Introduction
Inflammatory bowel diseases (IBDs) are chronic,
progressive immunologically-mediated diseases characterized by
chronic inflammation of the gastrointestinal tract in genetically
susceptible individuals exposed to environmental risk factors,
encompassing ulcerative colitis and Crohn's disease (1-3).
IBDs influence extensive populations and have been estimated to
affect 1.5 million Americans, 2.2 million people in Europe, and
several hundreds of thousands more worldwide, and these numbers
have been increasing with worsening of the environment and changing
habitats (3,4). Persistent, chronic inflammation of the
gastrointestinal tract is assumed to underlie the causes of
colitis-associated cancer (5),
fibrosis (6), heart diseases
(7) and even induces serious
behavioral symptoms reflecting the effect of colitis on the central
nerve system (8). The past decades
have witnessed notable progress in understanding the development of
IBDs, and various clinical trials aimed to interfere with IBDs have
been performed, but the therapy of IBDs still remains intractable
(9).
In the active state of IBDs, the intestinal
epithelial barrier breaks down and cells infiltrate into the lamina
propria. Cells from the innate immune system and adaptive immune
system, consisting of neutrophils, monocytes, macrophages and T
cells, release inflammatory cytokines (2,10) and
chemokines (11,12), such as interleukin 6 (IL-6), tumor
necrosis factor α (TNF-α), interleukin-1β (IL-1β), CXCL1 and
CXCL9(11). Persisting existence of
inflammatory cytokines and chemokines exacerbate the apoptosis of
epithelial cells leading to disruption of the epithelial barrier
(13,14). In accordance with the role of
inflammatory cytokines in the progression of IBDs, a great amount
of research and clinical trials have been conducted (15-17),
but the efficacy of anti-TNF-α was not promising and was even
accompanied with side effects, such as paradoxical psoriasis
(18). These challenges need to be
overcome.
Baicalin (5, 6,
7-trihydroxyflavone-7-b-D-glucuronide) is a major constituent
isolated from the herb Huangqin, found in the root of
Scutellaria baicalensis Georgi (19). Baicalin has been revealed to exhibit
many pharmacological activities, such as anti-inflammation
(20), antitumor (21), anti-apoptosis (22) and immunomodulation (23). It has been used to treat a series of
diseases, including acute myocardial infarction (24) and renal ischemia-reperfusion injury
(25). It has been revealed that
baicalin could ameliorate isoproterenol-induced acute myocardial
infarction through the p38/MAPK pathway (20), alleviate experimental colitis through
blockage of the TLR4/NF-κB pathway (26), and attenuate symptoms of experimental
autoimmune encephalomyelitis via activation of the SOCS3 pathway
(27), however, the molecular
mechanism of baicalin in treating IBDs still remains elusive.
In the present study, the underlying mechanisms of
baicalin in the treatment of IBDs were investigated in both an
animal model of 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced
rat colitis and an lipopolysaccharide (LPS)-induced HT-29 cell
inflammation model. In the present study, it was revealed that
baicalin reduced the levels of IL-1β, IL-6 and TNF-α and increased
the expression of IL-10, and ameliorated the apoptosis of
intestinal mucosal epithelial cells and promoted the expression of
tight-junction proteins in the PI3K/AKT-dependent pathway in
vivo and in vitro. Therefore, for the first time, our
results identified the mechanism by which baicalin affects the
development of colitis through the PI3K/AKT-dependent signaling
pathway.
Materials and methods
Chemicals and reagents
Baicalin (purity, 95%; CAS registry no. 21967-41-9;
molecular formula, C21H18O11; molecular weight, 446.36) and TNBS
were obtained from Sigma-Aldrich; Merck KGaA. LPS Escherichia
coli serotype 0111:B4 strain and LY294002 were purchased from
InvivoGen, Inc. Rabbit anti-phospho-AKT (1:1,000; cat. no. 4691),
anti-phospho-PI3K (1:1,000; cat. no. 17366), anti-total AKT
(1:1,000; cat. no. 9272), anti-total PI3K (1:1,000; cat. no. 4255),
anti-Bcl-2 (1:1,000; cat. no. 3498), anti-FasL (1:1,000; cat. no.
68405), anti-Bax (1:1,000; cat. no. 81740), anti-caspase-3
(1:1,000; cat. no. 14220), anti-caspase-9 (1:1,000; cat. no. 9508),
anti-ZO-1 (1:1,000; cat. no. 13663), anti-β-Catenin (1:1,000; cat.
no. 8480) and anti-GAPDH (1:1,000; cat. no. 5174) were purchased
from Cell Signaling Technology, Inc.
Animals
A total of 30 Sprague-Dawley rats (aged 6-8 weeks;
15 male and 15 female; weighing 200-250 g) were purchased from the
Model Animal Research Center of Nanjing University. All rats were
maintained under environmentally-controlled conditions (ambient
temperature, 22±2˚C; humidity 40%) in a pathogen-free facility with
a 12-h light/dark cycle, and had access to water and food ad
libitum.
All experimental procedures were performed in strict
accordance with the Institutional Animal Care and Use Committee of
Nanjing University of Chinese Medicine [approval no.
SYXK(Su)2014-0004].
Induction of colitis
Colitis was induced by intracolonic administration
of TNBS, as previously described (28). Briefly, rats that were fasted for 24
h with free access to water were lightly anesthetized with 350
mg/kg chloral hydrate, and a polyethylene catheter was inserted
rectally until the splenic flexure (6-8 cm from the anus). Then,
TNBS, dissolved in 50% ethanol for a dose of 100 mg/kg, 0.25 ml per
rat, was administered through the catheter. After removing the
catheter, the rats were held in a headfirst position for 60 sec to
prevent liquid outflow.
The rats were randomly divided into the following
six groups: Control group (rats received 0.9% saline.); model group
(TNBS-induced colitis without treatment, TNBS group); baicalin
group (TNBS-induced colitis treated with baicalin, 100 mg/kg/d, per
rat by gastric lavage); LY294002 group (TNBS-induced colitis
treated with LY294002, 50 µg/kg/d, per rat via intravenous (i.v.)
injection); IGF-1 group (TNBS-induced colitis treated with IGF-1,
1.5 µg/kg/d, per rat via intravenous (i.v.) injection); baicalin
and IGF-1 co-administration group (TNBS-induced colitis treated
with baicalin, 100 mg/kg/d, per rat by gastric lavage, and IGF-1,
1.5 µg/kg/d, per rat via intravenous (i.v.) injection). The animals
in each group were treated once a day for 14 days. In addition, the
defecation times and shape of the feces in each group were
observed. On the 15th day, the rats were sacrificed by cervical
dislocation under anesthesia with a solution of ketamine (100
mg/kg, i.p.) and xylazine (10 mg/kg, i.p.), and the entire colon
was removed from the cecum to the anus. The length and weight of
the colon were measured. Then, colonic specimens were fixed
immediately in a 10% (w/v) neutral formalin solution or frozen in
liquid nitrogen for further analyses.
TUNEL staining
TUNEL staining was performed to quantify cell
apoptosis using a TUNEL Detection kit, according to the
manufacturer's instructions. Briefly, sections were fixed with 4%
paraformaldehyde at 37˚C for 20 min, immersed in the 3%
H2O2 at 37˚C for 10 min, and dipped into 0.1%
TritonX-100 on ice for 2 min. FITC-labeled dUTP (50 µl) and
terminal deoxynucleotidyltransferase from the kit was added to each
section and then incubated at 37˚C for 60 min, followed by
incubation with 50 µl streptavidin-HRP from the kit at 37˚C for 30
min. After detection with the DAB kit for 10 min at 37˚C, sections
were sealed with neutral gum. A total of six images of randomly
selected visual fields were taken for each section with a
fluorescence microscope (Olympus Corporation) at x400
magnification. TUNEL-positive cells in the ipsilateral hippocampus
were observed and quantified with Image-Pro software (version
6.0.0.260). Brownish yellow particles in the nuclei of the cells in
the hippocampal sections indicated TUNEL-positive, apoptotic cells.
The apoptotic rate was calculated quantified with Image-Pro
software (version 6.0.0.260; Media Cybernetics, Inc.).
Histology and
immunohistochemistry
For hematoxylin and eosin (H&E) staining, colons
samples were fixed in 10% neutral formalin solution for 24 h,
dehydrated in increasing concentrations of ethanol, and embedded in
paraffin. Thereafter, sections of tissue were cut at a thickness of
3 µm. For immunohistochemical staining, paraffin-embedded colon
sections were deparaffinized, hydrated, and antigen-retrieved, and
endogenous peroxidase activity was quenched by 3%
H2O2 for 10 min. Sections were then blocked
with 5% bovine serum albumin for 20 min, followed by incubation
with anti-ZO-1 (cat. no. sc-33725; Santa Cruz Biotechnology, Inc.)
and anti-β-catenin (1:1,000; cat. no. 8480; Cell Signaling
Technology, Inc.) overnight at 4˚C and subsequently incubation with
a secondary antibody IgG (1:1,000; cat. no. 3900; Cell Signaling
Technology, Inc.) for 2 h. Positive cells were visualized by adding
DAB to the sections. Slides were viewed with a Nikon Eclipse 80i
microscope equipped with a digital camera (DS-Ri1; Nikon
Corporation).
Cell culture
The moderately differentiated human colon
adenocarcinoma cell line HT-29 was obtained from ATCC (HTB-38TM).
Cells were cultured in McCoy's 5A Medium supplemented with 10%
fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.),
100 U/ml penicillin G potassium, and 100 µg/ml streptomycin at 37˚C
in a humidified atmosphere with 5% CO2. Cells were
seeded on a six-well culture plate and were allowed to grow to
70-80% confluence in complete medium containing 10% FBS for 24 h,
and then the medium was changed to serum-free medium after washing
twice with serum-free medium. LPS (1 µg/ml) was used to stimulate
colonic adenocarcinoma HT-29 cells to induce inflammation.
Experimental cell groups
Growth-phase HT-29 cells were divided into six
groups: Normal control group (blank); model control group (LPS);
baicalin; LY294002; IGF-1 and baicalin + IGF-1 group. The blank
group was incubated in McCoy's 5A medium without FBS for 24 h; the
medium was changed to fresh serum-free medium after washing twice
with serum-free medium, and then LPS (1 mg/l) was added for 12 h.
The baicalin (316 µg/ml), LY29400 (0.158 µg/ml), IGF-1
(4.8x10-3 µg/ml) and baicalin (316 µg/ml) + IGF-1
(4.8x10-3 µg/ml) groups were based on the stated drug
interventions for 24 h.
Western blotting
After surgery, the rats were sacrificed by cervical
dislocation and the colons specimens were homogenized in
radioimmunoprecipitation assay lysis buffer (Sunshine Biotechnology
Co., Ltd.), then the supernatants were harvested by centrifugation
at 6,036 x g for 10 min at 4˚C. HT-29 cells were stimulated with
different concentrations of H2O2 (0, 100,
200, 300, 400, 500 and 1,000 µM) for 24 h, then washed twice with
PBS, and protein was extracted by adding radioimmunoprecipitation
assay lysis buffer on ice for 30 min, and centrifuging at 6,036 x g
for 10 min at 4˚C. Protein concentrations were determined using a
bicinchoninic acid kit (Beyotime Institute of Biotechnology).
Protein samples were homogenized with loading buffer and heated to
100˚C for 5 min, and 20 µg of each sample was then resolved by 10%
SDS-PAGE. Proteins were transferred to a polyvinylidene difluoride
membrane (EMD Millipore) and were probed with primary and secondary
anti-IgG (1:1,000; cat. no. 14708; Cell Signaling Technology,
Inc.). Immunoreactivity was visualized using an enhanced
chemiluminescence reaction kit (OriGene Technologies, Inc.) and
quantified using ImageJ 5.0 software (National Institutes of
Health).
Enzyme-linked immune absorbance assay
(ELISA)
The amount of TNF-α, IL-6, IL-1β and IL-10 in the
culture medium and in rat colon tissue extracts were measured with
a commercial ELISA kit (R&D Systems, Inc.) according to the
manufacturer's instructions.
Annexin V/PI binding assay
Briefly, HT-29 cells were seeded on 100-mm culture
dishes to 70-80% confluence in complete medium containing 10% FBS
for 24 h, and then changed to serum-free medium after washing twice
with serum-free medium. Cells were treated with
H2O2 (0, 100, 200, 300, 400, 500 and 1,000
µM) for 24 h, then harvested and washed twice, once in PBS and once
in binding buffer. An Annexin V-FITC Apoptosis Detection kit
(eBioscience, Inc.) was used to detect the translocation of
phosphatidylserine from inner membrane to the outer leaflet of the
plasma membrane. Cells were resuspended in Binding Buffer and the
concentration was adjusted to 106/ml. FITC-conjugated
Annexin V (5 µl) was added to 100 µl of the cell suspension. The
tubes were gently mixed and incubated for 15 min at room
temperature in the dark. The unconjugated Annexin V was removed by
a wash using binding buffer, and then 5 µl of PI was added to 200
µl of the binding buffer. Flow cytometric analysis was conducted
within 2 h (BD FACSCanto II; BD Biosciences), with the reagents
stored between 2 and 8˚C. Immediately after use, the remaining
reagents were returned to cold storage (2-8˚C).
Statistical analysis
All data examined are presented as the mean ± SEM.
Statistical analysis of the data was performed using SPSS software
(17.0 for Windows, IBM Inc.). Comparisons between two groups were
performed using Student's t-test. Comparisons among three or more
groups were performed using one-way ANOVA, followed by the Tukey
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Baicalin protects TNBS-induced colitis
in rats via inhibition of PI3K/AKT pathway activation
Sprague-Dawley rats (weighing 200-250 g) were
intracolonically administered TNBS to induce colitis, as previously
described (28). To investigate the
role of the PI3K/AKT pathway in the development of colitis, the
PI3K/AKT pathway was inhibited or activated by the use of LY294002
or IGF-1. The results of western blotting revealed that LY294002
could markedly inhibit the activation of the PI3K/AKT pathway, and
IGF-1 could activate the PI3K/AKT pathway by increasing the
expression of phosphorylated (p)-PI3K and p-AKT (Fig. 1A). H&E staining indicated that
severe mucosal injury was observed in TNBS-induced rats,
characterized by increased neutrophils, epithelial cell disruption,
massive bowel edema and distorted architecture of crypts, but the
PI3K inhibitor LY294002 could markedly ameliorate the morphological
change and histological damage of colon similar to baicalin
administration. Conversely, IGF-1 administration exacerbated
TNBS-induced colitis manifested by histological staining. However,
the accelerated colitis syndrome induced by the combination of TNBS
and IGF-1 could be suppressed by baicalin (Fig. 1B).
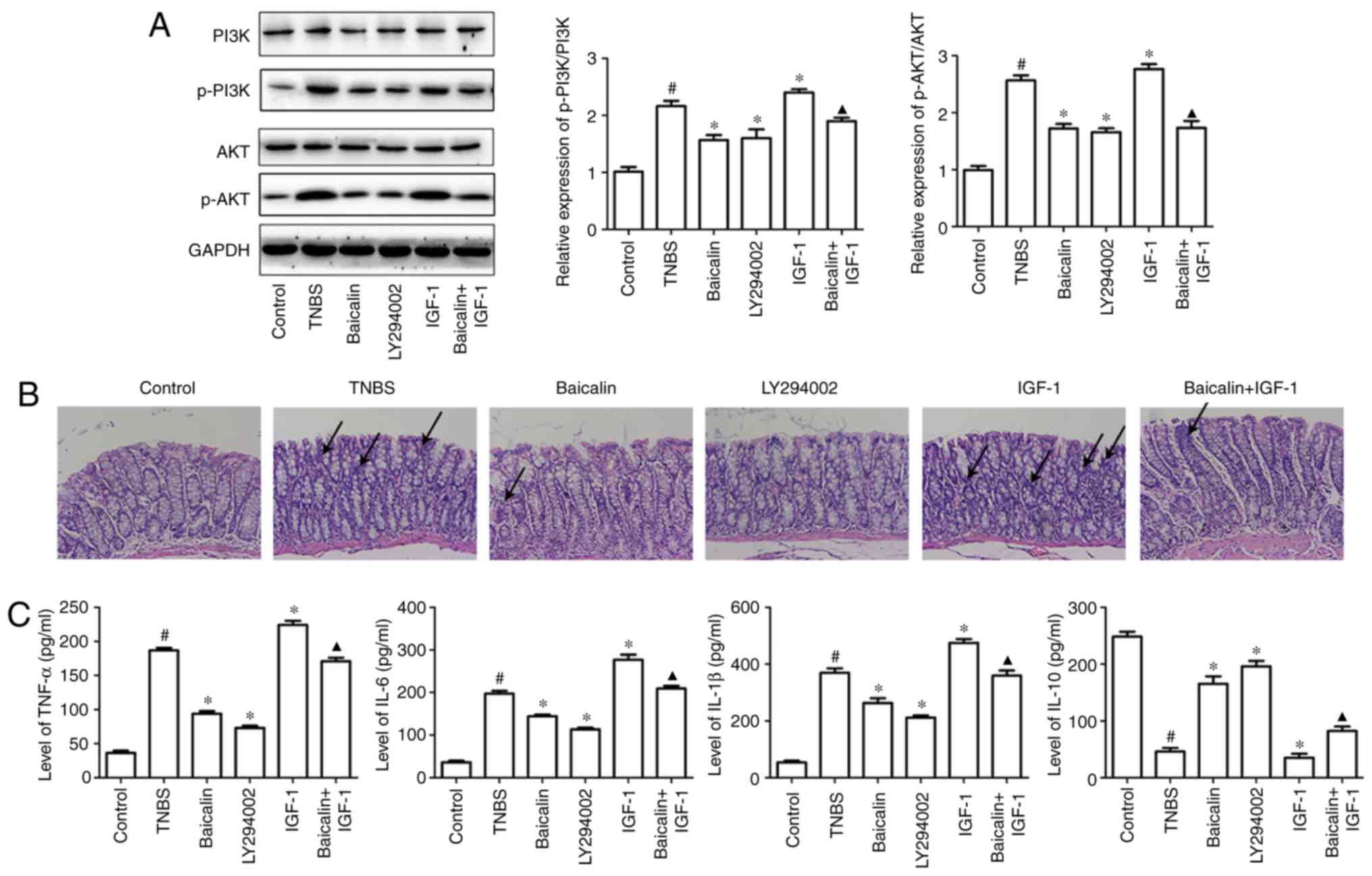 | Figure 1Baicalin protects TNBS-induced
colitis in rats by inhibiting PI3K pathway activation. Colitis was
induced by intracolonic administration of TNBS (100 mg/kg/d) for 14
days, then baicalin (100 mg/kg/d), LY294002 (50 µg/kg/d), IGF-1
(1.5 µg/kg/d) or IGF-1 along with baicalin were administrated for
14 days to investigate their role in TNBS-induced colitis. On the
15th day, the rats were sacrificed and colons were homogenized, and
20 µg of protein from lysates was analyzed for p-PI3K, p-AKT, PI3K
and AKT expression (A) by western blotting. The other colonic
specimens were fixed immediately in a 10% (w/v) neutral formalin
solution for H&E staining to observe the injury of the
intestine. (B) The colons of the mice in the control group
exhibited a normal structure without damage. However, in the TNBS
model group, the colons exhibited mucosal ulcerations and
inflammatory cell infiltration, however these alterations were
attenuated to varying degrees by baicalin and LY294002. In
addition, IGF significantly increased inflammatory cell
infiltration, however the effect could be reversed by baicalin, as
indicated by the arrows. (C) The supernatants of homogenized colons
were also used to perform ELISA to detect IL-6, TNF-α, IL-1β and
IL-10 production. The data are representative of at least three
independent experiments. Values are expressed as the mean ± SEM of
at least three independent experiments. #P<0.05 vs.
the control group; *P<0.05 vs. the TNBS group;
▲P<0.05 vs. the IGF-1 group. |
TNBS-induced colitis is characterized by the
breakdown of the intestinal epithelial barrier, infiltration of
both the innate and adaptive immune cells into the lamina propria,
and these immune cells synthesize and release a host of
proinflammatory and anti-inflammatory cytokines, including IL-6,
IL-1β and TNF-α. Furthermore, the levels of inflammatory cytokines
were detected in colitis tissues. As revealed in Fig. 1C, TNBS significantly increased the
expression of IL-6, TNF-α and IL-1β and inhibited the expression of
IL-10 in colon specimens, compared with the control group, however,
inhibition of the PI3K/AKT pathway using LY294002 predominantly
inhibited TNBS-induced inflammatory cytokine expression (IL-6,
TNF-α and IL-1β) and increased the expression of IL-10, compared
with the TNBS group. Furthermore, the efficacy of LY294002 in
suppressing inflammatory cytokine production was equivalent to
baicalin administration in the dosage of 100 mg/kg/d. By contrast,
IGF-1 efficiently increased the expression of IL-6, TNF-α and IL-1β
and inhibited the expression of IL-10, compared with the TNBS
group. However, this tendency could be rescued after baicalin
administration, compared with the IGF-1 group (Fig. 1C). These results demonstrated that
the PI3K/AKT pathway was involved in baicalin-alleviated colitis
response.
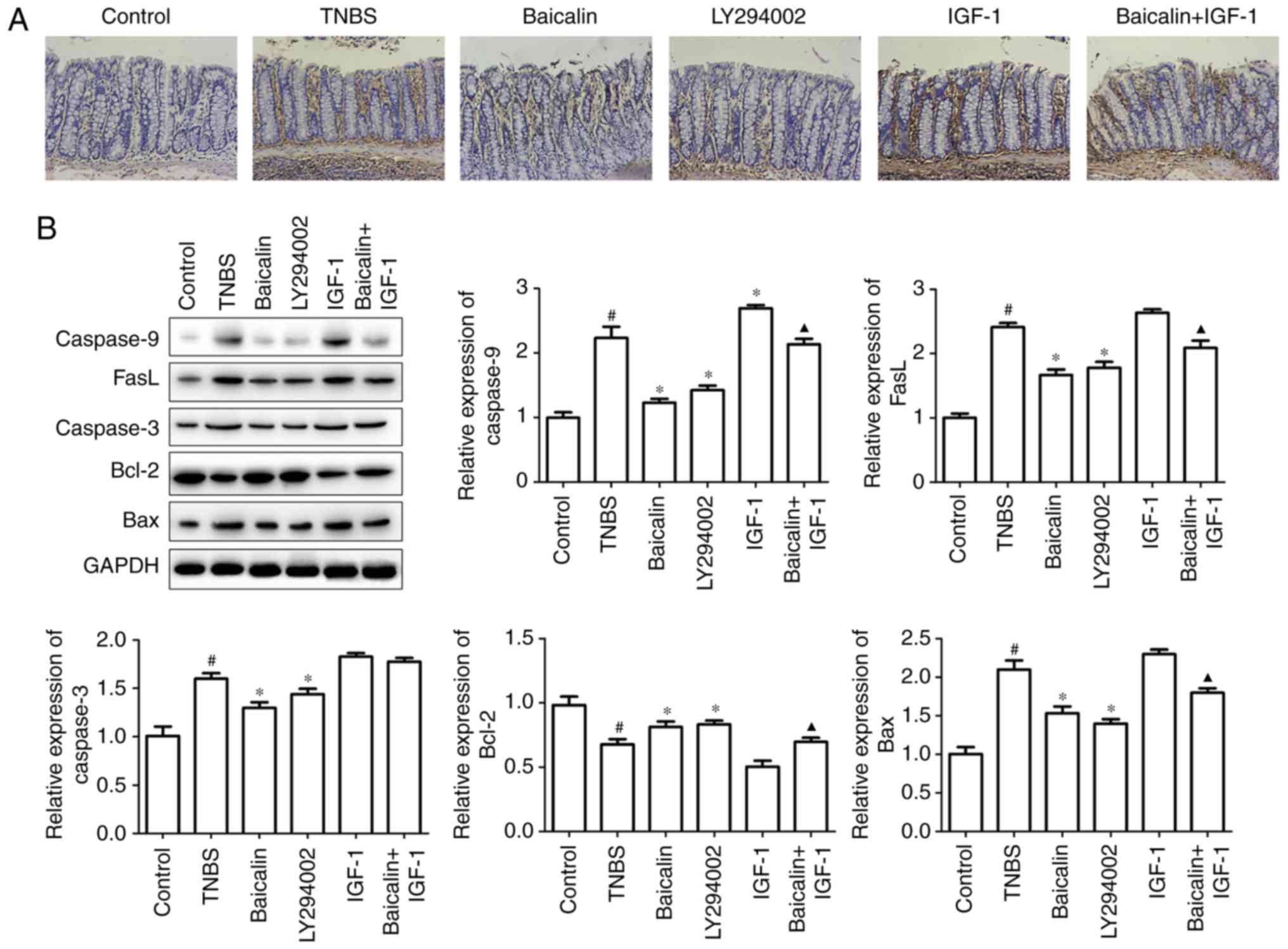
Baicalin ameliorates TNBS-induced
intestinal mucosal cell apoptosis through blockage of the PI3K/AKT
pathway
Apoptosis of the intestinal mucosal cell is a
hallmark of TNBS-induced colitis, which disrupts intestinal mucosal
integrity and barrier function, and leads to other changes
associated with colitis (29).
Excessive inflammation is considered to be a marker of apoptosis
(30). In line with the results of
inflammatory cytokine production in colon tissues, intestinal
mucosal cell apoptosis was then investigated using TUNEL staining.
As revealed in Fig. 2A, both
baicalin and LY204002 significantly inhibited intestinal mucosal
cell apoptosis, but IGF-1 induced cell apoptosis, which indicated
the role of the PI3K/AKT pathway in colitis development. Moreover,
when baicalin was administered in TNBS and IGF-1-treated rats,
intestinal mucosal cell apoptosis could be inhibited by baicalin
(Fig. 2A), which confirmed that
baicalin exerted the suppression partly through inhibition of the
PI3K/AKT pathway.
In order to ascertain the role of the PI3K/AKT
pathway in baicalin-suppressed cell apoptosis, colon tissues
lysates were used to detect the pro-apoptotic and anti-apoptotic
proteins by western blotting. It was revealed that baicalin or
LY294002 administration significantly suppressed TNBS-induced
pro-apoptotic caspase-3, caspase-9, Bax and FasL expression, but
increased the expression of Bcl-2, an anti-apoptotic protein, when
compared with the TNBS group. In addition, increased pro-apoptotic
protein caspase-9 expression was observed with IGF-1 treatment,
however this effect could be reversed by baicalin treatment
(Fig. 2B). Thus, baicalin suppressed
TNBS-induced cell apoptosis through inhibition of the PI3K/AKT
pathway.
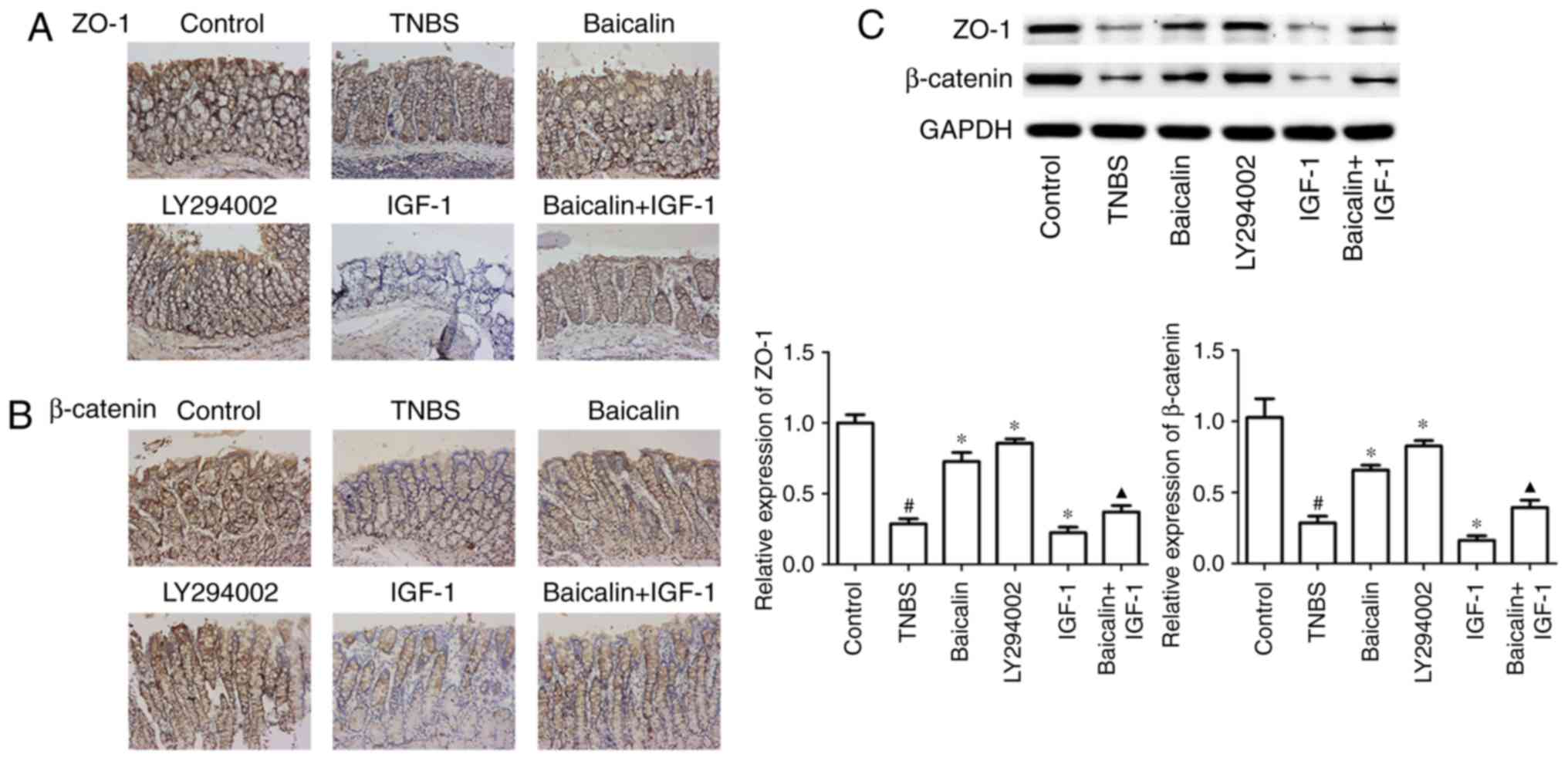
Baicalin protects TNBS-induced colitis
by increasing mucosal tight-junction proteins through inhibition of
the PI3K/AKT pathway
Integrity of the intestinal epithelial cell (IEC)
barrier plays an important role in maintaining mucosal immune
homeostasis. Dysregulated IEC barrier function appears to trigger
and perpetuate inflammation in IBD (31). To investigate the effects of baicalin
and the PI3K/AKT pathway on the integrity of the IEC barrier, colon
tissues underwent immunohistochemical staining for ZO-1 and
β-catenin, the marker proteins of tight-junctions. The present
results revealed that baicalin and LY294002 treatment could
markedly increase ZO-1 and β-catenin expression compared to the
TNBS-treated model group. In addition, the use of baicalin could
alleviate IGF-1-induced decrease of tight-junction proteins
(Fig. 3A), which indicated that
baicalin exerted the protection of the IEC barrier via inhibition
of PI3K/AKT pathway activation. Furthermore, in accordance with the
results of immunohistochemical staining, the results of western
blotting revealed that baicalin and LY294002 increased ZO-1 and
β-catenin expression, compared with the TNBS group, moreover,
baicalin also reversed IGF-1-induced reduction of ZO-1 and
β-catenin, compared with the TNBS group (Fig. 3C). These results indicated that
baicalin protected IEC barrier integrity, which was a PI3K/AKT
pathway inhibition process.
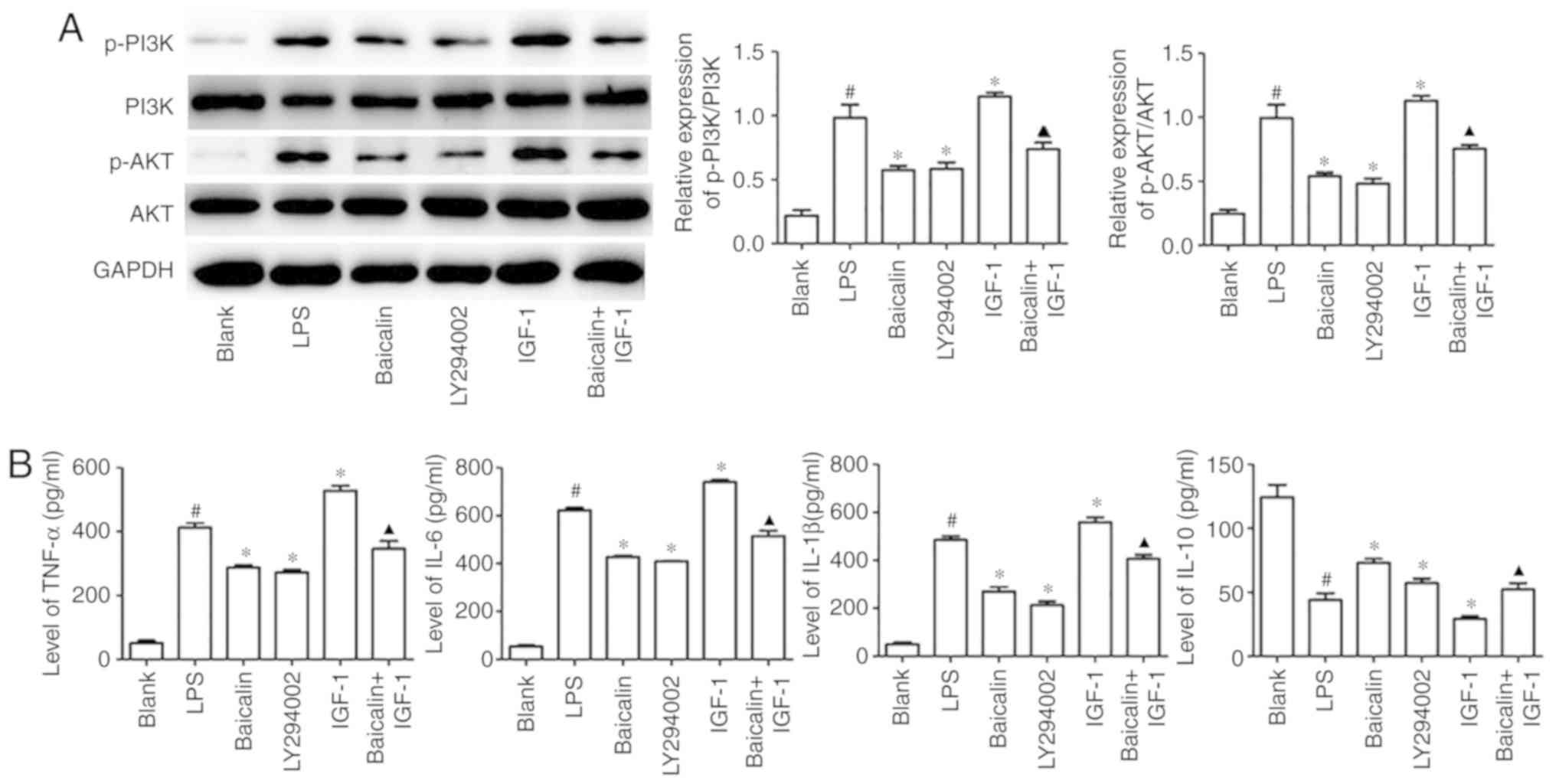
Baicalin suppresses LPS-induced
inflammation in HT-29 cells
Epithelial cells are the critical first barrier for
the intestine to defend itself from dangerous stimuli, and the most
sensitive cells respond to hazardous substances. To study the role
of baicalin in attenuating colitis, HT-29 cells were cultured, and
LPS was used to induce epithelial cell injury. As revealed in
Fig. 4A, when compared with the
blank group, LPS induced significant activation of the PI3K/AKT
pathway. However, when compared with the LPS group, baicalin
markedly suppressed the levels of p-PI3K and p-AKT. Furthermore,
IGF-1 was used to activate the pathway, and increased expression of
p-PI3K and p-AKT was revealed compared to the LPS group. However,
the influence of IGF-1 on LPS-stimulated epithelial cells could be
reversed by baicalin (Fig. 4A),
which indicated that baicalin was able to inhibit the PI3K/AKT
pathway in HT-29 cells. Inflammatory cytokine production is the
terminal result of pathway activation. Herein, baicalin
significantly inhibited the LPS-induced IL-6, TNF-α and IL-1β
production and promoted the expression of IL-10, and these effects
were also observed with LY294002 treatment. In addition, baicalin
treatment also reversed the IGF-1-induced increase of IL-6, TNF-α
and IL-1β (Fig. 4B). These results
indicated that baicalin ameliorated LPS-induced inflammation via
the inhibition of PI3K/AKT activation in HT-29 cells.
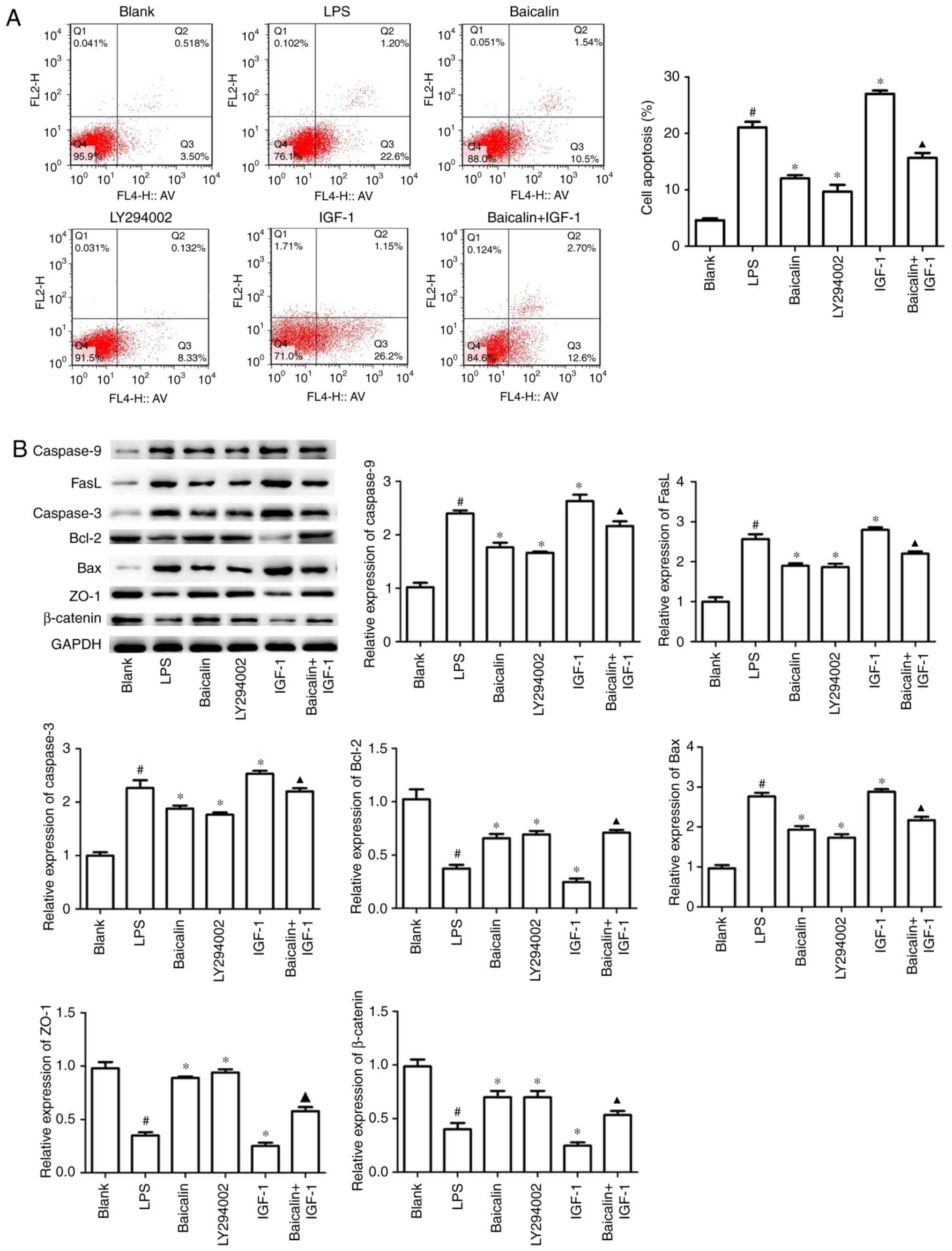
Baicalin ameliorates apoptosis and
tight-junction reduction by blockage of the PI3K/AKT pathway in
HT-29 cells
To further demonstrate that baicalin could
ameliorate cell death, flow cytometric analysis was employed to
assess early apoptosis and late apoptosis. As presented in Fig. 5A, LPS significantly triggered colon
cancer cell early apoptosis from 3.5 to 22.6% compared with the
control group, whereas baicalin and LY294002 treatment decreased
the early apoptosis rates from 22.6 to 10.5 and 8.33%,
respectively. Furthermore, baicalin markedly attenuated the early
apoptosis rate to 12.6%, which was significantly increased by IGF-1
treatment (26.2%), as compared to the control group. Notably, the
results also revealed that the various treatments did not cause any
significant changes in late cell apoptosis, compared with the
control group, indicating that the increase of apoptosis appeared
to be possibly due to early apoptosis rather than late apoptosis.
Western blotting results also revealed that when compared with the
LPS group, baicalin and LY294002 suppressed pro-apoptotic
caspase-3, caspase-9, Bax and FasL expression, but increased the
expression level of Bcl-2 in HT-29 cells (Fig. 5B). In addition, the role of baicalin
on tight-junction protein expression was explored in vitro,
and the results revealed that baicalin and LY294002 reversed
LPS-induced tight-junction protein decrease. These results
indicated the vital role of baicalin on alleviating inflammation,
apoptosis and tight-junction reduction, and this process involved
PI3K/AKT pathway inhibition.
Discussion
Ulcerative colitis (UC) and Crohn's disease (CD)
represent the two main forms of IBDs with clear-cut clinical and
histological features. UC involves the rectum and colon, whereas CD
generally involves the colon and ileum. Both of them cause great
pain and are costly to people and it has been estimated that 4.5
million people suffer from these diseases (32). Despite the recognized effect of IBD
conventional therapies such as aminosalicylates, corticosteroids,
and immunosuppressive agents, frequent adverse effects with these
agents have been documented (33).
Therefore, effective and safe treatments for IBDs are urgently
required. Chinese herbs have been used to treat diseases for a long
time. Nowadays, the use of their active ingredients is becoming an
increasingly attractive approach to deal with various inflammatory
disorders. Luan et al revealed that 50, 100 and 200 mg/kg of
baicalin could attenuate rat myocardial ischemia-reperfusion injury
through the AKT/NF-kB pathway (34).
In an in vitro HT-29 model, 50, 100, 150 and 200 µM of
baicalin was used to study its role in colon cancer and the results
revealed that baicalin could induce colon cancer cell apoptosis
(35).
In the present study, the role of baicalin in
treating TNBS-induced colitis in vitro and in vivo
was investigated. The results demonstrated that baicalin alleviated
the development of TNBS-induced colitis, reduced inflammation
release, ameliorated intestinal mucosal cell apoptosis and
increased the expression of tight-junction-associated proteins
in vivo and in vitro. Notably, baicalin exerted a
protective role in colitis via suppression of the PI3K/AKT
pathway.
TNBS has long been used as a valuable model to
explore the pathogenesis of IBDs. When TNBS is administered with
ethanol, the mucosal barrier of the intestine can be broken by
ethanol. TNBS is believed to haptenize colonic autologous or
microbiota proteins rendering them immunogenic to the host immune
system (34). The hapten-induced
colitis has been characterized by dense infiltration of adaptive
immune cells, predominantly CD4+T cells (35), and innate immune cell-like
macrophages (36). Based on
inclination towards Th1 immune response which involves IL-12 and
TNF-α as effector cytokines (35),
baicalin was firstly investigated on inflammatory cytokine
secretion. It was revealed that TNF-α, a typical Th1-type cytokine,
was significantly inhibited by the use of baicalin in rat colons,
which indicated that baicalin can be an effective regulator of
Th1-immune response. IL-6, produced by lamina propria T cells
(37) and macrophages in the
intestine, has been reported to be involved in the development of
IBDs (38,39). In the present study, it was reported
that baicalin can also be an efficient inhibitor of IL-6
secretion.
Aberrant apoptosis of IECs is a hallmark of
TNBS-induced colitis, which disrupts intestinal mucosal integrity
and barrier function and leads to other changes associated with
colitis (40,41). TNF-α, the main cytokine released in
TNBS-induced colitis, is believed to play a vital role in the
process of IEC apoptosis. Furthermore, anti-TNF-α therapies used
for the treatment IBD patients were revealed to inhibit IEC
apoptosis (30,42). In accordance with the present results
which revealed that baicalin inhibited TNF-α production, the
inhibitory effect of baicalin on IEC apoptosis was also revealed,
as observed by the fact that the expression of pro-apoptotic
proteins such as caspase-3, caspase-9, Bax and FasL were suppressed
in vivo and in vitro, and the anti-apoptotic molecule
Bcl-2 was increased. Herein, HT-29 cells were used to evaluate the
role of baicalin on inflammatory bowel diseases in vitro.
HT-29 cells have been reported in numerous studies as targets for
inflammatory bowel diseases in vitro (43-47).
In future experiments, other cell lines will be selected to study
the role of baicalin on inflammatory bowel diseases.
PI3K is an intracellular enzyme that catalyzes the
phosphorylation of membrane inositol lipids and is involved in many
biological processes, including cell growth, differentiation and
survival (48). In the present
study, it was reported that baicalin significantly suppressed the
TNBS- or LPS-induced phosphorylation of PI3K and AKT, which
indicated that baicalin exerted the protective effect in
TNBS-induced colitis partly by inhibiting PI3K/AKT pathway
activation. PI3K signaling was blocked by the PI3K specific
inhibitor LY294002, and it was revealed that the effect of LY294002
on the inhibition of inflammatory cytokine production and
intestinal epithelial cell apoptosis was equivalent to baicalin.
Numerous studies on tumors have revealed the vital role of PI3K
signaling in promoting tumor cell growth and resisting apoptosis.
PI3K signaling even helps tumor cells obtain the ability to
immortally grow (49,50). However, in other cells except tumor
cells, PI3K signaling appears to play an opposite role by promoting
cell apoptosis to maintain a steady state (51). IECs are essential parts of the
mucosal barrier. In order to maintain the balance and basic
functions of the barrier, epithelial cells often enter the process
of apoptosis upon various injury factors such as TNBS (52,53).
In conclusion, the present study demonstrated a
novel mechanism in which baicalin attenuated TNBS-induced colitis
via inhibition of PI3K/AKT activation, which was revealed by the
decreased inflammatory cytokine levels, reduced IEC apoptosis and
increased tight-junction proteins. These findings provide the first
evidence of a direct link between traditional Chinese herbal
ingredients and PI3K/AKT signaling in colitis, along with novel
insight into the mechanisms of baicalin in treating colitis.
Acknowledgements
Not applicable.
Funding
The research was supported by The Natural Science
Foundation of China (grant no. 81403344); the Special Scientific
Research for Traditional Chinese Medicine of State Administration
of Traditional Chinese Medicine of China (grant no. 201407001); the
Jiangsu Provincial Special Program of Medical Science (grant no.
BL2014100) and the National Chinese Medicine Clinical Research
Base.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
LeZ and HS designed the study and wrote the
manuscript. PQG, YJL and LuZ performed the experiments. LuZ and JFC
analyzed the data. HS supervised the work. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were performed in strict
accordance with the Institutional Animal Care and Use Committee of
Nanjing University of Chinese Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bitton A, Vutcovici M, Patenaude V,
Sewitch M, Suissa S and Brassard P: Epidemiology of inflammatory
bowel disease in Quebec: Recent trends. Inflamm Bowel Dis.
20:1770–1776. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bashashati M, Rezaei N, Andrews CN, Chen
CQ, Daryani NE, Sharkey KA and Storr MA: Cytokines and irritable
bowel syndrome: Where do we stand? Cytokine. 57:201–209.
2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cosnes J, Gower-Rousseau C, Seksik P and
Cortot A: Epidemiology and natural history of inflammatory bowel
diseases. Gastroenterology. 140:1785–1794. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ananthakrishnan AN: Epidemiology and risk
factors for IBD. Nat Rev Gastroenterol Hepatol. 12:205–217.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Beaugerie L and Itzkowitz SH: Cancers
complicating inflammatory bowel disease. N Engl J Med.
372:1441–1452. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fichtner-Feigl S, Strober W, Geissler EK
and Schlitt HJ: Cytokines mediating the induction of chronic
colitis and colitis-associated fibrosis. Mucosal Immunol. 1 (Suppl
1):S24–S27. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rungoe C, Nyboe Andersen N and Jess T:
Inflammatory bowel disease and risk of coronary heart disease.
Trends Cardiovasc Med. 25:699–704. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nyuyki KD and Pittman QJ: Toward a better
understanding of the central consequences of intestinal
inflammation. Ann NY Acad Sci. 1351:149–154. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Levesque BG, Sandborn WJ, Ruel J, Feagan
BG, Sands BE and Colombel JF: Converging goals of treatment of
inflammatory bowel disease from clinical trials and practice.
Gastroenterology. 148:37–51 e1. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fonseca-Camarillo G and Yamamoto-Furusho
JK: Immunoregulatory pathways involved in inflammatory bowel
disease. Inflamm Bowel Dis. 21:2188–2193. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Koelink PJ, Overbeek SA, Braber S, de
Kruijf P, Folkerts G, Smit MJ and Kraneveld AD: Targeting chemokine
receptors in chronic inflammatory diseases: An extensive review.
Pharmacol Ther. 133:1–18. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mitroulis I, Alexaki VI, Kourtzelis I,
Ziogas A, Hajishengallis G and Chavakis T: Leukocyte integrins:
Role in leukocyte recruitment and as therapeutic targets in
inflammatory disease. Pharmacol Ther. 147:123–135. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Merga Y, Campbell BJ and Rhodes JM:
Mucosal barrier, bacteria and inflammatory bowel disease:
Possibilities for therapy. Dig Dis. 32:475–483. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen SJ, Liu XW, Liu JP, Yang XY and Lu
FG: Ulcerative colitis as a polymicrobial infection characterized
by sustained broken mucus barrier. World J Gastroenterol.
20:9468–9475. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhu Y, Mahon BD, Froicu M and Cantorna MT:
Calcium and 1 alpha,25-dihydroxyvitamin D3 target the TNF-alpha
pathway to suppress experimental inflammatory bowel disease. Eur J
immunol. 35:217–224. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Danese S, Sans M, Scaldaferri F, Sgambato
A, Rutella S, Cittadini A, Piqué JM, Panes J, Katz JA, Gasbarrini A
and Fiocchi C: TNF-alpha blockade down-regulates the CD40/CD40L
pathway in the mucosal microcirculation: A novel anti-inflammatory
mechanism of infliximab in Crohn's disease. J Immunol.
176:2617–2624. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Condino G, Calabrese E, Zorzi F, Onali S,
Lolli E, De Biasio F, Ascolani M, Pallone F and Biancone L:
Anti-TNF-alpha treatments and obstructive symptoms in Crohn's
disease: A prospective study. Dig Liver Dis. 45:258–262.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pugliese D, Guidi L, Ferraro PM, Marzo M,
Felice C, Celleno L, Landi R, Andrisani G, Pizzolante F, De Vitis
I, et al: Paradoxical psoriasis in a large cohort of patients with
inflammatory bowel disease receiving treatment with anti-TNF alpha:
5-year follow-up study. Aliment Pharmacol Ther. 42:880–888.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ikemoto SK, Yoshida N, Yasumoto R, Wada S,
Yamamoto K and Kishimoto T: Antitumor effects of Scutellariae radix
and its components baicalein, baicalin, and wogonin on bladder
cancer cell lines. Urology. 55:951–955. 2000.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sun SJ, Wu XP, Song HL and Li GQ: Baicalin
ameliorates isoproterenol-induced acute myocardial infarction
through iNOS, inflammation, oxidative stress and P38MAPK pathway in
rat. Int J Clin Exp Med. 8:22063–22072. 2015.PubMed/NCBI
|
|
21
|
Li X, Zou K, Gou J, Du Q, Li D, He X and
Li Z: Effect of baicalin-copper on the induction of apoptosis in
human hepatoblastoma cancer HepG2 cells. Med Oncol.
32(72)2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Guo X, Chi S, Cong X, Li H, Jiang Z, Cao R
and Tian W: Baicalin protects sertoli cells from heat
stress-induced apoptosis via activation of the Fas/FasL pathway and
Hsp72 expression. Reprod Toxicol. 57:196–203. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zou Y, Dai SX, Chi HG, Li T, He ZW, Wang
J, Ye CG, Huang GL, Zhao B, Li WY, et al: Baicalin attenuates
TNBS-induced colitis in rats by modulating the Th17/Treg paradigm.
Arch Pharm Res. 38:1873–1887. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu X, Gu J, Fan Y, Shi H and Jiang M:
Baicalin attenuates acute myocardial infarction of rats via
mediating the mitogen-activated protein kinase pathway. Biol Pharm
Bull. 36:988–994. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lin M, Li L, Li L, Pokhrel G, Qi G, Rong R
and Zhu T: The protective effect of baicalin against renal
ischemia-reperfusion injury through inhibition of inflammation and
apoptosis. BMC Complement Altern Med. 14(19)2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cui L, Feng L, Zhang ZH and Jia XB: The
anti-inflammation effect of baicalin on experimental colitis
through inhibiting TLR4/NF-κB pathway activation. Int
Immunopharmacol. 23:294–303. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang Y, Li X, Ciric B, Ma CG, Gran B,
Rostami A and Zhang GX: Therapeutic effect of baicalin on
experimental autoimmune encephalomyelitis is mediated by SOCS3
regulatory pathway. Sci Rep. 5(17407)2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Morris GP, Beck PL, Herridge MS, Depew WT,
Szewczuk MR and Wallace JL: Hapten-induced model of chronic
inflammation and ulceration in the rat colon. Gastroenterology.
96:795–803. 1989.PubMed/NCBI
|
|
29
|
Siggers RH and Hackam DJ: The role of
innate immune-stimulated epithelial apoptosis during
gastrointestinal inflammatory diseases. Cell Mol Life Sci.
68:3623–3634. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Marini M, Bamias G, Rivera-Nieves J,
Moskaluk CA, Hoang SB, Ross WG, Pizarro TT and Cominelli F:
TNF-alpha neutralization ameliorates the severity of murine
Crohn's-like ileitis by abrogation of intestinal epithelial cell
apoptosis. Proc Natl Acad Sci USA. 100:8366–8371. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cario E: Barrier-protective function of
intestinal epithelial Toll-like receptor 2. Mucosal Immunol. 1
(Suppl 1):S62–S66. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kaplan GG: The global burden of IBD: From
2015 to 2025. Nat Rev Gastroenterol Hepatol. 12:720–727.
2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Baumgart DC and Sandborn WJ: Inflammatory
bowel disease: Clinical aspects and established and evolving
therapies. Lancet. 369:1641–1657. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wirtz S, Popp V, Kindermann M, Gerlach K,
Weigmann B, Fichtner-Feigl S and Neurath MF: Chemically induced
mouse models of intestinal inflammation. Nat Protoc. 12:1295–1309.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Randhawa PK, Singh K, Singh N and Jaggi
AS: A review on chemical-induced inflammatory bowel disease models
in rodents. Korean J Physiol Pharmacol. 18:279–288. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wallace KL, Zheng LB, Kanazawa Y and Shih
DQ: Immunopathology of inflammatory bowel disease. World J
Gastroenterol. 20:6–21. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Atreya R, Mudter J, Finotto S, Müllberg J,
Jostock T, Wirtz S, Schütz M, Bartsch B, Holtmann M, Becker C, et
al: Blockade of interleukin 6 trans signaling suppresses T-cell
resistance against apoptosis in chronic intestinal inflammation:
Evidence in Crohn disease and experimental colitis in vivo. Nat
Med. 6:583–588. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
38
|
Atreya R and Neurath MF: Involvement of
IL-6 in the pathogenesis of inflammatory bowel disease and colon
cancer. Clin Rev Allergy Immunol. 28:187–196. 2005.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Atreya R and Neurath MF: New therapeutic
strategies for treatment of inflammatory bowel disease. Mucosal
Immunol. 1:175–182. 2008.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Qiu W, Wu B, Wang X, Buchanan ME, Regueiro
MD, Hartman DJ, Schoen RE, Yu J and Zhang L: PUMA-mediated
intestinal epithelial apoptosis contributes to ulcerative colitis
in humans and mice. J Clin Invest. 121:1722–1732. 2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lin W, Ma C, Su F, Jiang Y, Lai R, Zhang
T, Sun K, Fan L, Cai Z, Li Z, et al: Raf kinase inhibitor protein
mediates intestinal epithelial cell apoptosis and promotes IBDs in
humans and mice. Gut. 66:597–610. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zeissig S, Bojarski C, Buergel N, Mankertz
J, Zeitz M, Fromm M and Schulzke JD: Downregulation of epithelial
apoptosis and barrier repair in active Crohn's disease by tumour
necrosis factor alpha antibody treatment. Gut. 53:1295–1302.
2004.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Gu L, Ge Z, Wang Y, Shen M and Zhao P:
Activating transcription factor 3 promotes intestinal epithelial
cell apoptosis in Crohn's disease. Pathol Res Pract. 214:862–870.
2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Rodrigues RC, Pocheron AL, Cappelier JM,
Tresse O and Haddad N: An adapted in vitro assay to assess
Campylobacter jejuni interaction with intestinal epithelial cells:
Taking into stimulation with TNFα. J Microbiol Methods. 149:67–72.
2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Gu L, Zhao J, Zhang S, Xu W, Ni R and Liu
X: Runt-related transcription factor 2 (RUNX2) inhibits apoptosis
of intestinal epithelial cells in Crohn's disease. Pathol Res
Pract. 214:245–252. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Li M, Zhang S, Qiu Y, He Y, Chen B, Mao R,
Cui Y, Zeng Z and Chen M: Upregulation of miR-665 promotes
apoptosis and colitis in inflammatory bowel disease by repressing
the endoplasmic reticulum stress components XBP1 and ORMDL3. Cell
Death Dis. 8(e2699)2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yan L, Wang L, Bai J, Miao X, Zeng W, Hua
X, Ni R, Zhang D and Tang Q: Chromosome region maintenance-1 (CRM1)
regulates apoptosis of intestinal epithelial cells via p27kip1 in
Crohn's disease. Clin Res Hepatol Gastroenterol. 41:445–458.
2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Baròn M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Chen W, Liu Y, Xue G, Zhang L, Zhang L and
Shao S: Diazoxide protects L6 skeletal myoblasts from H2O2-induced
apoptosis via the phosphatidylinositol-3 kinase/Akt pathway.
Inflamm Res. 65:53–60. 2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Schulzke JD, Ploeger S, Amasheh M, Fromm
A, Zeissig S, Troeger H, Richter J, Bojarski C, Schumann M and
Fromm M: Epithelial tight junctions in intestinal inflammation. Ann
NY Acad Sci. 1165:294–300. 2009.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Johansson ME: Mucus layers in inflammatory
bowel disease. Inflamm Bowel Dis. 20:2124–2131. 2014.PubMed/NCBI View Article : Google Scholar
|