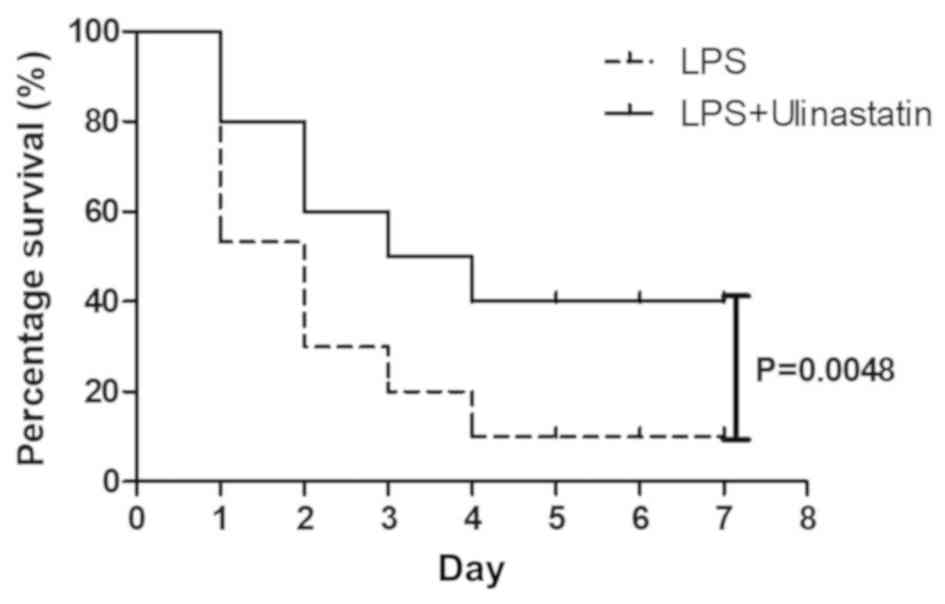
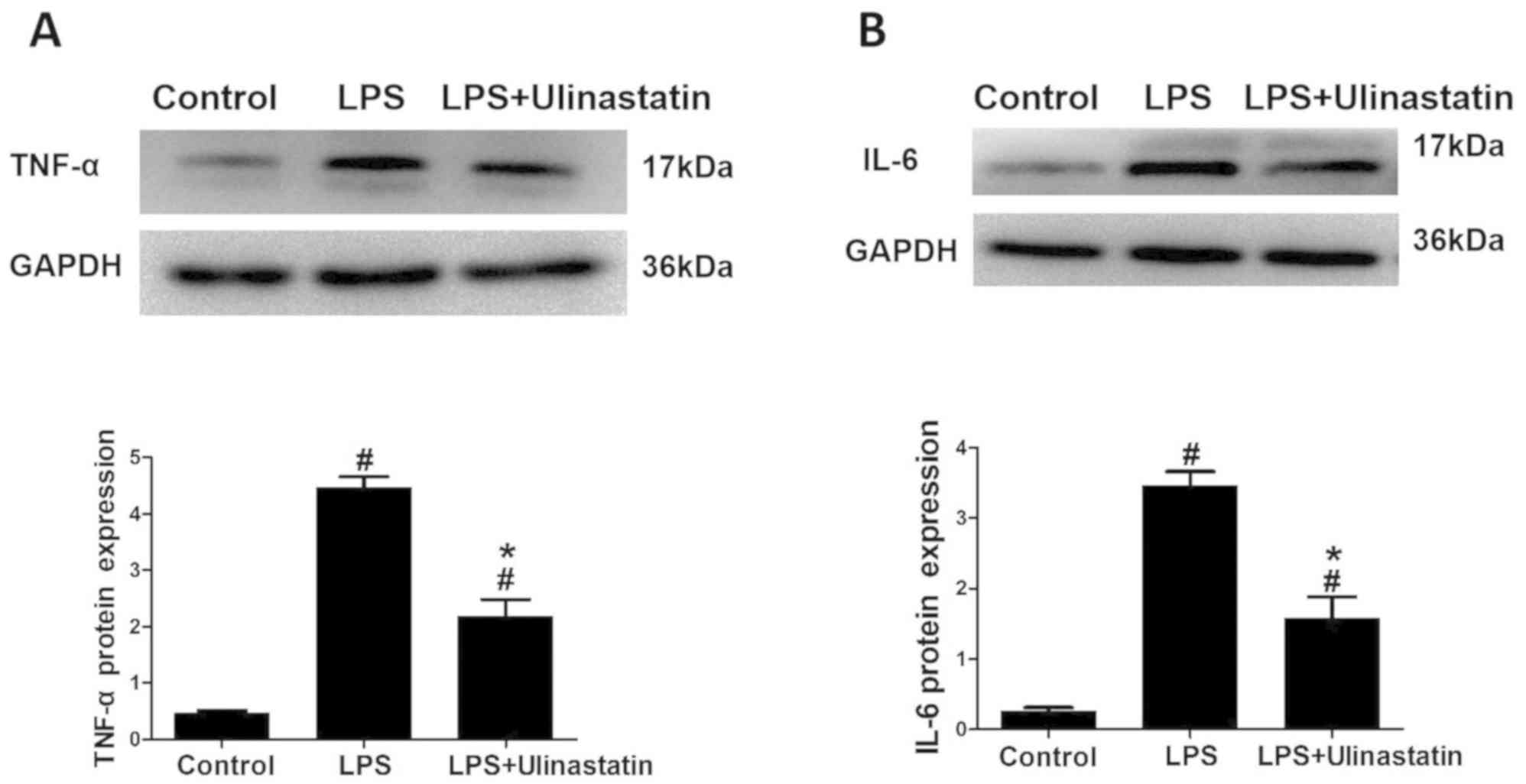
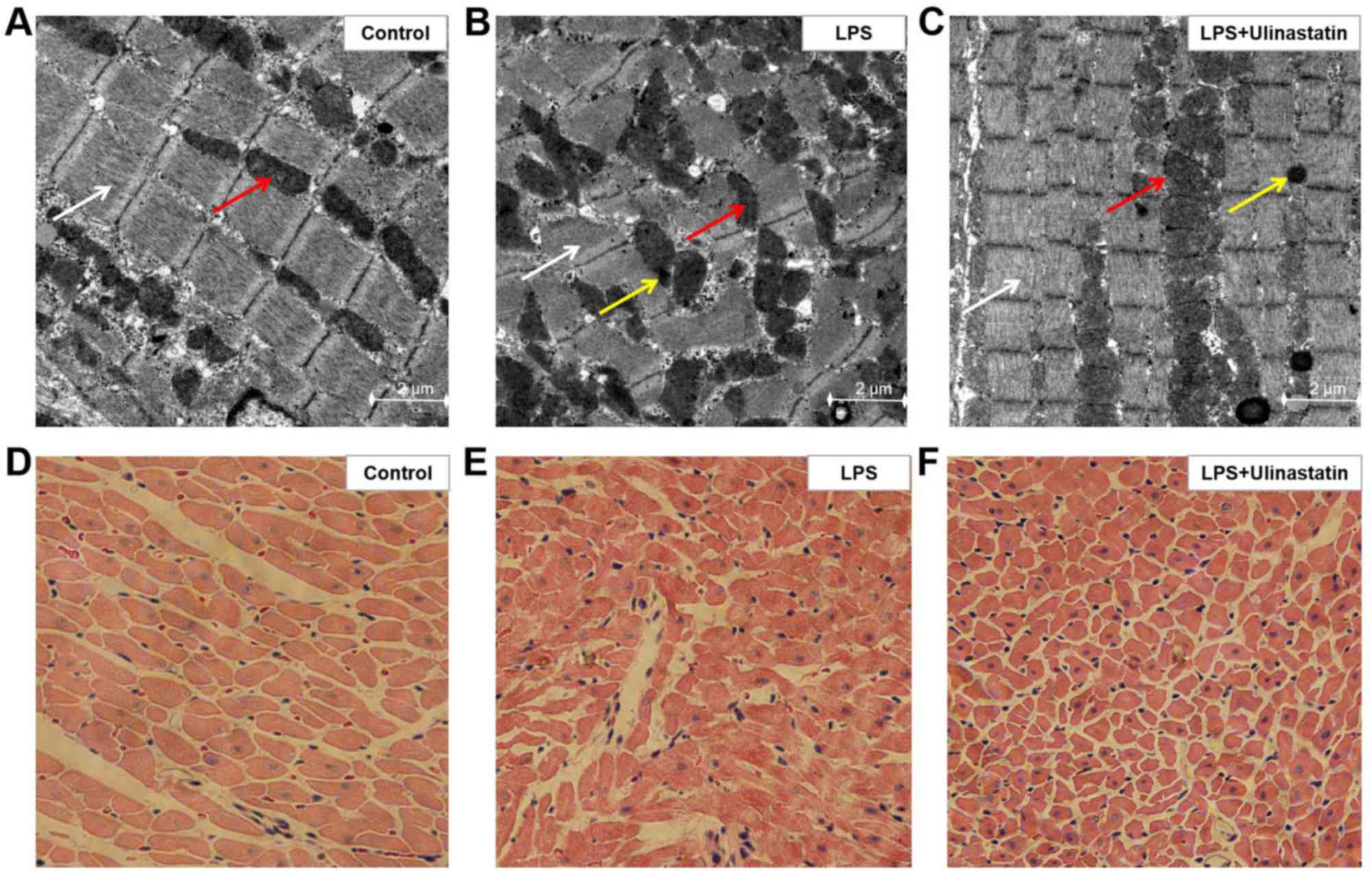
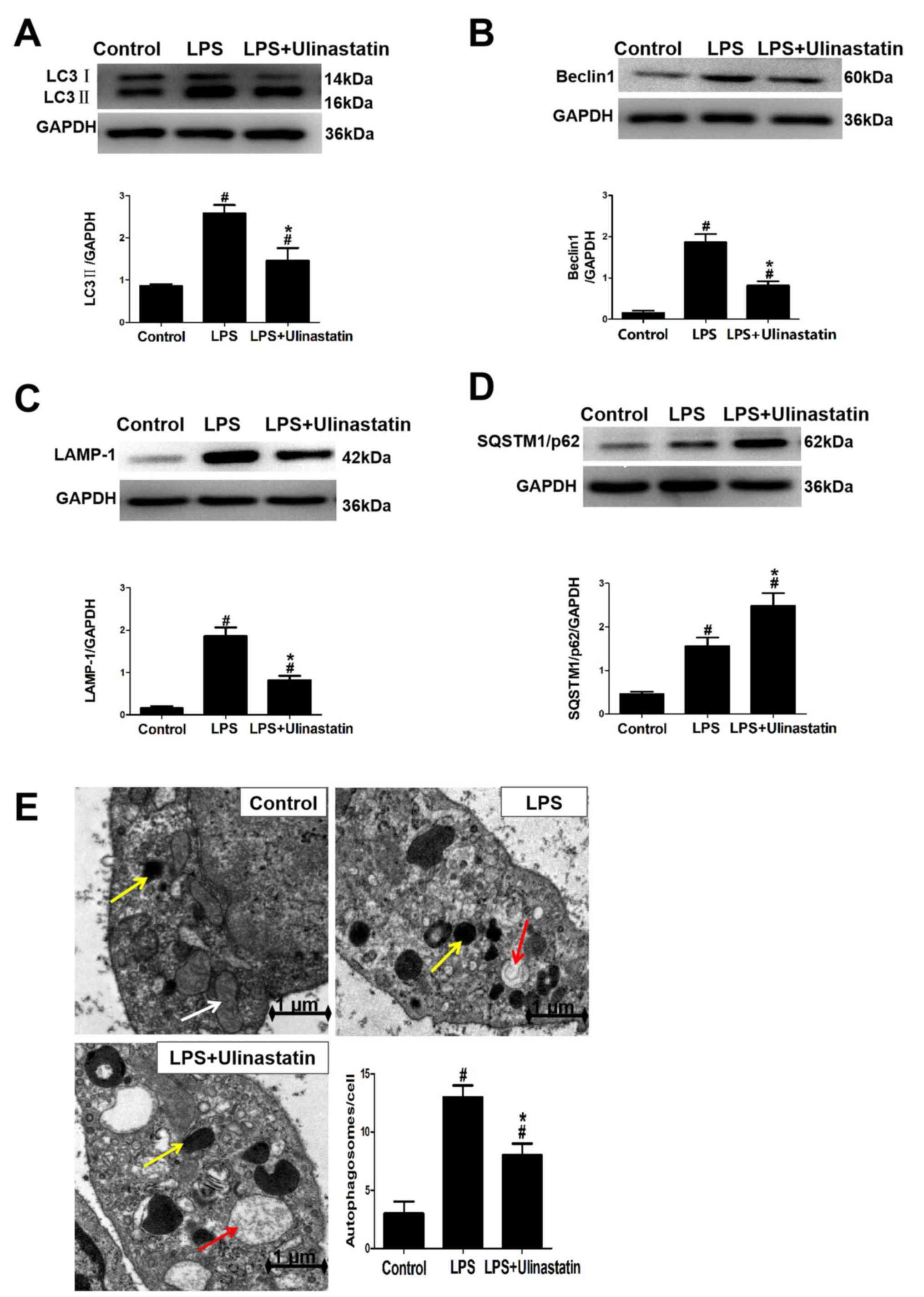
|
1
|
Hotchkiss RS and Karl IE: The
pathophysiology and treatment of sepsis. N Engl J Med. 348:138–150.
2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kumar A, Haery C and Parrillo JE:
Myocardial dysfunction in septic shock. Crit Care Clin. 16:251–287.
2000.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rudiger A and Singer M: Mechanisms of
sepsis-induced cardiac dysfunction. Crit Care Med. 35:1599–1608.
2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
You W, Min X, Zhang X, Qian B, Pang S,
Ding Z, Li C, Gao X, Di R, Cheng Y and Liu L: Cardiac-Specific
expression of heat shock protein 27 attenuated endotoxin-induced
cardiac dysfunction and mortality in mice through a
PI3K/Akt-dependent mechanism. Shock. 32:108–117. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fleischmann C, Scherag A, Adhikari NK,
Hartog CS, Tsaganos T, Schlattmann P, Angus DC and Reinhart K:
International Forum of Acute Care Trialists. Assessment of global
incidence and mortality of hospital-treated sepsis. Am J Respir
Crit Care Med. 193:259–272. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jönsson-Berling BM, Ohlsson K and
Rosengren M: Radioimmunological quantitation of the urinary trypsin
inhibitor in normal blood and urine. Biol Chem Hoppe Seyler.
370:1157–1161. 1989.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shin IW, Jang IS, Lee SM, Park KE, Ok SH,
Sohn JT, Lee HK and Chung YK: Myocardial protective effect by
ulinastatin via an anti-inflammatory response after regional
ischemia/reperfusion injury in an in vivo rat heart model. Korean J
Anesthesiol. 61:499–505. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Masuda T, Sato K, Noda C, Ikeda KM,
Matsunaga A, Ogura MN, Shimizu K, Nagasawa H, Matsuyama N and Izumi
T: Protective effect of urinary trypsin inhibitor on myocardial
mitochondria during hemorrhagic shock and reperfusion. Crit Care
Med. 31:1987–1992. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Koga Y, Fujita M, Tsuruta R, Koda Y,
Nakahara T, Yagi T, Aoki T, Kobayashi C, Izumi T, Kasaoka S, et al:
Urinary trypsin inhibitor suppresses excessive superoxide anion
radical generation in blood, oxidative stress, early inflammation,
and endothelial injury in forebrain ischemia/reperfusion rats.
Neurol Res. 32:925–932. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yu Z, Rayile A, Zhang X, Li Y and Zhao Q:
Ulinastatin protects against lipopolysaccharide-induced cardiac
microvascular endothelial cell dysfunction via downregulation of
lncRNA MALAT1 and EZH2 in sepsis. Int J Mol Med. 39:1269–1276.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mizushima N: The ATG conjugation systems
in autophagy. Curr Opin Cell Biol. 31:1–10. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cursio R, Colosetti P and Gugenheim J:
Autophagy and liver ischemia-reperfusion injury. Biomed Res Int.
2015(417590)2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ho J, Yu J, Wong SH, Zhang L, Liu X, Wong
WT, Leung CC, Choi G, Wang MH, Gin T, et al: Autophagy in sepsis:
Degradation into exhaustion? Autophagy. 12:1073–1082.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gurusamy N and Das DK: Is autophagy a
double-edged sword for the heart? Acta Physiol Hung. 96:267–276.
2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
De Meyer GR and Martinet W: Autophagy in
the cardiovascular system. Biochim Biophys Acta. 1793:1485–1495.
2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xiao J, Zhu X, Ji G, Yang Q, Kang B, Zhao
J, Yao F, Wu L, Ni X and Wang Z: Ulinastatin protects
cardiomyocytes against ischemiareperfusion injury by regulating
autophagy through mTOR activation. Mol Med Rep. 10:1949–1953.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhao P, Kuai J, Gao J, Sun L, Wang Y and
Yao L: Delta opioid receptor agonist attenuates
lipopolysaccharide-induced myocardial injury by regulating
autophagy. Biochem Biophys Res Commun. 492:140–146. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang WK, Lu QH, Wang X, Wang B, Wang J,
Gong HP, Wang L, Li H and Du YM: Ulinastatin attenuates
diabetes-induced cardiac dysfunction by the inhibition of
inflammation and apoptosis. Exp Ther Med. 14:2497–2504.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lan R, Zhang Y, Xiang J, Zhang W, Wang GH,
Li WW, Xu LL and Cai DF: Xiao-Xu-Ming decoction preserves
mitochondrial integrity and reduces apoptosis after f ocal cerebral
ischemiaand reperfusion via the mitochondrial p53 pathway. J
Ethnopharmacol. 151:307–316. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Qiu SQ, van Rooijen J, Nienhuis HH, van
derVegt B, Timmer-Bosscha H, van Leeuwen-Stok E, Walenkamp AME, van
Deurzen CHM, de Bock GH, et al: High hepatocyte growth factor
expression in primary tumor predicts better overall survival in
male breast cancer. Breast Cancer Res. 22(30)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lin CM, Chen CR, Wu XQ, Ren JH, Chen SZ,
Luo XF, Mei XQ, Shen LY, Guo MX, Ma XD and Yang T: Effects of blood
purification on serum levels of inflammatory cytokines and cardiac
function in a rat model of sepsis. Blood Purif. 44:40–50.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lim YP, Bendelja K, Opal SM, Siryaporn E,
Hixson DC and Palardy JE: Correlation between mortality and the
levels of inter-alpha inhibitors in the plasma of patients with
severe sepsis. J Infect Dis. 188:919–926. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Han D, Shang W, Wang G, Sun L, Zhang Y,
Wen H and Xu L: Ulinastatin and thymosin α1-based immunomodulatory
strategy for sepsis: A meta-analysis. Int Immunopharmacol.
29:377–382. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang FY, Fang B, Qiang XH, Yu TO, Zhong
JR, Cao J and Zhou LX: The efficacy and immunomodulatory effects of
ulinastatinand thymosin α1 for sepsis: A systematic review and
meta-analysis. Biomed Res Int. 2016(9508493)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gogos CA, Drosou E, Bassaris HP and
Skoutelis A: Pro-Versus anti-inflammatory cytokine profile in
patients with severe sepsis: A marker for prognosis and future
therapeutic options. J Infect Dis. 181:176–180. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Chen X, Wang Y, Luo H, Luo Z, Liu L, Xu W,
Zhang T, Yang N, Long X, Zhu N, et al: Ulinastatin reduces urinary
sepsis-related inflammation by upregulating IL-10 and
downregulating TNF-α levels. Mol Med Rep. 8:29–34. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Varvarousis D, Goulas N, Polytarchou K,
Psychari SN, Paravolidakis K, Konstantinidou A, Tsoukalas D, Vlad
D, Bouki K and Kotsakis A: Biomarkers of myocardial injury and
inflammation after permanent pacemaker implantation: The lead
fixation type effect. J Atr Fibrillation. 10(1798)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shires SE and Gustafsson ÅB: Mitophagy and
heart failure. J Mol Med (Berl). 93:253–262. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Joiner ML, Koval OM, Li JD, He B,
Allamargot C, Gao Z, Luczak ED, Hall DD, Fink BD, Chen B, et al:
CaMKII determines mitochondrial stress responses in heart. Nature.
491:269–273. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Nishida K, Kyoi S, Yamaguchi O, Sadoshima
J and Otsu K: The role of autophagy in the heart. Cell Death
Differ. 16:31–38. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Castillo K, Valenzuela V, Matus S, Nassif
M, Oñate M, Fuentealba Y, Encina G, Irrazabal T, Parsons G, Court
FA, et al: Measurement of autophagy flux in the nervous system in
vivo. Cell Death Dis. 4(e917)2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Eskelinen EL: Roles of LAMP-1 and LAMP-2
in lysosome biogenesis and autophagy. Mol Aspects Med. 27:495–502.
2006.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mathew R, Karp CM, Beaudoin B, Vuong N,
Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, et al:
Autophagy suppresses tumorigenesis through elimination of p62.
Cell. 137:1062–1075. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhao P, Gao JJ, Jiang J, Peng X, Wu W,
Zheng L and Yao L: Myocardial cells and mitochondrial autophagy in
sepsis mice induced by lipopolysaccharide. Xi Bao Yu Fen Zi Mian Yi
Xue Za Zhi. 32:177–181. 2016.PubMed/NCBI(In Chinese).
|