Introduction
The cornea is a transparent, avascular structure
that lacks lymphatic vessels. It is the first important refractive
medium in the eye and factors such as immune disease, infection and
trauma can lead to neovascularization from the corneal limbus to
the cornea. The clinical term for the formation of a new capillary
network in the cornea is corneal neovascularization. Neovascular
disease is an intractable disease in ophthalmology, and is the
major cause of blindness in a number of eye diseases such as
diabetic retinopathy, retinopathy of prematurity and occlusion of
the central or branch of the retina (1-4).
Retinal neovascularization is a complicated process. First,
vasodilation occurs, and permeability is increased. Subsequently,
basal membrane enzymes degrade the vascular wall, while vascular
basement membrane enzymes aid in endothelial cell chemotaxis and
migration. Mitosis in peripheral cells promotes the formation of a
new vascular cavity in the intraocular vascular endothelial cells
caused by ocular exudation. This gap increases bleeding and causes
hyperplasia and pathological changes, which leads to irreversible
damage to the structure and function of the eye, ultimately
resulting in a serious decline in vision and potentially blindness.
Keratitis is a common eye disorder that often leads to the
formation of new blood vessels (5,6).
Paxillin (PXN) is a focal adhesion protein, and is comprised of an
LD motif (containing the consensus peptide sequence LDXLLXXL), a
conserved cysteine-rich domain comprising of ~60 amino acids with
seven conserved cysteine residues and a histidine residue (LIM
domain), Src homology 2 (SH2) domain and Src homology 3 (SH3)
domains (7). A previous study
demonstrated that PXN also serves an important role in corneal
neovascularization (8). Vascular
endothelial growth factor (VEGF) is an important pro-angiogenic
factor required during the process of angiogenesis, and is a highly
specific vascular endothelial cell mitogen, which has been
previously reported to be regulated by PXN (9). The role of the PXN signaling pathway in
the process of corneal neovascularization induced by inflammation
is not clear. In the present study, human umbilical vein
endothelial cells (HUVECs) and mouse corneal neovascularization
models were used to investigate changes in the PXN signaling
pathway during corneal neovascularization induced by inflammation.
The results of the present study shed light on the role of PXN in
corneal neovascularization and provide a theoretical basis and
potential target for the treatment of corneal
neovascularization.
Materials and methods
HUVEC cell culture and grouping
HUVECs were cryopreserved at the Department of
Ophthalmology, Wuhan University (Wuhan, China), and seeded into
poly-L-lysine-coated flasks and maintained in endothelial complete
medium supplemented with 5% FBS, 1% penicillin/streptomycin and 1%
endothelial cell growth supplement (All from ScienCell Research
Laboratories, Inc.). The cells were maintained at 37˚C in a
humidified incubator with 5% CO2, and the medium was
replaced every 2-3 days until the cells were confluent. Cells were
harvested with 0.05% trypsin-ethylene glycol tetra-acetic acid
solution (Wuhan Boster Biological Technology, Ltd.) and were
further cultured in the poly-L-lysine-coated flasks for use in
subsequent experiments. Cell at passage five were used, when they
lost their cobblestone like appearance.
HUVECs were divided into 5 groups: Control group,
empty vector-transfected control group (EV group), PXN knockdown
group (shPXN group), PXN-negative control (NC) group (NC group) and
PXN over-expressed group (overExp group).
Lentivirus preparation
The positive-sense strand and antisense strands of
the oligonucleotide fragments of the PXN short hairpin (sh)RNA
(forward,
5'-GATCCAAAGTTTCGGGATCCAATCTCTTCAAGAGAGAGATTGGATCCCGAAACTTTTTTTTTCTCGAGG-3'
and reverse,
5'-AGCTCCTCGAGAAAAAAAAAGTTTCGGGATCCAATCTCTCTCTTGAAGAGATTGGATCCCGAAACTTTG-3';
synthesized by Suzhou GenePharma, Co., Ltd.) were annealed. They
were dissolved in double-steamed water, mixed in equal molar
amounts, heated at 90˚C for 3 min, and slowly cooled to 37˚C to
form double-stranded oligonucleotides. The synthesized shRNA was
inserted into the shRNA expression vector pSilencer 2.1-U6neo
(Suzhou GenePharma, Co., Ltd.) and digested with BamHI and
HindIII restriction enzymes to construct the shRNA
recombinant plasmid for PXN.
Ad-PXN was synthesized and identified by Hanbio
Biotechnology Co., Ltd. HUVECs in the logarithmic growth phase and
in a good growth state were digested with 0.25% trypsin and
cultured in growth medium (10% FBS + 1% double antibody). A cell
suspension was prepared and plated onto 6-well plates at a density
of 5x105 cells/well. Cells were cultured in saturated
humidity at 37˚C with 5% CO2 until they reached 70%
confluence. A total of 25 µl Ad-PXN was added to each well and
cells were further cultured for 24 h.
RNA extraction and reverse
transcription-quantitative (RT-q)PCR
Total RNA was extracted from corneal tissue or
HUVECs using TRIzol® reagent (Thermo Fisher Scientific,
Inc.). For each sample, 1 µg RNA was reverse transcribed using
PrimeScript® RT reagent kit with gDNA Eraser (Takara
Bio, Inc.) according to manufacturer's protocol. The following
temperature protocol was used: DNA extension at 42˚C for 60 min
followed by inactivation at 70˚C for 15 min and a holding
temperature of 16˚C, to obtain first-strand cDNA. Expression levels
of target genes Ras, FAK, Src, Rho, Rac and Cdc42 were analyzed
using RT-qPCR. Primer premier software (v5.0; Premier Biosoft
International) was used to design the fluorescent primers (Table I). The reaction contained 10 µl 2x
SYBR® Premix Ex Taq™ (Takara Bio, Inc.), 0.50 µmol/l of
each primer and 0.2±0.02 µg cDNA template, and made to a final
volume of 20 µl using nucleotide free water. The thermocycling
conditions were: Pre-denaturation at 95˚C for 30 sec; followed by
39 cycles of denaturation at 95˚C for 5 sec, annealing at 56˚C for
10 sec and elongation at 72˚C for 25 sec. Gene expression was
normalized to that of β-actin and quantified using the
2-ΔΔCq method (10).
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Primer | Sequence | Size, base
pairs |
|---|
| Rho-F |
5'-TCACGCTATCATGGGTGTGG-3' | 226 |
| Rho-R |
5'-CAGCTGCCCATAGCAGAAGA-3' | |
| Rac-F |
5'-CATTCCAGACTCACGACC-3' | 128 |
| Rac-R |
5'-CACAATCTCCGCACCATA-3' | |
| Cdc42-F |
5'-TGTTTCTCAGTGGTCTCTCCA-3' | 287 |
| Cdc42-R |
5'-GCAGCCAATATTGCTTCATCA-3' | |
| Src-F |
5'-CGCCTCACTACCGTATGTCC-3' | 250 |
| Src-R |
5'-CCAGTTTCTCGTGCCTCAGT-3' | |
| FAK-F |
5'-TGTCCTCCCACCCTCTAC-3' | 138 |
| FAK-R |
5'-CCTCATCCGTTCTTCTTG-3' | |
| PXN-F |
5'-CTTGACCGGCTGTTACTG-3' | 180 |
| PXN-R |
5'-TCGCTTTGGCTTCTCTTT-3' | |
| β-Actin-F |
5'-ACACTGTGCCCATCTACG-3' | 153 |
| β-Actin-R |
5'-TGTCACGCACGATTTCC-3' | |
Western blot analysis
Western blot analysis was performed to determine the
protein expression levels of Rho, Rac, Cdc42, SRC and FAK. The
cells were homogenized with lysis buffer (cat. no. AB100086,
Bio-swamp Life Science) and centrifuged at 12,000 x g at 4˚C for 20
min. The protein concentration was determined using a bicinchoninic
acid assay kit (Bio-swamp Life Science). Equal quantities of
protein (30 µg) were resolved on a 10% gel using SDS-PAGE and
subsequently transferred to a PVDF membrane (EMD Millipore; Merck
KGaA). The membranes were blocked for 2 h at room temperature with
5% skimmed milk in TBS-Tween 20 (20 mmol/l Tris, 500 mmol/l NaCl,
and 0.05% Tween 20). Subsequently, the membrane was incubated with
one of the following primary antibodies (all from Abcam) overnight
at 4˚C: Rabbit anti-BMP-2 antibody (cat. no. ab14933; 1:1,000),
rabbit anti-VEGF antibody (cat. no. ab32152; 1:4,000) and human
anti-CtⅡ antibody (cat. no. ab159157; 1:2,000). Anti-GAPDH antibody
(cat. no. ab181602; 1:10,000) was used as the loading control.
Following incubation with the primary antibody, the membranes were
washed with TBS and incubated in goat anti-rabbit secondary
antibody (cat. no. PAB160009; Bioswamp Life Science; 1:10,000) for
2 h at room temperature. The signals were visualized using enhanced
chemiluminescent substrate buffer (EMD Millipore). Membranes were
scanned using Tanon 5200 Chemiluminescent Imaging System (Tanon
Science and Technology Co., Ltd.).
Transwell migration assay
Prior to the experiment, cells were cultured in
serum-free medium, and cultured for 24 h. PBS was added to 24-well
plates with the Transwell inserts (Corning, Inc.) for 5 min to soak
the chambers, and the cells were washed with serum-free medium. A
0.5 ml cell suspension containing 1x105 cells/ml in 1%
FBS was added to the upper chamber, and 0.75 ml DMEM with 10% FBS
was added to the lower chamber. Cells were cultured at 37˚C with 5%
CO2 for 48 h. Subsequently, the media was removed and 1
ml 4% formaldehyde solution was added to fix the cells at 25˚C for
10 min. Following fixing, the cells were washed with PBS, and
stained with 1 ml 0.5% crystal violet solution for 30 min at 25˚C.
Cells which had not migrated were gently removed using a cotton
swab once the chambers had dried. The number of migrated cells were
observed and counted at a magnification, x200 under a inverted
fluorescence microscope (DMIL LED; Leica Microsystems GmbH). The
Transwell migration assays were performed in triplicate.
Experimental animals
Mice (age, 2 months) were obtained from Hubei
Provincial Center for Disease Control and Prevention. A total of 24
female mice, weighing 20-22 g, were used. To ensure a healthy
corneal surface, all eyes were carefully examined using an
operating microscope (Carl Zeiss AG) prior to any experimental
procedures.
Mice were placed under general anesthesia through an
intramuscular injection of a ketamine mixture (ketamine 60 mg/kg +
xylazine 8 mg/kg), supplemented with topical anesthesia with 0.1%
proparacaine hydrochloride. A 3 mm diameter filter paper disc was
soaked in 1.0 M NaOH for 1 min and used to cause a corneal burn.
The disc was applied to the cornea, 1-2 mm from the limbus and then
removed after 2 min. The corneal surface was rinsed thoroughly with
10 ml 0.9% NaCl solution. To ensure maximum reproducibility, all
alkali burns were performed by the same investigator. Knockdown and
overexpression model mice were established via a tail vein
injection of lentivirus with a titer of 1x108 TU/ml.
According to the criteria of animal pain judged by
the Welfare and Ethical Reference of Experimental Animal Center of
Zhejiang University, mild pain manifested as a weight loss of ~5%,
only 50-75% of normal intake within 72 h, a small amount of erect
hair, normal interactions with other mice, and normal sensitivity
to external stimuli (11,12).
In the present study, eye inflammation was observed
microscopically on the 14th day after modeling. The physiological
habits of the animals were continuously monitored. Although blood
vessels were observed in the eyes, the mice in each group showed no
abnormal symptoms, and the diet, body weight and behavior were
normal. On the 21st day, two mice presented few erect hairs,
exhibited normal interactions with other mice and remained
sensitive to external stimuli, although 2 mice were found
scratching their eyes with their front paws. If the mice suffered
from mild pain on the 21st day according to the aforementioned
criteria of animal pain, then the experiment was stopped
immediately. The mice were sacrificed at day 21 with an overdose of
sodium pentobarbital (100 mg/kg, intraperitoneally), and samples
were collected.
All experimental protocols were approved by the
Institutional Animal Care and Use Committee of Tongji Medical of
Huazhong University of Science and Technology and Animal
Experimental Ethical Inspection of Laboratory Animal Centre,
Huazhong Agriculture University; approval no. HZAUMO-2017-039.
Cell tube formation assay
Prior to the experiment, all products that were
exposed to the Matrigel matrix (BD Biosciences) adhesive were
placed in a -20˚C refrigerator for 6 h. Subsequently, the Matrigel
matrix glue was placed in a 4˚C refrigerator for 24 h. When the
matrix was in a liquid form, 60 µl Matrigel was added per well into
96-well plates, and placed at 37˚C for 1 h. The formation of HUVEC
capillary-like structures on a basement membrane matrix were used
to investigate the angiogenic activity of Paxillin. HUVECs were
then seeded onto the Matrigel bed (1.5x104 cells/well)
and cultured for 6 h. Tube formation was imaged and the tube
lengths were quantified using the Image-Pro® Plus
software (v6.0; Media Cybernetics, Inc.).
VEGF expression was detected by
immunohistochemistry
Following removal of the corneal tissue, the tissue
was fixed in 4% polyformaldehyde for 24 h, the paraffin embedded
sections were sliced into 4-5 µm samples. Subsequently, the samples
were dewaxed using dimethylbenzene, after which the samples were
incubated on a compound digestion solution for enzyme repair for 30
min at 25˚C. Samples were flushed with PBS and 3% hydrogen peroxide
was added to block peroxidase activity. Samples were blocked in
goat serum (cat. no. SL038, Beijing Solarbio Science &
Technology Co., Ltd.) at 37˚C for 30 min, and incubated with
anti-VEGF antibody (1:100; cat. no. PAB30096, Bio-swamp Life
Science), at 4˚C overnight. Subsequently, the samples were
biotinylated by incubating the samples with biotin (4 mg/ml; cat.
no. CYB167077-FTK, ZSGB-BIO; OriGene Technologies, Inc.) at room
temperature for 30 min to mark the goat anti-rabbit and horseradish
enzyme labelled chain enzyme working fluid (1:200; cat. no.
PAB160022; Bio-swamp Life Science), and 3,3'-diaminobenzidine color
reagent (cat. no. PAB180021; Bio-swamp Life Science) was added to
the enzyme. Samples were observed under a microscope, and brown or
yellow staining indicated positive expression. Samples were
counterstained with hematoxylin for 3 min at 4˚C. Subsequently an
alcohol series was used to dehydrate the solution and the slides
were sealed with neutral gum.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analysis was performed in SPSS version 22.0
(IBM, Corp.) and groups were compared using a one-way ANOVA with a
post-hoc Duncan's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
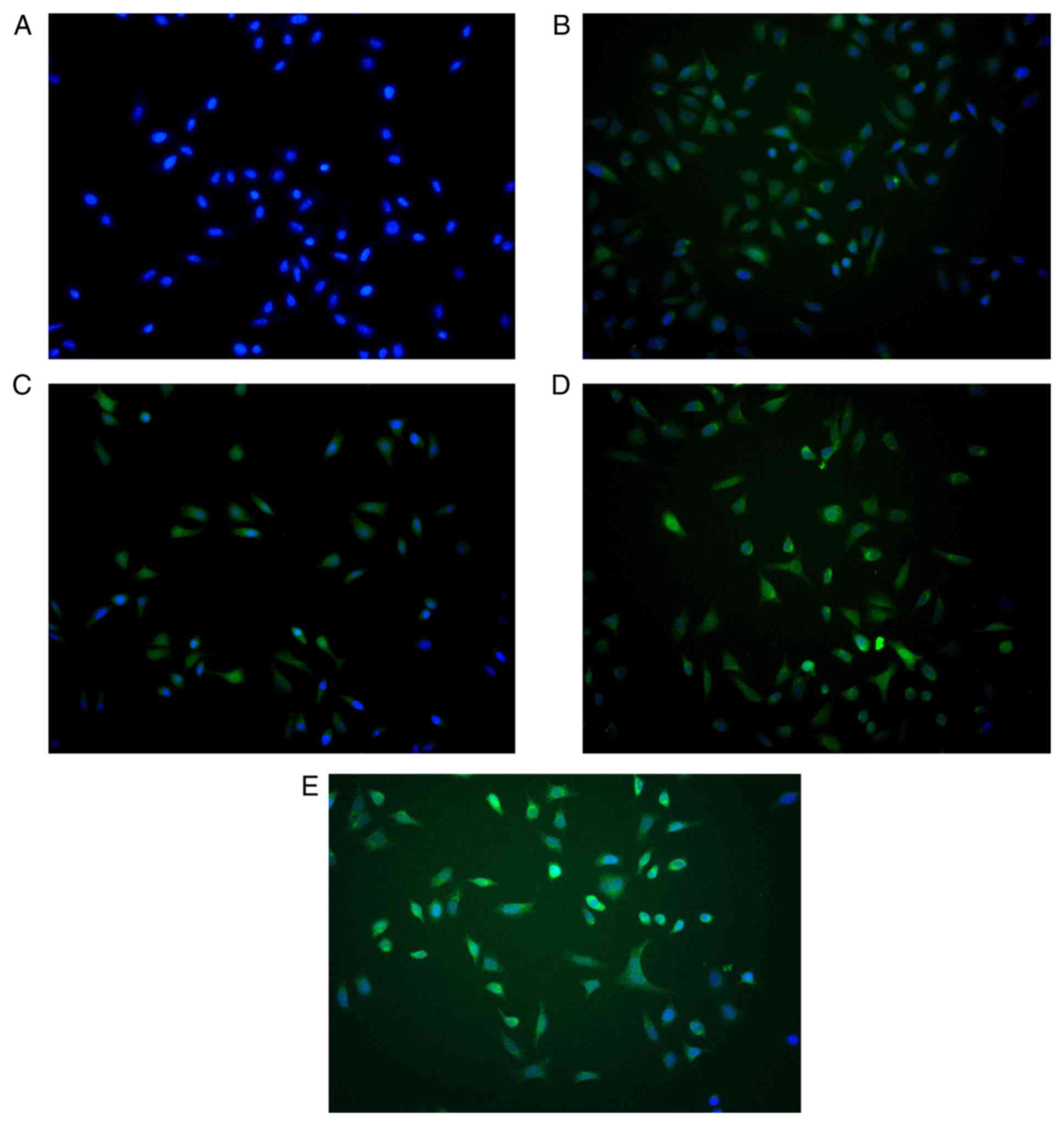
Expression of PXN in HUVECs
The lentiviral vector expression cassette allowed
for the expression of PXN in transduced cells (Fig. 1). There were no notable differences
in fluorescence intensity between the EV, shPXN, NC, and overExp
groups.
Involvement of PXN pathway in
angiogenesis
The protein expression levels of PXN were detected
by western blotting. Compared to the control group, the protein
expression levels of Rac, Rho, Cdc42, Src and PXN were decreased in
the shPXN group and increased in the overEXP group. Furthermore,
FAK expression levels were increased in the shPXN group (Fig. 2).
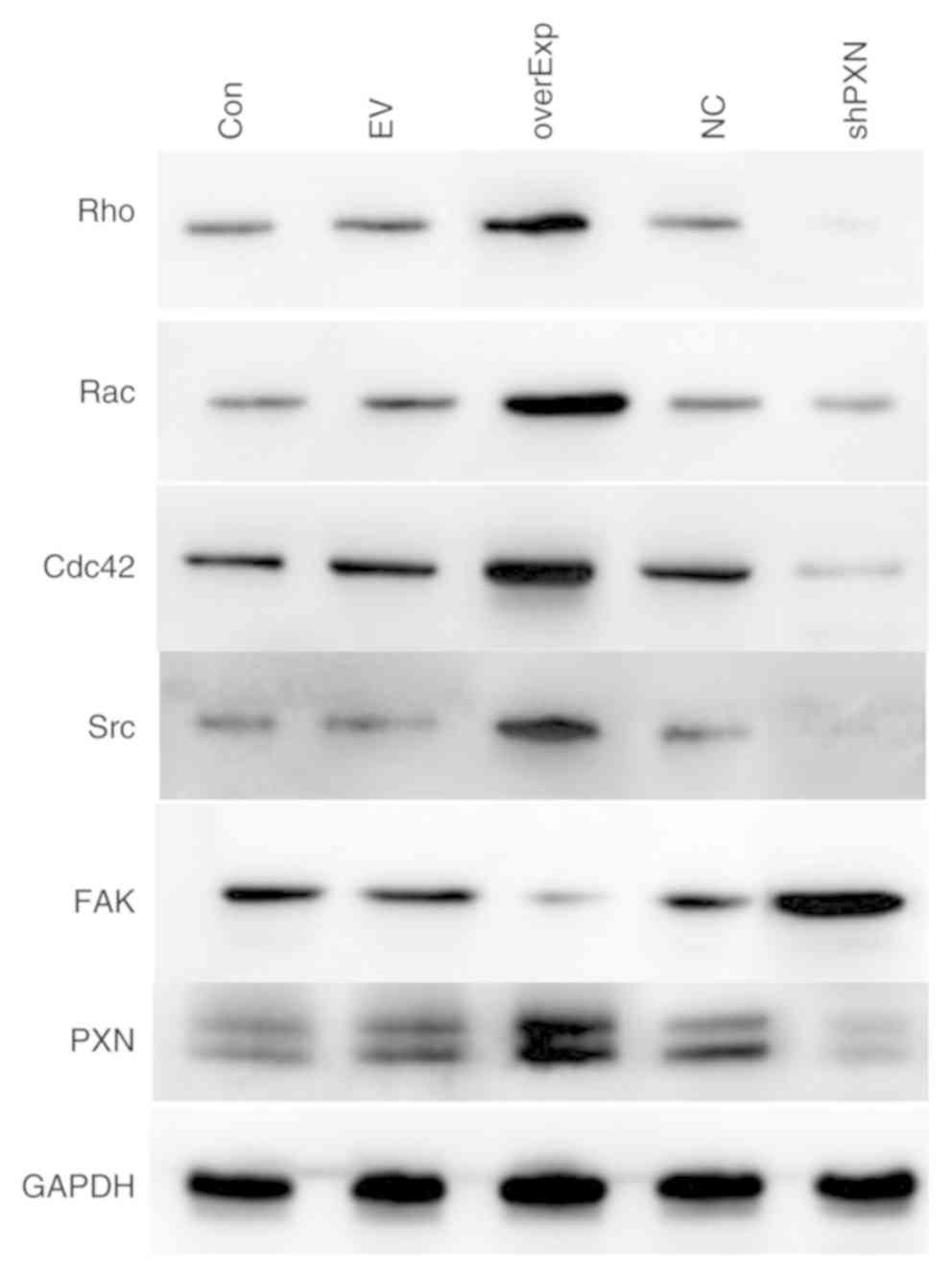 | Figure 2Protein expression levels of members
of the PXN signaling pathway. Protein expression levels of Rac,
Rho, Cdc42, Src and PXN were lower in the shPXN group and higher in
the overEXP group. FAK expression levels were higher in the shPXN
group. PXN, paxillin; Rac, AKT serine/threonine kinase 1; Cdc42,
cell division control protein 42; Src, SRC proto-oncogene,
non-receptor tyrosine kinase; Con, control group; EV, empty
vector-transfected control group; shPXN, PXN knockdown group; NC,
PXN-NC group; overExp, PXN over-expressed group. |
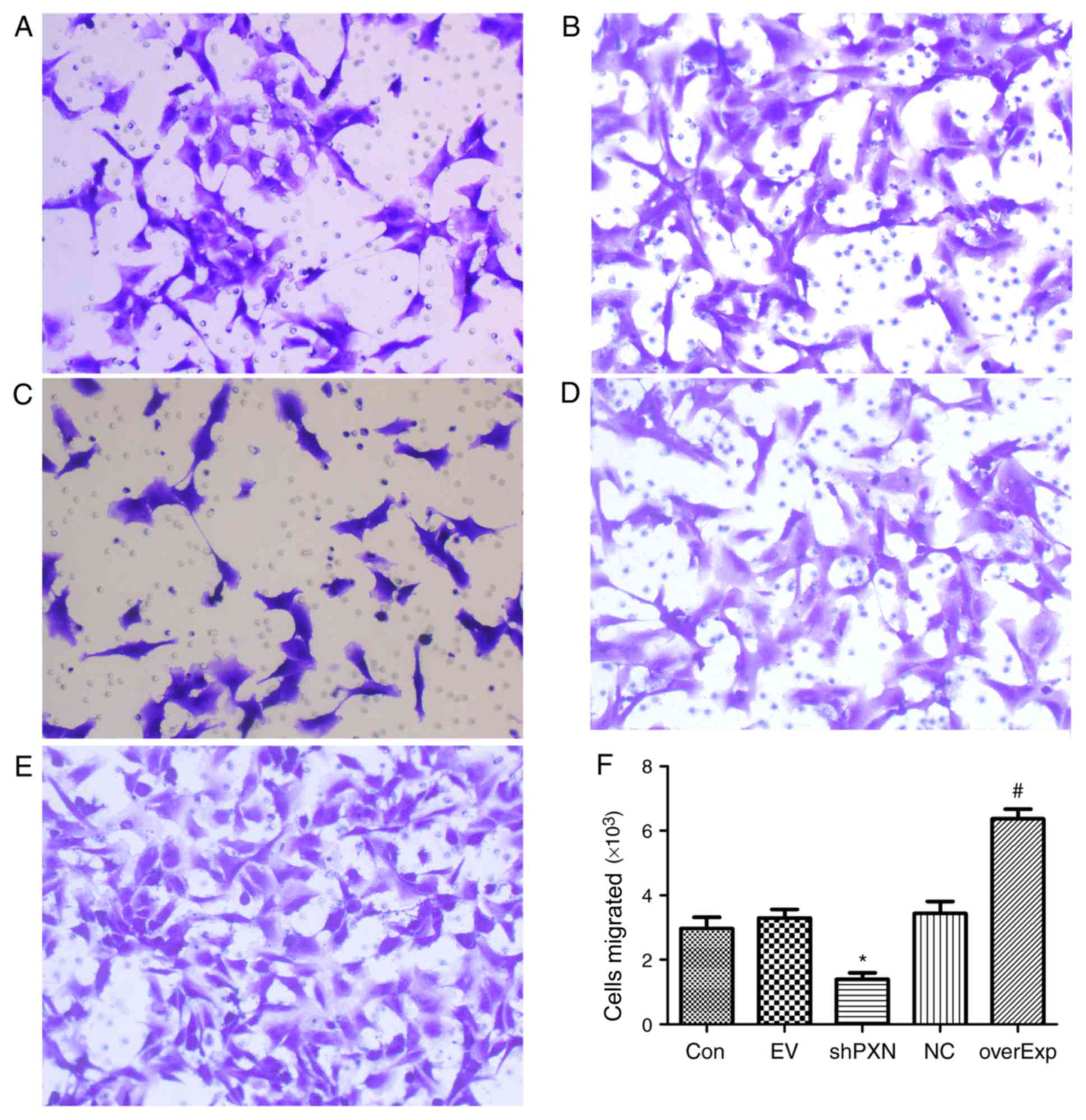
shPXN decreases the migratory capacity
of HUVECs
Migration of HUVEC was assessed using a Transwell
assay. As shown in Fig. 3, compared
with the empty vector-transfected control group, the number of
cells which had migrated was significantly higher in the
overexpression group (P<0.05; Fig.
3F). Compared with the PXN-NC group, migration in the shPXN
group was significantly lower (P<0.05; Fig. 3F).
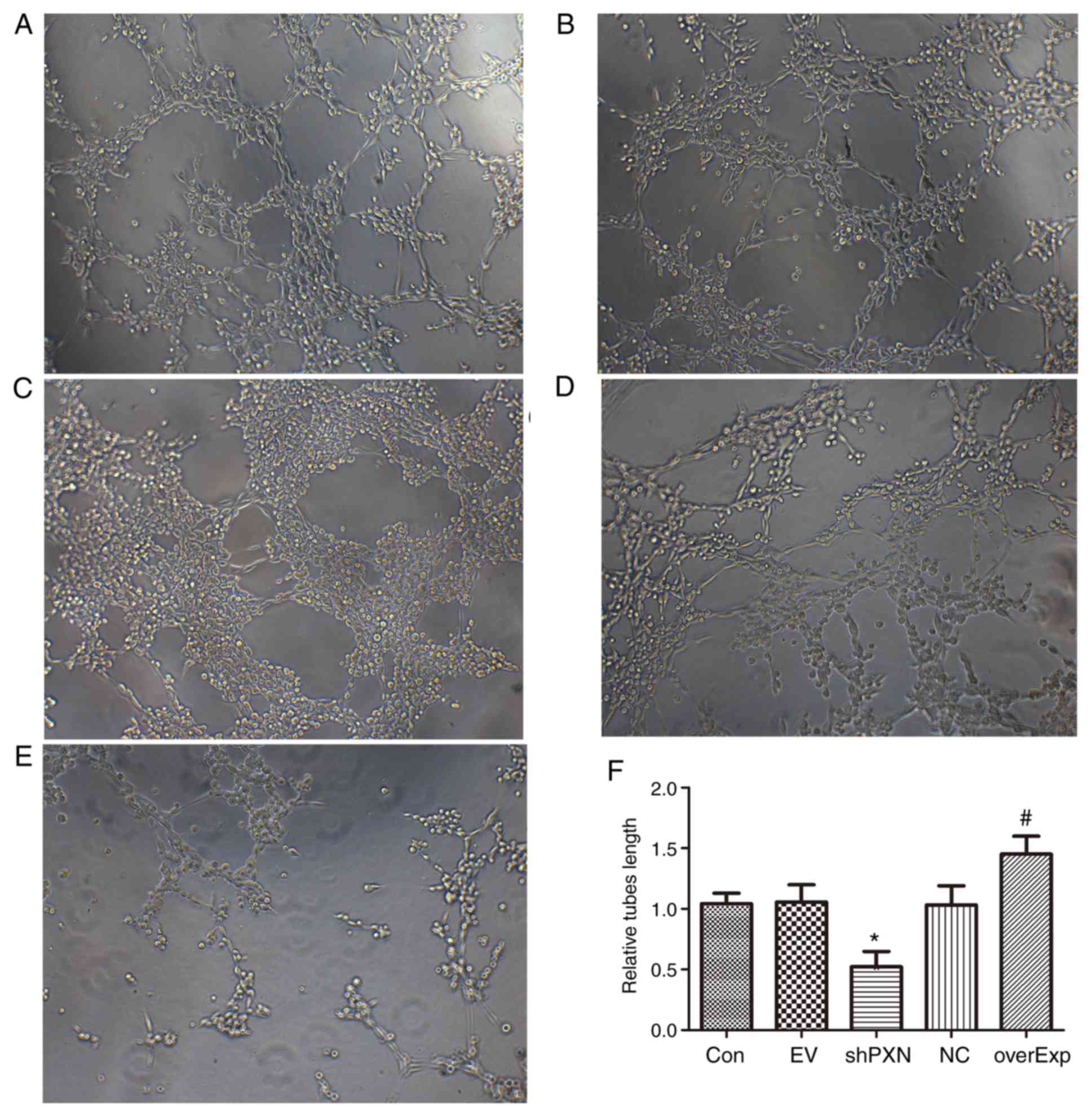
PXN enhances tube formation in
HUVECs
Tube formation was detected in HUVECs. As shown in
Fig. 4, tube formation was
significantly increased in the overexpression group compared with
the empty vector-transfected control group (P<0.05; Fig. 4F). Furthermore, tube formation was
significantly decreased in the shPXN group compared with the PXN-NC
group (P<0.05; Fig. 4F).
PXN regulates a series of genes in
vivo
PXN protein expression levels were determined by
western blotting. The levels of Rac, Rho, Cdc42, Src and PXN were
lower in the shPXN group compared with the model group, and higher
in the overEXP group (P<0.05; Fig.
5). Additionally, FAK expression levels were higher in the
shPXN group.
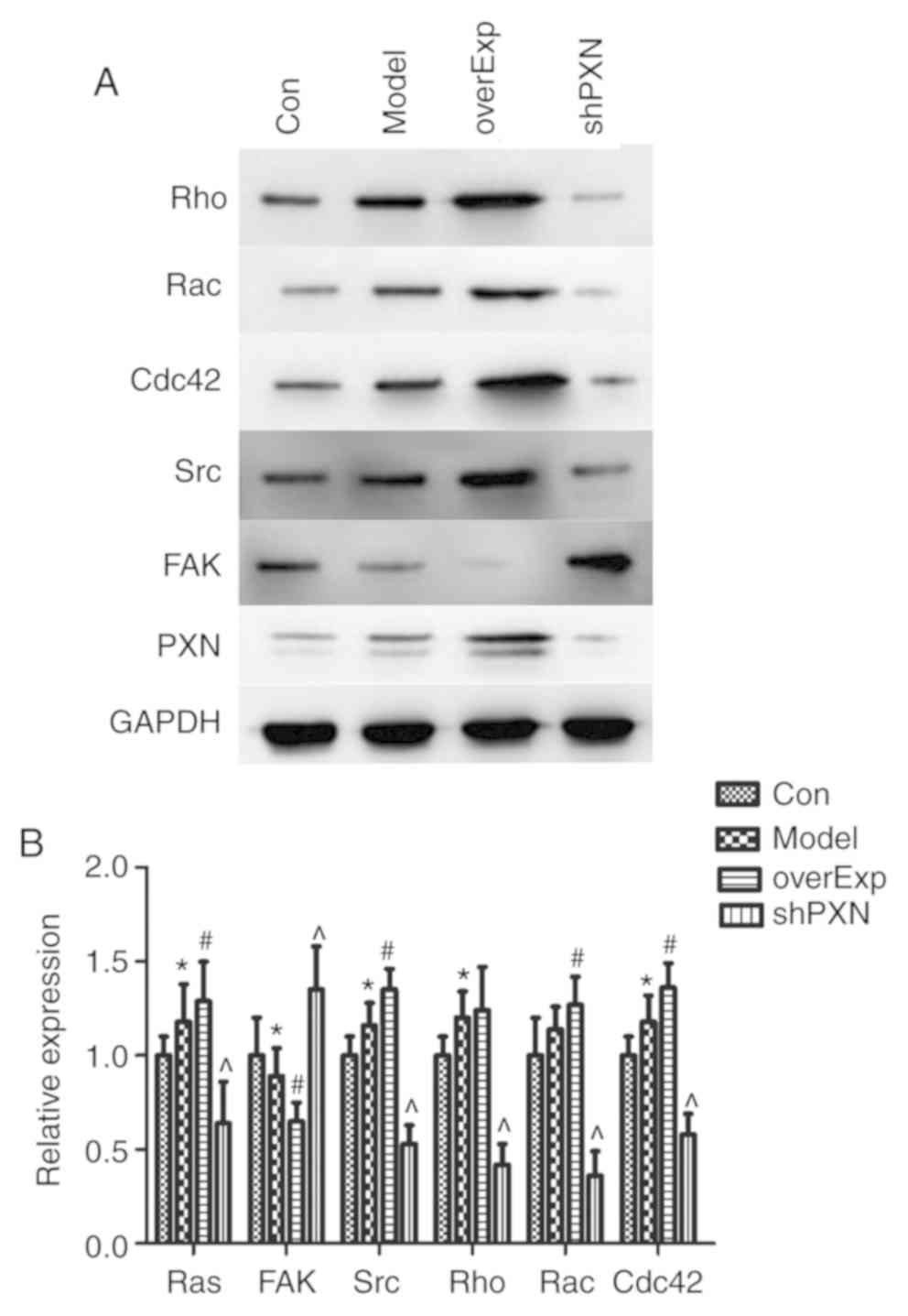 | Figure 5PXN regulates a series of genes in
vivo. (A) Representative western blots of Rho, Rac, Cdc42, Src,
FAK and PXN. (B) Expression of Rac, Rho, Cdc42, Src, and PXN was
lower in the shPXN group and higher in the overEXP group. FAK
levels were higher in the shPXN group. *P<0.05 vs.
Con; #P<0.05 vs. model and ^P<0.05 vs.
overExp. Con, control group; Model, model group; overExp, PXN
over-expressed group; shPXN, PXN knockdown group. |
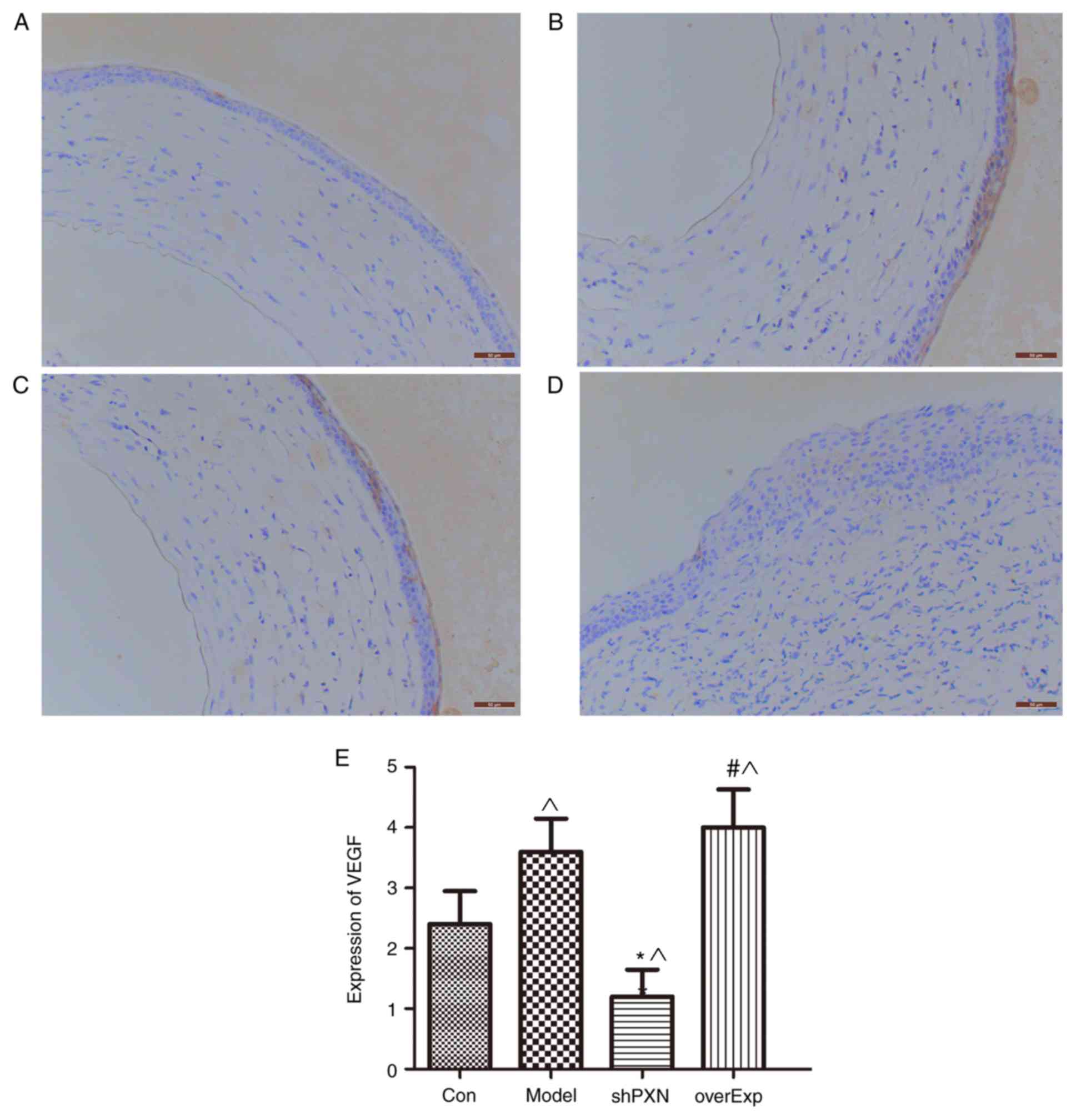
Expression of VEGF in vivo
Expression of VEGF was significantly higher in the
model group compared with the control group (P<0.05; Fig. 6). Compared with the control group,
VEGF expression was also significantly lower in the shPXN group and
significantly higher in the overEXP group.
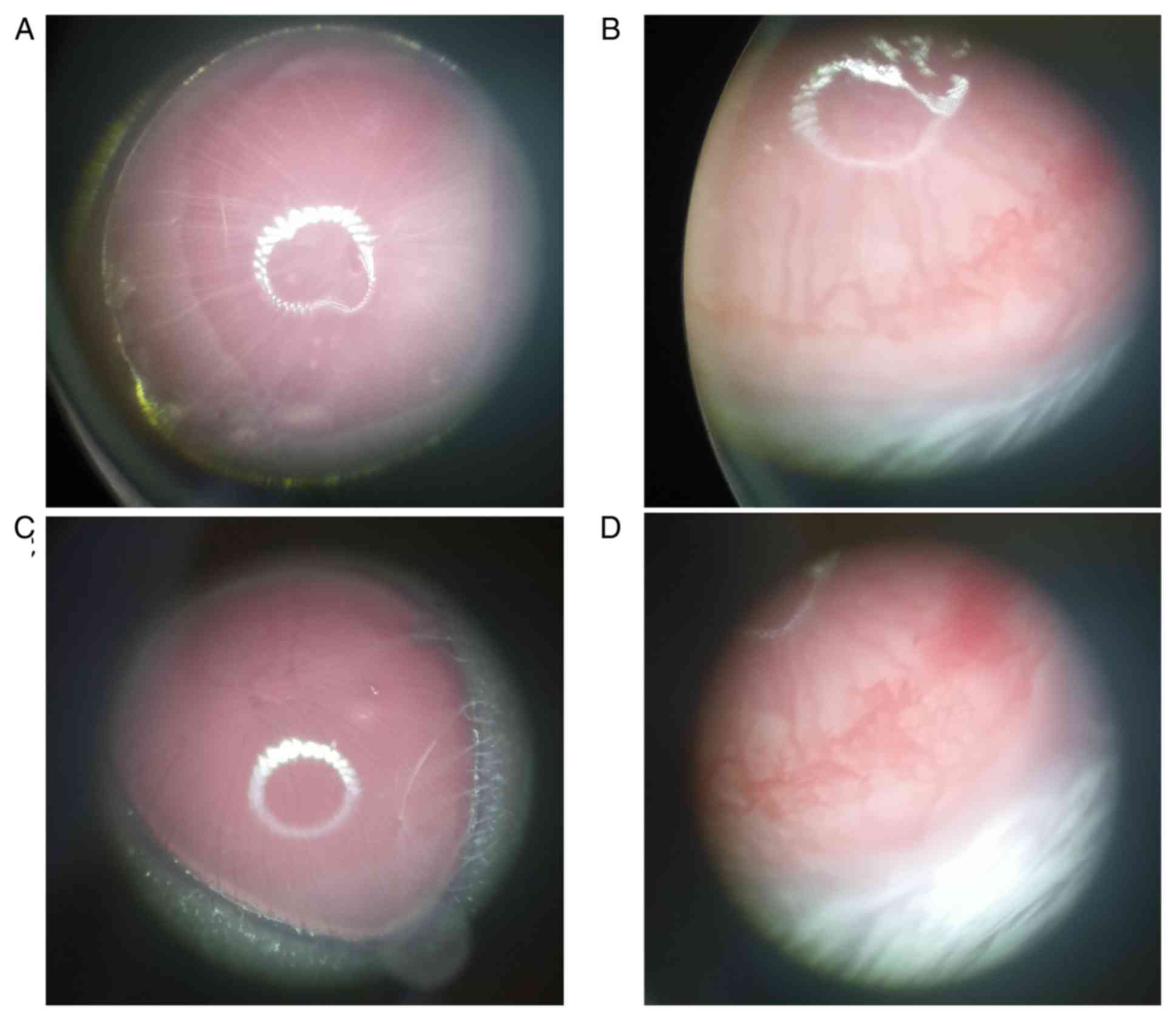
Angiogenesis in vivo
Capillaries could be seen growing from the corneal
limbus to the corneal stroma in the model and overexpression groups
(Fig. 7). No notable differences
were observed between the PXN knockdown and the control groups.
Discussion
Corneal avascularity is important for maintaining
corneal transparency and ensuring good eyesight. However, a number
of corneal diseases, such as infection, trauma and chemical injury,
can cause neovascularization to spread from the limbal cornea to
the corneal endothelium. This neovascularization will undermine the
transparency of the cornea and alter the physiological
microenvironment, causing damage to eyesight and even blindness
(13). According to records, ~4% of
ophthalmology patients in the United States are affected by corneal
neovascularization (14).
Neovascularization causes a variety of ophthalmological diseases
and eventual blindness, and has thus become a major issue in the
field of ophthalmology (15). At
present, there is still a lack of effective treatments for corneal
neovascularization (15,16). The product of the PXN gene is a
cytoskeletal phosphoric acid protein, primarily located in focal
adhesions (17,18). PXN is a complex protein that was
first discovered in v-Src-transfected cells (19), though its role in neovascularization
remains unclear and controversial. Shortly thereafter, purified
protein was obtained from smooth muscle tissue, and was identified
as a focal adhesion binding protein (20). PXN was proposed to function in the
uptake of muscle active proteins and at sites of cell adhesion.
Although PXN itself lacks enzymatic activity, it contains a variety
of structural domains that can bind signaling and structural
proteins (21,22). Therefore, PXN has been proposed to be
a cytoskeletal protein that can effectively transmit signals
(23-26).
Research has shown that paxillin mediates the adhesion, migration,
proliferation and cytoskeletal attachment of cells. Our previous
study demonstrated that PXN promotes VEGF-A-induced proliferation,
migration, adhesion and capillary formation in HUVECs (9). Minamiguchi et al (27) reported that PXN is essential for
neovascularization in tumor formation. Previous studies have shown
that PXN also serves an important role in corneal
neovascularization (8,9,28,29). In
the present study, HUVECs and a mouse corneal neovascularization
model were used to determine changes in PXN-mediated signaling
during corneal neovascularization induced by inflammation.
The establishment of adhesion junctions between
endothelial cells is a key component of angiogenesis (30). Formation of an endothelial network is
the premise of both initial angiogenesis as well as regeneration of
blood vessels. Node formation between endothelial cells is key to
various types of angiogenesis (31).
Cell migration involves dynamic spatial changes in the cytoskeleton
and cell attachment (32). Rho, Rac,
and Cdc42 are members of the GTP binding protein Rho subfamily
(Rho-GTPase). Rho-GTPases serve an important role in cell migration
by regulating the rearrangement of the cytoskeleton and stress
fiber formation (33-35).
Rac and Rho regulate the aggregation of agonist proteins, and
promote the production of plate pseudopods and stress fibers in
migrating cells (36). Cdc42
promotes the production of filamentous pseudopods and regulates the
direction of migration (37). Rho
can also increase the activity of myosin light chain kinase,
phosphorylate the myosin light chain, induce an increase in stress
fibers and promote cell migration (38). Rac regulates phosphorylation of the
myosin light chain, increases the tension of the cytoskeleton and
regulates cell contraction, which is the basis of focal adhesion
and stress fibers (38).
Furthermore, Cdc42 and Rac cooperate to regulate endothelial cell
lumen formation during vascular regeneration (39). In the present study, the levels of
Rac, Rho, Cdc42 and PXN protein were lower in the shPXN group and
higher in the overEXP group, suggesting that PXN downregulates Rac,
Rho and Cdc42, and promotes angiogenesis and cell migration. This
is consistent with the cell migration results, given the
involvement of Rac, Rho and Cdc42 in migration.
PXN is controlled by a series of genes, including
Ras, FAK, Src, GIT, PIX, NCK, PT538, PKC-δ, RAC, MEKK1 and MKK4/7,
both FAK and Src can modulate the phosphorylation status of
paxillin at Tyr31 and Tyr118(40).
In the present study, it was demonstrated that FAK promoted PXN
expression, whereas Src inhibited it. These results are consistent
with those of Sachdev et al (41) in which FAK was shown to promote the
phosphorylation of PXN, and increase the expression of Rho and Rac,
whilst inhibiting Cdc42. In the present study, compared with the
empty vector-transfected control group, cell migration was
significantly increased in the overexpression group. Therefore, PXN
promotes the migration of HUVECs. Lisiak et al (7) reached a similar conclusion, where it
was demonstrated that downregulation of PXN inhibited migration of
human breast cancer cells. Furthermore, German et al
(8) and Sero et al (28,29)
showed that a decrease in PXN expression enhanced the migration of
HUVECs. In addition, PXN has been shown to serve a key role in
tumor progression. Findings from the present study showed that tube
formation was significantly increased in HUVECs and mice in the
overexpression group, and thus PXN may facilitate angiogenesis.
In summary, the present study showed that during
corneal angiogenesis, PXN drove the migration of endothelial cells
and promoted angiogenesis. However, the detailed underlying
mechanism remains unknown. The results highlight the role of PXN in
corneal angiogenesis, and suggest that targeting the PXN signaling
pathway may inhibit corneal angiogenesis, providing a theoretical
basis for the prevention of corneal diseases.
Acknowledgements
The authors would like to pay their respects to ZMM,
who died in the fight against COVID-19 on 3rd March 2020.
Funding
The present study was supported by grants from the
National Natural Science Foundation Youth Project (grant no.
81800802) and the Wuhan Municipal Health and Family Planning
Committee Guidance Project (Wuhan, China; grant no. WX17Z02).
Availability of data and materials
All data generated or analyzed during this study are
included in the published article.
Authors' contributions
WJY and YQX designed the study and wrote the
manuscript. JBY, WY, YX, LZ, YNY, ZMM and FZ performed the
experiments. YX performed the statistical analysis. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Institutional Animal Care and Use Committee of Tongji Medical of
Huazhong University of Science and Technology and Animal
Experimental Ethical Inspection of Laboratory Animal Centre,
Huazhong Agriculture University (approval no. HZAUMO-2017-039).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gong X and Rubin LP: Role of macular
xanthophylls in prevention of common neovascular retinopathies:
Retinopathy of prematurity and diabetic retinopathy. Arch Biochem
Biophys. 572:40–48. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hackett SF, Seidel C, Abraham S, Chadha R,
Fortmann SD, Campochiaro PA and Cooke JP: The nicotinic cholinergic
pathway contributes to retinal neovascularization in a mouse model
of retinopathy of prematurity. Invest Ophthalmol Vis Sci.
58:1296–1303. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Choudhry N, Golding J and Rao RC: Vitreous
invasion: Neovascular frond in proliferative diabetic retinopathy.
Ophthalmology. 123(2625)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nguyen QD, De Falco S, Behar-Cohen F, Lam
WC, Li X, Reichhart N, Ricci F, Pluim J and Li WW: Placental growth
factor and its potential role in diabetic retinopathy and other
ocular neovascular diseases. Acta Ophthalmol. 96:e1–e9.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Singh NK, Kotla S, Kumar R and Rao GN:
Cyclic AMP response element binding protein mediates pathological
retinal neovascularization via modulating DLL4-NOTCH1 signaling.
EBioMedicine. 2:1767–1784. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nakama T, Yoshida S, Ishikawa K, Kubo Y,
Kobayashi Y, Zhou Y, Nakao S, Hisatomi T, Ikeda Y, Takao K, et al:
Therapeutic effect of novel single-stranded RNAi agent targeting
periostin in eyes with retinal neovascularization. Mol Ther Nucleic
Acids. 6:279–289. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lisiak N, Paszel-Jaworska A, Toton E,
Rubis B, Pakula M, Bednarczyk-Cwynar B, Zaprutko L and Rybczynska
M: Semisynthetic oleanane triterpenoids inhibit migration and
invasion of human breast cancer cells through downregulated
expression of the ITGB1/PTK2/PXN pathway. Chem Biol Interact.
268:136–147. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
German AE, Mammoto T, Jiang E, Ingber DE
and Mammoto A: Paxillin controls endothelial cell migration and
tumor angiogenesis by altering neuropilin 2 expression. J Cell Sci.
127:1672–1683. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang WJ, Yang YN, Cao J, Man ZH, Li Y and
Xing YQ: Paxillin regulates vascular endothelial growth factor
A-induced in vitro angiogenesis of human umbilical vein
endothelial cells. Mol Med Rep. 11:1784–1792. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Watanabe M, Da Fonseca CD and Vattimo Mde
F: Instrumental and ethical aspects of experimental research with
animal models. Rev Esc Enferm USP. 48:181–188. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Morton DB and Griffiths PH: Guidelines on
the recognition of pain, distress and discomfort in experimental
animals and hypothesis for assessment. Vet Rec. 116:431–436.
1985.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Voiculescu OB, Voinea LM and Alexandrescu
C: Corneal neovascularization and biological therapy. J Med Life.
8:444–448. 2015.PubMed/NCBI
|
|
14
|
Norooznezhad AH, Norooznezhad F and Ahmadi
K: Next target of tranilast: Inhibition of corneal
neovascularization. Med Hypotheses. 82:700–702. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hsu CC, Chang HM, Lin TC, Hung KH, Chien
KH, Chen SY, Chen SN and Chen YT: Corneal neovascularization and
contemporary antiangiogenic therapeutics. J Chin Med Assoc.
78:323–330. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gupta D and Illingworth C: Treatments for
corneal neovascularization: A review. Cornea. 30:927–938.
2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nikolopoulos SN and Turner CE: Actopaxin,
a new focal adhesion protein that binds paxillin LD motifs and
actin and regulates cell adhesion. J Cell Biol. 151:1435–1448.
2000.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kratimenos P, Koutroulis I, Marconi D,
Syriopoulou V, Delivoria-Papadopoulos M, Chrousos GP and Theocharis
S: Multi-targeted molecular therapeutic approach in aggressive
neuroblastoma: The effect of Focal Adhesion Kinase-Src-Paxillin
system. Expert Opin Ther Targets. 18:1395–1406. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Grgurevich S, Mikhael A and Mcvicar DW:
The Csk homologous kinase, Chk, binds tyrosine phosphorylated
Paxillin in Human Blastic T cells. Biochem Biophys Res Commun.
256:668–675. 1999.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Brown MC, Perrotta JA and Turner CE:
Identification of LIM3 as the principal determinant of paxillin
focal adhesion localization and characterization of a novel motif
on paxillin directing vinculin and focal adhesion kinase binding. J
Cell Biol. 135:1109–1123. 1996.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tsai WC, Yu TY, Lin LP, Lin MS, Tsai TT
and Pang JS: Platelet rich plasma promotes skeletal muscle cell
migration in association with up-regulation of FAK, paxillin, and
F-Actin formation. J Orthop Res. 35:2506–2512. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wu KH, Ho CT, Chen ZF, Chen LC, Whang-Peng
J, Lin TN and Ho YS: The apple polyphenol phloretin inhibits breast
cancer cell migration and proliferation via inhibition of signals
by type 2 glucose transporter. J Food Drug Anal. 26:221–231.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jagadeeswaran R, Zumba O, Yala S and
Salgia R: Paxillin and MET interactions promote lung cancer growth,
invasion, and angiogenesis. Cancer Res: 68, 2008.
|
|
24
|
Lyck R, Reiss Y, Gerwin N, Greenwood J,
Adamson P and Engelhardt B: T-cell interaction with ICAM-1/ICAM-2
double-deficient brain endothelium in vitro: The cytoplasmic tail
of endothelial ICAM-1 is necessary for transendothelial migration
of T cells. Blood. 102:3675–3683. 2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Stiegler AL, Draheim KM, Li X, Chayen NE,
Calderwood DA and Boggon TJ: Structural basis for paxillin binding
and focal adhesion targeting of β-parvin. J Biol Chem.
287:32566–32577. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Turner CE: Paxillin and focal adhesion
signalling. Nat Cell Biol. 2:E231–E236. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Minamiguchi K, Kumagai H, Masuda T, Kawada
M, Ishizuka M and Takeuchi T: Thiolutin, an inhibitor of HUVEC
adhesion to vitronectin, reduces paxillin in HUVECs and suppresses
tumor cell-induced angiogenesis. Int J Cancer. 93:307–316.
2001.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Sero JE, German AE, Mammoto A and Ingber
DE: Paxillin controls directional cell motility in response to
physical cues. Cell Adh Migr. 6:502–508. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sero JE, Thodeti CK, Mammoto A, Bakal C,
Thomas S and Ingber DE: Paxillin mediates sensing of physical cues
and regulates directional cell motility by controlling lamellipodia
positioning. PLoS One. 6(e28303)2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lampugnani MG and Dejana E: Adherens
junctions in endothelial cells regulate vessel maintenance and
angiogenesis. Thromb Res. 120 (Suppl 2):S1–S6. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
De Souza Junior DA, Mazucato VM, Santana
AC, Oliver C and Jamur MC: Mast cells interact with endothelial
cells to accelerate in vitro angiogenesis. Int J Mol Sci.
18:2674–2679. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ridley AJ, Allen WE, Peppelenbosch M and
Jones GE: Rho family proteins and cell migration. Biochem Soc Symp.
65:111–123. 1999.PubMed/NCBI
|
|
33
|
Krause-Gruszczynska M, Rohde M, Hartig R,
Genth H, Schmidt G, Keo T, Konig W, Miller WG, Konkel ME, Konkel ME
and Backert S: Role of the small Rho GTPases Rac1 and Cdc42 in host
cell invasion of Campylobacter jejuni. Cell Microbiol. 9:2431–2444.
2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hernández-Garcia R, Iruela-Arispe ML,
Reyes-Cruz G and Vazquez-Prado J: Endothelial RhoGEFs: A systematic
analysis of their expression profiles in VEGF-stimulated and tumor
endothelial cells. Vascul Pharmacol. 74:60–72. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kusuhara S, Fukushima Y, Fukuhara S, Jakt
LM, Okada M, Shimizu Y, Hata M, Nishida K, Negi A, Hirashima M, et
al: Arhgef15 promotes retinal angiogenesis by mediating
VEGF-induced Cdc42 activation and potentiating RhoJ inactivation in
endothelial cells. PLoS One. 7(e45858)2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nobes CD and Hall A: Rho, rac, and cdc42
GTPases regulate the assembly of multimolecular focal complexes
associated with actin stress fibers, lamellipodia, and filopodia.
Cell. 81:53–62. 1995.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Nakashima M and Lazo JS: Phosphatase of
regenerating liver-1 promotes cell migration and invasion and
regulates filamentous actin dynamics. J Pharmacol Exp Ther.
334:627–633. 2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Brzeska H, Szczepanowska J, Matsumura F
and Korn ED: Rac-induced increase of phosphorylation of myosin
regulatory light chain in HeLa cells. Cell Motil Cytoskeleton.
58:186–199. 2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Bayless KJ and Davis GE: The Cdc42 and
Rac1 GTPases are required for capillary lumen formation in
three-dimensional extracellular matrices. J Cell Sci.
115:1123–1136. 2002.PubMed/NCBI
|
|
40
|
Vindis C, Teli T, Cerretti DP, Turner CE
and Huynh-Do U: EphB1-mediated cell migration requires the
phosphorylation of paxillin at Tyr-31/Tyr-118. J Biol Chem.
279:27965–27970. 2004.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sachdev S, Bu Y and Gelman IH:
Paxillin-Y118 phosphorylation contributes to the control of
Src-induced anchorage-independent growth by FAK and adhesion. BMC
Cancer. 9(12)2009.PubMed/NCBI View Article : Google Scholar
|