Introduction
Delivery pain has been reported to be one of the
most painful experiences of the majority of women, which can cause
potential harm to both the mother and the baby (1-3).
Epidural blockade is the most effective method of labor analgesia,
which facilitates painless labor and can be customized for each
patient (4). However, there are also
a number of disadvantages associated with epidural labor analgesia,
including motor blockade, a lengthened second stage of labor and
hypotension (5). Anesthetists have
been seeking strategies to improve the effects of analgesia and
avoid the aforementioned side effects, and the use of opioids and
α2-adrenoreceptor agonists as adjuvant drugs is an example
(6,7).
Sufentanil and dexmedetomidine (Dex) have been used
individually as adjuvants to ropivacaine for epidural labor
analgesia to alleviate the side effects. Debon et al
(8) reported that the use of
sufentanil as an adjuvant increased the duration of epidural labor
analgesia. The use of opioids as adjuvants results in a high
incidence of respiratory depression, urinary retention, nausea,
vomiting and pruritus. The safety of epidural and spinal
administration of Dex has been demonstrated in humans, where
epidural administration was hypothesized to block sympathetic nerve
slower and therefore, increased safely (9,10).
Compared with ropivacaine alone, the addition of Dex as an adjuvant
to epidural ropivacaine can reduce the feeling of pain, but does
not result in motor blockage (11).
In addition, Zhang et al (12) reported that the analgesic effect and
duration of the first stage of labor during epidural analgesia (EA)
with 0.1% ropivacaine + Dex was superior compared with 0.1%
ropivacaine + sufentanil. In previous studies, intrathecal Dex
lengthened the sensory and motor blockage during hysteroscopic
surgery and cesarean sections (13,14).
Therefore, it has been hypothesized that the combination of Dex and
sufentanil as epidural adjuvants could enhance the beneficial
effects of each adjuvant. To the best of our knowledge, the present
study investigated for the first time whether the combination of
Dex and sufentanil in labor analgesia could improve the effects of
analgesia and decrease the incidence of associated side effects.
The results of the present study may provide insight into epidural
blockade and provide a novel strategy for labor analgesia.
Materials and methods
Study subjects
The present randomized, double-blinded, prospective,
controlled trial was performed at Shenzhen Maternity and Child
Healthcare Hospital, Southern Medical University. The present study
was approved by the Shenzhen Maternity and Child Healthcare
Hospital Ethics Committee (approval no. SZFY2018020798).
All parturient women undergoing vaginal delivery and
requesting labor analgesia in the hospital between March 2018 and
October 2018 were considered for inclusion. The inclusion criteria
were as follows: i) Aged 20-35 years; ii) American Society of
Anesthesiology Physical Status I/II (15); iii) a single fetus; iv) ≥37 gestation
weeks; v) cervical dilatation of 3 cm; and vi) provided written
informed consent. The exclusion criteria were as follows: i)
Refusal to participate; ii) aged <18 years; iii) endocrine
diseases, obesity, hypertension or hypotension; iv) fetal
compromise; v) allergy to study agents; or vi) an inability to
communicate. Furthermore, if the epidural anesthesia failed, the
epidural catheter was dislodged, an inadvertent epidural puncture
occurred or a rapid progress in labor occurred (delivery in <120
min), the patient was excluded from the final analysis.
Group allocation
Written informed consent was obtained from all
included parturient women. A total of 108 parturient women were
assigned to three groups: i) Group RD received 0.1% ropivacaine +
0.5 µg/ml Dex; ii) Group RS received 0.1% ropivacaine + 0.5 µg/ml
sufentanil; and iv) Group RDS received 0.1% ropivacaine + 0.25
µg/ml Dex + 0.25 µg/ml sufentanil. All treatments were administered
by epidural injection.
Parturient women were randomly assigned to the three
treatment groups by an independent investigator using a
computer-generated random number table. The grouping assignment was
sealed in envelopes and not opened until just before the anesthesia
was administered. To maintain blinding, the investigators and
patients were not informed of the group assignments.
Sample size
Based on the preliminary data (data not shown), the
visual analog scale (VAS) score (16) at 10 min after epidural placement
[mean (standard deviation)] in the RS group was 5.0 (2.5), which
was reduced to 3.7 (2.1) in the RDS group. By setting the VAS score
as the primary variable, 30 patients were assigned to each group
with a statistical significance of 0.05 and a power of 90%. To
compensate for possible dropouts or excluded cases, 36 parturient
women were assigned to each group.
Procedures
To eliminate any possible effects of anesthetic
technique, the same anesthetist group performed all procedures.
When cervical dilatation reached 3 cm, EA was performed at the
L2/L3 intervertebral space using a 16G epidural needle to insert an
epidural catheter 3-4 cm into the epidural space. Following the
administration of a test dose of 3 ml 1% lidocaine for 5 min,
parturients received 10 ml 0.5 µg/ml Dex (Group RD), 0.5 µg/ml
sufentanil (Group RS) or 0.25 µg/ml Dex + 0.25 µg/ml sufentanil
(Group RDS), together with 0.1% ropivacaine as the loading dose.
The maintenance of patient controlled EA was administered after the
loading dose using an Apon PCA pump (Jiangsu Apon Medical
Technology Co., Ltd.). The pumps were set at a rate of 7 ml/h with
a rescue bolus of 7 ml (lockout 25 min; limit 25 ml/h). Patients
experiencing inadequate analgesia could request an additional 5 ml
bolus of the medication solution, via epidural administration by
the nurse.
If hypotension (90/60 mmHg) occurred, the patient
was placed in a left-leaning position or phenylephrine was
administered as a vasoconstrictor active drug. After delivery, the
administration of the drugs was terminated and the epidural
catheter was removed.
Data collection
The demographic and baseline measurements, including
age, height, weight and gestational age, were recorded. In the
present study, the VAS score (0, no pain; 10, most serious pain)
was evaluated prior to epidural placement (baseline) and at 5, 10,
20, 30, 60, 90 and 120 min after the loading dose was injected. The
administration of the loading dose was considered to be 0 min. The
time of onset, which was defined as the duration between the end of
drug administration and the patient displaying a VAS score <3,
was observed. The duration of each labor period (active period,
second stage and third stage), Apgar score (11), umbilical vein pH, cesarean delivery
rate, bolus frequency and total volume of anesthetic solution were
also recorded. Evaluation of motor blockage was conducted using the
Bromage scoring system (1, able to lift the legs above the table;
2, able to bend the knees; 3, able to move the feet only; 4, no
movement in the feet or legs) (7).
Additionally, hypotension (systolic blood pressure <90 mmHg or
<30% of the base value), bradycardia (heart rate <60 bpm),
nausea, vomiting, shivering and pruritus were monitored.
In the present study, the primary outcome was the
VAS score, and the secondary outcomes were the onset time, duration
of each labor stage, Apgar score, cesarean delivery rate, bolus
frequency, total volume of anesthetic solution, Bromage score and
other side effects.
Statistical analysis
Statistical analyses were conducted using SPSS
software (version 20.0; IBM Corp.). The one-sample
Kolmogorov-Smirnov test was used to assess the normality of the
quantitative data. Quantitative variables are presented as the mean
± standard deviation and categorical variables are presented as
numbers (%; n, %). Quantitative variables were analyzed using
one-way ANOVA followed by the Bonferroni post hoc test. Categorical
variables were analyzed using the χ2 test or Fisher's
exact test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient variables
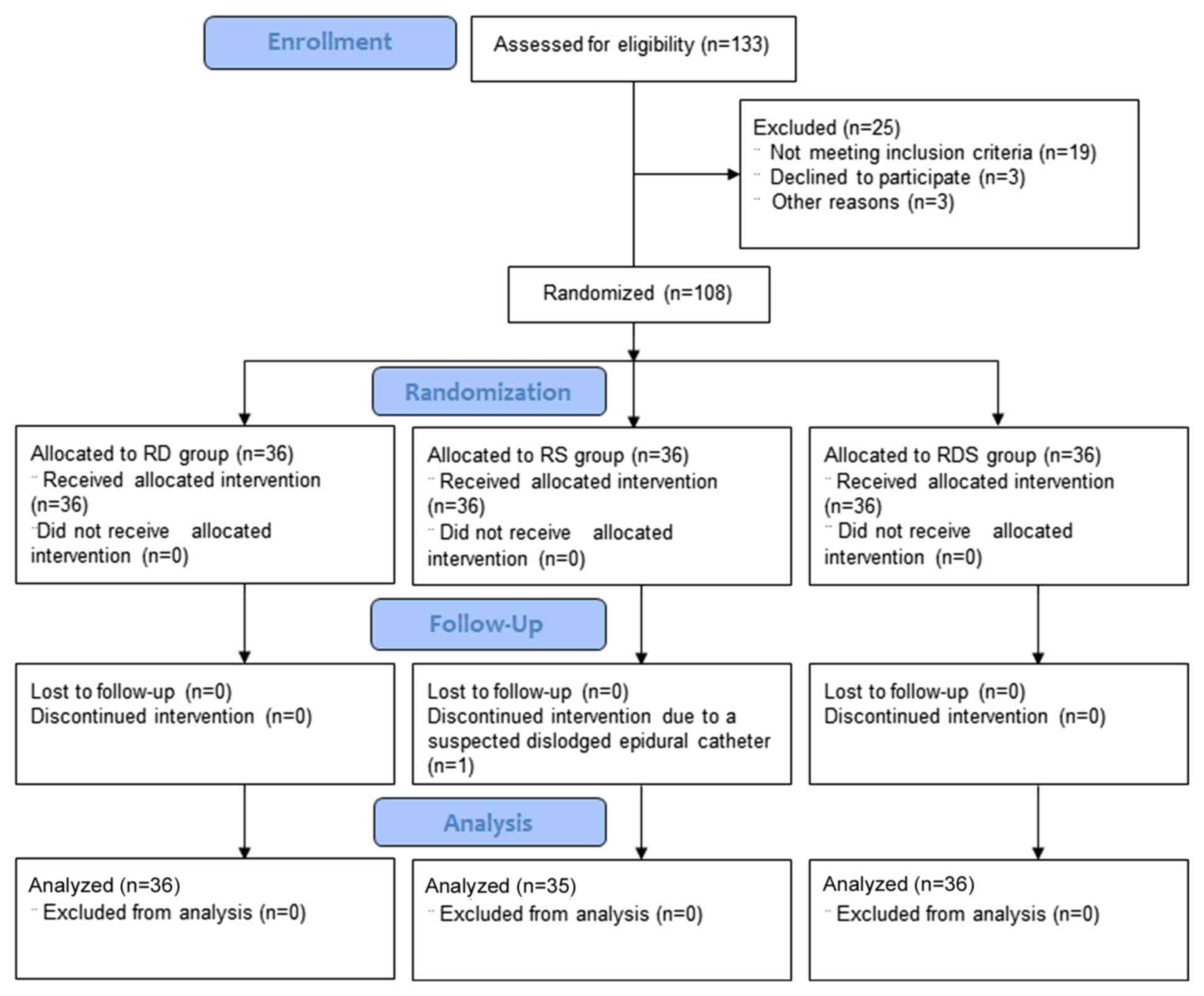
A total of 108 parturient women were recruited into
the present study; however, one woman was excluded during the study
due to a protocol deviation resulting in a suspected dislodged
epidural catheter. Therefore, 107 parturient women were included in
the final analysis among the RD, RS and RDS groups (Fig. 1). The demographic variables of the
parturient women in the three groups were comparable, including
age, height, weight and gestational age (P>0.05; Table I).
 | Table IDemographic baseline variables. |
Table I
Demographic baseline variables.
| Variable | Group RS
(n=35) | Group RD
(n=36) | Group RDS
(n=36) | P-value |
|---|
| Age (years) | 30.13±4.86 | 29.23±3.88 | 30.83±3.77 | 0.340 |
| Height (cm) | 159.03±4.49 | 159.61±5.13 | 159.70±4.42 | 0.829 |
| Weight (kg) | 64.73±8.85 | 65.21±7.15 | 66.35±9.01 | 0.756 |
| Gestational age
(weeks) | 37.41±3.94 | 39.10±0.91 | 38.22±2.92 | 0.063 |
Primary outcomes
The VAS scores at 10 min after epidural placement in
Groups RDS (2.44±1.27 vs. 5.13±2.74; P<0.001) and RD
(3.67±2.71 vs. 5.13±2.74; P<0.05) were significantly
decreased compared with Group RS (Table
II). Furthermore, at the 5, 10, 20, 30, 60, 90 and 120 min time
points, Group RDS displayed significantly lower VAS scores compared
with Group RS (P<0.05; Table II
and Fig. 2). In addition, the VAS
scores in Group RDS were significantly lower compared with Group RD
at 20 and 30 min (P<0.05; Table
II and Fig. 2). Group RD also
displayed significantly lower VAS scores compared with Group RS at
10, 60, 90 and 120 min (P<0.05; Table II and Fig. 2).
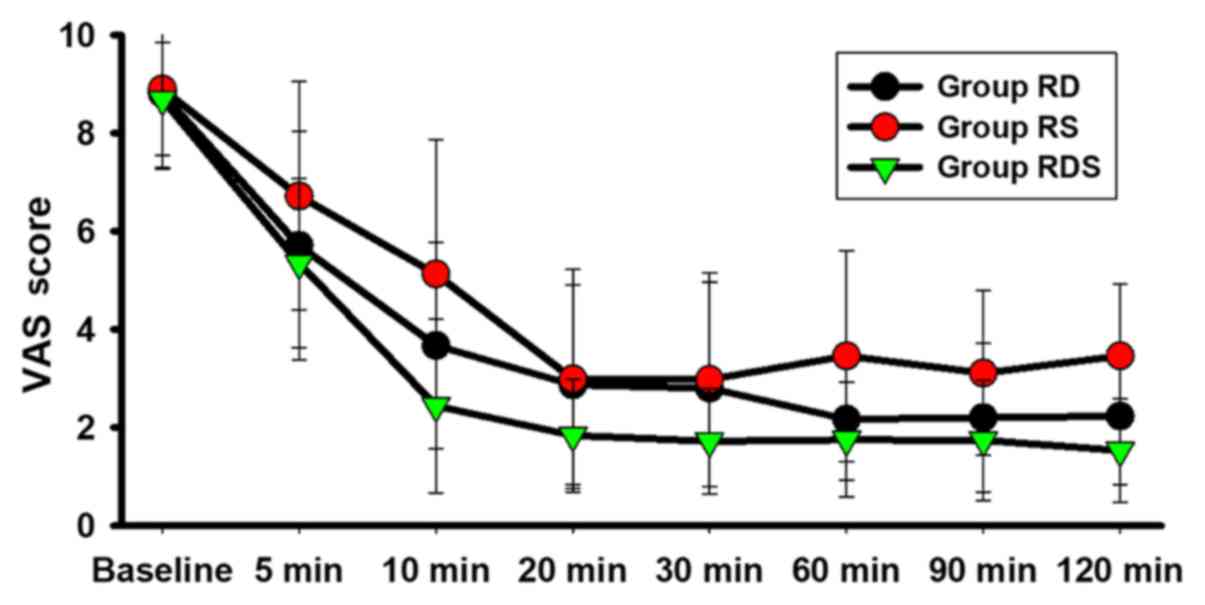 | Figure 2VAS among the three groups. Data are
presented as the mean ± SD. Patients were randomly assigned to the
three groups. The VAS score (0, no pain; 10, most serious pain) was
evaluated before epidural placement (baseline), and 5, 10, 20, 30,
60, 90 and 120 min after the loading dose was injected, which was
considered to be 0 min. VAS, Visual Analog Scale; RS, sufentanil +
ropivacaine epidural; RD, dexmedetomidine + ropivacaine epidural;
RDS, dexmedetomidine + sufentanil + ropivacaine epidural. |
 | Table IIVisual Analog scale at different time
points. |
Table II
Visual Analog scale at different time
points.
| Time (min) | Group RS
(n=35) | Group RD
(n=36) | Group RDS
(n=36) | P-value |
|---|
| Baseline | 8.90±1.60 | 8.81±1.54 | 8.69±1.15 | 0.822 |
| 5 | 6.72±2.33 | 5.71±3.33 |
5.35±1.72a | 0.103 |
| 10 | 5.13±2.74 |
3.67±2.71a |
2.44±1.27b | 0.000 |
| 20 | 2.99±1.44 | 2.87±1.53 |
1.84±1.15a,c | 0.013 |
| 30 | 2.98±1.75 | 2.80±2.16 |
1.72±1.07a,c | 0.011 |
| 60 | 3.46±2.18 |
2.17±1.24a |
1.75±1.17a | 0.004 |
| 90 | 3.11±1.68 |
2.20±1.51a |
1.74±1.22a | 0.009 |
| 120 | 3.46±1.47 |
2.23±1.40a |
1.53±1.05b | 0.000 |
Secondary outcomes
The parturient women in the three groups were
comparable for the following factors: Duration of each labor stage,
Apgar scores, umbilical vein pH and cesarean delivery rate
(P>0.05; Table III). In
addition, no patient experienced inadequate analgesia; therefore,
the additional 5 ml bolus analgesia was not administered to any of
the patients.
 | Table IIIData of parturient women and neonatal
outcome. |
Table III
Data of parturient women and neonatal
outcome.
| Variable | Group RS
(n=35) | Group RD
(n=36) | Group RDS
(n=36) | P-value |
|---|
| Duration of the
first labor stage (min) | 396.11±14.56 | 347.93±10.15 | 396.26±9.37 | 0.819 |
| Duration of the
second labor stage (min) | 30.59±7.67 | 52.54±6.49 | 42.74±6.799 | 0.127 |
| Duration of the
third labor stage (min) | 10.92±6.38 | 10.46±7.82 | 11.59±10.07 | 0.879 |
| Apgar
scorea |
|
1 min
≥7 | 35(100) | 36(100) | 36(100) | 1.000 |
|
5 min
≥9 | 35(100) | 36(100) | 36(100) | 1.000 |
| Umbilical vein
pH | 7.21±0.08 | 7.21±0.02 | 7.20±0.05 | 0.793 |
| Cesarean delivery
(%) | 13.3 | 10.0 | 10.0 | 0.897 |
The onset time of Group RDS was significantly
shorter compared with Groups RS and RD (P<0.05), and the
parturient women in Groups RDS and RD required a reduced injection
volume and fewer local anesthetic administrations compared with
Group RS (P<0.05; Table IV).
Motor blockage in Group RD was more severe compared with Groups RS
(9/19/2/0 vs. 27/2/1/0; P<0.001) and RDS (9/19/2/0 vs. 22/8/0/0;
P<0.001; Table IV).
Additionally, the incidence of pruritus was significantly lower in
Groups RD and RDS compared with Group RS (P<0.05; Table V).
 | Table IVOnset time, local anesthetic
requirement and Bromage score. |
Table IV
Onset time, local anesthetic
requirement and Bromage score.
| Variable | Group RS
(n=35) | Group RD
(n=36) | Group RDS
(n=36) | P-value |
|---|
| Onset time
(min) | 15.50±2.67 | 12.97±3.13 |
9.68±1.26a,c | 0.037 |
| Total volume of
anesthetic solution (ml) | 65.44±5.64 | 42.65±6.44 |
50.34±6.56a | 0.043 |
| Bolus
frequency | 2.80±0.92 |
0.10±0.31a |
0.80±0.78a | 0.026 |
| Bromage score
(1/2/3/4) | 27/2/1/0 |
9/19/2/0b |
22/8/0/0d | 0.000 |
 | Table VAdverse events among the three
groups. |
Table V
Adverse events among the three
groups.
| Event | Group RS (n=35)
(%) | Group RD (n-36)
(%) | Group RDS (n=36)
(%) | P-value |
|---|
| Hypotension | 0 (0.0) | 1 (2.8) | 0 (0.0) | 1.000 |
| Bradycardia | 0 (0.0) | 1 (2.8) | 0 (0.0) | 1.000 |
| Nausea | 1 (2.9) | 0 (0.0) | 0 (0.0) | 0.327 |
| Vomiting | 1 (2.9) | 1 (2.8) | 0 (0.0) | 0.771 |
| Shivering | 2 (5.7) | 3 (8.3) | 2 (5.6) | 1.000 |
| Pruritus | 5 (14.3) | 0
(0.0)a | 0
(0.0)a | 0.003 |
| Urinary
retention | 2 (5.7) | 2 (5.6) | 1 (2.8) | 0.869 |
There were no statistical differences in the
proportion of patients with nausea, vomiting, shivering,
bradycardia, hypotension and urinary retention among the three
groups (P>0.05; Table V).
Discussion
Labor pain is a complicated sensory reaction that
occurs during delivery. EA with local anesthetics is the main
strategy used to induce labor analgesia, and the most
representative adjuvants used in clinical practice for labor
analgesia are opioids and α2-adrenergic receptor agonists (17,18).
Compared with the use of 0.1% ropivacaine + 0.5 µg/ml Dex or 0.5
µg/ml sufentanil, 0.1% ropivacaine in combination with 0.25 µg/ml
Dex and 0.25 µg/ml sufentanil resulted in an improved labor
analgesia effect, quicker onset time, reduced need for local
anesthetics and fewer side effects.
Sufentanil has been used as local anesthetic
adjuvant in epidural labor analgesia in a number of previous
studies (1,12); therefore, sufentanil in combination
with ropivacaine was used as the positive control in the present
study. Dex has also been reported to display hemodynamic stability,
pain alleviation, improved stress responses without respiratory
depression when administered intravenously and intratracheally
(19,20), and improved epidural and neuraxial
blocks (21,22). Zhao et al (11) reported that compared with ropivacaine
alone, the addition of Dex to epidural ropivacaine reduced the
feeling of pain and did not result in motor blockage. Abdallah
et al (23) also reported
that both perineural and intravenous Dex effectively prolonged the
interscalene brachial plexus block analgesic duration and reduced
sufentanil consumption without prolonging motor blockade. By
contrast, certain studies have indicated that intrathecal Dex
lengthens sensory and motor blockage during hysteroscopic surgery
and cesarean sections (13,14), and the addition of epidural opioids
results in a high incidence of respiratory depression, urinary
retention, nausea, vomiting and pruritus (24,25). Qin
et al (26) also reported
that the combination of Dex and sufentanil for postoperative
analgesia in patients with partial laryngectomy resulted in
significantly reduced sufentanil consumption, improved analgesia, a
reduced frequency of coughing episodes and improved sleep quality.
To the best of our knowledge, the present study was the first study
to investigate the combination of epidural Dex and sufentanil in
labor analgesia. In the present study, Group RDS displayed lower
VAS scores compared with Group RS at all time points, and compared
with Group RD at 20 and 30 min after epidural placement. The
results indicated that the combined administration of Dex and
sufentanil as adjuvants to local anesthetic displayed an improved
analgesic effect compared with the use of either drug alone.
Both 0.5 µg/ml Dex and 0.5 µg/ml sufentanil have
been used as adjuvants in clinical practice (12,27). In
the present study, the combination of 0.25 µg/ml Dex + 0.25 µg/ml
sufentanil + 0.1% ropivacaine was administered via an epidural for
labor analgesia, and the efficiency and safety of the combined
treatment was compared with 0.1% ropivacaine + 0.5 µg/ml Dex or 0.5
µg/ml sufentanil. The dose used in the present study was determined
according to a preliminary study, which indicated that for epidural
labor analgesia the optimal concentration of Dex was 0.5 µg/ml when
combined with 0.1% ropivacaine; therefore, 0.5 µg/ml Dex was used
as an adjuvant to epidural ropivacaine in labor analgesia.
Furthermore, the addition of 5 µg intrathecal Dex to 10 µg fentanyl
lengthened the analgesia duration and lowered the incidence of
adverse effects compared with the use of intrathecal 10 µg Dex or
intrathecal 20 µg fentanyl alone (28). Therefore, 0.25 µg/ml Dex and 0.25
µg/ml sufentanil were used as adjuvants in combination with 0.1%
ropivacaine epidurally for labor analgesia in the present
study.
Koraki et al (29) reported that the onset time of
epidural Dex combined with ropivacaine was ~15 min, which was
longer compared with the results of the present study. The
inconsistency could be explained by the different ropivacaine
concentrations used in each study. The combined use of ropivacaine,
0.25 µg/ml Dex and 0.25 µg/ml sufentanil displayed a quicker onset
time, enhanced the analgesic effect, decreased the VAS scores,
reduced the bolus frequency and limited motor blockage without
causing adverse side effects compared with the use of either
adjuvants alone. The results indicated that Dex synergized with
sufentanil systemically and regionally, which was consistent with
previously reported clinical results (26,30).
The analgesic effect of Dex is not completely
understood. Eisenach et al (31) reported that Dex is present in the
cerebrospinal fluid rapidly after administration and binds highly
to α2-receptors in the spinal cord. Marhofer et al (32) demonstrated that the analgesic effect
of Dex was primarily mediated at the spinal level; therefore,
epidural administration is recommended. Yang et al (33) reported that intraperitoneal Dex
displayed a dose-dependent analgesic effect by inhibiting
hyperpolarization-activated cyclic nucleotide-gated currents.
Recently, Sun et al (34)
reported that the analgesic effects of Dex were associated with its
anti-inflammatory effect. The aforementioned studies indicated that
Dex exerts analgesic effects not only via α2-adrenergic receptors,
but also by direct channel inhibition via an α2-independent
mechanism, which enables Dex to serve as an analgesic adjuvant. A
recent systematic review and meta-analysis demonstrated that the
use of Dex as an adjuvant in epidural procedures is generally safe
and well tolerated (35).
Compared with the combination of Dex and sufentanil,
0.5 µg/ml epidural Dex weakened muscle strength and induced more
severe motor block, which was consistent with previous studies
(13,14). Furthermore, the incidence of pruritus
observed in the present study was similar to the incidence reported
by Boselli et al (36). No
significant intergroup differences were detected for the three
stages of labor, umbilical vein pH or Apgar scores, which was
consistent with previous studies (7,37,38).
The present study had a number of limitations.
Firstly, the present study only investigated the efficiency and
safety of 0.25 µg/ml Dex and 0.25 µg/ml sufentanil as adjuvants to
0.1% ropivacaine; therefore, further studies should be performed
using different doses of epidural Dex and sufentanil. Secondly, the
present study was a single-center clinical trial and the
preliminary results should be verified by a large-scale multicenter
study. Thirdly, although Dex has been widely used as an epidural
drug in clinical practice and has been reported to display no
significant adverse reactions, it is still not licensed for
epidural use. In particular, the safety of Dex needs to be
investigated in a large-scale phase IV clinical trial. Finally, the
Ramsay sedation scale (39) was not
assessed in the present study; therefore the effect of Dex on the
sedative state of an individual requires further investigation.
In summary, the present study investigated the
effects of using 0.25 µg/ml Dex and 0.25 µg/ml sufentanil as
adjuvants to 0.1% ropivacaine for labor analgesia. The combined
adjuvant group displayed an improved analgesia effect, quicker
onset time, reduced need for local anesthetics and decreased rate
of pruritus compared with sufentanil (0.5 µg/ml). In addition,
compared with Dex (0.5 µg/ml), the combined adjuvant group
displayed reduced motor blockage. However, Dex is not licensed for
epidural use and its safety requires further investigation. The
results of the present study indicated that the combined use of Dex
and sufentanil increased the effectiveness of the local anesthetic
agent during epidural labor.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request
Authors' contributions
YUL designed and supervised the study. GL, YX, XW,
JS and YOL conducted the study and collected the data. XQ and HW
performed the data analysis. GL wrote the paper. XW, JS and YOL
help revised the manuscript. GL and YX contributed equally to the
work. All authors read and approved the final manuscript.
Ethical approval and consent to
participate
The present study was approved by the Shenzhen
Maternity and Child Healthcare Hospital Ethics Committee (approval
no. SZFY2018020798) and registered with the Chinese Clinical Trial
Registry Center on Feb 23, 2018 (registration no.
ChiCTR-IOR-1800014943). Written informed consent was obtained from
all parturient women.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Veenhof AA, Vlug MS, van der Pas MH,
Sietses C, van der Peet DL, de Lange-de Klerk ES, Bonjer HJ,
Bemelman WA and Cuesta MA: Surgical stress response and
postoperative immune function after laparoscopy or open surgery
with fast track or standard perioperative care: A randomized trial.
Ann Surg. 255:216–221. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Keskin HL, Keskin EA, Avsar AF, Tabuk M
and Caglar GS: Pethidine versus tramadol for pain relief during
labor. Int J Gynaecol Obstet. 82:11–16. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Elbohoty AE, Abd-Elrazek H, Abd-El-Gawad
M, Salama F, El-Shorbagy M and Abd-El-Maeboud KH: Intravenous
infusion of paracetamol versus intravenous pethidine as an
intrapartum analgesic in the first stage of labor. Int J Gynaecol
Obstet. 118:7–10. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lebovits AH, Zenetos P, O'Neill DK, Cox D,
Dubois MY, Jansen LA and Turndorf H: Satisfaction with epidural and
intravenous patient-controlled analgesia. Pain Med. 2:280–286.
2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Okholm C, Goetze JP, Svendsen LB and
Achiam MP: Inflammatory response in laparoscopic vs. open surgery
for gastric cancer. Scand J Gastroenterol. 49:1027–1034.
2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bajwa SJ, Arora V, Kaur J, Singh A and
Parmar SS: Comparative evaluation of dexmedetomidine and fentanyl
for epidural analgesia in lower limb orthopedic surgeries. Saudi J
Anaesth. 5:365–370. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Selim MF, Elnabtity AM and Hasan AM:
Comparative evaluation of epidural bupivacaine-dexmedetomidine and
bupivacaine-fentanyl on Doppler velocimetry of uterine and
umbilical arteries during labor. J Prenat Med. 6:47–54.
2012.PubMed/NCBI
|
|
8
|
Debon R, Allaouchiche B, Duflo F, Boselli
E and Chassard D: The analgesic effect of sufentanil combined with
ropivacaine 0.2% for labor analgesia: A comparison of three
sufentanil doses. Anesth Analg. 92:180–183. 2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Salgado PF, Sabbag AT, Silva PC, Brienze
SL, Dalto HP, Módolo NS, Braz JR and Nascimento P Jr: Synergistic
effect between dexmedetomidine and 0.75% ropivacaine in epidural
anesthesia. Rev Assoc Med Bras (1992). 54:110–115. 2008.(In
Portuguese). PubMed/NCBI View Article : Google Scholar
|
|
10
|
Al-Mustafa MM, Abu-Halaweh SA, Aloweidi
AS, Murshidi MM, Ammari BA, Awwad ZM, Al-Edwan GM and Ramsay MA:
Effect of dexmedetomidine added to spinal bupivacaine for
urological procedures. Saudi Med J. 30:365–370. 2009.PubMed/NCBI
|
|
11
|
Zhao Y, Xin Y and Liu Y, Yi X and Liu Y:
Effect of epidural dexmedetomidine combined with ropivacaine in
labor analgesia: A randomized double-blinded controlled study. Clin
J Pain. 33:319–324. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang T, Yu Y, Zhang W and Zhu J:
Comparison of dexmedetomidine and sufentanil as adjuvants to local
anesthetic for epidural labor analgesia: A randomized controlled
trial. Drug Des Devel Ther. 13:1171–1175. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Qi X, Li Y, Rahe-Meyer N, Huang X, Gu Y,
Wang X, Li Y and Wen Y: Intrathecal dexmedetomidine as adjuvant to
ropivacaine in hysteroscopic surgery: A prospective, randomized
control study. Int J Clin Pharmacol Ther. 54:185–192.
2016.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Qi X, Chen D, Li G, Huang X and Li Y, Wang
X and Li Y: Comparison of intrathecal dexmedetomidine with morphine
as adjuvants in cesarean sections. Biol Pharm Bull. 39:1455–1460.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen C, Hei Z, Xing J, Zhu Q, Qiu R, Liu
J, Gong C, Cheng N, Zhou S and Shen N: Laryngoscopic techniques
modulate anaesthesiologists' perception of halitosis in patients: A
randomised controlled trial. Eur J Anaesthesiol. 36:918–923.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li X, Chen CJ, Tan F, Pan JR, Xing JB, Zhu
QQ, Hei ZQ and Zhou SL: Effect of dexmedetomidine for attenuation
of propofol injection pain in electroconvulsive therapy: A
randomized controlled study. J Anesth. 32:70–76. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Congedo E, Sgreccia M and De Cosmo G: New
drugs for epidural analgesia. Curr Drug Targets. 10:696–706.
2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Park SJ, Shin S, Kim SH, Kim HW, Kim SH,
Do HY and Choi YS: Comparison of dexmedetomidine and fentanyl as an
adjuvant to ropivacaine for postoperative epidural analgesia in
pediatric orthopedic surgery. Yonsei Med J. 58:650–657.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen C, Huang P, Lai L, Luo C, Ge M, Hei
Z, Zhu Q and Zhou S: Dexmedetomidine improves gastrointestinal
motility after laparoscopic resection of colorectal cancer: A
randomized clinical trial. Medicine (Baltimore).
95(e4295)2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang F, Zhong H, Xie X, Sha W, Li C, Li Z,
Huang Z and Chen C: Effect of intratracheal dexmedetomidine
administration on recovery from general anaesthesia after
gynaecological laparoscopic surgery: A randomised double-blinded
study. BMJ Open. 8(e020614)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zeng X, Jiang J, Yang L and Ding W:
Epidural dexmedetomidine reduces the requirement of propofol during
total intravenous anaesthesia and improves analgesia after surgery
in patients undergoing open thoracic surgery. Sci Rep.
7(3992)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Helal SM, Eskandr AM, Gaballah KM and
Gaarour IS: Effects of perineural administration of dexmedetomidine
in combination with bupivacaine in a femoral-sciatic nerve block.
Saudi J Anaesth. 10:18–24. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Abdallah FW, Dwyer T, Chan VW, Niazi AU,
Ogilvie-Harris DJ, Oldfield S, Patel R, Oh J and Brull R: IV and
perineural dexmedetomidine similarly prolong the duration of
analgesia after interscalene brachial plexus block: A randomized,
three-arm, triple-masked, placebo-controlled trial. Anesthesiology.
124:683–695. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sztain JF, Gabriel RA and Said ET:
Thoracic epidurals are associated with decreased opioid consumption
compared to surgical infiltration of liposomal bupivacaine
following video-assisted thoracoscopic surgery for lobectomy: A
retrospective cohort analysis. J Cardiothorac Vasc Anesth.
33:694–698. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Peterson NW, Buote NJ and Bergman P:
Effect of epidural analgesia with opioids on the prevalence of
urinary retention in dogs undergoing surgery for cranial cruciate
ligament rupture. J Am Vet Med Assoc. 244:940–943. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Qin M, Chen K, Liu T and Shen X:
Dexmedetomidine in combination with sufentanil for postoperative
analgesia after partial laryngectomy. BMC Anesthesiol.
17(66)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shukla U, Prabhakar T, Malhotra K and
Srivastava D: Dexmedetomidine versus midazolam as adjuvants to
intrathecal bupivacaine: A clinical comparison. J Anaesthesiol Clin
Pharmacol. 32:214–219. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wangping Z and Ming R: Optimal dose of
epidural dexmedetomidine added to ropivacaine for epidural labor
analgesia: A pilot study. Evid Based Complement Alternat Med.
2017(7924148)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Koraki E, Stachtari C, Kapsokalyvas I,
Stergiouda Z, Katsanevaki A and Trikoupi A: Dexmedetomidine as an
adjuvant to 0.5% ropivacaine in ultrasound-guided axillary brachial
plexus block. J Clin Pharm Ther. 43:348–352. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bazin M, Bonnin M, Storme B, Bolandard F,
Vernis L, Lavergne B, Pereira B, Bazin JE and Dualé C: Addition of
clonidine to a continuous patient-controlled epidural infusion of
low-concentration levobupivacaine plus sufentanil in primiparous
women during labour. Anaesthesia. 66:769–779. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Eisenach JC, Shafer SL, Bucklin BA,
Jackson C and Kallio A: Pharmacokinetics and pharmacodynamics of
intraspinal dexmedetomidine in sheep. Anesthesiology. 80:1349–1359.
1994.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Marhofer P and Brummett CM: Safety and
efficiency of dexmedetomidine as adjuvant to local anesthetics.
Curr Opin Anaesthesiol. 29:632–637. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yang YC, Meng QT, Pan X, Xia ZY and Chen
XD: Dexmedetomidine produced analgesic effect via inhibition of HCN
currents. Eur J Pharmacol. 740:560–564. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sun L, Zhou J and Sun C: MicroRNA-211-5p
enhances analgesic effect of dexmedetomidine on inflammatory
visceral pain in rats by suppressing ERK signaling. J Mol Neurosci.
68:19–28. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang X, Wang D, Shi M and Luo Y: Efficacy
and safety of dexmedetomidine as an adjuvant in epidural analgesia
and anesthesia: A systematic review and meta-analysis of randomized
controlled trials. Clin Drug Investig. 37:343–354. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Boselli E, Debon R, Duflo F, Bryssine B,
Allaouchiche B and Chassard D: Ropivacaine 0.15% plus sufentanil
0.5 microg/ml and ropivacaine 0.10% plus sufentanil 0.5 microg/ml
are equivalent for patient-controlled epidural analgesia during
labor. Anesth Analg. 96:1173–1177, Table of contents.
2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Illuzzi JL, Greenberg JT and Mancini PA:
Epidural analgesia during the second stage of labor: A randomized
controlled trial. Obstet Gynecol. 131(742)2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Shen X, Li Y, Xu S, Wang N, Fan S, Qin X,
Zhou C and Hess PE: Epidural analgesia during the second stage of
labor: A randomized controlled trial. Obstet Gynecol.
130:1097–1103. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yan MJ, Wang T, Wu XM and Zhang W:
Comparison of dexmedetomidine or sufentanil combined with
ropivacaine for epidural analgesia after thoracotomy: A randomized
controlled study. J Pain Res. 12:2673–2678. 2019.PubMed/NCBI View Article : Google Scholar
|