Introduction
Pancreatic cancer was the fourth leading cause of
cancer-related death in the United States in 2017, with an
estimated 53,670 new cases and 43,090 estimated deaths (1). The incidence of pancreatic cancer has
been on the increase in recent years and it is estimated that it
will be the second leading cause of cancer-related death by
2030(2). Although constituting only
approximately 30% volume of the whole pancreas, approximately 70%
of pancreatic cancers are located in the head of the pancreas
(3,4). Additionally, 20-25% of pancreatic
cancers reside in the body and tail of the pancreas (3). Previously, several studies reported
that patients with pancreatic body/tail cancer had poorer prognosis
than patients with head cancer, which was generally attributed to
the advanced stages of body/tail cancers upon detection (5-8).
However, the different prognosis of patients with head or body/tail
pancreatic cancers weighed by stages is largely unidentified. Thus,
whether diversities in clinical features exist between head and
body/tail pancreatic cancers have not been systematically
examined.
The differences between distal and proximal colon
cancers in terms of incidence, molecular, immunological,
pathological, clinical features, and therapeutic response are well
recognized (9-14).
The differences have a great impact on the clinical management of
colorectal cancer. For example, proximal colon carcinomas were
comprised of B-Raf proto-oncogene, and microsatellite instable-high
and distal colon carcinomas were more commonly identified in
chromosome instable and EGFR or HER2 overexpression (11). Additionally, only patients with
metastatic distal colon carcinoma were sensitive to anti-EGFR
therapy, which is in agreement with the molecular features of
distal colon cancers (11). However,
the differences between head and body/tail pancreatic cancers in
terms of incidence, clinical features, and therapeutic response
were not systematically analyzed.
The current study was performed to examine the
differences in terms of prognosis and incidence between patients
with head or body/tail pancreatic cancers using the SEER dataset.
In addition, a literature review was performed to identify
differences between head and body/tail cancers in terms of
embryology, histology, and therapeutic response.
Materials and methods
Incidence and survival analysis
The SEER database (1973-2014) was used to collect
cases with primary pancreatic adenocarcinoma. Subjects were
retrieved using the International Classification of Diseases for
Oncology, 3rd editions (ICD-O-3) for tumors of the pancreas. The
following tumors of the pancreas were included: 8140/3:
adenocarcinoma, nos. Only cases with pathology and/or cytology
confirmation were included. Cases with tumor locations of
C25.0-head of pancreas, C25.1-body of pancreas, and C25.2-tail of
pancreas were included. Patients with ampullary cancer, intraductal
papillary mucinous neoplasm, pancreatic neuroendocrine tumor, or
adenosquamous carcinoma were excluded. Subjects with multiple
primary lesions in the pancreas were excluded. Cases with tumor
locations of C25.3-pancreatic duct, C25.4-Islets of Langerhans,
C25.7-other specified parts of pancreas, C25.8-overlapping lesion
of pancreas, and C25.9-pancreas numbers, were also excluded.
Patients with clinical diagnosis only, direct visualization without
microscopy, positive laboratory test/marker study, or radiography
without microscopic confirmation, were excluded. Subjects with
unknown information of follow-up data were also excluded.
The primary examined factor was tumor location.
Tumor grade was classified as low (well differentiated),
intermediate (moderately differentiated), and high (poorly
differentiated or undifferentiated) grade. The 7th edition of
American Joint Commission on Cancer Staging (AJCC) staging system
for pancreatic cancer was used (15). Informed consent was waived in the
study. The study protocol was approved by the ethics committee of
Fundan University Shanghai Cancer Center.
Literatures review
Available literature about differences in embryology
and histology between head and body/tail pancreas was reviewed.
Additionally, a search was conducted for literature concerning the
clinical presentation of pancreatic cancers divided by primary
tumor locations. The response of pancreatic cancers to therapeutic
modalities including systemic and adjuvant chemotherapy by site was
also reviewed.
Statistical analysis
The distribution of categorical variables was
assessed by Pearson's χ2 test by tumor locations (head
vs body/tail pancreatic cancers). Continuous variables were
examined by Student's t-test or rank sum tests. Comparison of
overall survival between head and body/tail pancreatic cancers was
performed for all stages combined and within each stage (i.e.,
stages I to IV) by univariate Kaplan-Meier survival analysis and
log-rank test. Adjusted HRs and 95% CIs were examined by Cox
proportional hazards regression analysis. Statistical analysis was
assessed using STATA 12.0 software (STATA, College Station).
Statistical significance was considered as two-sided P<0.05.
Results
Patient characteristics
In total, 85,715 patients were retrieved from the
SEER database, including 60,015 (70.0%) patients with head
pancreatic tumor and 25,700 (30.0%) patients with body/tail tumor
(Table I). The size of body/tail
tumor was larger than that of head tumor (mean size, 4.6 vs. 3.8
cm, P<0.001). Body/tail pancreatic tumor had a higher proportion
of high-grade tumors (poorly differentiated or undifferentiated)
than that of head tumor (49.6 vs. 44.4%, P<0.001). For resected
tumors, body/tail tumor had less lymph nodes harvested than head
tumor (median number, 7 vs. 10, P<0.001). Body/tail cancer had
lower proportion of T1/T2 tumors (24.2%) than that of head cancer
(35.2%, P<0.001). The proportion of metastatic tumor was higher
in body/tail pancreatic cancer (79.4%) than that in head cancer
(46.3%) and the surgical resection rate of body/tail cancer (9.4%)
was lower than that of head cancer (20.4%).
 | Table ICharacteristics of patients with head
and body/tail pancreatic cancer. |
Table I
Characteristics of patients with head
and body/tail pancreatic cancer.
| Characteristics | Overall
(n=85,715) | Head (n=60,015) | Body/tail
(n=25,700) | P-value |
|---|
| Survival (months),
median | 6.0 | 7.0 | 5.0 | <0.001 |
| Sex | | | | <0.001 |
|
Male
(%) | 43,925 (51.2) | 30,190 (50.3) | 13,735 (53.4) | |
|
Female
(%) | 41,790 (48.8) | 29,825 (49.7) | 11,965 (46.6) | |
| Age (years) | | | | <0.001 |
|
Mean ±
SD | 68.2±11.5 | 68.4±11.6 | 67.7±11.3 | |
| Race (%) | | | | <0.001 |
|
Caucasian | 69,663 (81.3) | 49,035 (81.7) | 20,628 (80.3) | |
|
African
descent | 10,368 (12.1) | 7,136 (11.9) | 3,232 (12.6) | |
|
Others | 5,684 (6.6) | 3,844 (6.4) | 1,840 (7.2) | |
| Tumor size (cm) | n=40,677 | n=26,778 | n=13,899 | <0.001 |
|
Mean ±
SD | 4.0±2.8 | 3.8±2.7 | 4.6±2.9 | |
| Surgical
resection | 83,568 | 58,408 | 25,160 | <0.001 |
|
Yes (%) | 14,304 (17.1) | 11,930 (20.4) | 2,374 (9.4) | |
|
No (%) | 69,264 (82.9) | 46,478 (79.6) | 22,786 (90.6) | |
| Tumor grade
(%) | 35,691 | 26,847 | 8,844 | <0.001 |
|
Low | 4,680 (13.1) | 3,721 (13.9) | 959 (10.8) | |
|
Intermediate | 14,706 (41.2) | 11,211 (41.8) | 3,495 (39.5) | |
|
High | 16,305 (45.7) | 11,915 (44.4) | 4,390 (49.6) | |
| No. of nodes
resecteda | 12,553 | 10,470 | 2,083 | <0.001 |
|
Median | 10 | 10 | 7 | |
|
95% CI | (9, 10) | (10, 10) | (6, 7) | |
| T stage | 40,956 | 27,904 | 13,052 | <0.001 |
|
T1 | 1,553 (3.8) | 1,110 (4.0) | 443 (3.4) | |
|
T2 | 9,789 (23.9) | 5,637 (20.2) | 4,152 (31.8) | |
|
T3 | 19,912 (48.6) | 15,194 (54.5) | 4,718 (36.1) | |
|
T4 | 9,702 (23.7) | 5,963 (21.4) | 3,739 (28.6) | |
| Nodal status | 41,382 | 28,112 | 13,270 | <0.001 |
|
N0 | 25,059 (60.6) | 16,498 (58.7) | 8,561 (64.5) | |
|
N1 | 16,323 (39.4) | 11,614 (41.3) | 4,709 (35.5) | |
| Distant
metastasis | 81,832 | 56,858 | 24,974 | <0.001 |
|
M0 | 35,712 (43.6) | 30,556 (53.7) | 5,156 (20.6) | |
|
M1 | 46,120 (56.4) | 26,302 (46.3) | 19,818 (79.4) | |
| Stageb | 45,406 | 29,676 | 15,730 | <0.001 |
|
I | 3,132 (6.9) | 2,475 (8.3) | 657 (4.2) | |
|
II | 12,894 (28.4) | 11,118 (37.5) | 1,776 (11.3) | |
|
III | 4,949 (10.9) | 3,544 (11.9) | 1,405 (8.9) | |
|
IV | 24,431 (53.8) | 12,539 (42.3) | 11,892 (75.6) | |
Survival analysis
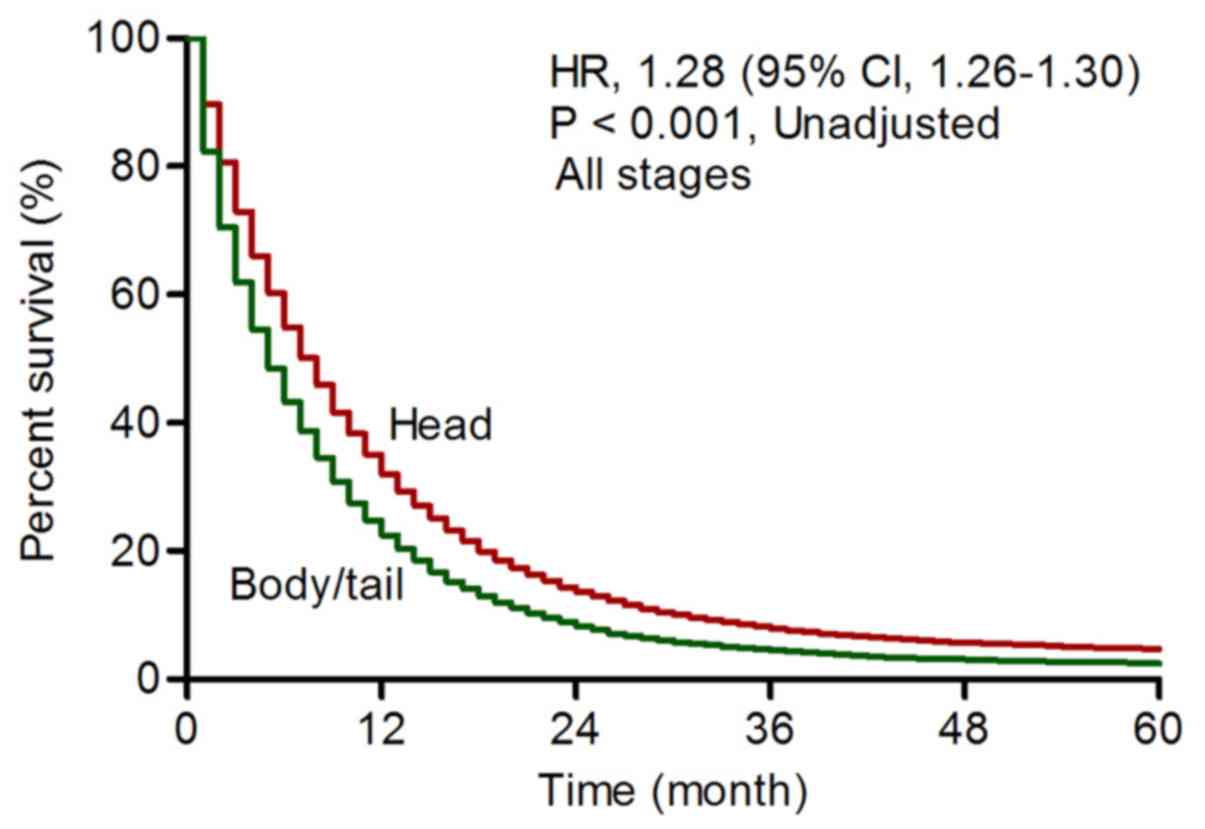
Overall, patients with body/tail pancreatic cancer
had poorer prognosis than patients with head cancer (unadjusted HR,
1.28, 95% CI, 1.26-1.30, P<0.001, Fig. 1; adjusted HR, 1.03, 95% CI,
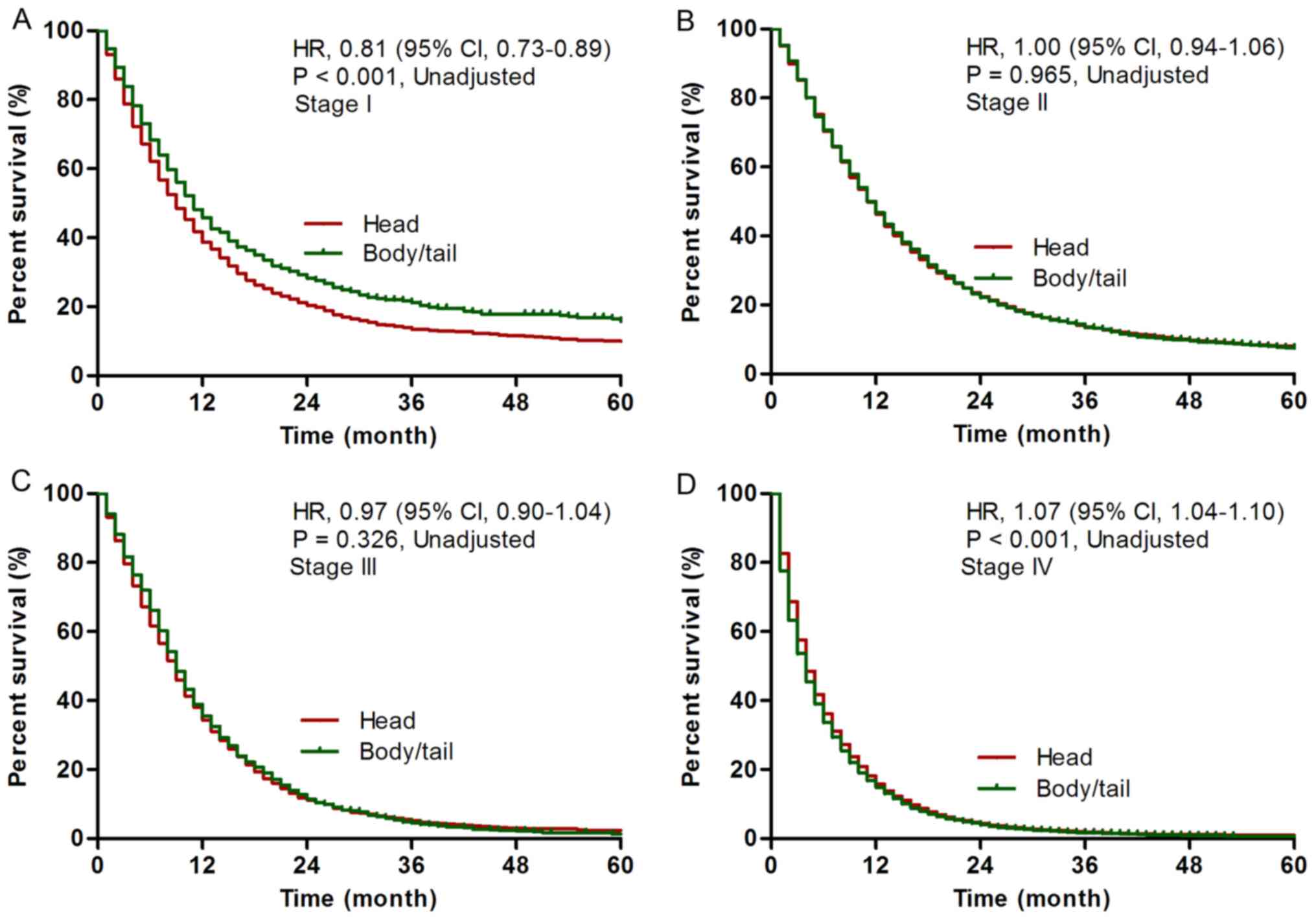
1.00-1.05, P=0.025, Table II). For
stage I disease, patients with body/tail cancer had superior
prognosis compared with patients with head cancer (adjusted HR,
0.85, 95% CI, 0.76-0.94, P=0.001; Fig.
2A). For stage II/III disease, no statistical significance was
found between patients with body/tail cancer and patients with head
cancer [stage II, adjusted HR, 1.00, 95% CI, 0.95-1.06, P=0.965
(Fig. 2B); stage III, 0.97, 95% CI,
0.91-1.04, P=0.398 (Fig. 2C)
(Table II)]. For stage IV disease,
patients with body/tail cancer had poorer prognosis than patients
with head cancer (adjusted HR, 1.07, 95% CI, 1.04-1.10, P<0.001;
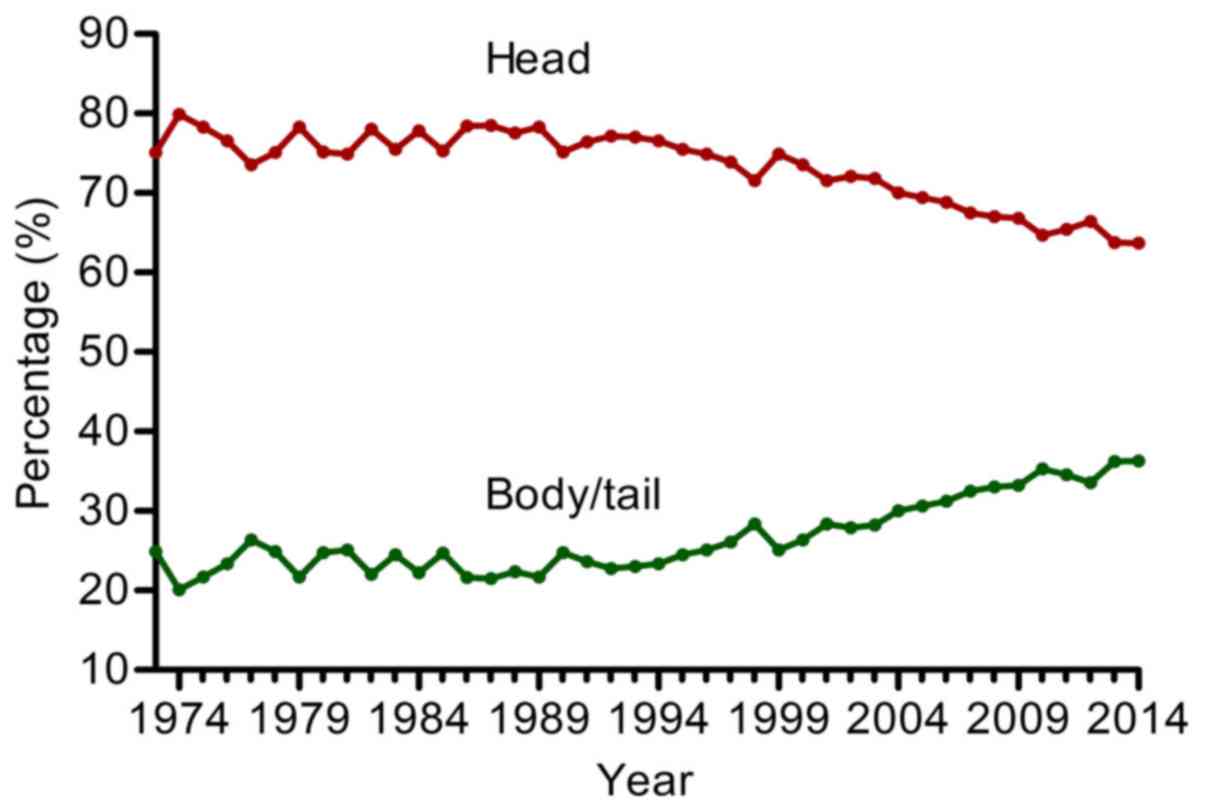
Table II). The proportion of
body/tail tumor showed a steady increase from 24.9% in 1973 to
36.3% in 2014, with an annual increasing rate of 0.27% (Fig. 3).
 | Table IIMultivariate analysis of prognostic
factors. |
Table II
Multivariate analysis of prognostic
factors.
| | All stages | Stage I | Stage II | Stage III | Stage IV |
|---|
|
Characteristics | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years |
|
≤70 | 1 | | 1 | | 1 | | 1 | | 1 | |
|
>70 | 1.41
(1.38-1.44) | <0.001 | 1.54
(1.41-1.69) | <0.001 | 1.46
(1.40-1.52) | <0.001 | 1.44
(1.35-1.54) | <0.001 | 1.33
(1.30-1.37) | <0.001 |
| Sex |
|
Male | 1 | | 1 | | 1 | | 1 | | 1 | |
|
Female | 0.95
(0.93-0.97) | <0.001 | 0.98
(0.90-1.06) | 0.623 | 0.96
(0.93-1.00) | 0.067 | 0.89
(0.83-0.94) | <0.001 | 0.95
(0.93-0.98) | 0.001 |
| Race (%) |
|
Caucasian | 1 | | 1 | | 1 | | 1 | | 1 | |
|
African
descent | 1.17
(1.13-1.21) | <0.001 | 1.14
(1.01-1.29) | 0.037 | 1.13
(1.06-1.21) | <0.001 | 1.13
(1.03-1.24) | 0.010 | 1.18
(1.13-1.23) | <0.001 |
|
Others | 1.00
(0.96-1.05) | 0.863 | 0.92
(0.78-1.09) | 0.327 | 1.04
(0.96-1.12) | 0.350 | 0.99
(0.88-1.11) | 0.818 | 1.00
(0.95-1.06) | 0.876 |
| Grade |
|
Low | 1 | | 1 | | 1 | | 1 | | 1 | |
|
Medium | 1.13
(1.07-1.20) | <0.001 | 1.33
(1.09-1.62) | 0.005 | 1.02
(0.93-1.12) | 0.645 | 1.18
(1.01-1.39) | 0.042 | 1.24
(1.11-1.38) | <0.001 |
|
High | 1.50
(1.41-1.60) | <0.001 | 1.85
(1.51-2.27) | <0.001 | 1.32
(1.21-1.45) | <0.001 | 1.73
(1.48-2.04) | <0.001 | 1.53
(1.38-1.71) | <0.001 |
| Stage |
|
I | 1 | | / | | / | | / | | / | |
|
II | 1.05
(1.00-1.09) | 0.053 | / | | / | | / | | / | |
|
III | 1.33
(1.26-1.40) | <0.001 | / | | / | | / | | / | |
|
IV | 2.25
(2.15-2.35) | <0.001 | / | | / | | / | | / | |
| Location |
|
Head | 1 | | 1 | | 1 | | 1 | | 1 | |
|
Body/tail | 1.03
(1.00-1.05) | 0.025 | 0.85
(0.76-0.94) | 0.001 | 1.00
(0.95-1.06) | 0.965 | 0.97
(0.91-1.04) | 0.398 | 1.07
(1.04-1.10) | <0.001 |
Literature review
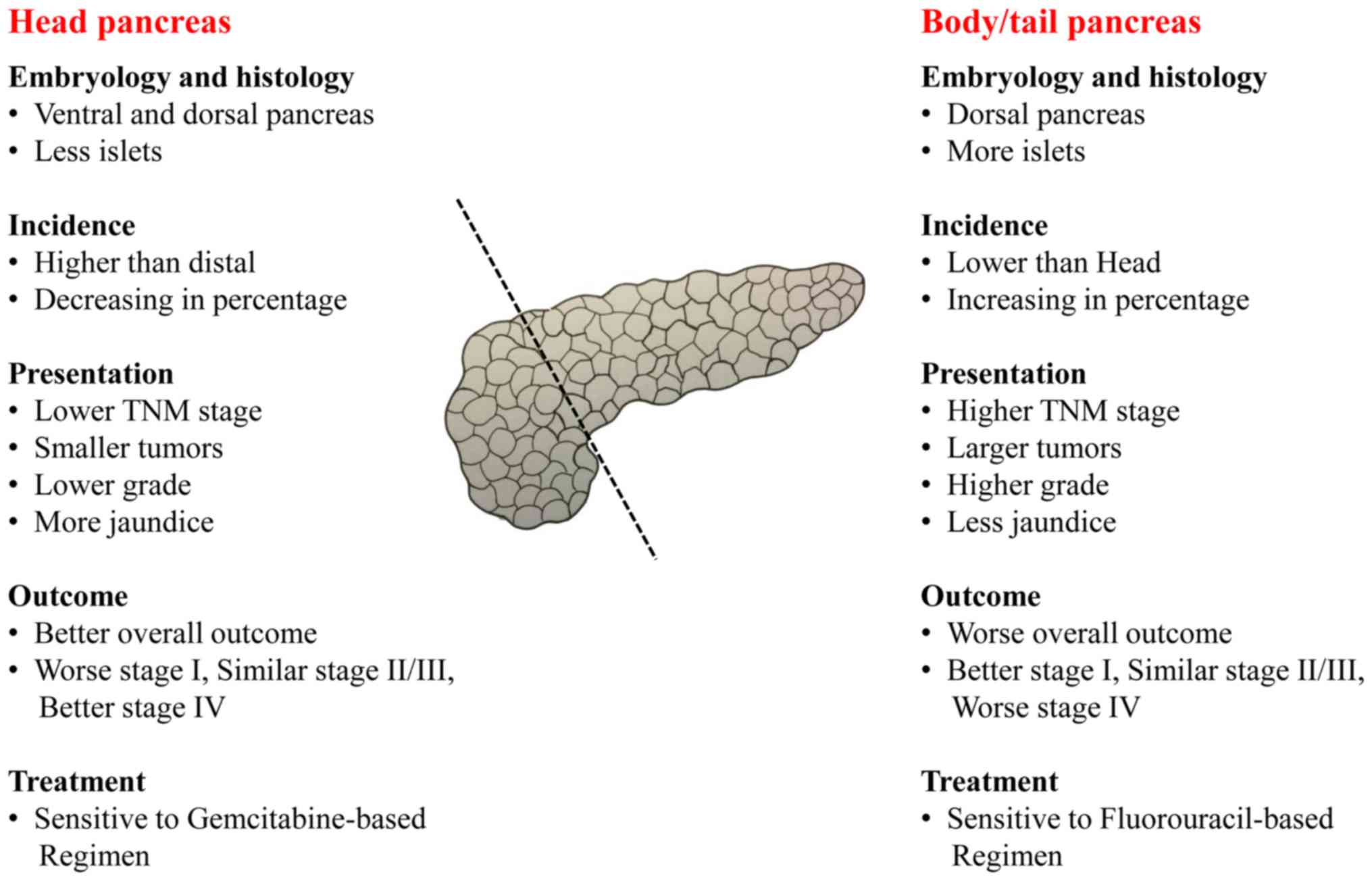
A systematic literature review was performed to
identify potential differences between body/tail and head pancreas
and pancreatic cancers, which is summarized in Fig. 4. The ventral pancreas forms the
posterior part of the head of the pancreas and the uncinate
process, while the dorsal pancreas forms the body and tail of the
pancreas and the anterior part of the head of the gland (16). The ventral pancreas has macroscopic,
microscopic and molecular features differing from the dorsal
pancreas (16,17). In addition, islets mainly reside in
the body/tail of the pancreas (18).
Patients with body/tail cancer usually undergo distal
pancreatectomy and experience less jaundice, while patients with
head cancer typically undergo pancreaticoduodenectomy and exhibit
more jaundice (3,19). Previous findings have shown the
adjuvant and systemic chemotherapy for pancreatic cancer by
anatomic sites (20-24).
For body/tail pancreatic cancer, metastatic diseases were sensitive
to Nanoliposomal irinotecan, 5-FU/Leucovorin (Nanoliposomal
irinotecan, 5-FU/Leucovorin vs. 5-FU/Leucovorin, HR, 0.51, 95% CI,
0.31-0.85) (23) and resected
diseases were sensitive to both S-1 and Gemcitabine (S-1 vs.
Gemcitabine, HR, 0.59, 95% CI, 0.37-0.94) (21). For head pancreatic cancer, metastatic
diseases were sensitive to Nab-Paclitaxel plus Gemcitabine
(Nab-Paclitaxel plus Gemcitabine vs. Gemcitabine, HR, 0.59, 95% CI,
0.46-0.75) (22) and resected
diseases were sensitive to Gemcitabine (Gemcitabine vs. 5-FU, HR,
0.80, 95% CI, 0.63-1.00) (24).
Discussion
It is well known that the majority of pancreatic
cancers reside in the head of the pancreas and that body/tail
pancreatic cancers are less common (3,19).
However, we found that the proportion of head pancreatic cancer was
steadily decreased from 75.1 to 63.7% and the proportion of
body/tail cancer was gradually increased from 24.9 to 36.3%
according to the SEER database from 1973 to 2014. The increased
proportion of body/tail pancreatic may be attributed to the wide
use of modern detection methods. Notably, a similar trend occurred
in colorectal cancer, with the incidence of proximal colorectal
cancer mildly decreasing (-6.37%) and distal colorectal cancer
obviously decreasing (-37.79%) from 1976 to 2005 based on the SEER
database (25).
Patients with body/tail pancreatic cancer usually
have worse outcome than patients with head cancer, which is largely
attributed to the fact that body/tail pancreatic cancers typically
present at a more advanced stage than head cancers (5,6,26). Our study confirms that body/tail
cancers had a higher proportion of metastatic diseases than head
cancers (75.6 vs. 42.3%). However, when controlled for stages and
other prognostic factors, patients with body/tail pancreatic cancer
still had worse prognosis than patients with head cancer. Moreover,
body/tail pancreatic cancers showed lower mortality for stage I
cancers, no significant difference in mortality for stages II and
III, and a higher mortality for stage IV cancer compared with head
cancers by adjusted Cox regression, indicating that the biology of
body/tail cancers is different from that of head cancers. Of note,
right-sided colon cancers also had a worse outcome than left-sided
ones (14). In addition, within
stage I, no difference in prognosis was observed between right- and
left-sided colon cancers; within stage II disease, right-sided
cancers had an improved outcome; within stage III, right-sided
cancers had worse outcome (12).
For body/tail pancreatic cancer, metastatic diseases
were sensitive to Nanoliposomal irinotecan, 5-FU/Leucovorin and
resected diseases were sensitive to adjuvant S-1 or Gemcitabine
(21,23). For head pancreatic cancer, metastatic
diseases were sensitive to Nab-Paclitaxel plus Gemcitabine and
resected diseases were sensitive to adjuvant Gemcitabine (22,24).
Those findings indicate that pancreatic head cancers may be
sensitive to Gemcitabine-based regimen and body/tail cancers may be
sensitive to 5-FU based regimen. However, future studies concerning
the response to systematic therapy divided by tumor locations are
needed.
This study systematically demonstrated that major
differences exist between head and body/tail pancreatic cancers in
terms of incidence, prognosis, molecular characteristics,
embryology and histology, clinical presentation and therapeutic
response. These diversities between head and body/tail pancreatic
cancers may have a great impact on clinical management. However, in
spite of employing a well-recognized public database and
systematically reviewing the literature, the current study was
mainly limited by its retrospective nature. Further studies are
imperative to identify potential molecular diversities between head
and body/tail pancreatic cancers and explore therapeutic potential.
Prospective clinical trials regarding the different therapeutic
response between head and body/tail pancreatic cancers are
required.
Acknowledgements
This study was jointly funded by the National
Science Foundation for Distinguished Young Scholars of China (no.
81625016), the National Natural Science Foundation of China (nos.
81372649, 81172276, 81370065, 81372653 and 81871940), the Shanghai
Cancer Center Foundation for Distinguished Young Scholars (no.
YJJQ201803), Shanghai Natural Science Foundation (no. 17ZR1406300),
and basic research projects of the Science and Technology
Commission of Shanghai Municipality (no. 15JC1401200). We thank
Professor Qi Feng from Shanghai Institutes for Biological Sciences,
Chinese Academy of Sciences for his technical assistance.
Funding
This study was jointly funded by the National
Science Foundation for Distinguished Young Scholars of China (grant
no. 81625016), the National Natural Science Foundation of China
(grant nos. 81372649, 81172276, 81370065, 81372653 and 81871940),
the Shanghai Cancer Center Foundation for Distinguished Young
Scholars (no. YJJQ201803), Shanghai Natural Science Foundation (no.
17ZR1406300), and basic research projects of the Science and
Technology Commission of Shanghai Municipality (no.
15JC1401200).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GL and XY contributed to the study design. CL, HC,
MG, YG, ZF, CY, QH, QN, and KJ contributed to the acquisition of
data. CL, GL, and XY contributed to the analysis and
interpretation. CL, HC, KJ, MG, YG, ZF, CY, QH, and QN contributed
to the manuscript drafting. GL, CL, HC, GM, and KJ provided
statistical advice. All authors critically reviewed the manuscript
and approved the final revision.
Ethics approval and consent to
participate
The study protocol was approved by the ethics
committee of Fundan University Shanghai Cancer Center.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rahib L, Smith BD, Aizenberg R, Rosenzweig
AB, Fleshman JM and Matrisian LM: Projecting cancer incidence and
deaths to 2030: The unexpected burden of thyroid, liver, and
pancreas cancers in the United States. Cancer Res. 74:2913–2921.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ryan DP, Hong TS and Bardeesy N:
Pancreatic adenocarcinoma. N Engl J Med. 371:1039–1049.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Feng L, Gu S, Wang P, Chen H, Chen Z, Meng
Z and Liu L: Pretreatment values of bilirubin and albumin are not
prognostic predictors in patients with advanced pancreatic cancer.
Cancer Med. 7:5943–5951. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Johnson CD, Schwall G, Flechtenmacher J
and Trede M: Resection for adenocarcinoma of the body and tail of
the pancreas. Br J Surg. 80:1177–1179. 1993.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shoup M, Conlon KC, Klimstra D and Brennan
MF: Is extended resection for adenocarcinoma of the body or tail of
the pancreas justified? J Gastrointest Surg. 7:946–952; discussion
952. 2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Artinyan A, Soriano PA, Prendergast C, Low
T, Ellenhorn JD and Kim J: The anatomic location of pancreatic
cancer is a prognostic factor for survival. HPB (Oxford).
10:371–376. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lau MK, Davila JA and Shaib YH: Incidence
and survival of pancreatic head and body and tail cancers: A
population-based study in the United States. Pancreas. 39:458–462.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee GH, Malietzis G, Askari A, Bernardo D,
Al-Hassi HO and Clark SK: Is right-sided colon cancer different to
left-sided colorectal cancer? - a systematic review. Eur J Surg
Oncol. 41:300–308. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Loupakis F, Yang D, Yau L, Feng S,
Cremolini C, Zhang W, Maus MK, Antoniotti C, Langer C, Scherer SJ,
et al: Primary tumor location as a prognostic factor in metastatic
colorectal cancer. J Natl Cancer Inst. 107(107)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Missiaglia E, Jacobs B, D'Ario G, Di Narzo
AF, Soneson C, Budinska E, Popovici V, Vecchione L, Gerster S, Yan
P, et al: Distal and proximal colon cancers differ in terms of
molecular, pathological, and clinical features. Ann Oncol.
25:1995–2001. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Weiss JM, Pfau PR, O'Connor ES, King J,
LoConte N, Kennedy G and Smith MA: Mortality by stage for right-
versus left-sided colon cancer: Analysis of surveillance,
epidemiology, and end results - Medicare data. J Clin Oncol.
29:4401–4409. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Weiss JM, Schumacher J, Allen GO, Neuman
H, Lange EO, Loconte NK, Greenberg CC and Smith MA: Adjuvant
chemotherapy for stage II right-sided and left-sided colon cancer:
Analysis of SEER-medicare data. Ann Surg Oncol. 21:1781–1791.
2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Meguid RA, Slidell MB, Wolfgang CL, Chang
DC and Ahuja N: Is there a difference in survival between right-
versus left-sided colon cancers? Ann Surg Oncol. 15:2388–2394.
2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: the 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474.
2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Uchida T, Takada T, Ammori BJ, Suda K and
Takahashi T: Three-dimensional reconstruction of the ventral and
dorsal pancreas: A new insight into anatomy and embryonic
development. J Hepatobiliary Pancreat Surg. 6:176–180.
1999.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Malaisse-Lagae F, Orci L and Perrelet A:
Anatomic and hormonal markers for the ventral primordium in the
human pancreas? N Engl J Med. 300(436)1979.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Baetens D, Malaisse-Lagae F, Perrelet A
and Orci L: Endocrine pancreas: Three-dimensional reconstruction
shows two types of islets of langerhans. Science. 206:1323–1325.
1979.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kamisawa T, Wood LD, Itoi T and Takaori K:
Pancreatic cancer. Lancet. 388:73–85. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: Groupe Tumeurs Digestives of Unicancer;
PRODIGE Intergroup: FOLFIRINOX versus gemcitabine for metastatic
pancreatic cancer. N Engl J Med. 364:1817–1825. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Uesaka K, Boku N, Fukutomi A, Okamura Y,
Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto
H, et al: JASPAC 01 Study Group: Adjuvant chemotherapy of S-1
versus gemcitabine for resected pancreatic cancer: A phase 3,
open-label, randomised, non-inferiority trial (JASPAC 01). Lancet.
388:248–257. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang-Gillam A, Li CP, Bodoky G, Dean A,
Shan YS, Jameson G, Macarulla T, Lee KH, Cunningham D, Blanc JF, et
al: NAPOLI-1 Study Group: Nanoliposomal irinotecan with
fluorouracil and folinic acid in metastatic pancreatic cancer after
previous gemcitabine-based therapy (NAPOLI-1): A global,
randomised, open-label, phase 3 trial. Lancet. 387:545–557.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Regine WF, Winter KA, Abrams RA, Safran H,
Hoffman JP, Konski A, Benson AB, Macdonald JS, Kudrimoti MR, Fromm
ML, et al: Fluorouracil vs gemcitabine chemotherapy before and
after fluorouracil-based chemoradiation following resection of
pancreatic adenocarcinoma: A randomized controlled trial. JAMA.
299:1019–1026. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cheng L, Eng C, Nieman LZ, Kapadia AS and
Du XL: Trends in colorectal cancer incidence by anatomic site and
disease stage in the United States from 1976 to 2005. Am J Clin
Oncol. 34:573–580. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Christein JD, Kendrick ML, Iqbal CW,
Nagorney DM and Farnell MB: Distal pancreatectomy for resectable
adenocarcinoma of the body and tail of the pancreas. J Gastrointest
Surg. 9:922–927. 2005.PubMed/NCBI View Article : Google Scholar
|