Introduction
Lung cancer is becoming one of the most prevalent
malignancies in China, which is associated with a particularly high
morbidity (17.1% of cancers) and high mortality (21.6% of
cancer-related deaths) (1). Smoking,
environmental pollution, and fumes from fuel burning are among the
reported risk factors for this disease (1,2). The
majority of lung cancer cases are diagnosed at the latter stages,
which precludes curative surgery and leads to poor prognosis
(3). In China, the estimated
five-year survival rate for lung cancer is only 16.1% (4). To promote the early diagnosis and
subsequent therapeutic intervention of lung cancer, low-dose spiral
computed tomography (CT) is used as a screen. However, peripheral
pulmonary lesions (PPL), which are pre-cursors to lung cancer,
occasionally develop in positions that are difficult to visualize,
which increases the difficulty of CT-guided diagnosis.
Recently, minimally invasive methods, including
percutaneous transthoracic and thoracoscopic biopsy, have been
commonly applied for the diagnosis of PPL (5,6).
However, limitations persist with such techniques, including the
risk of pneumothorax and bleeding. Radial endobronchial
ultrasound-guided transbronchial lung biopsy (rEBUS-D-TBLB)
potentially offers a major contribution to the diagnosis of PPL
(7).
Theoretically, although the entire chest cavity is
within the detection range of ultrasound, the diagnostic positive
rate by rEBUS-TBLB is not constant throughout the chest in clinical
practice (8,9). To increase the diagnostic yield of
rEBUS-TBLB for PPL, analysis of its indications at different
locations of the lung would be of particular clinical significance.
Therefore, the present study retrospectively analyzed the data
collected from 774 patients with PPL, to explore the indications in
using rEBUS-D-TBLB for the diagnosis of PPL at various
bronchopulmonary segments and subsegments, providing a theoretical
basis for its clinical application.
Materials and methods
Subjects
The present retrospective study was approved by the
Ethics Committee of Changzhou No. 1 Hospital (Changzhou, China).
All patients provided written informed consent.
Data were collected from 774 patients (sex, 516
males and 258 females; age range, 26-85 years; mean age, 62.81±9.19
years) who underwent rEBUS-D-TBLB at the Department of Respiratory
Medicine, Changzhou No. 1 Hospital between January 1, 2015 and
August 31, 2017. General clinical data of the patients are shown in
Table I.
 | Table IClinical data from the patients. |
Table I
Clinical data from the patients.
| Indicators | No. of cases [n
(%)] |
|---|
| Sex |
|
Male | 516 |
|
Female | 258 |
| Age |
|
≤60
years | 288 |
|
>60
years | 486 |
| TNM stage |
|
IA | 25 (2.23) |
|
IIB | 29 (3.75) |
|
IIA | 16 (2.01) |
|
IIB | 11 (1.42) |
|
IIIA | 63 (8.14) |
|
IIIB | 35 (4.52) |
|
IVA | 183 (23.64) |
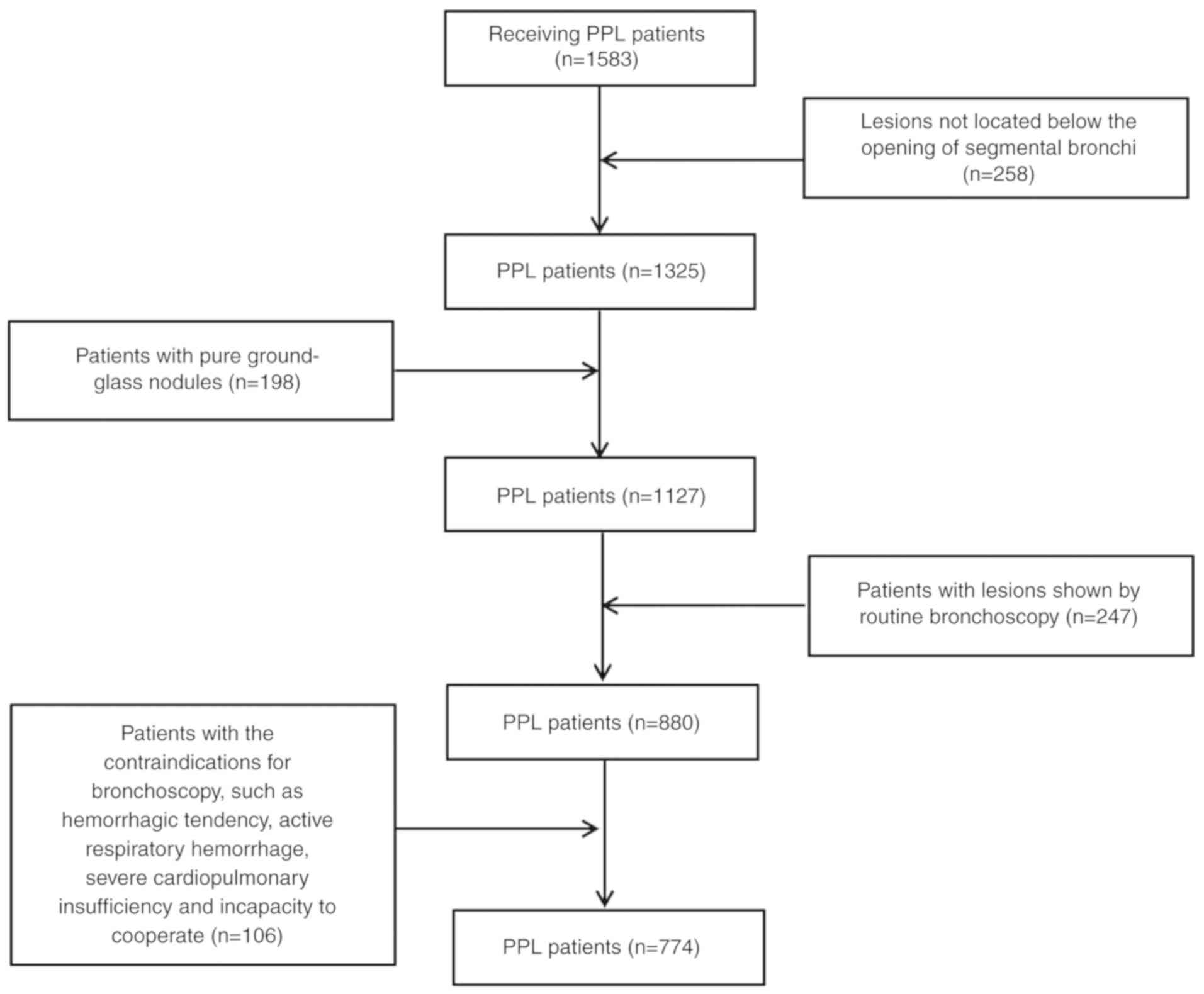
The inclusion criterion was as follows: Patients
with lesions located below the opening of segmental bronchi as
revealed by chest CT. The exclusion criteria were as follows: i)
Patients without a blurred opaque shadow of bronchial structure or
pulmonary vascular sign on chest high resolution CT; ii) patients
with lesions that were revealed by routine bronchoscopy; and iii)
patients with contraindications for bronchoscopy, including
hemorrhagic tendency, active respiratory hemorrhage, severe
cardiopulmonary insufficiency, and incapacity to cooperate. A
schematic of the selection criteria for the present study is shown
in Fig. 1.
Materials
The following equipment was used: BF-P260 flexible
bronchoscope; EU-ME1 ultrasonic mainframe; MAJ-935 ultrasonic probe
driving unit; UM-S20-17S 20 MHz ultrasonic miniprobe (1.4 mm); and
UM-S20-20R 20 MHz ultrasonic miniprobe (1.7 mm). All equipment was
purchased from Olympus Corporation.
rEBUS-D-TBLB
Prior to the rEBUS-D-TBLB operation, results of the
CT scan, including the diameter and localization of the lesion,
were carefully recorded; there was also a record of the bronchial
path through which the ultrasonic probe was to reach the
lesion.
After 6 h preoperative fasting and water
deprivation, the patients were anesthetized through inhalation of
lidocaine for 5 min. A routine bronchoscopy was conducted to search
for and assess the lesion; rEBUS-D-TBLB was subsequently performed
in cases where no definite lesion was found. Using the imaging
system as a guide, the ultrasonic probe was delivered to the
inspection location through the biopsy channel. On meeting
resistance, the ultrasonic scan was initiated, and the ultrasonic
probe was slowly pulled backwards; changes in the ultrasonic images
were closely observed until a clear ultrasonic image of the
bronchial wall where the lesion is located becomes visible. The
adjacent bronchi were then inspected to determine the optimal site
for performing the biopsy. After the ultrasonic probe was
withdrawn, a pair of biopsy forceps was inserted which followed the
same path down to the site of the lesion, guided by the ultrasonic
distance measurements (10,11). No more than eight biopsies were
performed until ideal diagnostic rates, accurate subtypes and
molecular detection were obtained . If no lesion was detected by
the ultrasound probe within 30 min, rEBUS was terminated and was
considered ineffective for that case. All patients suspected to
have PPL were all diagnosed with PPL.
Final diagnosis
Pathological diagnoses of chronic mucosal
inflammation, fibrous hyperplasia and idioblast (cells different in
shape, size, structure and contents of surrounding cells) were all
considered negative for PPL, whilst every diagnosis of suspected
cancer was considered positive for PPL.
If the use of data from rEBUS-D-TBLB could not reach
a diagnosis, other methods were performed, including transthoracic
needle biopsy, surgical operation, metastatic biopsy or follow-up,
to find the definitive diagnosis.
Statistical analysis
Statistical analysis was performed using SPSS 16.0
software (SPSS, Inc). Measurement data are presented as the mean ±
SD and enumeration data as number/percentage (n/%). The Wilcoxon
signed-rank sum test was used to analyze the diagnostic yield of
lesions in the subsegments that are below the lesion diameter limit
of 3 cm. P<0.05 was considered indicate a statistically
significant difference.
Results
Diagnoses of the 774 patients with PPL
using rEBUS-D-TBLB
In the 774 PPL patients, there were 802 lesions, 336
in the left lung and 466 in the right. The diagnostic yield of
rEBUS-D-TBLB for all patients was 67.18% (520/774). Overall, 362
cases of malignant disease, including adenocarcinoma and squamous
carcinoma, were found, whilst 158 cases of benign diseases were
diagnosed; the diagnostic sensitivity of malignancy was 70.98%
(181/255 patients) and the diagnostic sensitivity of benign tumors
was 79.00% (79/100 patients).
Pathological findings in the 520
patients with PPL diagnosed by rEBUS-D-TBLB
Through rEBUS-D-TBLB, the histopathological findings
of 520 PPL patients were obtained from the biopsies, including 362
cases of malignant disease and 158 cases of benign disease.
Among the 362 cases of malignant diseases
adenocarcinoma was the most common at 260 cases, accounting for
71.28% of all the malignant diseases. There were 66 cases of
squamous carcinoma (18.23%), 10 cases with malignant tumors not
pathologically classified (2.76%), 8 cases of adenosquamous
carcinoma (2.21%), 4 cases of small-cell carcinoma and 4 cases of
mucosa-associated lymphoid tissue lymphoma. Additionally, 2 cases
each of spindle cell malignancy, sarcomatoid carcinoma,
adenocarcinoma combined with sarcomatoid carcinoma, adenocarcinoma
combined with small cell carcinoma and poorly-differentiated
neuroendocrine carcinoma were found (Table II).
 | Table IIPathological findings in 362 cases of
malignant disease. |
Table II
Pathological findings in 362 cases of
malignant disease.
| Diagnosis | No. of cases (%) |
|---|
| Adenocarcinoma | 260 (71.28) |
| Squamous
carcinoma | 66 (18.23) |
| Malignant tumors not
pathologically classified | 10 (2.76) |
| Adenosquamous
carcinoma | 8 (2.21) |
| Small cell
carcinoma | 4 (1.10) |
| Mucosa associated
lymphoid tissue lymphoma | 4 (1.10) |
| Spindle cell
malignancy | 2 (0.55) |
| Sarcomatoid
carcinoma | 2 (0.55) |
| Adenocarcinoma
combined with sarcomatoid carcinoma | 2 (0.55) |
| Adenocarcinoma
combined with small cell carcinoma | 2 (0.55) |
| Poorly differentiated
neuroendocrine carcinoma | 2 (0.55) |
Among the 158 cases of benign disease, nonspecific
pneumonia (72 cases, 45.57%) was the most frequently diagnosed,
followed by tuberculosis (32 cases, 20.25%). There were 18 cases of
inflammatory myofibroblastic tumor (11.39%), 16 cases of lung
abscess (10.13%), 6 cases each of pulmonary aspergillosis and
pulmonary cryptococcosis (3.80%), 4 cases of organizing pneumonia
(2.53%) and 2 cases each of pulmonary mucormycosis and radiation
pneumonitis (1.27%; Table
III).
 | Table IIIPathological findings in 158 cases of
benign diseases. |
Table III
Pathological findings in 158 cases of
benign diseases.
| Diagnosis | No. of cases (%) |
|---|
| Pneumonia | 72 (45.57) |
| Tuberculosis | 32 (20.25) |
| Inflammatory
pseudotumor | 18 (11.39) |
| Lung abscess | 16 (10.13) |
| Pulmonary
aspergillosis | 6 (3.80) |
| Pulmonary
cryptococcosis | 6 (3.80) |
| Organizing
pneumonia | 4 (2.53) |
| Pulmonary
mucormycosis | 2 (1.27) |
| Radiation
pneumonitis | 2 (1.27) |
Distribution of lesions among the
bronchopulmonary segments
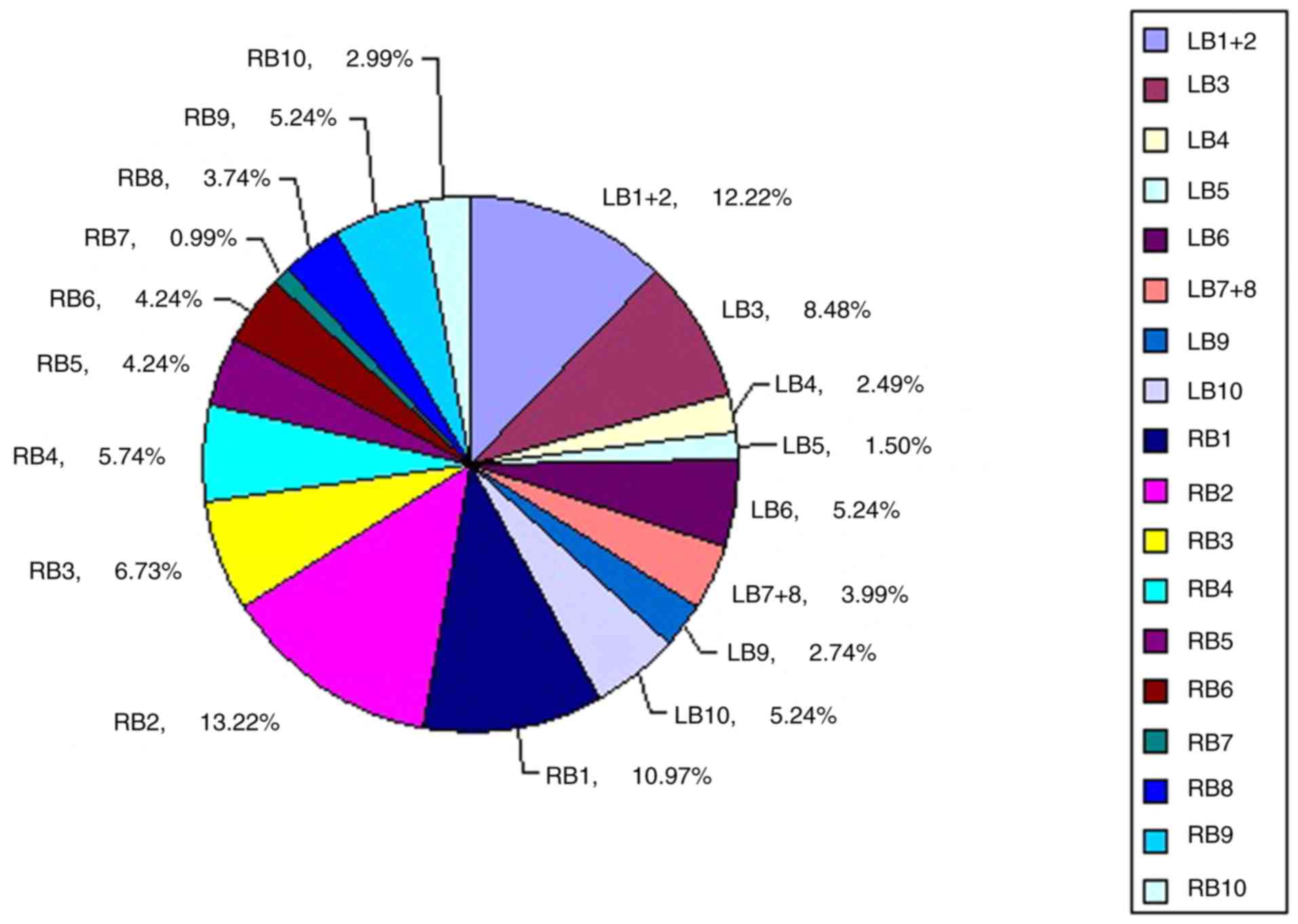
The 802 lesions were distributed in all 18
bronchopulmonary segments of the bilateral lungs (Fig. 2). Sorting by the number of lesions
found in each bronchopulmonary segment with >5% of lesions gave
the following results: 106 lesions were found in the posterior
segment of the right upper lobe bronchus (RB2; 13.22%), 98 in the
apical posterior segment of the left upper lobe bronchus (LB1+2;
12.22%), 88 in the apical segment of the right upper lobe bronchus
(RB1; 10.97%), 68 in the anterior segment of the left upper lobe
bronchus (LB3; 8.48%), 54 in the anterior segment of the right
upper lobe bronchus (RB3; 6.73%), 46 in the lateral segment of the
middle lobe bronchus (RB4; 5.74%), 42 in the superior segment of
the left lower lobe bronchus (LB6; 5.24%), 42 in the posterior
basal segment of the left lower lobe bronchus (LB10; 5.24%) and 42
in the lateral basal segment of right lower lobe bronchus (RB9;
5.24%).
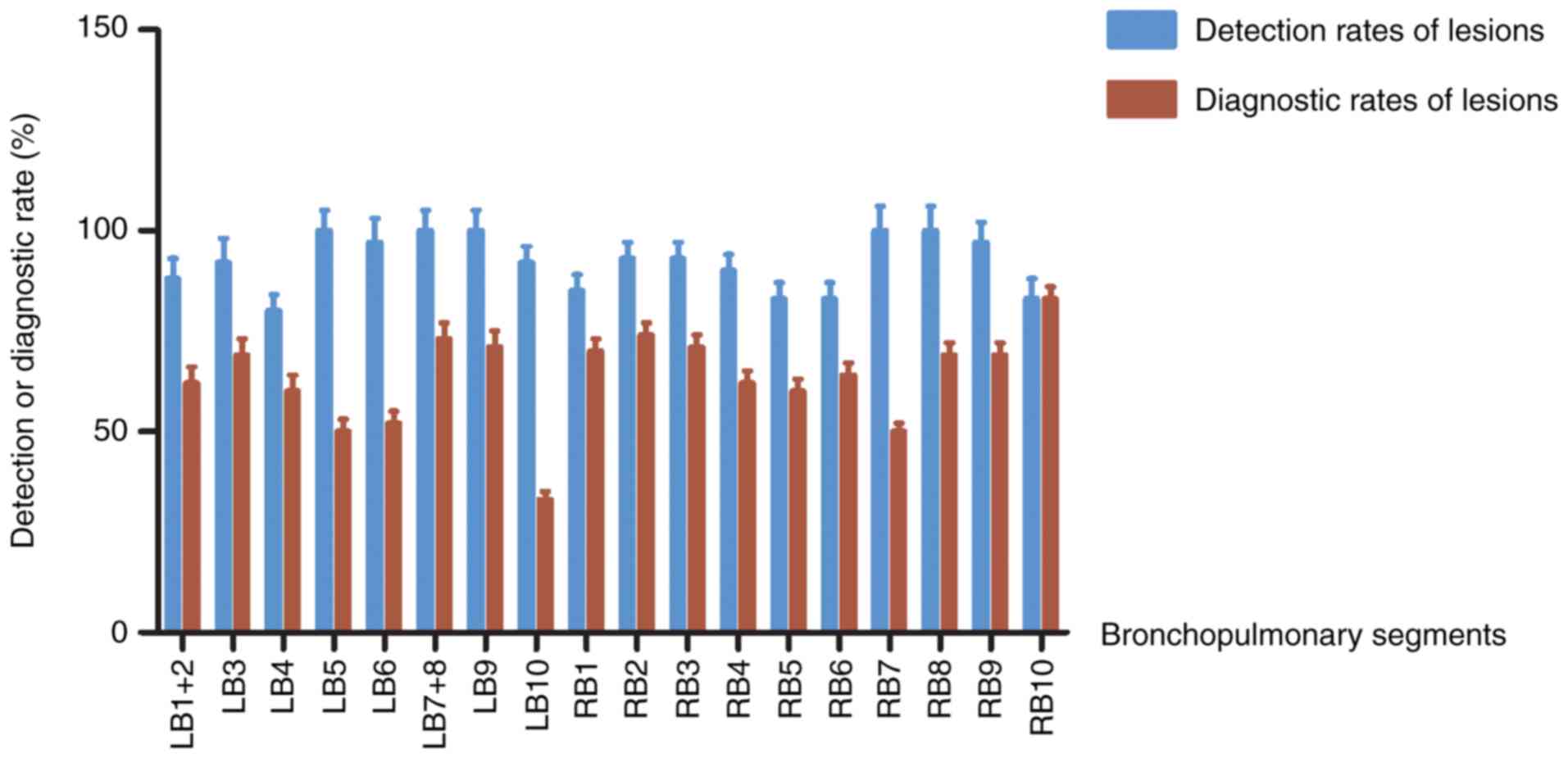
Detectivity and diagnostic ability of
rEBUS-D-TBLB in the 802 lesions
‘Detected’ refers to the ability of rEBUS to detect
the lesion and obtain the tissue through rEBUS-D-TBLB, with
negative pathological diagnosis results. ‘Diagnosed’ refers to the
ability of rEBUS to detect the lesion and obtain the tissue through
rEBUS-D-TBLB, with positive pathological diagnosis results.
According to the aforementioned results, 106 of the lesions were
found in the RB2 segment, in which rEBUS-D-TBLB detected 98
(92.45%) of these and diagnosed 80 (75.47%). In the RB3 segment,
where 54 lesions were found, rEBUS-D-TBLB detected 50 (92.95%) and
diagnosed 38 (70.37%). There were 88 lesions in the RB1 segment,
where rEBUS-D-TBLB detected 74 (84.09%) and diagnosed 60 (68.18%).
In the LB3 segment, 68 lesions were confirmed, where rEBUS-D-TBLB
detected 62 (91.18%) and diagnosed 46 (67.75%). In the RB9 segment,
42 lesions were found, where rEBUS-D-TBLB detected 40 (95.24%) and
diagnosed 28 (66.67%). The lesion detection rates for other
bronchopulmonary segments were all found to be >80%, whereas the
rates of diagnosis were considered low at <65% (Fig. 3).
Lesion analysis in the
bronchopulmonary LB1+2, LB6, LB10 and RB4 subsegments
Although >5% of the 802 lesions were found in the
LB1+2, LB6, LB10 and RB4 segments, the capability of rEBUS-D-TBLB
to diagnose the lesions was lower in these segments compared with
other segments. Therefore, the lesions found in these
bronchopulmonary subsegments were analyzed further to confirm the
diagnostic yields.
The diagnostic yields of the lesions were
comparatively high; the yield in the LB1+2 apical branch (LB1+2a)
was found to be 81.25%, whereas that in the LB6 superior branch
(LB6a) was 66.67 and 71.43% in the RB4 internal branch (RB4b). The
diagnostic yields in the LB6 external branch (LB6b), LB10 posterior
branch (LB10a), LB10 external branch (LB10b) and LB10 internal
branch (LB10c) were found to be low, with few lesions. The
diagnostic yields in the LB1+2 posterior branch (LB1+2b), LB1+2
external branch (LB1+2c), LB6 internal branch (LB6c) and RB4
external branch (RB4a) were also found to be low, although many
lesions were found (Fig. 4).
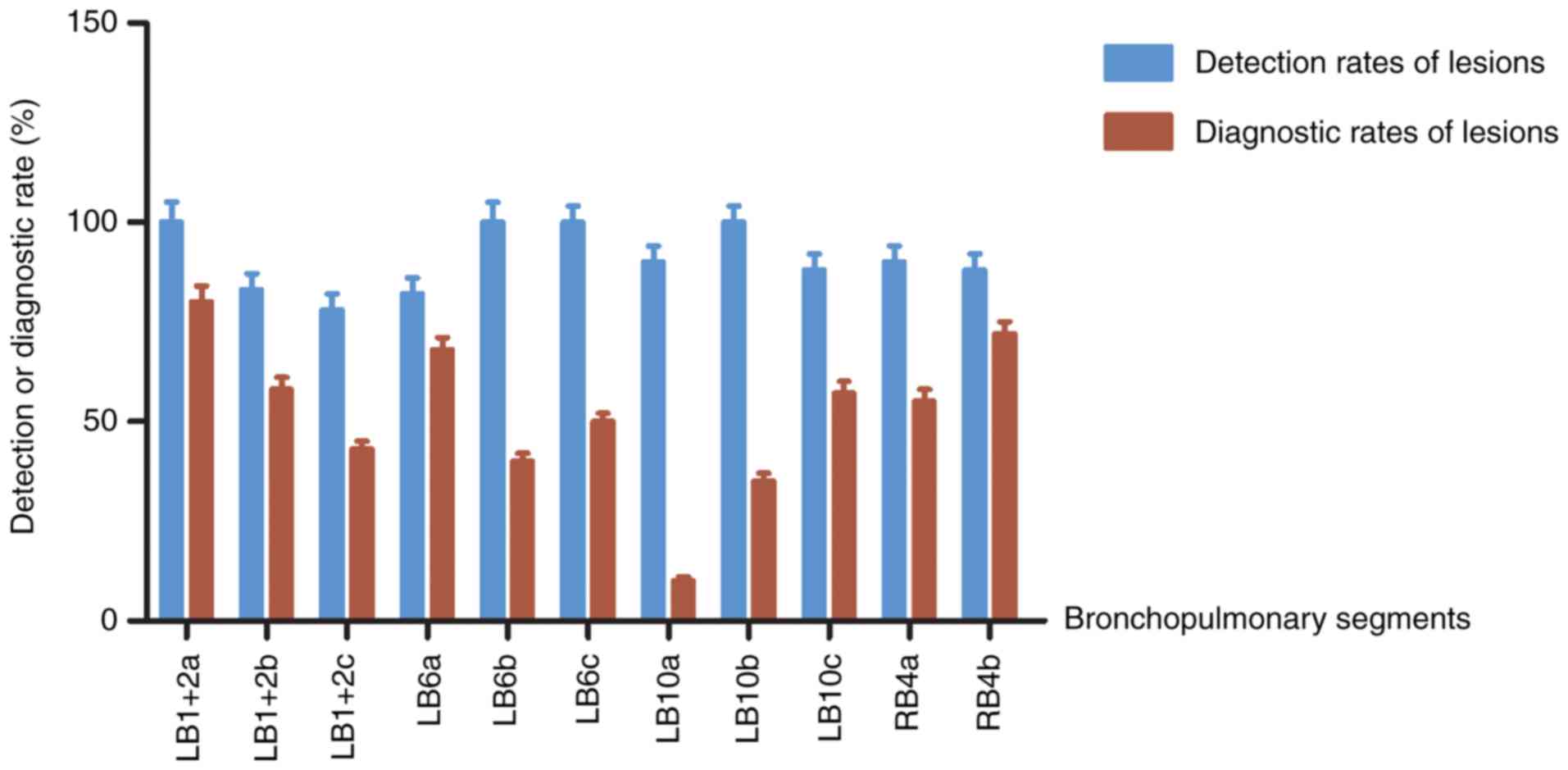 | Figure 4Rates of lesion detection by radial
endobronchial ultrasound and diagnostic ability of subsequent
transbronchial lung biopsy in lesions located at the
bronchopulmonary LB1+2, LB6, LB10 and RB4 subsegments. LB1+2a,
LB1+2 apical branch; LB1+2b, LB1+2 posterior branch; LB1+2c, LB1+2
external branch; LB6a, LB6 superior branch; LB6b, LB6 external
branch; LB6c, LB6 internal branch; LB10a, LB10 posterior branch;
LB10b, LB10 external branch; LB10c, LB10 internal branch; RB4a, RB4
external branch; RB4b, RB4 internal branch. |
Analysis of diagnostic yield using
Wilcoxon signed-rank test
Wilcoxon signed-rank test was used to analyze the
diagnostic yields of rEBUS-D-TBLB between lesions with a diameter
of ≥3 and <3 cm at the LB1+2b, LB1+2c, LB6c and RB4a segments.
The overall diagnostic yields of rEBUS-D-TBLB for lesions were
lower compared with other segments. The diagnostic yields could not
be increased by distinguishing the size of lesions in LB1+2b,
LB1+2c or LB6c segments with the exception of RB4a, in which the
diagnostic yield of rEBUS-D-TBLB was higher for lesions with a
diameter of >3 cm compared with that for <3 cm (Table IV).
 | Table IVAnalysis of diagnostic rates of
lesions using Wilcoxon signed-rank test. |
Table IV
Analysis of diagnostic rates of
lesions using Wilcoxon signed-rank test.
| Subsegments | Diagnostic rates of
lesions (%) | Subgroups | Number of
lesions | Diagnostic
rates | t | t range | P-value |
|---|
| LB1+2b | 57.89 | <3 cm | 16 | 50% (8/16) | 64.5 | 55-105 | P>0.05 |
| | | ≥3 cm | 22 | 63.64% (14/22) | | | |
| LB1+2c | 42.86 | <3 cm | 14 | 28.57% (4/14) | 46.5 | 36-69 | P>0.05 |
| | | ≥3 cm | 14 | 57.14% (8/14) | | | |
| LB6c | 50 | <3 cm | 6 | 0% (0/6) | 17 | 7-26 | P>0.05 |
| | | ≥3 cm | 14 | 100% (14/14) | | | |
| RB4a | 56.25 | <3 cm | 18 | 22.22% (4/18) | 38.5 | 40-79 | P<0.05 |
| | | ≥3 cm | 14 | 100% (14/14) | | | |
Discussion
A number of techniques have been developed for
diagnosing PPL over recent decades, including blind bronchoscopic
examination, and thoracic, thoracoscopic and percutaneous
transthoracic biopsies. However, these techniques are associated
with limitations. Blind bronchoscopic examination has a low success
rate (12), whereas thoracic and
thoracoscopic biopsies result in significant trauma in patients
(13,14). For percutaneous transthoracic biopsy,
although it remains the principal approach for the PPL diagnosis,
it is less effective for lesions near the major vessels of the
heart and diaphragm, in addition to those distant from the body
surface (15,16); importantly, percutaneous
transthoracic biopsy is performed through the non-natural lumina,
leading to a high incidence of postoperative complications such as
pneumothorax and bleeding (17).
Hospitalization is therefore required for all patients undergoing
percutaneous transthoracic biopsy, increasing the treatment cost
and the use of medical resources (18).
With the development of ultrafine bronchoscopes and
endobronchial ultrasound, rEBUS-TBLB is becoming increasingly
important for diagnosing PPL. is conducted through the natural
lumina of the tracheal bronchus, a safe route that causes minimal
damage (19). Additionally, the
positive diagnostic rate increases when it is combined with other
technologies, including X-ray fluoroscopy, guiding sheath and
virtual navigation. A previous meta-analysis demonstrated that the
diagnostic yield of rEBUS-TBLB was as high as 69%, with only 27% of
the postoperative complications experienced with percutaneous
transthoracic biopsy (20). The use
of rEBUS-TBLB is extended due to its high diagnostic yield and
superior safety. The ultrasonic characteristics of rEBUS-TBLB
lesions have high clinical value, as parameters including lesion
properties, airway structure and filtration range can be
characterized. A previous report revealed that rEBUS-TBLB and
transbronchial brushing confer advantages in the evaluation of PPL,
but cannot access lesions located adjacent to the proximal
segmental bronchus (21). The high
point specificity (95% CI=0.99-1.00) and point sensitivity (95%
CI=0.70-0.76) of rEBUS-TBLB renders it suitable for the diagnosis
of peripheral lung cancer (22).
Additionally, the application of transbronchial needle aspiration
has drastically improved the diagnostic yield, especially when the
EBUS probe cannot reach the PPL (23). A previous study has shown that the
diagnostic yield of EBUS-guided bronchoscopy alone was 69.0%, 50.6%
when combined with TBLB, 42.0% when combined with brush smear and
44.3% in combination with bronchoalveolar lavage fluid analysis
(24). The present study found the
diagnostic yield of rEBUS-D-TBLB for all lesions to be 67.18%,
consistent with this previous observation (24). In addition, the present study also
found the diagnostic yield of rEBUS-D-TBLB in the LB1+2a segment to
be 81.25%, in comparison with 66.67% in the LB6a segment and 71.43%
in RB4b the segment, suggesting that rEBUS-D-TBLB confers
advantages for the diagnosis of lesions in LB1+2a compared with
percutaneous lung puncture.
The outer diameter (1.4-2.0 mm) of the rEBUS probe
allows it to reach into the sixth subsegmental bronchus with a scan
range of 4 cm (19). In ideal
situations, all lesions in the pulmonary field should be
detectable. However, the diagnostic ability of rEBUS-TBLB was lower
compared with percutaneous transthoracic biopsy (25). In clinical practice, the positive
diagnostic rate of rEBUS-TBLB was significantly lower in
bronchiolar segments compared with others. Although rEBUS-TBLB
detected central lesions with typical ultrasonic images, the
positive diagnostic rate remained low, possibly due to the degree
of curvature of the rEBUS-TBLB path to the lesion and variable
sensitivities of lesions with differing properties to pathological
examination. To increase the diagnostic rate of PPL by rEBUS-TBLB,
analysis of indications at each bronchopulmonary segment and
subsegment would be of marked clinical benefit, since information
regarding this issue is lacking. Previous studies have demonstrated
the effects of lesion location on the positive diagnostic rate of
rEBUS-TBLB at the pulmonary lobe level. One study reported that
distinguishing lesions did not improve the positive diagnostic rate
of rEBUS-TBLB (10), whilst another
study showed that the diagnostic rates for lesions in the left
upper pulmonary lobe were demonstratively lower compared with those
for lesions in other lobes (9).
Another previous finding demonstrated that although the diagnostic
rates of lesions in the right middle lobe and left lingular lobe
were relatively high, the diagnostic rates of lesions between the
right middle lobe and left lingular lobe had no significant
difference (26). In the present
study, therefore, the sample size was increased to 774 patients,
allowing for further exploration of the indications for
rEBUS-D-TBLB in the diagnosis of PPL at different locations in the
lung.
In the present study, 802 lesions were found in 774
patients. The diagnostic yield of rEBUS-D-TBLB for all patients was
67.18%, which lies within the range of that reported by previous
studies (27,28). In addition, through rEBUS-D-TBLB, 362
cases of malignant diseases and 158 cases of benign diseases were
identified with sensitivities of 70.98 and 79.00%, respectively.
This high percentage demonstrates the importance of rEBUS-D-TBLB
for pathological diagnosis, which can potentially prevent false
positive diagnoses, facilitating prompt treatment and improved
prognoses.
To study the indications for rEBUS-D-TBLB in the
diagnosis of PPL at the bronchopulmonary segments and subsegments,
the general distribution of lesions in the whole pulmonary field
was first examined. The results showed that the 802 lesions were
distributed throughout the 18 bronchopulmonary segments of the
bilateral lungs. Those with >5% of lesions were LB1+2, LB3, LB6,
LB10, RB1, RB2, RB3, RB4 and RB9, whereas other bronchopulmonary
segments found with fewer lesions were not explored further in the
present study.
As previously reported, the diagnostic yield of
rEBUS-TBLB for PPL was found to be >65% (29). According to this criterion,
rEBUS-TBLB is recommended for bronchopulmonary segments with >5%
of lesions and rEBUS-TBLB diagnostic yields for PPL of >65%. In
the aforementioned nine bronchopulmonary segments, the diagnostic
yields in RB2, RB3, RB1, LB3 and RB9 by rEBUS-TBLB were >65%.
However, those in the four remaining bronchopulmonary segments with
>5% of lesions, namely LB1+2, LB6, LB10 and RB4, were found to
be low. Therefore, lesions in these four bronchopulmonary
subsegments were analyzed further to improve the diagnostic yield.
It was found that the rEBUS-TBLB diagnosis yields for PPL in
subsegments LB1+2a (81.25%), LB6a (66.67%) and RB4b (71.43%) were
sufficient for the recommendation of rEBUS-TBLB. The rEBUS-TBLB
diagnostic yields for PPL in LB6b, LB10a, LB10b and LB10c
subsegments were low with small numbers of lesions, which were not
studied further. In the LB1+2b, LB1+2c, LB6c and RB4a subsegments,
although the diagnostic rates were low, a number of lesions were
found. Therefore, the Wilcoxon signed-rank test was subsequently
performed to analyze the rEBUS-TBLB diagnostic yields further for
PPL under the lesion diameter limit of 3 cm in the LB1+2b, LB1+2c,
LB6c and RB4a segments. The diagnostic yields in LB1+2b, LB1+2c and
LB6c cannot be increased by distinguishing the size of lesion,
though the diagnostic ability of rEBUS-D-TBLB was comparatively
high for lesions >3 cm in diameter in RB4a compared with those
<3 cm.
A number of limitations remain attached to the
present study. Although it presents the diagnostic rates of lesions
by rEBUS-D-TBLB, which provide a theoretical basis for the
diagnosis of PPL using rEBUS-D-TBLB, the present study did not
associate the pathological findings with the clinical endpoints. In
addition, the Wilcoxon signed-rank test was performed to analyze
the diagnostic yield of lesions, but not multivariate or univariate
analysis, which may be more suitable for statistical comparisons
across different parameters.
In conclusion, the diagnostic yields of rEBUS-D-TBLB
for PPL, especially those distributed at the LB1+2a, LB3, LB6a,
RB1, RB2, RB3, RB4a (diameter >3 cm), RB4b and RB9 subsegments,
were higher compared with those for other segments, providing the
theoretical basis for the clinical application of rEBUS-D-TBLB in
the diagnosis of PPL.
Acknowledgements
Not applicable.
Funding
The present study was supported by the guiding
science and technology project of the Health Planning Commission of
Changzhou in 2017 (grant no. WZ201710).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GSH and ZJ designed this study. SHG, JZ and ZM
performed the experiments and collected the data. GSH, ZSJ, ZQD and
XQQ analyzed the data. GSH wrote the manuscript. All authors read,
revised and approved the manuscript.
Ethics approval and consent to
participate
The present retrospective study was approved by the
Ethics Committee of Changzhou No. 1 Hospital (Changzhou,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zheng R, Zeng H, Zhang S and Chen W:
Estimates of cancer incidence and mortality in China, 2013. Chin J
Cancer. 36(66)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wong MCS, Lao XQ, Ho KF, Goggins WB and
Tse SLA: Incidence and mortality of lung cancer: Global trends and
association with socioeconomic status. Sci Rep.
7(14300)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zeng H, Zheng R, Guo Y, Zhang S, Zou X,
Wang N, Zhang L, Tang J, Chen J, Wei K, et al: Cancer survival in
China, 2003-2005: A population-based study. Int J Cancer.
136:1921–1930. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang T, Luo L and Zhou Q: Risk of pleural
recurrence in early stage lung cancer patients after percutaneous
transthoracic needle biopsy: A meta-analysis. Sci Rep.
7(42762)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Luo K, Lin Y, Lin X, Yu X, Wen J, Xi K,
Lin P and Zhang L: Localization of peripheral pulmonary lesions to
aid surgical resection: A novel approach for electromagnetic
navigation bronchoscopic dye marking. Eur J Cardiothorac Surg.
52:516–521. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Han Y, Kim HJ, Kong KA, Kim SJ, Lee SH,
Ryu YJ, Lee JH, Kim Y, Shim SS and Chang JH: Diagnosis of small
pulmonary lesions by transbronchial lung biopsy with radial
endobronchial ultrasound and virtual bronchoscopic navigation
versus CT-guided transthoracic needle biopsy: A systematic review
and meta-analysis. PLoS One. 13(e0191590)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Durakovic A, Andersen H, Christiansen A
and Hammen I: Retrospective analysis of radial EBUS outcome for the
diagnosis of peripheral pulmonary lesion: Sensitivity and
complications. Eur Clin Respir J. 2(28947)2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kurimoto N, Miyazawa T, Okimasa S, Maeda
A, Oiwa H, Miyazu Y and Murayama M: Endobronchial ultrasonography
using a guide sheath increases the ability to diagnose peripheral
pulmonary lesions endoscopically. Chest. 126:959–965.
2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu J, Han J, Lv H and Li B: An ultrasonic
sensor system based on a two-dimensional state method for highway
vehicle violation detection applications. Sensors (Basel).
15:9000–9021. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Park J, Je Y, Lee H and Moon W: Design of
an ultrasonic sensor for measuring distance and detecting
obstacles. Ultrasonics. 50:340–346. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Paradis TJ, Dixon J and Tieu BH: The role
of bronchoscopy in the diagnosis of airway disease. J Thorac Dis.
8:3826–3837. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Durheim MT, Kim S, Gulack BC, Burfeind WR,
Gaissert HA, Kosinski AS and Hartwig MG: Mortality and respiratory
failure after thoracoscopic lung biopsy for interstitial lung
disease. Ann Thorac Surg. 104:465–470. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lieberman S, Gleason JB, Ilyas MIM,
Martinez F, Mehta JP and Savage EB: Assessing the safety and
clinical impact of thoracoscopic lung biopsy in patients with
interstitial lung disease. J Clin Diagn Res. 11:OC57–OC59.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jo Y, Han DH, Beck KS, Park JS and Kim TJ:
Practice pattern of transthoracic needle biopsy: 2016 survey in the
members of Korean society of thoracic radiology. Korean J Radiol.
18:1005–1011. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Anzidei M, Porfiri A, Andrani F, Di
Martino M, Saba L, Catalano C and Bezzi M: Imaging-guided chest
biopsies: Techniques and clinical results. Insights Imaging.
8:419–428. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Heerink WJ, de Bock GH, de Jonge GJ, Groen
HJ, Vliegenthart R and Oudkerk M: Complication rates of CT-guided
transthoracic lung biopsy: Meta-analysis. Eur Radiol. 27:138–148.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Accordino MK, Wright JD, Buono D, Neugut
AI and Hershman DL: Trends in use and safety of image-guided
transthoracic needle biopsies in patients with cancer. J Oncol
Pract. 11:e351–e359. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kim EJ and Kim KC: Utility of radial probe
endobronchial ultrasound-guided transbronchial lung biopsy in
diffuse lung lesions. Tuberc Respir Dis (Seoul). 82:201–210.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhan P, Zhu QQ, Miu YY, Liu YF, Wang XX,
Zhou ZJ, Jin JJ and Li Q: Comparison between endobronchial
ultrasound-guided transbronchial biopsy and CT-guided transthoracic
lung biopsy for the diagnosis of peripheral lung cancer: A
systematic review and meta-analysis. Transl Lung Cancer Res.
6:23–34. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dhooria S, Sehgal IS, Gupta N, Aggarwal
AN, Behera D and Agarwal R: Role of radial endobronchial
ultrasound-guided transbronchial needle aspiration in the diagnosis
of pulmonary nodules: Case report and literature review. Lung
India. 34:61–64. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Steinfort DP, Khor YH, Manser RL and
Irving LB: Radial probe endobronchial ultrasound for the diagnosis
of peripheral lung cancer: Systematic review and meta-analysis. Eur
Respir J. 37:902–910. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hayama M, Izumo T, Chavez C, Matsumoto Y,
Tsuchida T and Sasada S: Additional transbronchial needle
aspiration through a guide sheath for peripheral pulmonary lesions
that cannot be detected by radial EBUS. Clin Respir J. 11:757–764.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Boonsarngsuk V, Kanoksil W and
Laungdamerongchai S: Diagnosis of peripheral pulmonary lesions with
radial probe endobronchial ultrasound-guided bronchoscopy. Arch
Bronconeumol. 50:379–383. 2014.(In English, Spanish). PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang Q, Zhang S, Xu X, Xu Q and Zhou J:
Value of radial probe endobronchial ultrasound-guided
transbronchial biopsy and computer tomography-guided transthoracic
needle aspiration in the diagnosis of peripheral pulmonary lesions.
Medicine (Baltimore). 96(e7843)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang S, Zhou J, Zhang Q, Xu Q and Xu X:
Diagnosis of peripheral lung diseases by combined measurement with
ultrasound guidance under bronchoscope. Chin J Tuberc Respir.
38:566–569. 2015.(In Chinese).
|
|
27
|
Fukusumi M, Ichinose Y, Arimoto Y, Takeoka
S, Homma C, Matsuoka H, Mouri A, Hamamoto Y, Matsumoto J and
Kamimura M: Bronchoscopy for pulmonary peripheral lesions with
virtual fluoroscopic preprocedural planning combined with EBUS-GS:
A pilot study. J Bronchology Interv Pulmonol. 23:92–97.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hsia DW, Jensen KW, Curran-Everett D and
Musani AI: Diagnosis of lung nodules with peripheral/radial
endobronchial ultrasound-guided transbronchial biopsy. J
Bronchology Interv Pulmonol. 19:5–11. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Eberhardt R, Anantham D, Ernst A,
Feller-Kopman D and Herth F: Multimodality bronchoscopic diagnosis
of peripheral lung lesions: A randomized controlled trial. Am J
Respir Crit Care Med. 176:36–41. 2007.PubMed/NCBI View Article : Google Scholar
|