Introduction
MicroRNA (miR) is a type of non-coding short-chain
RNA of 22nt in length. In eukaryotes, it can regulate the
expression of target genes by binding to the downstream target gene
3'UTR, 5'UTR and coding region, thus participating in intercellular
signal modulation (1,2). At present, approximately 1,500 genes
encoded by miR have been identified in the human genome (3). Along with the continuous exploration of
miRs, there is emerging evidence showing that miRs play an
important part in synaptic plasticity, learning and memory function
(4). Among them, miR-132 is an
important member of the miR family, which has been widely studied
in a variety of neurological diseases. It has been reported that
downregulation of miR-132/21 disrupts the S-nitrosation balance of
Alzheimer's disease (AD) and induces tau phosphorylation, thereby
promoting the pathogenesis of AD (5). Other studies have pointed out that
elevated levels of miR-132 are associated with visual memory
dysfunction in patients with depression (6). All the above studies have shown that
miR-132 is closely related to learning and memory function and
related diseases, however, its specific mechanisms of action remain
a subject of investigation.
To explore the pathways in which miR-132 affects
learning and memory function, we predicted the presence of a
targeted binding site between glycogen synthase kinase-3β (GSK-3β)
and miR-132 by targetscan, an online biological prediction
software. GSK-3β and GSK-3α constitute the GSK-3 family, a
ubiquitously expressed and highly conserved serine/threonine kinase
that was first discovered in 1980 and is implicated in a variety of
central intracellular signaling pathways, including glucose
metabolism, inflammation and immune response as well as cell
biological functions (7). Numerous
scholar in the past have found that GSK-3β is closely related to
learning and memory function. For example, it is stated that the
activation of GSK-3β is closely related to aluminum-induced
long-term potentiation injury in rats (8). Others have shown that
tetramethylpyrazine can protect the memory loss of AD patients by
inhibiting GSK-3β activity (9). In
recent years, studies have also found that there is a certain
regulatory relationship between GSK-3β and miR. For example, it is
reported that upregulation of miR-26a can promote apoptosis of
neonatal cardiomyocytes in hypoxic rats by inhibiting the
expression of GSK-3β protein (10).
Still, some others have revealed that miR-199a can inhibit the
proliferation and survival of renal cancer cells by targeting
GSK-3β (11). All these findings
suggest that miR has a regulatory relationship with GSK-3β and may
affect learning and memory function.
Thus, it is hypothesized that miR-132 might affect
the learning and memory function by targeting the activity of
GSK-3β, and the present study was conducted.
Materials and methods
Source of experimental animals and
cell lines
Two batches of C57/BL male mice, aged 3-4 months and
24-26 months, respectively, were purchased from Beijing Charles
River Laboratory Animal Technology Co., Ltd., and the animals were
cultured in an animal room at 21-26˚C with relative humidity of
51-57%. They were allowed to eat freely under natural light for 15
days for subsequent experiments. The experiment was conducted in
strict accordance with the Guide for the Care and Use of
Experimental Animals (12). 293
cells were purchased from ATCC (USA).
Main reagents and instruments
Radio immunoprecipitation assay (RIPA) reagent,
bicinchoninic acid (BCA) protein assay kit and
electrochemiluminescence (ECL) kit were purchaced from Thermo
Fisher Scientific, Inc. GSK-3β (cat. no. MAB2506), β-actin antibody
(cat. no. MAB8929), Dulbecco's modified Eagle's medium (DMEM) and
fetal bovine serum, all from R&D Systems, Inc. Goat anti-rabbit
IgG secondary antibody (cat. no. BA1032) from Wuhan Boster
Biological Technology, Co., Ltd. TRIzol extraction kit (CDLG-4396;
Wuhan Chundu Biotechnology Co., Ltd.). Reverse transcription kit
[FP209; Tiangen Biotechnology (Beijing) Co., Ltd.]. Dual luciferase
reporter assay kit (D0010; Beijing Solarbio Science and Technology
Co., Ltd.). PCR instrument (7500; Applied Biosystems; Thermo Fisher
Scientific, Inc.). The primers were designed and synthesized by
Shanghai GenePharma Co., Ltd., and the lentiviral vectors were
synthesized by Guangzhou Ribo Biotechnology Co., Ltd.
Grouping and treatment of mice
Virus injection: Adult mice were anesthetized with
5% chloral hydrate, and their scalp was fixed and exposed. Then,
the hippocampus of the mice was injected with miR-132-inhibitor or
GSK-3β overexpression agent (sh-GSK-3β) using stereoscopic
localization technique, and disinfection was performed after the
injection was completed. While the negative control group was
injected with normal saline in the same manner, and the unrelated
control group was healthy adult mice without any treatment.
Follow-up studies were conducted 2 weeks after viral infection.
Cell culture
The cells were placed in DMEM (containing 10% bovine
fetal serum) medium at 37˚C in a constant temperature incubator
with 5% CO2, and cultured until the cells were adherent
to approximately 90%. Then cells were digested and passaged with
conventional trypsin. One day before the experiment, DMEM medium
was replaced with DMEM medium without bovine fetal serum and
further cultured overnight.
Morris water maze test
Ten mice from each group were subjected to Morris
water maze test. The mice were placed in a water-filled cylindrical
bucket, which was divided into four quadrants (one of which hid a
platform that could hold the mice in the quadrant), and randomly
selected one of them as the entry point. After putting the mice
into the water, the time of finding the platform was recorded, and
the mice were allowed to stay on the platform for 30 sec. The
operation was repeated every 1 h until the mice could find the
platform in all the four quadrants. After 6 days of continuous
training, the mice were allowed to rest for 1 day before spatial
probe test. The steps were as follows: The underwater platform was
removed and the mouse was placed in the quadrant opposite the
platform to record the duration of its stay in the target quadrant
and the number of times of crossings the original platform within
90 sec.
Western blot analysis
The hippocampus was isolated from the mouse brain,
ground and pulverized in a grinder, and the total protein was
extracted by RIPA lysis. Then the protein concentration was
adjusted to 4 µg/µl by BCA and separated by 6% SDS-PAGE
electrophoresis before transferring to the PVDF membrane and
staining in Ponceau S working solution. Followed by washing after
immersion in PBST, sealed with 5% skim milk powder for 2 h, then
added with GSK-3β and β-actin primary antibody (1:1,000), and
closed in a refrigerator at 4˚C overnight. After that, the primary
antibody was washed and the horseradish peroxidase-labeled goat
anti-rabbit secondary antibody (1:5,000) was added, incubated at
37˚C for 1 h, and rinsed 3 times with PBS, 5 min each. Finally,
development was carried out in the dark, the excess liquid on the
film was blotted with filter paper, and then illuminated by use of
an ECL kit and developed. The protein bands were scanned and the
gray value was analyzed using Quantity One software, wherein the
relative expression level of the protein = the gray value of the
target protein band/the gray value of the β-actin protein band.
RT-qPCR detection
The total RNA was extracted from the hippocampal
tissues of mice with TRIzol kit, whose purity, concentration and
integrity were detected by UV spectrophotometer and agarose gel
electrophoresis. Then RNA was reversely transcribed into cDNA
according to the instructions of the reverse transcription kit
(one-step method), followed by PCR amplification, with the PCR
reaction system as follows: 2xTalent qPCR PreMix: 10 μl,
upstream and downstream primers each 1.25 μl, cDNA: 100 ng,
and water was added to reach 20 μl. PCR reaction conditions:
Pre-denaturation at 95˚C for 3 min, denaturation at 95˚C for 5 sec,
and annealing at 60˚C for 15 sec, totaling for 40 cycles. With U6
as the internal parameter of miR-132, the upstream of miR-132 was
5'-TAACAGTCTACAGCCATGGTCG-3', and the downstream was
5'-CTTCTTGCTGGTCTTGCCATTCC-3', while the upstream of U6 was
5'-GCTTCGGCAGCACATATACTAAA AT-3', and the downstream was
5'-CGCTTCACGAATTTGC GTGTCAT-3'. The data was analyzed using
2-ΔΔCq (13).
Double luciferase report
The downstream target genes of miR-132 were
predicted by Targetscan 7.2. Lipofectamine™ 2000 kit was used to
transfect GSK-3β-3'UTR wild-type (Wt) and GSK-3β-3'UTR mutant (Mut)
and miR-132-inhibitor, miR-NC into target cells. Luciferase
activity was determined using a dual luciferase reporter assay kit
2 days after transfection.
Statistical analysis
The data processing of the experiments was analyzed
by SPSS 22.0 (IBM Corp.), and the illustrations were plotted using
the GraphPad 7. Student's t-test was employed for pairwise
comparison of the mean, while one-way ANOVA was applied for
multi-group comparison of the mean. Dunnett's test was adopted for
post-hoc pairwise comparison. P<0.05 was considered to indicate
a statistically significant difference.
Results
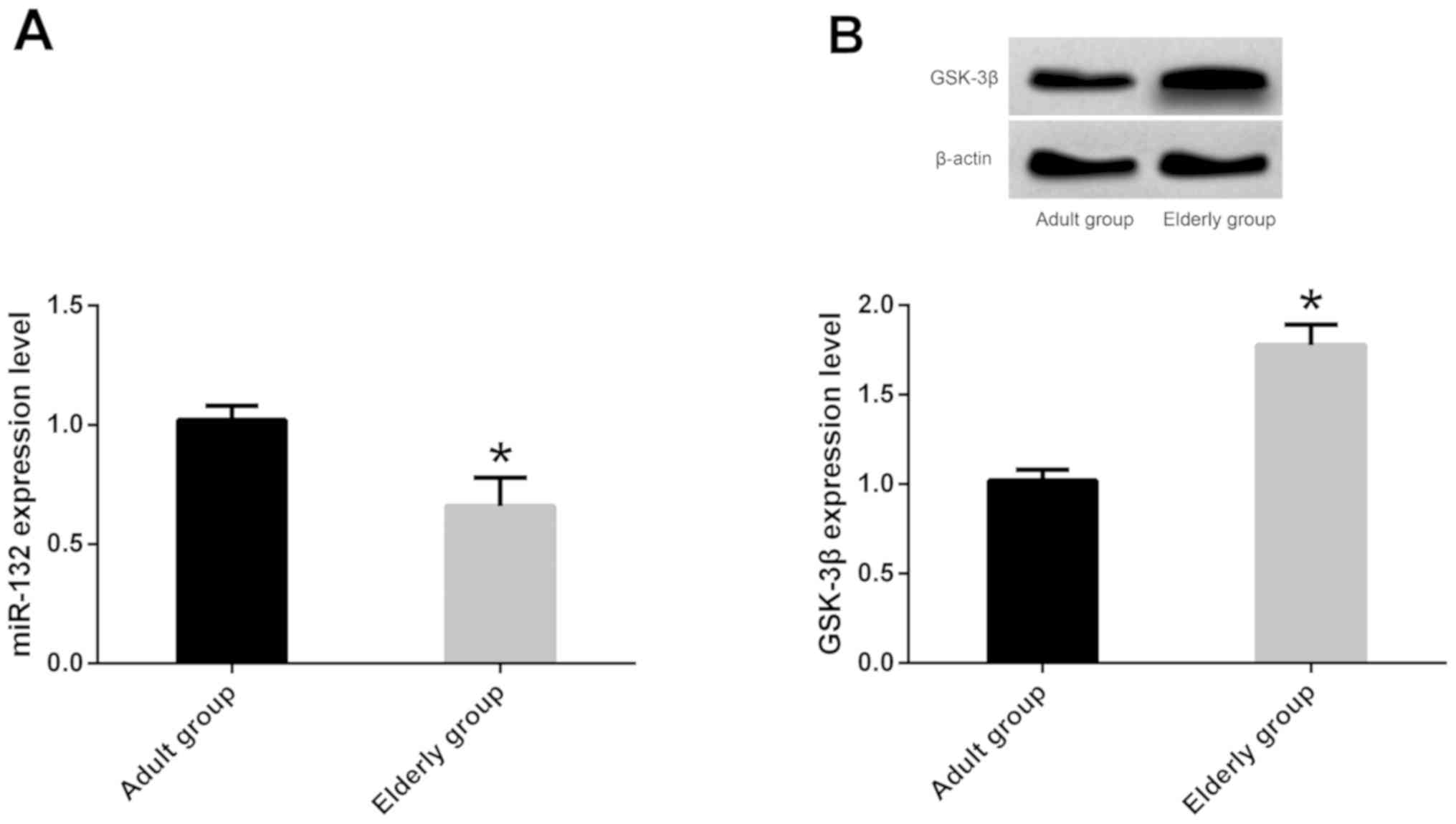
Expression of miR-132 and GSK-3β in
mice of different ages
RT-qPCR showed that the expression of miR-132 in
hippocampus of elderly mice was downregulated compared with that of
adult mice (P<0.05). While WB revealed that the expression of
GSK-3β in hippocampus of elderly mice was upregulated compared with
that of adult mice (P<0.05) (Fig.
1).
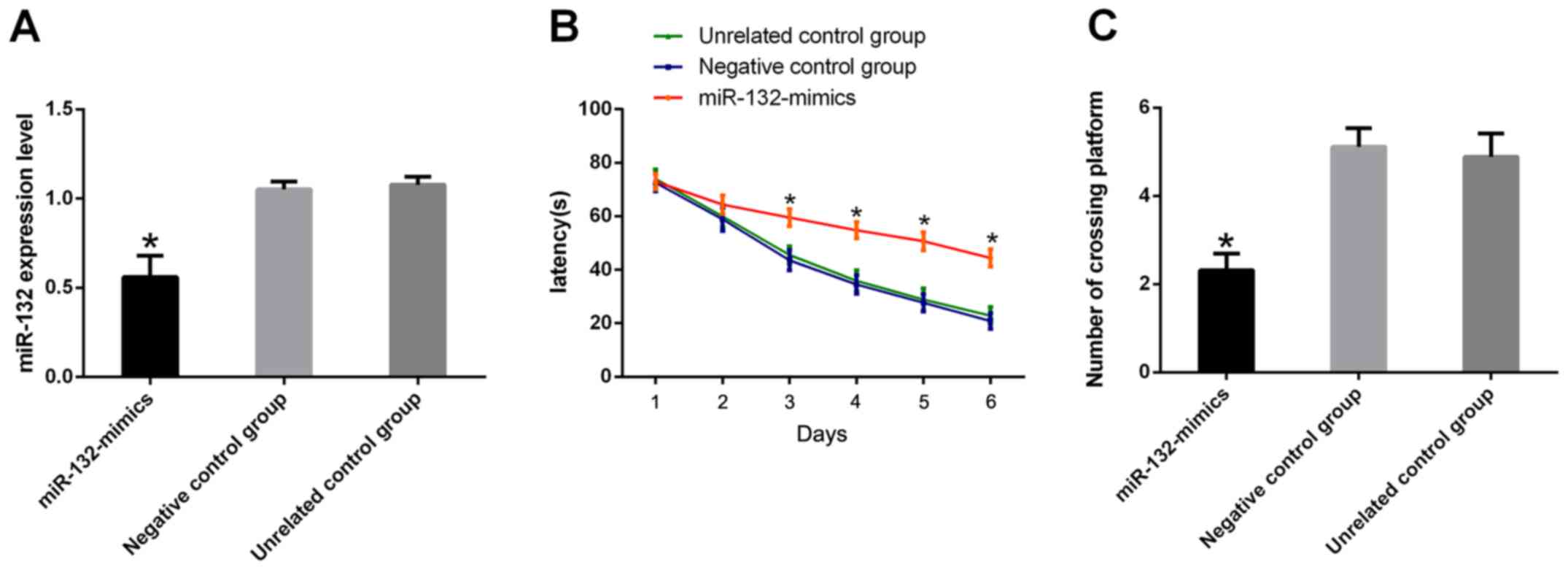
Downregulation of miR-132 impairs
learning and memory in mice
The expression of miR-132 in the hippocampus of the
miR-132-inhibitor group was significantly higher than that of the
two control groups (P<0.05). Morris water maze test results
showed that compared with the two control groups, the
miR-132-inhibitor group had a significant increase in escape
latency and a significant decrease in the number of times of
crossing platforms (P<0.05) (Fig.
2).
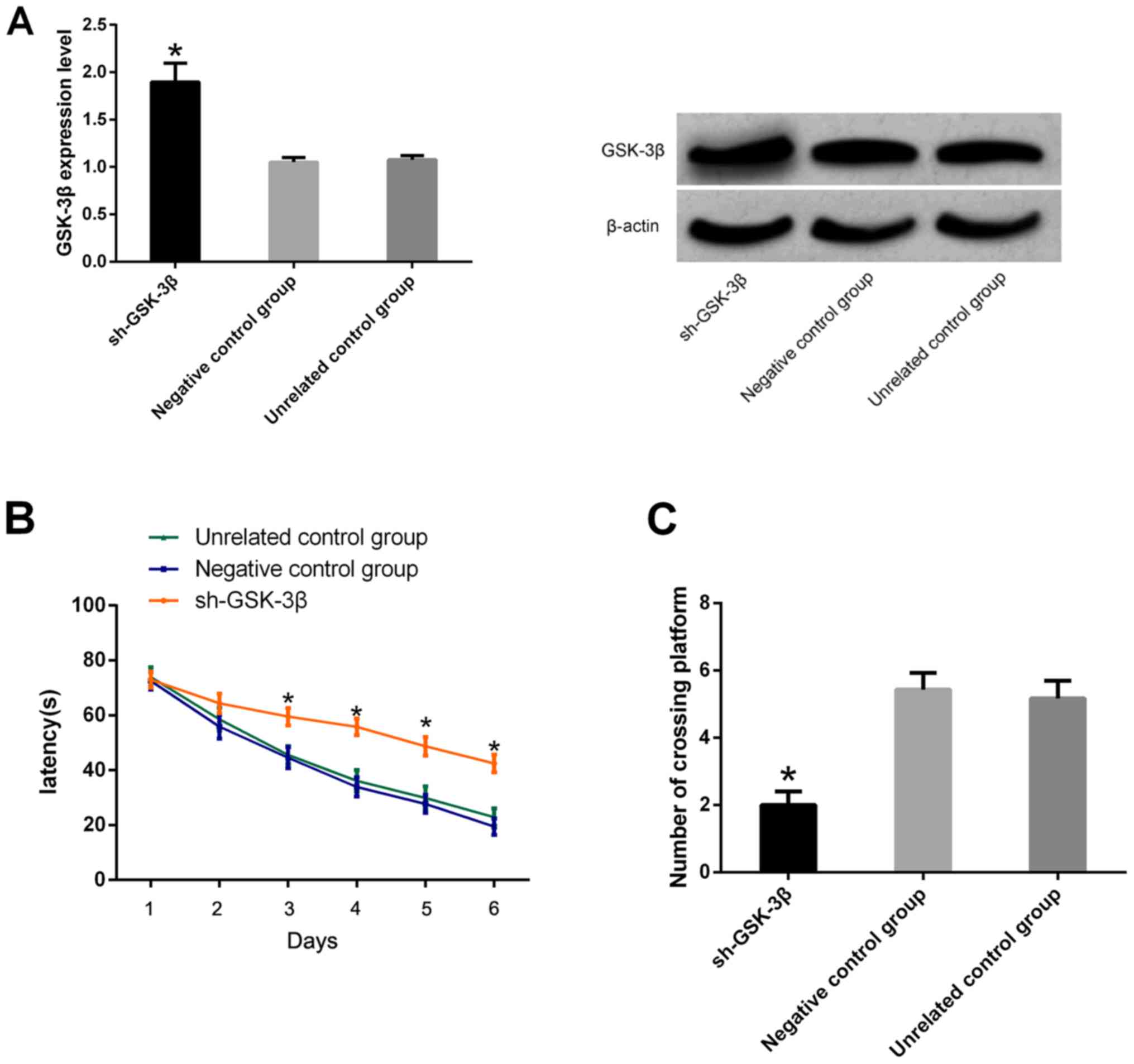
Upregulation of GSK-3β impairs
learning and memory in mice
It was found that the expression of GSK-3β in
hippocampus of sh-GSK-3β group was significantly higher than that
of the two control groups (P<0.05). Morris water maze test
results indicated that compared with the two control groups, the
escape latency of sh-GSK-3β mice increased significantly and the
number of times of crossing platforms declined significantly
(P<0.05) (Fig. 3).
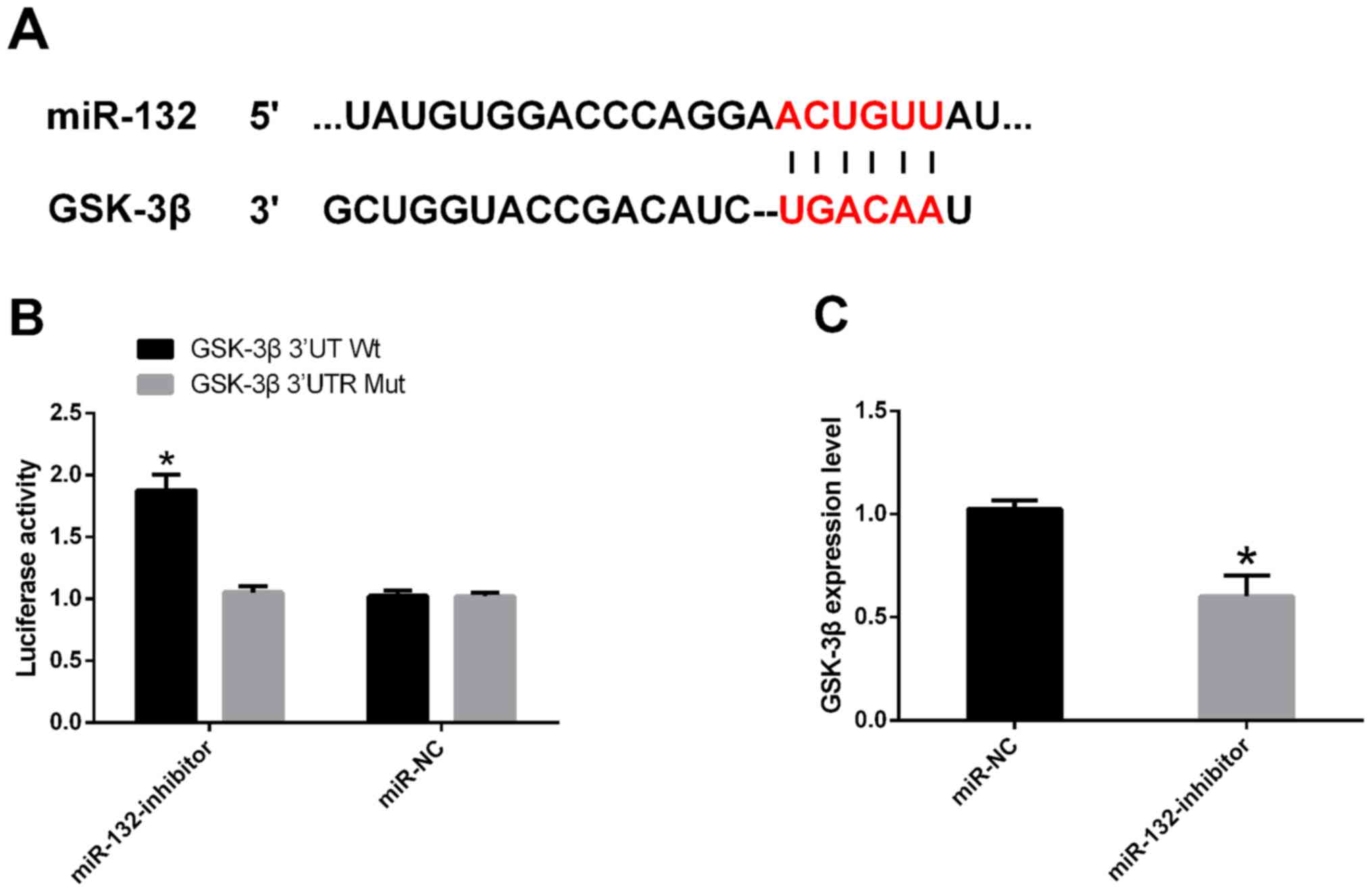
Double luciferase report
The downstream target gene of miR-132 was predicted
by Targetscan 7.2, and GSK-3β was found to have a target binding
site with miR-132. The double luciferase activity test revealed
that the luciferase activity of GSK-3β 3'UT Wt was markedly
elevated after miR-132 was inhibited (P<0.05), while had no
effect on GSK-3β 3'UTR Mut luciferase activity (P>0.05). WB
assay showed that the expression of GSK-3β was significantly
decreased in cells transfected with miR-132-inhibitor compared with
those transfected with miR-NC (P<0.05) (Fig. 4).
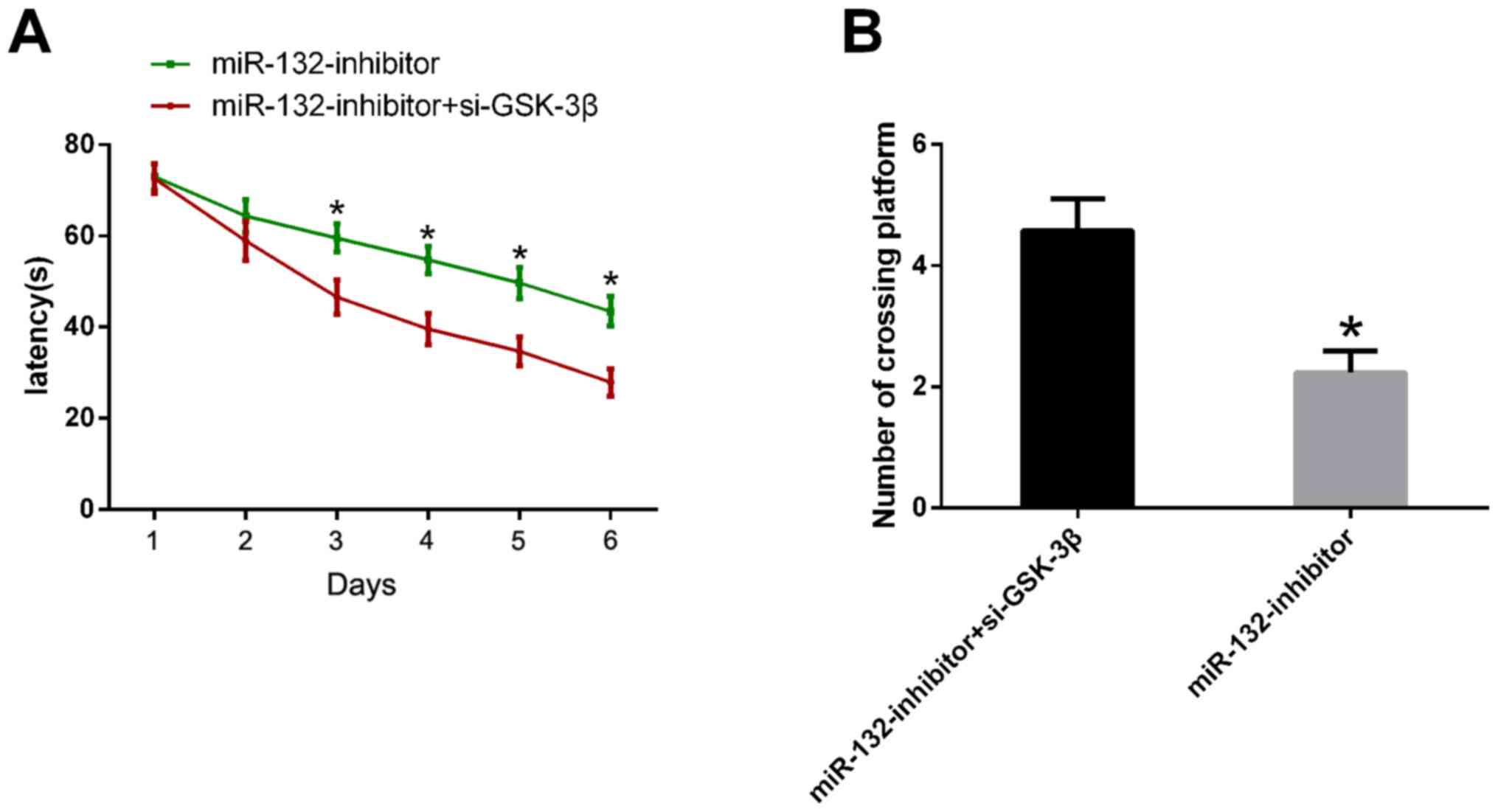
Downregulation of GSK-3β reverses the
decline in learning and memory in mice caused by downregulation of
miR-132 expression
Adult mice were also taken as the
miR-132-inhibitor+si-GSK-3β group. After injecting miR-132
inhibitor into the hippocampus for 2 days, GSK-3β inhibitor was
further injected into mice before conducting the Morris water maze
test. The test results demonstrated that the escape latency of mice
in the miR-132-inhibitor+si-GSK-3β group was significantly
shortened and the number of times of crossing the platform was
significantly increased compared with those in the Mi-132-inhibitor
group (P<0.05) (Fig. 5).
Discussion
Learning and memory is an essential basic function
of human survival. Many diseases related to neurological function
can lead to the loss of learning and memory function, which leads
to serious social burden. Therefore, understanding the relevant
mechanism of learning and memory has been the research hotspot in
the field of neuroscience. As a class of short non-coding RNA, miRs
participate in the biological functions of cell growth,
proliferation and apoptosis (13).
In recent years, extensive studies have demonstrated that miR is
involved in neuroplasticity and memory formation, and that miR
dysregulation is part of the pathophysiology of neurological
diseases and neurodevelopmental disorders (14-16).
Among them, miR-132 is a frequent visitor in neurological related
research and has been shown to be closely related to
neurodevelopment and neurosy-related diseases (17-19).
Yet, how miR-132 affects learning and memory function has not been
elucidated.
As miR-132 is highly expressed in hippocampal tissue
(20), our study selected
hippocampus as the very place to examine the expression of miR-132.
It is well known that the learning and memory ability of animals
gradually declines with aging, as was validated in the current
study that miR-132 was downregulated in the hippocampus of the
elderly group compared with that of the adult group. Subsequently,
in order to explore the role of miR-132 in learning and memory, we
observed the performance of adult mice in Morris water maze test
after injecting miR-132-inhibitor. The results showed that compared
with the two control groups, the miR-132-inhibitor group had a
significant increase in escape latency and a significant decrease
in the number of times of crossing platforms, indicating that the
expression level of miR-132 was closely related to the learning and
memory function of mice. Previous studies have explored the
relationship between miR-132 and learning and memory function. For
example, studies have revealed that repeated anesthesia with
propofol in neonatal rats can lead to a significant decrease in
miR-132 expression and a decrease in the number of dendritic spines
in the hippocampus of rats, resulting in learning and memory
disorders in rats (21). Other
studies have shown that knockout of miR-132 expression can
significantly reduce spatial memory and learning ability in mice
(22). Although here we validated
that miR-132 expression disorder can cause changes in learning and
memory ability, its mechanisms of action remains a mystery.
GSK-3β was found to have target binding sites with
miR-132 when predicting the downstream target gene of miR132 by
Targetscan7.2. GSK-3β, is a multifunctional protein kinase
implicated in a variety of cellular processes (23). Previous studies have shown that
GSK-3β can affect the memory learning ability of animals. For
example, some studies have found that memory retrieval in the
passive avoidance task activates the activity of GSK-3β in the
hippocampus of mice, and they also found that injection of GSK-3β
inhibitor into mice can impair the memory capacity, while it is
without impact on memory reconstruction (24). Other studies have exhibited that
inhibition of GSK-3β activity can significantly improve cognitive
dysfunction in mice caused by chronic unpredictable stress
(25). While in our study, the
expression of GSK-3β was upregulated in hippocampus of elderly
mice, and the decrease of GSK-3β level could significantly increase
the escape latency of mice and decrease the number of times of
crossing the platform in the maze test, suggesting that the
expression of GSK-3β was closely related to the learning and memory
ability of mice. Subsequently, we injected the hippocampus of mice
with miR-132 inhibitor, and then with GSK-3β inhibitor to explore
the relationship between miR-132 and GSK-3β. The results indicated
that the downregulation of GSK-3β could reverse the decline in
learning and memory ability of mice caused by the downregulation of
miR-132 expression, indicating a certain link between miR-132 and
GSK-3β. Therefore, double luciferase report was further performed
to verify the relationship between miR-132 and GSK-3β, and the
results showed that the luciferase activity of GSK-3β 3'UT Wt was
significantly decreased after overexpression of miR-132, while
without influence on the luciferase activity of GSK-3β 3'UTR Mut,
and GSK-3β expression was significantly decreased in cells
overexpressed by miR-132. From the above experimental results, we
can rudimentarily prove that miR-132 overexpression can inhibit the
expression of GSK-3β and affect the learning and memory ability of
mice.
In summary, this study preliminarily demonstrated
that miR-132 overexpression can inhibit the expression of GSK-3β
and affect the learning and memory ability of mice, which provides
some enlightenment for the learning and understanding of learning
and memory disorders.
Acknowledgements
Not applicable.
Funding
This study is supported by 1 The effect of Tau and
GSK-3β expression changes on the learning and memory function of
young rats and its mechanism. 2018 Bengbu Medical College Natural
Science Key Project (Science and Technology Development Fund
Project). Project Number: BYKF18100.2. Regulation of different
end-expiratory carbon dioxide partial pressure on the expression of
c-fos and PARP-1 proteins in young rats and the mechanism of
changes in learning and memory function. 2017 Anhui Provincial
Universities Natural Science Research Key Project. Project Number:
KJ2017A246."
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
GL wrote the manuscript. GL and FY conceived and
designed the study. CZ and YZ were responsible for the collection
and analysis of the experimental data. CZ and XL interpreted the
data and drafted the manuscript. YZ and YL revised the manuscript
critically for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the First Affiliated Hospital of Bengbu Medical College (Bengbu,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lin S and Gregory RI: MicroRNA biogenesis
pathways in cancer. Nat Rev Cancer. 15:321–333. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
eLife. 4(e05005)2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Qian Y, Song J, Ouyang Y, Han Q, Chen W,
Zhao X, Xie Y, Chen Y, Yuan W and Fan C: Advances in roles of
miR-132 in the nervous system. Front Pharmacol.
8(770)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wei CW, Luo T, Zou SS and Wu AS: Research
progress on the roles of microRNAs in governing synaptic
plasticity, learning and memory. Life Sci. 188:118–122.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang Y, Veremeyko T, Wong AHK, El Fatimy
R, Wei Z, Cai W and Krichevsky AM: Downregulation of miR-132/212
impairs S-nitrosylation balance and induces tau phosphorylation in
Alzheimer's disease. Neurobiol Aging. 51:156–166. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu Y, Yang X, Zhao L, Zhang J, Li T and
Ma X: Increased miR-132 level is associated with visual memory
dysfunction in patients with depression. Neuropsychiatr Dis Treat.
12:2905–2911. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Guo Y, Gupte M, Umbarkar P, Singh AP, Sui
JY, Force T and Lal H: Entanglement of GSK-3β, β-catenin and TGF-β1
signaling network to regulate myocardial fibrosis. J Mol Cell
Cardiol. 110:109–120. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang H, Yang X, Qin X and Niu Q:
Caspase-3 is involved in aluminum-induced impairment of long-term
potentiation in rats through the Akt/GSK-3β pathway. Neurotox Res.
29:484–494. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lu F, Li X, Li W, Wei K, Yao Y, Zhang Q,
Liang X and Zhang J: Tetramethylpyrazine reverses
intracerebroventricular streptozotocin-induced memory deficits by
inhibiting GSK-3β. Acta Biochim Biophys Sin (Shanghai). 49:722–728.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Suh JH, Choi E, Cha MJ, Song BW, Ham O,
Lee SY, Yoon C, Lee CY, Park JH, Lee SH, et al: Up-regulation of
miR-26a promotes apoptosis of hypoxic rat neonatal cardiomyocytes
by repressing GSK-3β protein expression. Biochem Biophys Res
Commun. 423:404–410. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tsukigi M, Bilim V, Yuuki K, Ugolkov A,
Naito S, Nagaoka A, Kato T, Motoyama T and Tomita Y: Re-expression
of miR-199a suppresses renal cancer cell proliferation and survival
by targeting GSK-3β. Cancer Lett. 315:189–197. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wolfensohn S and Lloyd M: Handbook of
laboratory animal management and welfare. John Wiley & Sons.
2008. View Article : Google Scholar
|
|
13
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Busto GU, Guven-Ozkan T, Chakraborty M and
Davis RL: Developmental inhibition of miR-iab8-3p disrupts mushroom
body neuron structure and adult learning ability. Dev Biol.
419:237–249. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xu XF, Jing X, Ma HX, Yuan RR, Dong Q,
Dong JL, Han XF, Chen ZY, Li XZ and Wang Y: miR-181a participates
in contextual fear memory formation via activating mTOR signaling
pathway. Cereb Cortex. 28:3309–3321. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tian F, Yuan C and Yue H: MiR-138/SIRT1
axis is implicated in impaired learning and memory abilities of
cerebral ischemia/reperfusion injured rats. Exp Cell Res.
367:232–240. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang RY, Phang RZ, Hsu PH, Wang WH, Huang
HT and Liu IY: In vivo knockdown of hippocampal miR-132 expression
impairs memory acquisition of trace fear conditioning. Hippocampus.
23:625–633. 2013. View Article : Google Scholar
|
|
18
|
Huang S, Zhao J, Huang D, Zhuo L, Liao S
and Jiang Z: Serum miR-132 is a risk marker of post-stroke
cognitive impairment. Neurosci Lett. 615:102–106. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Numakawa T, Yamamoto N, Chiba S, Richards
M, Ooshima Y, Kishi S, Hashido K, Adachi N and Kunugi H: Growth
factors stimulate expression of neuronal and glial miR-132.
Neurosci Lett. 505:242–247. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Magill ST, Cambronne XA, Luikart BW, Lioy
DT, Leighton BH, Westbrook GL, Mandel G and Goodman RH:
microRNA-132 regulates dendritic growth and arborization of newborn
neurons in the adult hippocampus. Proc Natl Acad Sci USA.
107:20382–20387. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang S, Liang Z, Sun W and Pei L:
Repeated propofol anesthesia induced downregulation of hippocampal
miR-132 and learning and memory impairment of rats. Brain Res.
1670:156–164. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hernandez-Rapp J, Smith PY, Filali M,
Goupil C, Planel E, Magill ST, Goodman RH and Hébert SS: Memory
formation and retention are affected in adult miR-132/212 knockout
mice. Behav Brain Res. 287:15–26. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sun A, Li C, Chen R, Huang Y, Chen Q, Cui
X, Liu H, Thrasher JB and Li B: GSK-3β controls autophagy by
modulating LKB1-AMPK pathway in prostate cancer cells. Prostate.
76:172–183. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hong JG, Kim DH, Lee CH, Park SJ, Kim JM,
Cai M, Jang DS and Ryu JH: GSK-3β activity in the hippocampus is
required for memory retrieval. Neurobiol Learn Mem. 98:122–129.
2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hui J, Zhang J, Pu M, Zhou X, Dong L, Mao
X, Shi G, Zou J, Wu J, Jiang D, et al: Modulation of
GSK-3β/β-catenin signaling contributes to learning and memory
impairment in a rat model of depression. Int J
Neuropsychopharmacol. 21:858–870. 2018.PubMed/NCBI View Article : Google Scholar
|