 for the
rapamycin-treated group (n=6; P<0.01 vs. placebo group)
(Fig. 2D). Furthermore, the
inflammation score of the rapamycin-treated group was significantly
lower compared with that of the MP-treated group (P<0.01).
Muscle strength was assessed by measuring the
time-to-fall in the inverted screen test for each mouse. Our
preliminary experiments indicated that under normal circumstances,
a healthy BALB/c mouse (weight 15-17 g, age 6-8 weeks) can stay on
the inverted screen for >30 min before falling (data not shown).
By contrast, the average time-to-fall was 20.50±2.27 sec for the
placebo group, 88.13±4.77 sec for the MP-treated group (P<0.01
vs. placebo group), and 102.20±7.83 sec for the rapamycin-treated
group (P<0.01 vs. placebo group). The time-to-fall in the
rapamycin-treated group was significantly longer compared with that
in the MP-treated group (P<0.01; Fig.
2E).
Assessment of plasma TGF-β
and IL-10 levels
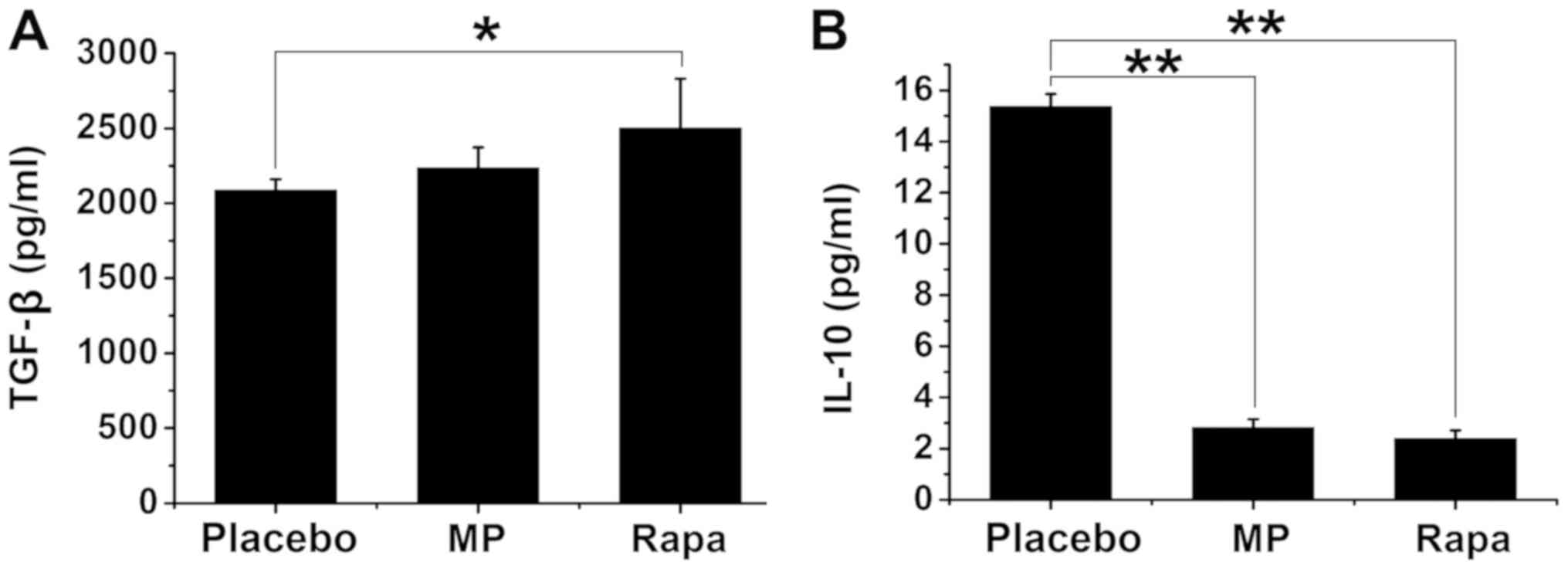
To investigate the mechanisms by which rapamycin
suppresses the immune response, the plasma levels of TGF-β and
IL-10 were subsequently measured, both of which are known to
suppress immune responses (29). The
plasma TGF-β concentration was 2,087.00±74.27 pg/ml in the placebo
group, 2,238.50±134.29 pg/ml in the MP-treated group, and
2,501.75±329.11 pg/ml in the rapamycin-treated group (P<0.05 vs.
placebo group; Fig. 3A). No
significant differences were found between the placebo and
MP-treated groups or between the MP- and rapamycin-treated groups
(P>0.05; Fig. 3A). The plasma
IL-10 concentration was 15.36±0.50 pg/ml in the placebo group,
2.82±0.33 pg/ml in the MP-treated group (P<0.01 vs. placebo
group), and 2.39±0.32 pg/ml in the rapamycin-treated group
(P<0.01 vs. placebo group) (Fig.
3B). The plasma IL-10 levels were not significantly different
between the MP-treated and the rapamycin-treated groups (P>0.05;
Fig. 3B).
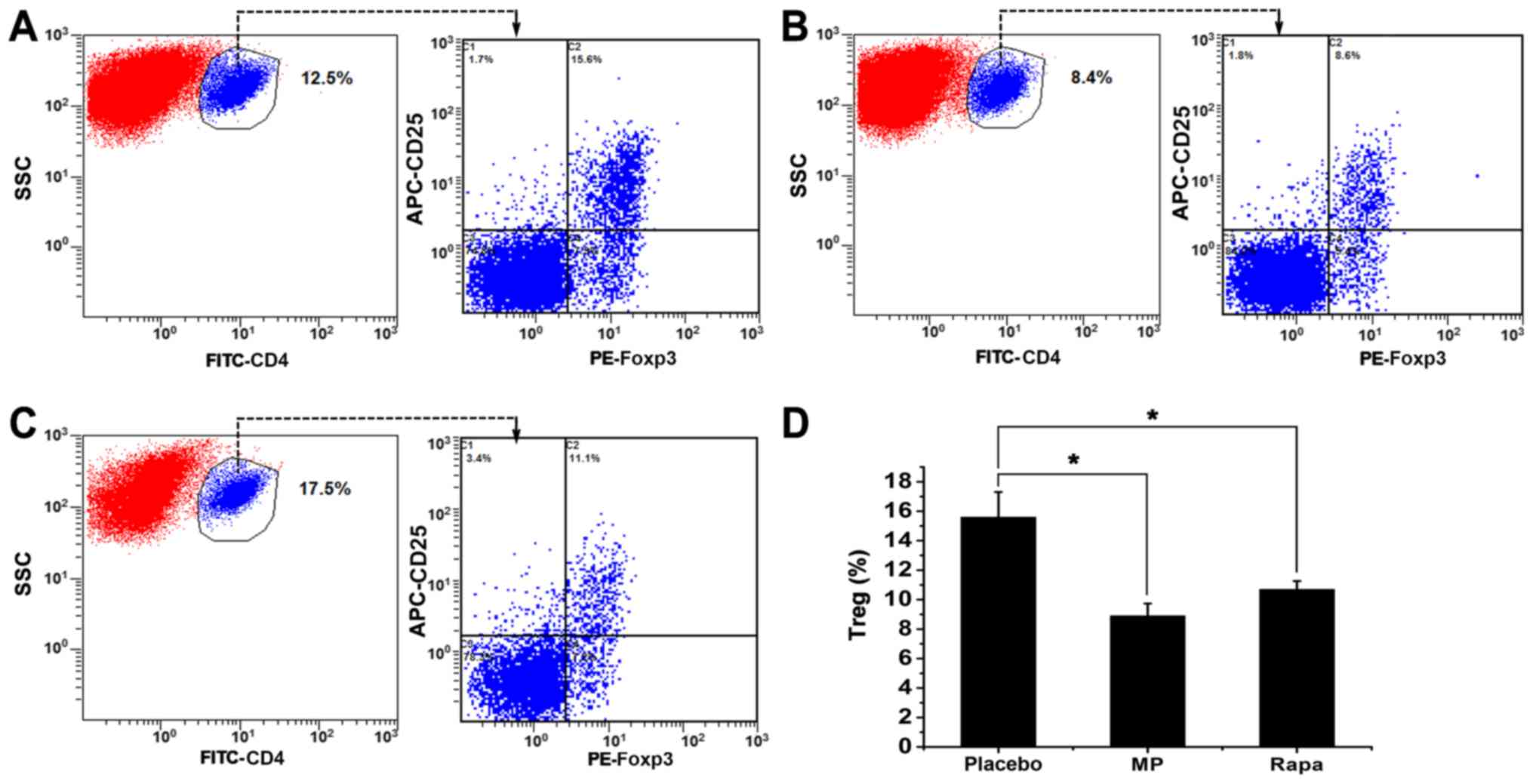
Treg cell frequency in the spleen
Finally, the frequency of splenic Treg cells was
investigated in the three groups. Splenocytes were analyzed using
flow cytometry, and the percentages of Treg cells
(Foxp3+ cells), calculated as the percentage of
CD4+ CD25+ Foxp3+ cells out of
CD4+ cells, were measured in all three groups (Fig. 4A-C). The percentage of splenic Treg
cells was 15.6±1.71% in the placebo group, 8.9±0.83% in the
MP-treated group (P<0.05 vs. placebo group), and 10.7±0.56% in
the rapamycin-treated group (P<0.05 vs. placebo group) (Fig. 4D). All three groups had significantly
higher splenic Treg cell ratios compared with the normal mice
(4.2%; data not shown).
Discussion
In a previous study, a murine model of EAM was
established to investigate the pathogenesis of autoimmune myositis
(24). The present study further
confirmed the validity of this model. Mice that underwent the EAM
induction protocol exhibited symptoms that were typical of
myositis, including a deteriorated body condition, muscle weakness,
and inflammatory lesions in the muscles. Subsequently, the efficacy
of MP and rapamycin for treating EAM was assessed, which
demonstrated that treatment with rapamycin was significantly more
beneficial compared with treatment with MP, in terms of improving
the general body condition, muscle strength and alleviating
inflammation. Since rapamycin is known to suppress the immune
response by inhibiting mTOR, the effects of rapamycin on two key
immune suppressive molecules, TGF-β and IL-10, were compared with
those of MP. The rapamycin-treated mice exhibited a significant
increase in plasma TGF-β levels, but not plasma IL-10 levels.
Moreover, treatment with either MP or rapamycin was not associated
with an increase in the proportion of splenic Treg cells. These
results suggested that rapamycin possibly exerts its
anti-inflammatory effects via the TGF-β signaling pathway.
Previous studies on the role of rapamycin in
regulating TGF-β expression have been inconsistent. Li et al
(13) reported that rapamycin
decreased the expression of TGF-β in mononuclear cells in an
autoimmune hepatitis model. Furthermore, in a rat model of
unilateral ureteral obstruction, rapamycin was reported to decrease
both the protein and mRNA expression of TGF-β (14). In contrast, Yamane et al
(30) reported that rapamycin
promoted TGF-β signaling and thereby increased ceramide synthesis
in keratinocytes. In the present EAM model, treatment with
rapamycin induced a moderate but significant increase in the plasma
TGF-β level. Moreover, rapamycin ameliorated inflammation in TGF-β
knockout mice (31), indicating a
TGF-β-independent mechanism of immunoregulation by rapamycin. In
summary, it is likely that the modulation of the inflammatory
response and TGF-β signaling by rapamycin are context- and
disease-dependent.
In the present study, it was found that the
proportion of splenic Treg cells among total CD4+ T
cells in normal mice was slightly lower than the reported normal
range of 5-10% (32,33). Treg cells are known to counteract T
cells that are responsible for muscle degradation (34). In a previous study on a murine model
of EAM, the depletion of Treg cells was associated with more severe
myositis, whereas the injection of expanded polyclonal Treg cells
improved myositis (22). In
addition, Treg cell frequency was inversely correlated with disease
(35). Thus, the finding that the
proportion of Treg cells was the highest in the placebo group was
unexpected and inconsistent with a previous study, which found that
rapamycin increased Treg frequency (23). Hence, as the placebo group of animals
exhibited the most severe inflammation, it is possible that the
higher ratio of Treg cells might represent elevated disease
severity. Additionally, since the immune response is a complex
process, multiple signaling pathways are often activated
concurrently in response to the same stimuli (36). Furthermore, individual immune systems
respond to the same immune insults differently, which adds another
layer of complexity to the study of immune regulation. Therefore,
further studies are necessary to fully understand the regulation
and function of Treg cells in myositis.
The mTOR signaling pathway is a master regulator of
cell growth and metabolism, and this pathway is activated
downstream of the PI3K-AKT axis. The PI3K/AKT/mTOR pathway has been
implicated in autoimmune diseases and cancer, and patients with
these diseases can benefit from rapamycin treatment. For example,
in a preclinical Dark Agouti rat model of multiple sclerosis, oral
administration of rapamycin for 28 consecutive days significantly
ameliorated protracted relapsing experimental allergic
encephalomyelitis (11). Mammana
et al (37) also reported
that the PI3K/AKT/mTOR pathway was significantly involved in the
etiopathogenesis of a murine model of multiple sclerosis, and that
rapamycin treatment could be a potential therapeutic approach to
treating multiple sclerosis clinically. Furthermore, the
therapeutic potential of targeting the PI3K/AKT/mTOR pathway has
also been highlighted in multiple malignancies, such as T-cell
acute lymphoblastic leukemia (38).
Since upregulated PI3K/AKT/mTOR signaling could also occur in
inflammatory myopathies, a novel therapeutic approach consisting of
dual inhibitors of the PI3K/AkT/mTOR pathway might be more potent
than using rapamycin alone. For instance, NVP-BEZ235, an inhibitor
of both PI3K and mTOR complex 1/2, exhibited superior anti-cancer
activity in an orthotopic bladder cancer model (39) Recently, p70S6 kinase, a downstream
target of the PI3K/AKT/mTOR pathway, has gained attention for its
potential as a therapeutic target for treating autoimmune diseases
and cancer (40,41). In addition, a class of nitric-oxide
derivatives of antiretroviral protease inhibitors, such as
Saquinavir-NO and Lopinavir-NO (42-44),
can specifically inhibit p70S6 kinase, and is now under further
investigation. Therefore, the results of the present study
substantiate the superior efficacy of rapamycin in the mouse model
of EAM, opening new avenues to study p70S kinase and its inhibitors
in inflammatory muscle diseases.
TGF-β is an anti-inflammatory cytokine and,
consistent with the results of the present study, increased
circulating levels of TGF-β have been reported in patients treated
with immunomodulatory drugs (15,45).
Nicoletti et al (45)
reported that TGF-β levels were elevated in patients with both
relapsing-remitting and chronic progressive multiple sclerosis, and
treatment with interferon-β augmented the increase in serum TGF-β
levels. Moreover, T cell cytokine profiling in patients with
multiple sclerosis treated with rapamycin demonstrated a
significant increase in serum TGF-β levels (15). Taken together, TGF-β induction might
be a pharmacological mode of action by which rapamycin and other
immunomodulators exert beneficial effects in inflammatory and
autoimmune diseases. However, in the present study, only the
impacts of rapamycin or MP on the plasma levels of TGF-β and IL-10
in EAM mice were investigated; it is possible that other
pro-inflammatory or anti-inflammatory cytokines might also be
modulated by rapamycin. For example, the pro-inflammatory cytokine
macrophage migration inhibitory factor (MIF) has been shown to
activate the mTOR and AMP-activated protein kinase (AMPK) pathways
in the pathogenesis of autoimmune diseases and cancer (46-49).
Whether rapamycin treatment can also decrease the production of MIF
to attenuate the activation of the PI3K/Akt/mTOR and AMPK pathways
remains to be investigated.
In conclusion, using the established model of EAM,
it was demonstrated that rapamycin had better efficacy than MP for
treating myositis. Thus, the administration of rapamycin is a
potential treatment option for patients with IIMs. However, since
this is a preliminary in vivo animal study, the molecular
signaling pathways involved in immunosuppression were not
specifically examined. Further studies will be directed towards
investigating the underlying molecular mechanisms of muscle
inflammation and immunosuppression mediated by rapamycin.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JK and HJ conceived and designed the experiments; DF
and FY performed the experiments; XT and WH analyzed the data; JK
wrote the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The experimental protocols were approved by the
Institutional Animal Care and Use Committee of The Fourth Military
Medical University. All animal procedures were in accordance with
the ethical standards and practice of The Fourth Military Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dalakas MC: Therapeutic advances and
future prospects in immune-mediated inflammatory myopathies. Ther
Adv Neurol Disord. 1:157–166. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dalakas MC and Hohlfeld R: Polymyositis
and dermatomyositis. Lancet. 362:971–982. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hilton-Jones D: Inflammatory muscle
diseases. Curr Opin Neurol. 14:591–596. 2001.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mastaglia FL and Phillips BA: Idiopathic
inflammatory myopathies: Epidemiology, classification, and
diagnostic criteria. Rheum Dis Clin North Am. 28:723–741.
2002.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zeng L, Maruyama S, Nakamura K,
Parker-Duffen JL, Adham IM, Zhong X, Lee HK, Querfurth H and Walsh
K: The injury-induced myokine insulin-like 6 is protective in
experimental autoimmune myositis. Skelet Muscle.
4(16)2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kojima T, Tanuma N, Aikawa Y, Shin T,
Sasaki A and Matsumoto Y: Myosin-Induced autoimmune polymyositis in
the rat. J Neurol Sci. 151:141–148. 1997.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gunawardena H: The clinical features of
myositis-associated autoantibodies: A review. Clin Rev Allergy
Immunol. 52:45–57. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mozaffar T and Pestronk A: Myopathy with
anti-Jo-1 antibodies: Pathology in perimysium and neighbouring
muscle fibres. J Neurol Neurosurg Psychiatry. 68:472–478.
2000.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gelardi C, Paolini L and Danieli MG:
Subcutaneous immunoglobulin G in idiopathic inflammatory
myopathies: Therapeutic implications. Isr Med Assoc J. 16:646–647.
2014.PubMed/NCBI
|
|
10
|
Tournadre A: Therapeutic strategy in
inflammatory myopathies (polymyositis, dermatomyositis, overlap
myositis, and immune-mediated necrotizing myopathy). Rev Med
Interne. 35:466–471. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Donia M, Mangano K, Amoroso A, Mazzarino
MC, Imbesi R, Castrogiovanni P, Coco M, Meroni P and Nicoletti F:
Treatment with rapamycin ameliorates clinical and histological
signs of protracted relapsing experimental allergic
encephalomyelitis in dark agouti rats and induces expansion of
peripheral CD4+CD25+Foxp3+ regulatory T cells. J Autoimmun.
33:135–140. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gu L, Deng WS, Sun XF, Zhou H and Xu Q:
Rapamycin ameliorates CCl4-induced liver fibrosis in mice through
reciprocal regulation of the Th17/Treg cell balance. Mol Med Rep.
14:1153–1161. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li WW, Sun P, Chen DD, Wang WQ, Jiao GH,
Wang YJ, Zhou L, Wang BM and Zhang J: Preventive and therapeutic
effects of rapamycin against autoimmune hepatitis and liver
fibrosis and possible mechanisms. Zhonghua Gan Zang Bing Za Zhi.
24:368–374. 2016.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
14
|
Liu CF, Liu H, Fang Y, Jiang SH, Zhu JM
and Ding XQ: Rapamycin reduces renal hypoxia, interstitial
inflammation and fibrosis in a rat model of unilateral ureteral
obstruction. Clin Invest Med. 37(E142)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Salehi M, Bagherpour B, Shayghannejad V,
Mohebi F and Jafari R: Th1, Th2 and Th17 cytokine profile in
patients with multiple sclerosis following treatment with
rapamycin. Iran J Immunol. 13:141–147. 2016.PubMed/NCBI
|
|
16
|
Wang B, Ding W, Zhang M, Li H and Gu Y:
Rapamycin attenuates aldosterone-induced tubulointerstitial
inflammation and fibrosis. Cell Physiol Biochem. 35:116–125.
2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang GY, Zhang Q, Yang Y, Chen WJ, Liu W,
Jiang N and Chen GH: Rapamycin combined with allogenic immature
dendritic cells selectively expands CD4+CD25+Foxp3+ regulatory T
cells in rats. Hepatobiliary Pancreat Dis Int. 11:203–208.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gambineri E, Torgerson TR and Ochs HD:
Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked
inheritance (IPEX), a syndrome of systemic autoimmunity caused by
mutations of FOXP3, a critical regulator of T-cell homeostasis.
Curr Opin Rheumatol. 15:430–435. 2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li X, Liang Y, LeBlanc M, Benner C and
Zheng Y: Function of a Foxp3 cis-element in protecting regulatory T
cell identity. Cell. 158:734–748. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shevach EM: Regulatory T cells in
autoimmmunity. Ann Rev Immunol. 18:423–449. 2000.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cohen JL, Trenado A, Vasey D, Klatzmann D
and Salomon BL: CD4(+)CD25(+) immunoregulatory T cells: New
therapeutics for graft-versus-host disease. J Exp Med. 196:401–406.
2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Allenbach Y, Solly S, Grégoire S, Dubourg
O, Salomon B, Butler-Browne G, Musset L, Herson S, Klatzmann D and
Benveniste O: Role of regulatory T cells in a new mouse model of
experimental autoimmune myositis. Am J Pathol. 174:989–998.
2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Prevel N, Allenbach Y, Klatzmann D,
Salomon B and Benveniste O: Beneficial role of rapamycin in
experimental autoimmune myositis. PLoS One.
8(e74450)2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kang J, Zhang HY, Feng GD, Feng DY and Jia
HG: Development of an improved animal model of experimental
autoimmune myositis. Int J Clin Exp Pathol. 8:14457–14464.
2015.PubMed/NCBI
|
|
25
|
Contet C, Rawlins JN and Deacon RM: A
comparison of 129S2/SvHsd and C57BL/6JOlaHsd mice on a test battery
assessing sensorimotor, affective and cognitive behaviours:
Implications for the study of genetically modified mice. Behav
Brain Res. 124:33–46. 2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kohyama K and Matsumoto Y: C-Protein in
the skeletal muscle induces severe autoimmune polymyositis in lewis
rats. J Neuroimmunol. 98:130–135. 1999.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Matsumoto Y, Kohyama K, Park IK, Nakajima
M and Hiraki K: Characterization of pathogenic T cells and
autoantibodies in C-protein-induced autoimmune polymyositis. J
Neuroimmunol. 190:90–100. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Okiyama N, Hasegawa H, Oida T, Hirata S,
Yokozeki H, Fujimoto M, Miyasaka N and Kohsaka H: Experimental
myositis inducible with transfer of dendritic cells presenting a
skeletal muscle C protein-derived CD8 epitope peptide. Int Immunol.
27:327–332. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Taylor A, Verhagen J, Blaser K, Akdis M
and Akdis CA: Mechanisms of immune suppression by interleukin-10
and transforming growth factor-beta: The role of T regulatory
cells. Immunology. 117:433–442. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yamane T, Muramatsu A, Yoshino S, Matsui
S, Shimura M, Tsujii Y, Iwatsuki K, Kobayashi-Hattori K and Oishi
Y: MTOR inhibition by rapamycin increases ceramide synthesis by
promoting transforming growth factor-β1/Smad signaling in the skin.
FEBS Open Bio. 6:317–325. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Borkowski TA, Letterio JJ, Farr AG and
Udey MC: A role for endogenous transforming growth factor beta 1 in
langerhans cell biology: The skin of transforming growth factor
beta 1 null mice is devoid of epidermal langerhans cells. J Exp
Med. 184:2417–2422. 1996.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Horwitz DA, Zheng SG and Gray JD: The role
of the combination of IL-2 and TGF-beta or IL-10 in the generation
and function of CD4+ CD25+ and CD8+ regulatory T cell subsets. J
Leukoc Biol. 74:471–478. 2003.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yagi H, Nomura T, Nakamura K, Yamazaki S,
Kitawaki T, Hori S, Maeda M, Onodera M, Uchiyama T, Fujii S and
Sakaguchi S: Crucial role of FOXP3 in the development and function
of human CD25+CD4+ regulatory T cells. Int Immunol. 16:1643–1656.
2004.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Waschbisch A, Schwab N, Ruck T, Stenner MP
and Wiendl H: FOXP3+ T regulatory cells in idiopathic inflammatory
myopathies. J Neuroimmunol. 225:137–142. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Banica L, Besliu A, Pistol G, Stavaru C,
Ionescu R, Forsea AM, Tanaseanu C, Dumitrache S, Otelea D, Tamsulea
I, et al: Quantification and molecular characterization of
regulatory T cells in connective tissue diseases. Autoimmunity.
42:41–49. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Mai J, Wang H and Yang XF: Th 17 cells
interplay with Foxp3+ Tregs in regulation of inflammation and
autoimmunity. Front Biosci (Landmark Ed). 15:986–1006.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
37
|
Mammana S, Bramanti P, Mazzon E, Cavalli
E, Basile MS, Fagone P, Petralia MC, McCubrey JA, Nicoletti F and
Mangano K: Preclinical evaluation of the PI3K/Akt/mTOR pathway in
animal models of multiple sclerosis. Oncotarget. 9:8263–8277.
2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Evangelisti C, Evangelisti C, Chiarini F,
Lonetti A, Buontempo F, Bressanin D, Cappellini A, Orsini E,
McCubrey JA and Martelli AM: Therapeutic potential of targeting
mTOR in T-cell acute lymphoblastic leukemia (review). Int J Oncol.
45:909–918. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Matsushima M, Kikuchi E, Matsumoto K,
Hattori S, Takeda T, Kosaka T, Miyajima A and Oya M: Intravesical
dual PI3K/mTOR complex 1/2 inhibitor NVP-BEZ235 therapy in an
orthotopic bladder cancer model. Int J Oncol. 47:377–383.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Pai C, Walsh CM and Fruman DA:
Context-specific function of S6K2 in th cell differentiation. J
Immunol. 197:3049–3058. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Babchia N, Calipel A, Mouriaux F, Faussat
AM and Mascarelli F: The PI3K/Akt and mTOR/P70S6K signaling
pathways in human uveal melanoma cells: Interaction with B-Raf/ERK.
Invest Ophthalmol Vis Sci. 51:421–429. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Paskas S, Mazzon E, Basile MS, Cavalli E,
Al-Abed Y, He M, Rakocevic S, Nicoletti F, Mijatovic S and
Maksimovic-Ivanic D: Lopinavir-NO, a nitric oxide-releasing HIV
protease inhibitor, suppresses the growth of melanoma cells in
vitro and in vivo. Invest New Drugs. 37:1014–1028. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Basile MS, Mazzon E, Krajnovic T, Draca D,
Cavalli E, Al-Abed Y, Bramanti P, Nicoletti F, Mijatovic S and
Maksimovic-Ivanic D: Anticancer and differentiation properties of
the nitric oxide derivative of lopinavir in human glioblastoma
cells. Molecules. 23(E2463)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Maksimovic-Ivanic D, Fagone P, McCubrey J,
Bendtzen K, Mijatovic S and Nicoletti F: HIV-Protease inhibitors
for the treatment of cancer: Repositioning HIV protease inhibitors
while developing more potent NO-hybridized derivatives? Int J
Cancer. 140:1713–1726. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Nicoletti F, Di Marco R, Patti F, Reggio
E, Nicoletti A, Zaccone P, Stivala F, Meroni PL and Reggio A: Blood
levels of transforming growth factor-beta 1 (TGF-beta1) are
elevated in both relapsing remitting and chronic progressive
multiple sclerosis (MS) patients and are further augmented by
treatment with interferon-beta 1b (IFN-beta1b). Clin Exp Immunol.
113:96–99. 1998.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Cui J, Zhang F, Wang Y, Liu J, Ming X, Hou
J, Lv B, Fang S and Yu B: Macrophage migration inhibitory factor
promotes cardiac stem cell proliferation and endothelial
differentiation through the activation of the PI3K/Akt/mTOR and
AMPK pathways. Int J Mol Med. 37:1299–1309. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Gunther S, Fagone P, Jalce G, Atanasov AG,
Guignabert C and Nicoletti F: Role of MIF and D-DT in
immune-inflammatory, autoimmune, and chronic respiratory diseases:
From pathogenic factors to therapeutic targets. Drug Discov Today.
24:428–439. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Richard V, Kindt N and Saussez S:
Macrophage migration inhibitory factor involvement in breast cancer
(Review). Int J Oncol. 47:1627–1633. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Mangano K, Mazzon E, Basile MS, Di Marco
R, Bramanti P, Mammana S, Petralia MC, Fagone P and Nicoletti F:
Pathogenic role for macrophage migration inhibitory factor in
glioblastoma and its targeting with specific inhibitors as novel
tailored therapeutic approach. Oncotarget. 9:17951–17970.
2018.PubMed/NCBI View Article : Google Scholar
|