Introduction
The intestinal epithelium functions as a natural
barrier against the invasion of intestinal bacteria, toxins and
other harmful substances, and serves and important role in the
occurrence and development of multiple organ dysfunction syndrome
(MODS) (1). Intestinal injury is the
most common organ damage during the early stages of severe burn
injuries (2). Severe burns can
directly or indirectly result in the overgrowth of pathogenic
bacteria in the intestines and the disruption of intestinal
mechanical barriers, which in turn can trigger the translocation of
intestinal bacteria or toxins, leading to systemic inflammatory
response syndrome, sepsis and MODS (3,4).
Meanwhile, apoptotic and necrotic intestinal epithelial cells
enhance intestinal mucosal permeability, oxidative stress and the
inflammatory response, thereby damaging the mechanical and immune
barriers of the intestinal tissues (5,6).
Therefore, intestinal protection plays an important role in the
treatment of severe burns (7).
It has become increasingly clear that the
inflammatory response and oxidative stress play crucial roles in
the pathological process of burn injury (8). Toll-like receptor 4 (TLR4) is an innate
immunity pattern recognition receptor, which initiates the immune
response and oxidative damage by activating NF-κB, a crucial
proinflammatory transcription factor (9,10).
Therefore, it has been proposed that therapeutic targets that
abolish intestinal inflammation and oxidative stress may function
by mediating the TLR4 signaling pathway (11). For example, Zhou et al
(12) reported that pharmacological
administration of the mTOR inhibitor AZD8055 or the autophagy
activator rapamycin significantly attenuated lipopolysaccharide
(LPS)-induced intestinal inflammation and oxidative stress by
inhibiting the TLR4-MyD88-mitogen-activated protein kinase and the
NF-κB signaling pathways.
Loganin is an iridoid glycoside extracted from the
crude herb Cornus officinalis Sieb. et Zucc. (13). A number of previous studies have
reported that loganin displays anti-inflammatory, neuroprotective,
antiatherosclerotic and antidiabetic activities in acute
pancreatitis (14),
neurodegenerative disorders (15),
atherosclerosis (16) and diabetes
(17), respectively. However, the
role of loganin in the treatment of burn injury is not completely
understood. The aim of the present study was to establish an
experimental model of burn injury in rats to evaluate the effects
of loganin on intestinal inflammation and oxidative stress.
Therefore, the present study provided novel insights into the
potential anti-inflammatory and antioxidant effects of loganin
in vivo.
Materials and methods
Animals
Male Sprague-Dawley rats aged 4-6 weeks and weighing
150-200 g (n=18) were purchased from the Laboratory Animal Center
of The Affiliated Hospital of Chengde Medical University. Before
experimentation, all rats were allowed to acclimatize for one week
under specific pathogen-free conditions. The rats were maintained
at 25˚C with the humidity of 55% and 12-h light/dark cycles and
were fed a standard chow diet with free access to food and water.
All animals were handled according to the Animal Welfare Guidelines
issued by The Affiliated Hospital of Chengde Medical University for
the Care and Use of Laboratory Animals. The experimental procedures
were approved by the Animal Care and Use Committee of The
Affiliated Hospital of Chengde Medical University.
Burn procedure
Burn injuries were established by scalding the skin
of the rats' back using boiling water as described in previously
published studies (5). Briefly, the
rats were anesthetized by the intraperitoneal injection of 30 mg/kg
sodium pentobarbital. The dorsal area was then dehaired and
completely immersed in 100˚C water for 15 sec to create a 20% total
body surface area (TBSA) full-thickness burn.
Experimental design
A total of 18 rats were randomly divided into three
groups as follows: i) Sham-operated rats, which served as the
control group (n=6); ii) rats subjected to burn injury, which
served as the burn group (n=6); and iii) rats intragastrically
administered 50 mg/kg loganin (Sigma-Aldrich; Merck KGaA; 5 mg
loganin dissolved in 1 ml normal saline; extrasynthese) daily for 7
days before burn injury, which served as the burn + loganin group
(n=6). Additionally, untreated rats were used as blank normal
controls (n=6). All rats were anesthetized by the intraperitoneal
injection of 30 mg/kg sodium pentobarbital, placed in room
temperature water for 15 sec and administered an equal volume of
normal saline to the loganin dose. At 24 h after burn injury, all
rats were euthanized by the intraperitoneal injection of 120 mg/kg
sodium pentobarbital, as previously described (18). Following euthanasia, intestinal
tissues were surgically resected and immediately immersed in 10%
formalin overnight at 4˚C.
Histopathological examination
Intestines were fixed in 10% formalin overnight at
4˚C and were subsequently embedded in paraffin. After
deparaffinization with xylene and rehydration using a graded
ethanol series, the 5-µm thick sections were stained for 5 min with
hematoxylin, rinsed, and stained for 30 sec using 0.5% eosin at
room temperature for histological assessment of intestinal damage.
The images were observed by a pathologist from the Affiliated
Hospital of Chengde Medical University, and five randomly selected
areas were examined using a light microscope (magnification, x400;
Leica DM1000, Leica Microsystems, Inc.).
ELISA
The intestinal levels of tumor necrosis factor
(TNF)-α (cat. no. 550610), interleukin (IL)-6 (cat. no. 550799) and
IL-1β (cat. no. 557966) were determined by commercial ELISA kits
(BD Biosciences), according to the manufacturer's instructions.
Expression levels were determined at a wavelength of 450 nm. Each
sample was assayed in duplicate.
Reverse transcription-quantitative PCR
(RT-qPCR)
The RT-qPCR experiments were independently carried
out three times, with three repeats each. Total RNA was extracted
from intestinal tissues using TRIzol® reagent (Thermo
Fisher Scientific, Inc.), according to the manufacturer's
instructions. RNA was then reverse transcribed to cDNA using the
PrimeScript™ 1st Strand cDNA Synthesis kit (Takara Biotechnology
Co., Ltd.) according to manufacturer's protocol. qPCR was
subsequently performed using the Primer-ScriptTM One Step RT-PCR
kit (Takara Biotechnology Co., Ltd.) on a 7900 HT Fast Real-Time
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The thermocycling conditions were as follows: pre-denaturation at
95˚C for 30 sec, followed by 95˚C for 5 sec, annealing at 60˚C for
20 sec, extension at 72˚C for 30 sec for a total of 40 cycles. The
primer sequences used were as follows: TNF-α forward,
5'-GCCCACGTCGTAGCAA-3' and reverse, 5'-GTCTTTGAGATCCATGCCAT-3';
IL-6 forward, 5'-AGAAGACCAGAGCAGATTTT-3' and reverse,
5'-GAGAAAGAGTTGTGCAATG-3'; IL-1β forward,
5'-GAGCTGAAAGCTCTCCACCT-3' and reverse, 5'-TTCCATCTTCTTCTTTGGGT-3';
and β-actin forward, 5'-GAAGATCAAGATCATTGCTCCT-3' and reverse,
5'-TACTCCTGCTTGCTGATCCA-3'. The relative expression levels of
TNF-α, IL-6 and IL-1β were analyzed by the 2-ΔΔCq method
(19) and normalized to β-actin.
Measurement of antioxidative
activities
The experiments were independently carried out three
times, with three repeats each. Intestinal tissues were centrifuged
at 1,500 x g for 15 min at 4˚C and homogenized in normal saline.
The supernatant was transferred into new tubes for evaluation of
reactive oxygen species (ROS) accumulation and superoxide dismutase
(SOD), catalase (CAT), glutathione peroxidase (GSH-Px) and
malondialdehyde (MDA) activities, according to the manufacturer's
protocol. Intracellular ROS accumulation (Reactive oxygen species
Assay kit; cat. no. E004-1-1; Nanjing Jiancheng Bioengineering
Institute) was measured using a spectrofluorophotometer at
excitation/emission wavelengths of 488/525 nm. SOD level was
detected by a water-soluble tetrazolium salt method (Superoxide
Dismutase assay kit; cat. no. A001-3-2; Nanjing Jiancheng
Bioengineering Institute), and the sample absorbance was analyzed
using a microplate reader at a wavelength of 450 nm. The levels of
CAT (Catalase assay kit; cat. no. A007-1-1; Nanjing Jiancheng
Bioengineering Institute), GSH-Px (Glutathione Peroxidase assay
kit; cat. no. A005-1-2; Nanjing Jiancheng Bioengineering Institute)
and MDA (Malondialdehyde assay kit; cat. no. A003-1-2; Nanjing
Jiancheng Bioengineering Institute) were determined
colorimetrically using a spectrophotometer at a wavelength of 405,
412 and 532 nm, respectively.
Apoptosis detection
The experiments were independently carried out three
times, with three repeats each. Following trypsinization for 2 h
using 0.05% trypsin at 4˚C, resected segments of intestines were
washed with PBS to collect the intestinal epithelial cells. Cell
apoptosis was assessed using the Annexin V-FITC/PI detection kit
(Sigma-Aldrich; Merck KGaA), according to the manufacturer's
instructions. Briefly, the intestinal epithelial cells
(1x106 cells) were resuspended in the binding buffer and
stained with the Annexin V/FITC for 15 min and PI solution for 5
min at 4˚C. The early and late apoptosis was analyzed using a flow
cytometer and estimated using the ModFit software (version 3.0; BD
Biosciences).
Western blotting
The frozen intestines were lysed in RIPA buffer
(Santa Cruz Biotechnology, Inc.). The lysates were centrifuged at
12,000 x g at 4˚C for 20 min. The protein content in the
supernatant was measured by the bicinchoninic acid protein assay
(Pierce; Thermo Fisher Scientific, Inc.). Protein samples (30 µg)
were resolved in 10% SDS-PAGE gels and transferred onto PVDF
membranes in 5% non-fat dry milk in tris-buffered saline containing
0.1% Tween 20 for 2 h at room temperature. Immunoblotting was
performed on the membranes using the following primary antibodies:
Anti-Bcl-2 (cat. no. 3498; 1:1,000; Cell Signaling Technology,
Inc.), anti-Bax (cat. no. 5023; 1:1,000; Cell Signaling Technology,
Inc.), anti-cleaved caspase-3 (cat. no. 9661; 1:1,000; Cell
Signaling Technology, Inc.), anti-TLR4 (cat. no. 14358; 1:1,000;
Cell Signaling Technology, Inc.), anti-phosphorylated (p)-inhibitor
of κB-α (IκB-α; cat. no. 2859; 1:1,000; Cell Signaling Technology,
Inc.), anti-IκB-α (cat. no. 4812; 1:1,000; Cell Signaling
Technology, Inc.), anti-p-NF-κB p65 (cat. no. 8214; 1:1,000; Cell
Signaling Technology, Inc.) and anti-NF-κB p65 (cat. no. 8242;
1:1,000; Cell Signaling Technology, Inc.) overnight at 4˚C.
Subsequently, membranes were incubated with horseradish
peroxidase-conjugated secondary antibodies (cat. no. 7076; 1:1,000;
Cell Signaling Technology, Inc.) at room temperature for 2 h.
Immunoreactive bands were visualized using the ECL western blot
detection system (Abcam). The same membranes were probed with GAPDH
(cat. no. 5176; 1:1,000; Cell Signaling Technology, Inc.) as a
loading control. Quantitative results of Western blotting were
obtained by densitometry using ImageJ software (version 1.46;
National Institutes of Health). The experiments were independently
carried out three times, with three repeats each.
Immunohistochemical staining
Formalin-fixed, paraffin-embedded intestines were
cut into 5-µm thick slices and permeabilized in 0.3% Triton X-100
for 20 min at 4˚C. The sections were incubated overnight at 4˚C
with primary antibodies against TLR4 (cat. no. ab22048; 1:100;
Abcam) and NF-κB p65 (cat. no. ab207297; 1:2,000; Abcam).
Subsequently, the sections were incubated with horseradish
peroxidase-conjugated secondary antibodies (cat. no. 3900; 1:1,000;
Cell Signaling Technology, Inc.) for 30 min at 37˚C. After sections
were washed three times with PBS, slides were stained with
3,3'-diaminobenzidine for 5 min at 37˚C, counterstained with
hematoxylin for 2 min at 37˚C, and imaged under a light microscope
(x400 magnification).
Statistical analysis
All data are expressed as mean ± standard deviation.
SPSS software (version 19.0; IBM Corp.) was used for statistical
analysis. The results were analyzed by one-way ANOVA with
Student-Newman-Keuls or Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
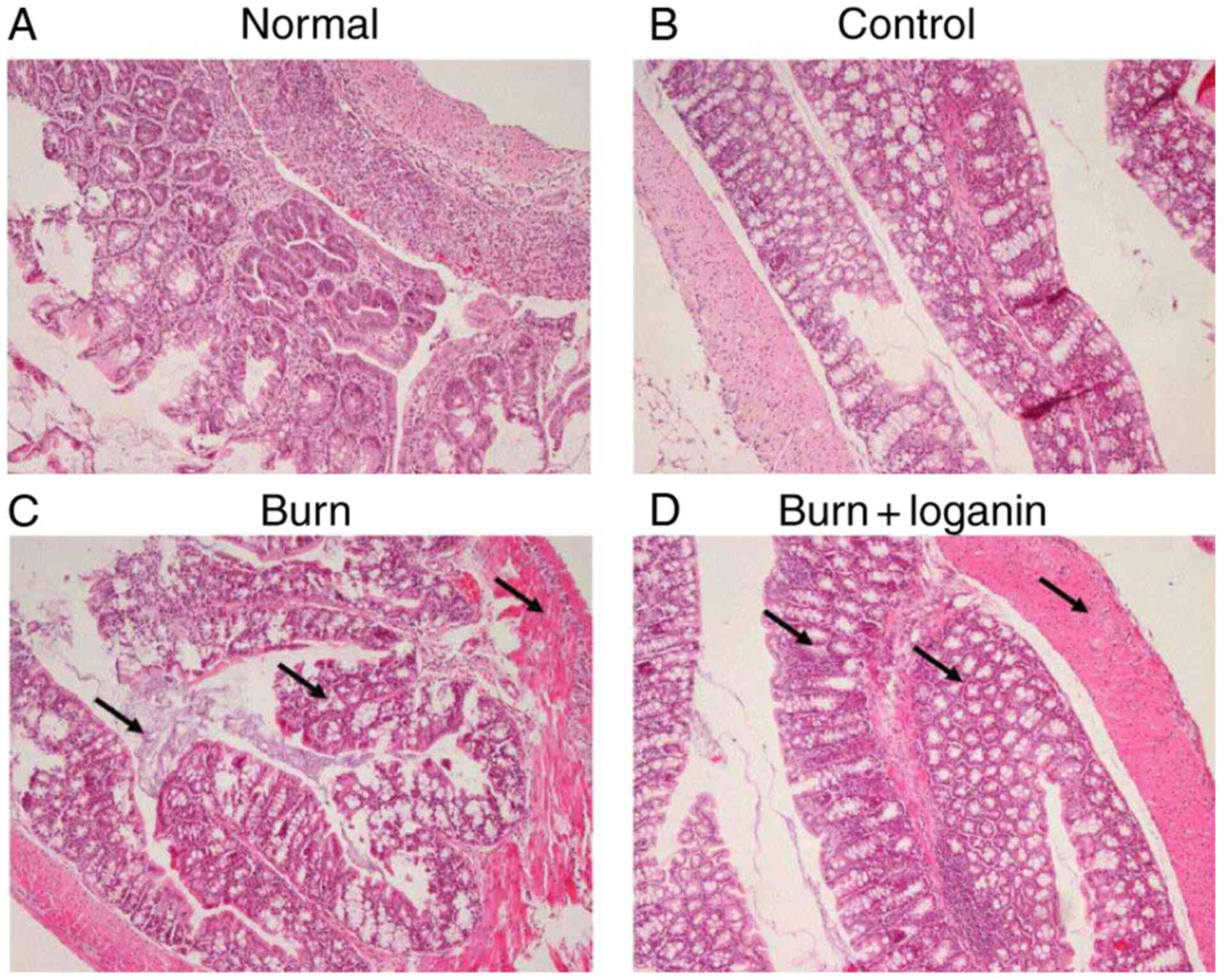
Loganin ameliorates intestinal
histopathology caused by severe burn in rats
The normal group displayed normal histopathology
(Fig. 1). The control group also
displayed no destructive changes in the hematoxylin and
eosin-stained intestinal tissues (Fig.
1). At 24 h after 20% TBSA burn injury, rats displayed
intestinal damage characterized by intestinal edema, the loss of
villi integrity and inflammatory cell infiltration (Fig. 1). By contrast, intragastric injection
of loganin to rats with severe burns resulted in less histological
inflammation compared with the burn group (Fig. 1).
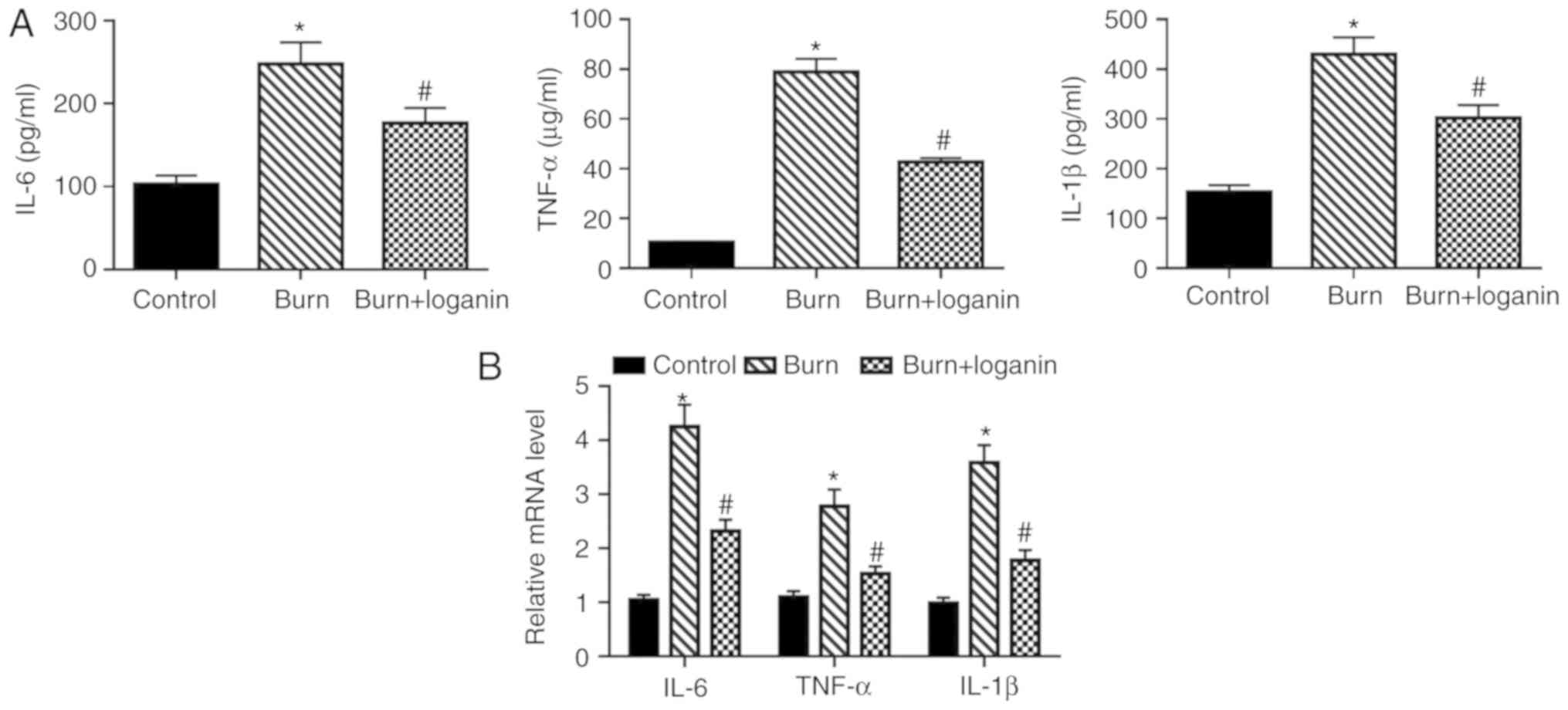
Loganin inhibits TNF-α, IL-6 and IL-1β
production induced by burn injury
Inflammation plays a critical role in the
pathogenesis of burn injury (20).
To determine the effect of loganin on the inflammatory response
induced by severe burns in rats, the expression levels of the
proinflammatory cytokines TNF-α, IL-6 and IL-1β in the intestinal
tissues were examined by ELISA and RT-qPCR. The protein levels of
TNF-α, IL-6 and IL-1β in the intestinal tissues from rats with
severe burns were significantly elevated compared with those from
the control rats (Fig. 2A;
P<0.05). However, compared with the burned rats, loganin
treatment significantly decreased the intestinal expression of
TNF-α, IL-6 and IL-1β at the protein level (P<0.05).
Furthermore, the administration of loganin significantly reduced
burns-induced upregulation of TNF-α, IL-6 and IL-1β mRNA levels
compared with the burned rats (Fig.
2B; P<0.05).
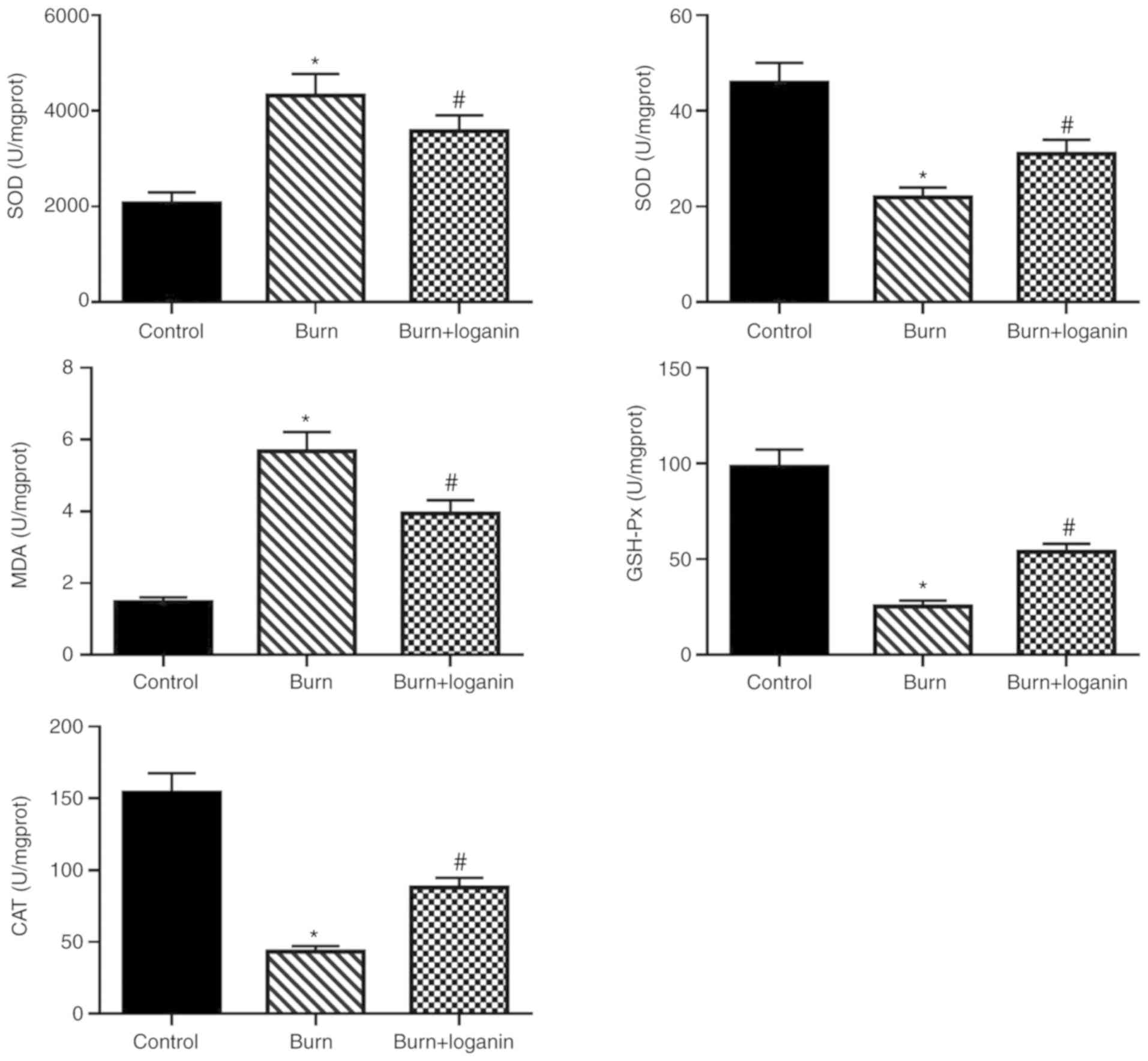
Loganin ameliorates oxidative stress
in intestinal tissues in the rat burn model
Subsequently, changes in oxidative stress marker
activity in the intestinal tissues were measured. The results
suggested that loganin treatment suppressed the burn-induced
production of ROS (Fig. 3) and
oxidative damage of the intestines, as indicated by increased SOD,
CAT and GSH-Px levels, as well as reduced MDA level, compared with
the burn group (Fig. 3).
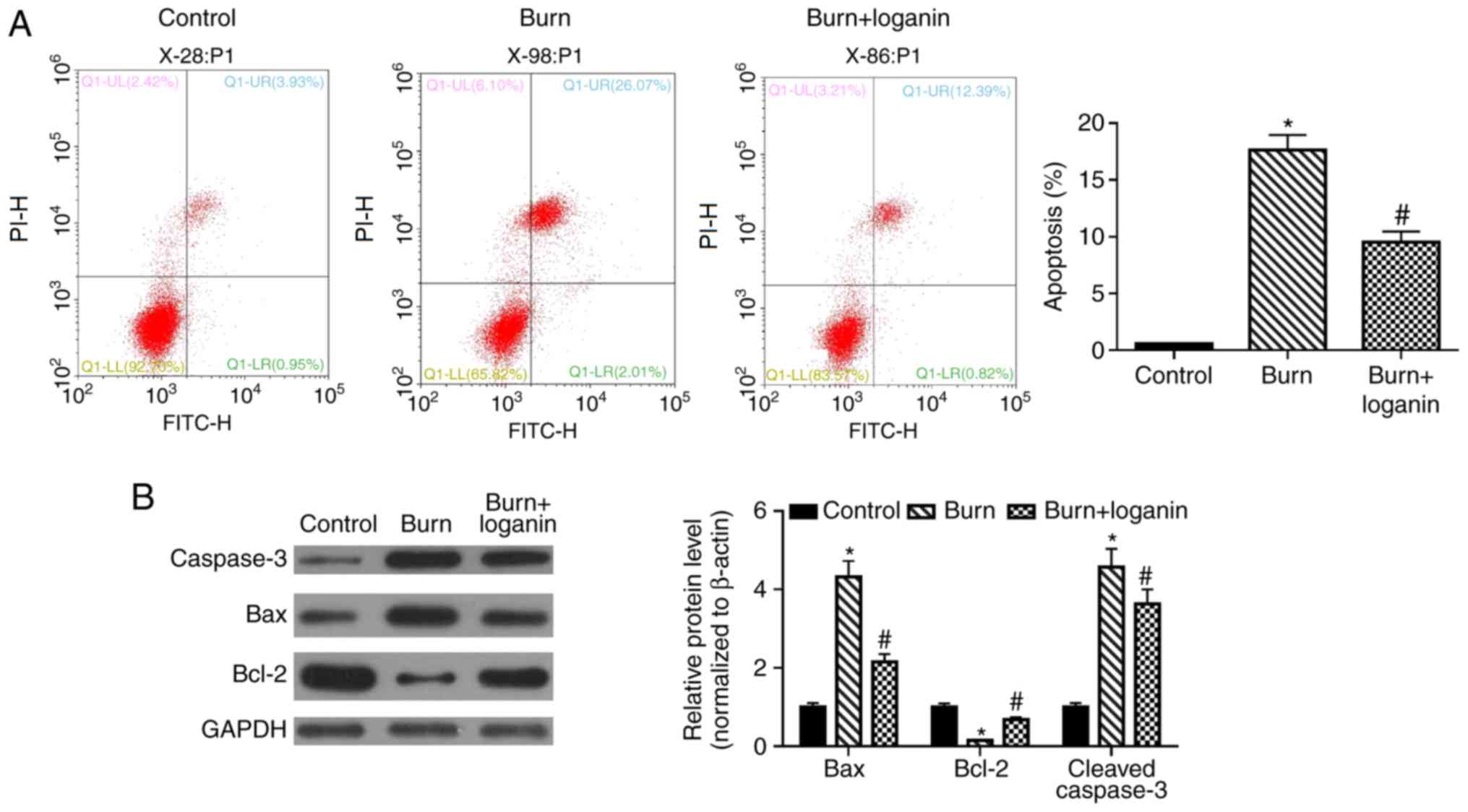
Loganin reduces intestinal epithelial
cell apoptosis in severely burned rats
Apoptosis of intestinal epithelial cells was then
assessed by Annexin V-FITC/PI double staining and flow cytometry.
The percentage of apoptotic intestinal epithelial cells was
significantly increased in the burn group compared with the control
group, while loganin treatment reduced the level of burns-induced
apoptosis of intestinal epithelial cells compared with the burn
group (Fig. 4A; P<0.05). To
further investigate the antiapoptotic effects of loganin, the
effects of loganin on the expression of the apoptosis-related
proteins Bax, Bcl-2 and cleaved-caspase-3 were analyzed by western
blotting. Loganin significantly reduced the burn injury-induced
upregulation of Bax and cleaved-caspase-3 and the downregulation of
Bcl-2, compared with the burn group (Fig. 4B; P<0.05).
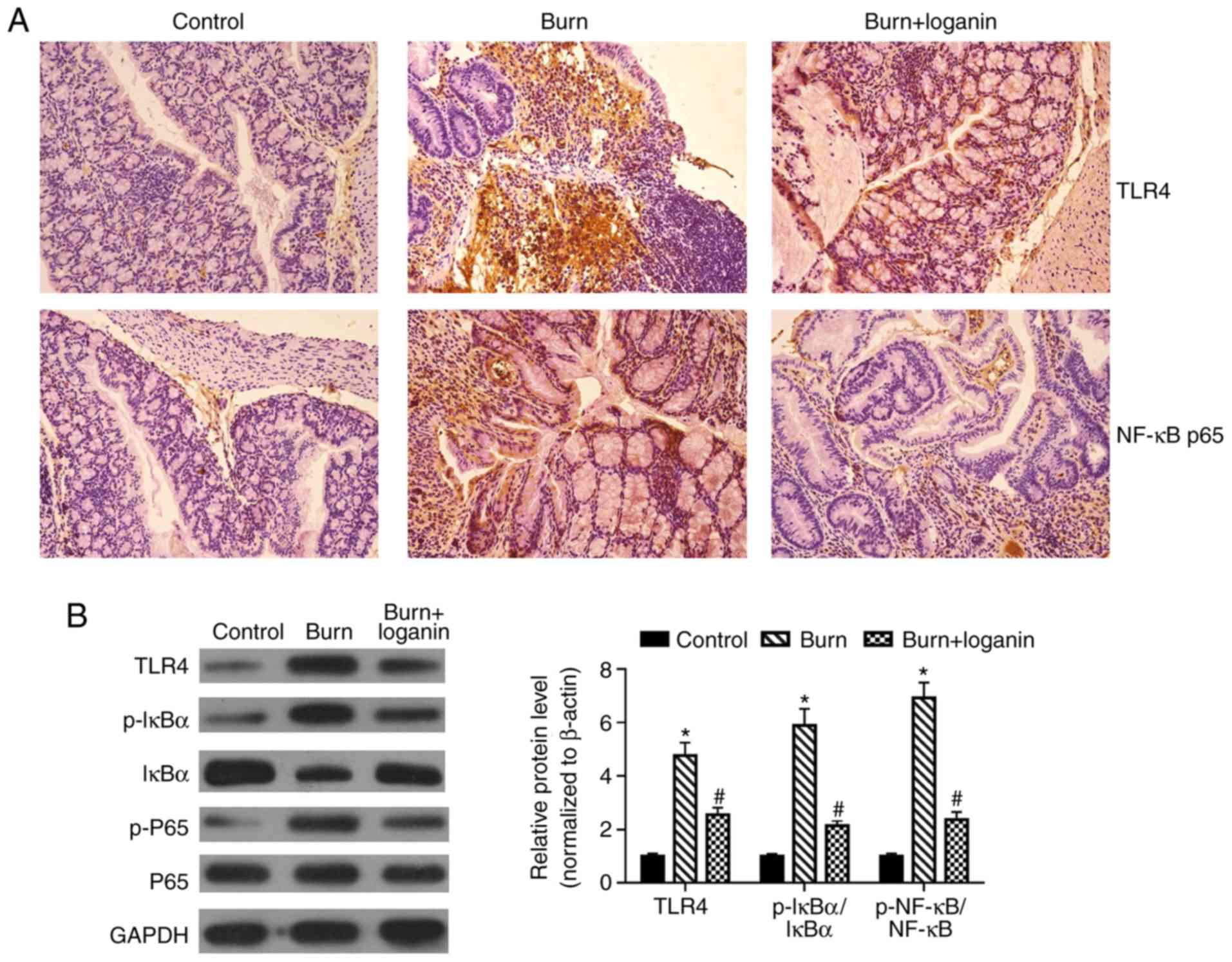
Loganin suppresses severe burn-induced
TLR4/NF-κB signaling pathway activation in intestinal tissues
The TLR/NF-κB signaling pathway plays an important
role in inflammation and oxidative stress (9,10).
Hence, the present study investigated whether loganin could inhibit
burns-induced TLR4/NF-κB signaling pathway activation.
Immunohistochemical analysis suggested that loganin limited the
expression of TLR4 and NF-κB p65 in intestinal tissues of burned
rats (Fig. 5A). Furthermore, the
burn group displayed significant TLR4 upregulation and induction of
IκBα and NF-κB p65 phosphorylation in intestinal tissues compared
with the control group (P<0.05). By contrast, compared with the
burn group, administration of loganin downregulated TLR4 protein
levels, impaired IκBα degradation and reduced the phosphorylation
of NF-κB p65 (Fig. 5B;
P<0.05).
Discussion
The present study demonstrated that the
administration of loganin inhibited severe burn-induced intestinal
pathology, intestinal inflammation, oxidative stress and intestinal
epithelial cell apoptosis by targeting the TLR4/NF-κB signaling
pathway.
Thermal burn injury is a leading cause of mortality
and disability worldwide (21,22).
Previous studies support the existence of intestinal injury
following severe burn in rats (23,24).
Histopathological examination in the present study further
suggested that the intestinal tissues were noticeably damaged at 24
h after 20% TBSA full-thickness burn wound induction. Severe burns
result in intestinal inflammation and oxidative stress, eventually
leading to intestinal barrier damage and gut dysfunction (8,25,26). The
present study suggested that damaged intestines of Sprague-Dawley
rats following severe burn injuries displayed intracellular ROS
overproduction, subsequent excessive consumption of SOD, CAT,
GSH-Px and increased lipid peroxidation compared with control rats.
Oxidative stress can provoke the production of inflammatory
cytokines, which contribute to chronic and acute inflammatory
reactions (27). The present study
observed a significant increase in the mRNA and protein levels of
the proinflammatory cytokines TNF-α, IL-6 and IL-1β in the
intestinal tissues of rats with burns (P<0.05). Growing evidence
suggests that gut epithelial apoptosis is implicated in changes in
mucosal integrity and impaired intestinal barrier function after
severe burns, provoking further oxidative stress and inflammation
(28,29). The present study demonstrated that
the percentage of apoptotic cells in the intestinal tissues
significantly increased 24 h after the burn injury (P<0.05). The
TLR4/NF-κB signaling pathway is involved in the regulation of the
inflammatory response and cell apoptosis (9,10). Wang
et al (8) suggested that
isoquercetin mitigated myocardial inflammation and apoptosis by
suppressing the TLR4/NF-κB signaling pathway. Furthermore,
Minden-Birkenmaier et al (26) indicated that harmine attenuated
LPS-induced acute kidney injury by reducing oxidative stress and
inflammatory responses via inhibition of the TLR4-NF-κB signaling
pathway. Similarly, the present study suggested that severe burn
injury induced NF-κB signaling pathway activation and TLR4
upregulation.
It is generally accepted that loganin is a bioactive
component with anti-inflammatory and antioxidant effects (13,16). For
example, Fei et al (27)
illustrated that loganin could attenuate neuroinflammation in BV-2
microglia cells by inhibiting the activation of the TLR4 signaling
pathway. Furthermore, Carter et al (28) suggested that loganin exerted a
neuroprotective effect by decreasing neuronal apoptosis and
oxidative stress. The present study used a rat burn injury model to
evaluate the role of loganin in intestinal protection following
burn injury. The results revealed that the intragastric injection
of loganin (50 mg/kg) after severe burn injury ameliorated
intestinal pathological changes. Following the intragastric
administration of loganin in the burn + loganin group, the
production of ROS, MDA, TNF-α, IL-6 and IL-1β were significantly
reduced, while SOD, CAT and GSH-Px levels improved in intestinal
tissue samples, compared with the untreated burn group (P<0.05).
Loganin has been well documented to antagonize the oxidative
stress-induced apoptosis of various cell types, including renal
mesangial cells (13), neurons
(30) and hepatocytes (31) in vivo. The present study
demonstrated that loganin treatment significantly decreased the
percentage of apoptotic intestinal epithelial cells following
severe burn injury (P<0.05). This suggested that loganin
protected intestinal epithelial cells from burns-induced apoptosis.
Additionally, loganin inhibited the burn injury-induced
upregulation of cleaved caspase-3 and Bax expression, and
downregulation of Bcl-2 expression. Furthermore, treatment with
loganin significantly attenuated the burn injury-induced TLR4
activation and phosphorylation of NF-κB p65 (P<0.05). These
results suggested that the protective effects of loganin against
burns-induced apoptosis in the intestinal epithelium may be due to
mediation of the TLR4/NF-κB signaling pathway.
Despite the lack of clinical data and in
vitro experiments, the present study suggested that the
anti-inflammatory and antioxidant effects of loganin in severe burn
injury may be mediated by targeting the TLR4/NF-κB signaling
pathway. Further investigations are required to verify the
therapeutic action of loganin in severe burn injury.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW and LX designed the study. KS, CX and XM
conducted the experiments and analyzed the data. JY conducted the
experiments and wrote the manuscript.
Ethics approval and consent to
participate
This study was approved by the Animal Care and Use
Committee of The Affiliated Hospital of Chengde Medical University.
The patients provided consent to participate.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fay KT, Ford ML and Coopersmith CM: The
intestinal microenvironment in sepsis. Biochim Biophys Acta Mol
Basis Dis. 1863:2574–2583. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
He W, Wang Y, Wang P and Wang F:
Intestinal barrier dysfunction in severe burn injury. Burns Trauma.
7(24)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Qin Y, Hamilton JL, Bird MD, Chen MM,
Ramirez L, Zahs A, Kovacs EJ and Makowski L: Adipose inflammation
and macrophage infiltration after binge ethanol and burn injury.
Alcohol Clin Exp Res. 38:204–213. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Earley ZM, Akhtar S, Green SJ, Naqib A,
Khan O, Cannon AR, Hammer AM, Morris NL, Li X, Eberhardt JM, et al:
Burn injury alters the intestinal microbiome and increases gut
permeability and bacterial translocation. PLoS One.
10(e0129996)2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gong ZY, Yuan ZQ, Dong ZW and Peng YZ:
Glutamine with probiotics attenuates intestinal inflammation and
oxidative stress in a rat burn injury model through altered iNOS
gene aberrant methylation. Am J Transl Res. 9:2535–2547.
2017.PubMed/NCBI
|
|
6
|
Haines RJ, Wang CY, Yang CGY, Eitnier RA,
Wang F and Wu MH: Targeting palmitoyl acyltransferase ZDHHC21
improves gut epithelial barrier dysfunction resulting from
burn-induced systemic inflammation. Am J Physiol Gastrointest Liver
Physiol. 313:G549–G557. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li JY, Sheng ZY, Lu Y, Yu Y and Zhou BT:
Severe trauma induced intestinal barrier function injury and
protection. World Chin J Digestol. 8:1093–1096. 2000.
|
|
8
|
Wang Z, Chen R, Zhu Z, Zhang X and Wang S:
Effects of insulin combined with ethyl pyruvate on inflammatory
response and oxidative stress in multiple-organ dysfunction
syndrome rats with severe burns. Am J Emerg Med. 34:2154–2158.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang L, Liu XH, Chen H, Chen ZY, Weng XD,
Qiu T and Liu L: Picroside II protects rat kidney against
ischemia/reperfusion-induced oxidative stress and inflammation by
the TLR4/NF-κB pathway. Exp Ther Med. 9:1253–1258. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang L, Sun S, Li W, Zhang W, Wang X and
Yang SY: Effect of Scutellarin inhibits collagen-induced arthritis
through TLR4/NF-κB-mediated inflammation. Mol Med Rep.
16:5555–5560. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Latorre E, Mendoza C, Layunta E, Alcalde
AI and Mesonero JE: TLR2, TLR3, and TLR4 activation specifically
alters the oxidative status of intestinal epithelial cells. Cell
Stress Chaperones. 19:289–293. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhou M, Xu W, Wang J, Yan J, Shi Y, Zhang
C, Ge W, Wu J, Du P and Chen Y: Boosting mTOR-dependent autophagy
via upstream TLR4-MyD88-MAPK signalling and downstream NF-κB
pathway quenches intestinal inflammation and oxidative stress
injury. EBioMedicine. 35:345–360. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xu H, Shen J, Liu H, Shi Y, Li L and Wei
M: Morroniside and loganin extracted from Cornus officinalis have
protective effects on rat mesangial cell proliferation exposed to
advanced glycation end products by preventing oxidative stress. Can
J Physiol Pharmacol. 84:1267–1273. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Kim MJ, Bae GS, Jo IJ, Choi SB, Kim DG,
Shin JY, Lee SK, Kim MJ, Shin S, Song HJ and Park SJ: Loganin
protects against pancreatitis by inhibiting NF-κB activation. Eur J
Pharmacol. 765:541–550. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tseng YT, Chen CS, Jong YJ, Chang FR and
Lo YC: Loganin possesses neuroprotective properties, restores SMN
protein and activates protein synthesis positive regulator Akt/mTOR
in experimental models of spinal muscular atrophy. Pharmacol Res.
111:58–75. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li Y, Li Z, Shi L, Zhao C, Shen B, Tian Y
and Feng H: Loganin inhibits the inflammatory response in mouse
3T3L1 adipocytes and mouse model. Int Immunopharmacol. 36:173–179.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rajabi M, Mohaddes G, Farajdokht F, Nayebi
Rad S, Mesgari M and Babri S: Impact of loganin on pro-inflammatory
cytokines and depression- and anxiety-like behaviors in male
diabetic rats. Physiol Int. 105:199–209. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhou Q, Price DD, Caudle RM and Verne GN:
Visceral and somatic hypersensitivity in a subset of rats following
TNBS-induced colitis. Pain. 134:9–15. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nakazawa H, Chang K, Shinozaki S, Yasukawa
T, Ishimaru K, Yasuhara S, Yu YM, Martyn JA, Tompkins RG, Shimokado
K and Kaneki M: iNOS as a driver of inflammation and apoptosis in
mouse skeletal muscle after burn injury: Possible involvement of
Sirt1 S-Nitrosylation-mediated acetylation of p65 NF-κB and p53.
PLoS One. 12(e0170391)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Möller H, Falster K, Ivers R, Clapham K,
Harvey L and Jorm L: High rates of hospitalised burn injury in
Indigenous children living in remote areas: A population data
linkage study. Aust N Z J Public Health. 42:108–109.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Botchey IM Jr, Hung YW, Bachani AM, Paruk
F, Mehmood A, Saidi H and Hyder AA: Epidemiology and outcomes of
injuries in Kenya: A multisite surveillance study. Surgery. 162
(Suppl):S45–S53. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yuan ZQ, Peng YZ, Li XL, Huang YS and Yang
ZC: Induction of heat shock protein 70 by sodium arsenite
attenuates burn-induced intestinal injury in severe burned rats.
Burns. 34:247–253. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sun YX, Han LN, Gao Z, Wu XS, Zhou M, Wang
F, Peszel A and Chen XL: 200 mM hypertonic saline resuscitation
attenuates intestinal injury and inhibits p38 signaling in rats
after severe burn trauma. Burns. 43:1693–1701. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
O'Dea KP, Porter JR, Tirlapur N, Katbeh U,
Singh S, Handy JM and Takata M: Circulating microvesicles are
elevated acutely following major burns injury and associated with
clinical severity. PLoS One. 11(e0167801)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Minden-Birkenmaier BA, Meadows MB,
Cherukuri K, Smeltzer MP, Smith RA, Radic MZ and Bowlin GL: The
effect of manuka honey on dHL-60 cytokine, chemokine, and
matrix-degrading enzyme release under inflammatory conditions. Med
One. 4(e190005)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fei Y, Sun L, Yuan C, Jiang M, Lou Q and
Xu Y: CFTR ameliorates high glucose-induced oxidative stress and
inflammation by mediating the NF-κB and MAPK signaling pathways in
endothelial cells. Int J Mol Med. 41:3501–3508. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Carter SR, Zahs A, Palmer JL, Wang L,
Ramirez L, Gamelli RL and Kovacs EJ: Intestinal barrier disruption
as a cause of mortality in combined radiation and burn injury.
Shock. 40:281–289. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li YM, Wang HB, Zheng JG, Bai XD, Zhao ZK,
Li JY and Hu S: Dimethyl sulfoxide inhibits zymosan-induced
intestinal inflammation and barrier dysfunction. World J
Gastroenterol. 21:10853–10865. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kwon SH, Kim JA, Hong SI, Jung YH, Kim HC,
Lee SY and Jang CG: Loganin protects against hydrogen
peroxide-induced apoptosis by inhibiting phosphorylation of JNK,
p38, and ERK 1/2 MAPKs in SH-SY5Y cells. Neurochem Int. 58:533–541.
2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Park CH, Tanaka T, Kim JH, Cho EJ, Park
JC, Shibahara N and Yokozawa T: Hepato-protective effects of
loganin, iridoid glycoside from Corni Fructus, against
hyperglycemia-activated signaling pathway in liver of type 2
diabetic db/db mice. Toxicology. 290:14–21. 2011.PubMed/NCBI View Article : Google Scholar
|