Introduction
Liver fibrosis is a common pathological
manifestation of various chronic liver diseases, which can advance
to cirrhosis and hepatocellular carcinoma (1) Liver biopsy is the gold standard
approach to assess fibrosis progression in chronic liver diseases,
such as chronic hepatitis B (CHB), chronic hepatitis C (CHC),
autoimmune liver diseases and non-alcoholic fatty liver disease,
even though it is an invasive procedure (2). As the progression of liver fibrosis can
be reversed with treatment, serial hepatic biopsy analysis is
important for chronic liver diseases (3). However, repetitive procedures are
difficult to perform because of various limitations regarding the
principle, cost and sampling error (3). Therefore, non-invasive methods for
assessing liver fibrosis are required to overcome these
limitations. Non-invasive techniques, such as magnetic resonance
imaging (MRI) (4) and
ultrasound-based transient elastography, are considered helpful for
estimating the advanced stages of fibrosis (5). Serum surrogate biomarkers can also be
employed and they are broadly classified as direct markers, which
reflect alterations in the contents of extracellular matrix
proteins, and indirect markers, which reflect changes in liver
function. Direct markers include hyaluronan (HA), 7S domain of type
4 collagen (7S collagen) and procollagen type III N-terminal
peptide (PIIINP), and with all these markers, there are
difficulties in discriminating accurately between the early and
adjacent stages of fibrosis (6,7).
Conversely, indirect markers involve routine laboratory parameters
and are calculated from these laboratory parameters [for example,
fibrosis index based on four factors (FIB-4 index) and aspartate
aminotransferase (AST)-to-platelet ratio index (APRI)] (8). These are inefficient in detecting early
stage fibrosis in patients with CHB (9).
The early diagnosis of liver fibrosis is important
to control disease progression. The clinical practice guidelines
for the management of hepatitis B proposed by the American
Association for the Study of Liver Diseases (10) and the Japan Society of Hepatology
(11) state that the decision to
initiate antiviral therapy should be taken if a patient has F1
fibrosis without necroinflammation. The underlying mechanisms of
histological progression vary among patients with chronic liver
diseases (12,13). Recently, plasma N-terminal type III
collagen propeptide (Pro-C3) has been introduced as a novel
non-invasive marker for the assessment of liver fibrosis in
patients with CHC (14) and
non-alcoholic steatohepatitis (15).
The authors of the present study have previously demonstrated that
the serum angiotensin-converting enzyme level is a beneficial
non-invasive marker for evaluating significant fibrosis in patients
with CHB without fatty liver or habitual alcoholic consumption
(16). To date, no surrogate marker
that accurately quantifies hepatic fibrosis has been identified in
patients with CHB. The present study aimed to compare the
performances of fibrosis biomarkers for diagnosing significant
liver fibrosis that indicate the need for antiviral therapy in
patients with CHB to identify the most appropriate biomarker in
these patients.
Materials and methods
Patients
The present study enrolled 96 treatment-naïve
patients who were diagnosed with CHB serologically and
histologically between September 2005 and May 2017 (Table I). The typical characteristics of CHB
infection were as follows: i) Hepatitis B surface antigen (HBsAg)
positivity for at least 6 months; and ii) serum hepatitis B virus
(HBV) DNA level ≥1.3 log IU/ml. Detections of HB envelope antigen,
anti-HBe IgG and anti-HB core IgG were not considered as inclusion
criteria in the present assessment. The exclusion criteria were
clinical findings suggestive of concomitant liver diseases
(including CHC), autoimmune hepatitis, primary biliary cholangitis,
alcoholic liver disease, non-alcoholic fatty liver disease and
hepatocellular carcinoma. The present study was performed in
accordance with the standards of the Declaration of Helsinki and
written informed consent was obtained from all the study
participants. The Ethics Committee of Nara Medical University
affiliated Hospital approved this study (approval no. 1077).
 | Table IBaseline characteristics of patients
with CHB. |
Table I
Baseline characteristics of patients
with CHB.
| Variable | CHB patients
(n=96) |
|---|
| Sex | |
|
Male | 49 |
|
Female | 47 |
| Age, years | 51.1±13.7 |
| Fibrosis stage | |
|
F0 | 25 |
|
F1 | 44 |
|
F2 | 14 |
|
F3 | 10 |
|
F4 | 3 |
| Inflammatory
activity | |
|
A0 | 36 |
|
A1 | 37 |
|
A2 | 20 |
|
A3 | 3 |
| Platelet
(104/µl) | 19.7±5.4 |
| AST (IU/l) | 37.7±27.2 |
| ALT (IU/l) | 46.8±57.3 |
| Serum Albumin
(g/dl) | 4.2±0.3 |
| Total Bilirubin
(mg/dl) | 0.9±0.3 |
| HBV DNA (Log
copies/ml) | 4.8±2.6 |
| HBs Antigen
(IU/ml) | 13,782±30,650 |
| Hyaluronic acid
(ng/ml) | 83.7±15.0 |
| Type 4 collagen 7S
(ng/ml) | 4.3±2.2 |
| PIIINP (ng/ml) | 10.4±6.4 |
| TIMP-1 (ng/ml) | 224.9±73.8 |
| M2BPGi (cutoff
index) | 0.87±0.51 |
| Pro C3 (ng/ml) | 16.6±5.2 |
| FIB-4 | 1.77±1.31 |
| APRI | 0.71±0.73 |
| ELF score | 9.34±1.10 |
Laboratory analysis and measurement of
clinical laboratory parameters
Variables, including age, platelet (PLT) count and
levels of AST, alanine aminotransferase (ALT), albumin (ALB), total
bilirubin, HBV DNA, and HBsAg, were assessed and recorded on
admission (Table I). Additionally,
the HA level, 7S collagen level, PIIINP level, tissue inhibitor of
metalloproteinases 1 (TIMP-1) level, Mac-2 binding protein
glycosylation isomer (M2BPGi) level, Pro-C3 level, FIB-4 index,
APRI and enhanced liver fibrosis (ELF) score were used as
non-invasive biomarkers for the assessment of liver fibrosis. The
following formulas were used: FIB-4 index=(age x AST)/[(PLT count)
x (ALT)1/2]; APRI=[(sample AST/reference AST) x100]/PLT count; ELF
score=2.278 + [0.851 ln(HA) + 0.751 ln(PIIINP) + 0.394 ln(TIMP-1)].
The levels of HA, TIMP-1 and PIIINP were measured using
chemiluminometric immunoassays performed on the ADVIA Centaur XP
Immunoassay System (Siemens Healthineers) (17,18).
Serum 7S collagen was determined using radioimmunoassay kits
(7S-RIA; Nippon DPC Corporation) (19). The Wisteria floribunda
agglutinin-positive Mac-2 binding protein assay was performed using
an automated chemiluminescence enzyme immunoassay analyzer
(HISCL-5000; Sysmex Corporation) (20). Pro-C3 level was measured using UniQ
PIIINP RIA assay (Orion Diagnostica Ltd.) (21). HBs antigen and HBV DNA levels were
measured as previously described (22).
Liver biopsy
Percutaneous liver biopsy was performed before the
initiation of therapy, using ultrasound localization. Liver samples
were fixed in formalin at a room temperature of 20-22˚C, embedded
in paraffin and sectioned to 5 µm. Each section was stained with
hematoxylin-eosin and reticular fiber stain for 30 sec at a room
temperature of 20 to 22˚C or Masson's stain for 60 min at 54-64˚C.
Professor Chiho Obayashi and Dr Kohei Morita (Department of
Diagnostic Pathology, Nara Medical University) independently
reviewed all cases for validation of the histological features of
CHB. The METAVIR scoring system (23) was used to evaluate fibrosis and
necroinflammation. The degree of hepatic fibrosis was scored from
F0 to F4 (F0, no fibrosis; F1, portal fibrosis without septa; F2,
portal fibrosis with few septa; F3, numerous septa without
cirrhosis; and F4, cirrhosis) (11,24). The
degree of necroinflammatory activity was scored from A0 to A3 (A0,
no histological necroinflammatory activity; A1, minimal
necroinflammatory activity; A2, moderate necroinflammatory
activity; and A3, severe necroinflammatory activity) (25). F0-F1 and A0-A1 were considered to
indicate no to mild fibrosis and no to mild necroinflammation,
whereas F2-F4 and A2-A3 were considered to indicate moderate to
severe fibrosis and cirrhosis and moderate to severe
necroinflammation, respectively. Significant liver fibrosis and
necroinflammation were defined as the fibrosis stage ≥F2 and
necroinflammation grade ≥A2, respectively.
Statistical analysis
Patient characteristics are presented as the mean ±
standard error of the mean. Differences in continuous variables
were assessed using Student's t-tests or one-way ANOVAs followed by
Tukey's post-hoc tests. The Mann-Whitney U test was used to compare
two groups of nonparametric data. Chi-square test was used to
analyze categorical variables. Correlations were evaluated using
Spearman's correlation coefficient for continuous variables. All
statistical tests were two-tailed and P<0.05 was considered to
indicate a statistically significant difference. The areas under
the receiver operating characteristic (ROC) curves (AUCs) were used
to evaluate the diagnostic values of the fibrosis biomarkers with
regard to the correct identification of significant liver fibrosis.
The sensitivities, specificities, positive-predictive values
(PPVs), negative-predictive values (NPVs), diagnostic accuracies
and cut-off values of the fibrosis biomarkers were calculated from
the ROC curves. All analyses were performed using SPSS software
version 24 (IBM Corp.).
Results
Baseline clinical characteristics of
patients with different fibrosis stages
The demographic and baseline characteristics of the
patients are summarized in Table I.
The fibrosis stages were F0, F1, F2, F3 and F4 in 25 (26.0%), 44
(45.8%), 14 (14.6%), 10 (10.4%) and 3 (3.2%) patients, respectively
(Table II). Spearman's rank
correlation coefficients between the fibrosis stage and PLT count,
HA level, 7S collagen level, PIIINP level, TIMP-1 level, M2BPGi
level, Pro-C3 level, FIB-4 index, APRI and ELF score were -0.43,
0.38, 0.52, 0.54, 0.38, 0.61, 0.08, 0.42, 0.58 and 0.55,
respectively (Fig. 1A-J). All
fibrosis biomarkers, except the Pro-C3 level, were significantly
associated with the liver fibrosis stage in patients with CHB
(Table II).
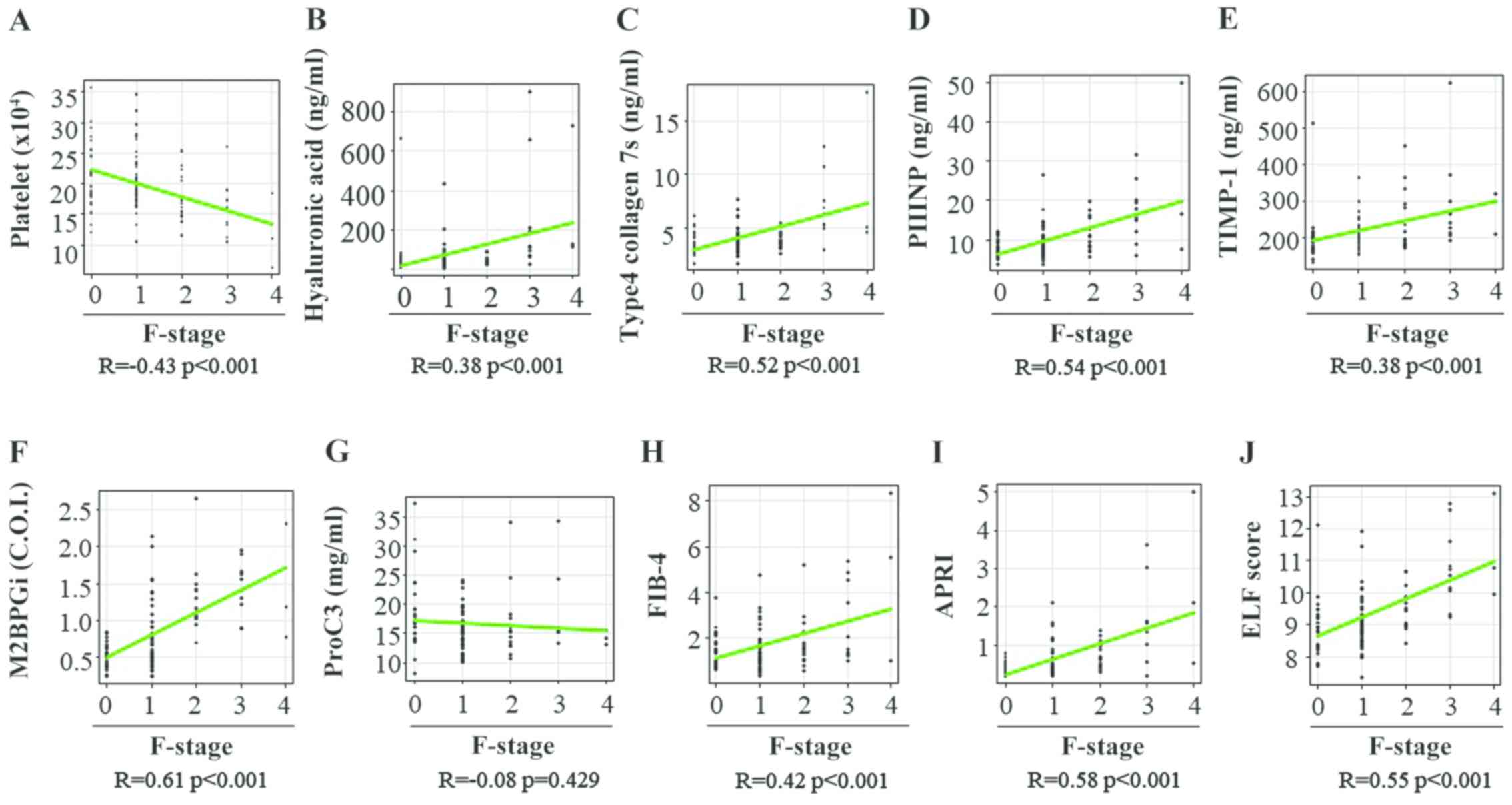 | Figure 1Correlation between 10 liver fibrosis
biomarkers and fibrosis stage in patients with chronic hepatitis B.
(A) Platelet count, (B) hyaluronan acid, (C) 7S domain of type 4,
(D) PIIINP, (E) TIMP-1, (F) M2BPGi, (G) Pro-C3, (H) FIB-4 index,
(I) APRI and (J) ELF score. PIIINP, procollagen type III N-terminal
peptide; TIMP-1, tissue inhibitor of metalloproteinases 1; M2BPGi,
Mac-2 binding protein glycosylation isomer; Pro-C3, N-terminal type
III collagen propeptide; FIB-4 index, fibrosis index based on four
factors; APRI, aspartate aminotransferase-to-platelet ratio index;
ELF, enhanced liver fibrosis; F, fibrosis. |
 | Table IIBaseline characteristics of patients
with chronic hepatitis B stratified according to liver fibrosis
stages. |
Table II
Baseline characteristics of patients
with chronic hepatitis B stratified according to liver fibrosis
stages.
| Variable (reference
range) | F0 (n=25) | F1 (n=44) | F2 (n=14) | F3 (n=10) | F4 (n=3) | Overall
P-value |
|---|
| Male/female | 11/14 | 23/21 | 7/7 | 6/4 | 2/1 | NS |
| Age (years) | 53.7±2.4 | 50.0±2.1 | 49.1±3.4 | 51.9±5.4 | 51.6±6.7 | NS |
| Platelet
(104/µl) (14.0-37.9) | 21.6±1.1 | 20.6±0.7 | 18.0±1.0 | 15.4±4.8 | 12.0±2.9 | <0.01 |
| AST (IU/l)
(10-40) | 26.1±2.5 | 34.1±3.4 | 42.9±6.0 | 66.3±46.3 | 67.3±15.9 | <0.01 |
| ALT (IU/l)
(5-45) | 22.0±2.3 | 42.4±5.4 | 65.9±19.9 | 97.4±118.3 | 60.7±3.9 | <0.01 |
| Serum albumin
(g/dl) (3.7-5.5) | 4.2±0.0 | 4.2±0.0 | 4.1±0.0 | 4.0±0.4 | 3.9±0.0 | NS |
| Total Bilirubin
(mg/dl) (0.3-1.2) | 0.8±0.0 | 0.8±0.0 | 0.9±0.0 | 1.0±0.3 | 1.8±0.0 | NS |
| HBV DNA (Log
copies/ml) | 3.5±0.4 | 4.8±0.4 | 5.8±0.5 | 6.3±2.9 | 3.0±1.4 | 0.01 |
| HBsAg (IU/ml)
(<0.05) | 12,128±5,088 | 42,946±24,337 | 11,051±5,832 | 11,801±24,431 | 754±340 | NS |
| Hyaluronic acid
(ng/ml) (<50.0) | 61.7±24.9 | 52.5±10.2 | 53.4±6.3 |
245.9±88.1b | 325.1±165.6 | <0.01 |
| Type 4 collagen 7S
(ng/ml) (<6.0) | 3.6±0.2 | 3.8±0.2 | 4.0±0.2 |
6.9±0.9b | 9.1±3.5 | <0.01 |
| PIIINP (ng/ml)
(3.62-9.52) | 7.9±0.4 | 8.9±0.6 | 11.4±1.1 |
17.3±2.3b | 24.7±10.5 | <0.01 |
| TIMP-1 (ng/ml) | 198.6±13.7 | 213.7±5.9 | 247.9±22.1 | 291.2±38.3 | 277.7±27.1 | <0.01 |
| M2BPGi (cutoff
index) (<1.00) | 0.53±0.03 | 0.75±0.07 |
1.29±0.12a | 1.44±0.11 | 1.42±0.38 | <0.01 |
| ProC3 (ng/ml) | 18.5±1.3 | 15.5±0.5 | 16.5±1.6 | 18.0±1.9 | 14.3±0.5 | NS |
| FIB-4 | 1.52±0.14 | 1.46±0.13 | 1.85±0.30 |
2.71±0.51c | 4.97±1.74 | 0.05 |
| APRI | 0.43±0.03 | 0.56±0.06 | 0.77±0.09 |
1.52±0.33b | 2.56±1.07 | <0.01 |
| ELF score | 8.91±0.18 | 9.04±0.13 | 9.51±0.18 |
10.79±0.36b | 11.28±0.77 | <0.01 |
Levels of serum fibrosis biomarkers
according to the degree of liver fibrosis in patients with CHB
It was identified that the M2BPGi level was markedly
higher in patients with F2 fibrosis compared with those with F1
fibrosis [F1, 0.75±0.45 vs. F2, 1.29±0.46 Cutoff index (COI)
P<0.01; Fig. 2F]. Unlike the
M2BPGi findings, there were significant differences between
patients with F2 fibrosis and those with F3 fibrosis with regard to
the HA level (F2, 53.4±23.6 vs. F3, 245.9±278.9 ng/ml; P<0.01;
Fig. 2B), 7S collagen level (F2,
4.0±0.9 vs. F3, 6.9±2.7 ng/ml; P<0.01; Fig. 2C), PIIINP level (F2, 11.4±4.2 vs. F3,
17.3±7.2 ng/ml; P<0.05; Fig. 2D),
APRI (F2, 0.77±0.34 vs. F3, 1.52±1.03; P<0.01; Fig. 2I) and ELF score (F2, 9.51±0.68 vs.
F3, 10.79±1.15; P<0.01; Fig. 2J)
and between patients with F3 fibrosis and those with F4 fibrosis
with regard to the FIB-4 index (F3, 2.71±1.62 vs. F4, 4.97±3.02;
P<0.05; Fig. 2H). However, no
differences were found among the fibrosis groups with regard to the
PLT count and serum TIMP-1 and Pro-C3 levels (Fig. 2A, E
and G).
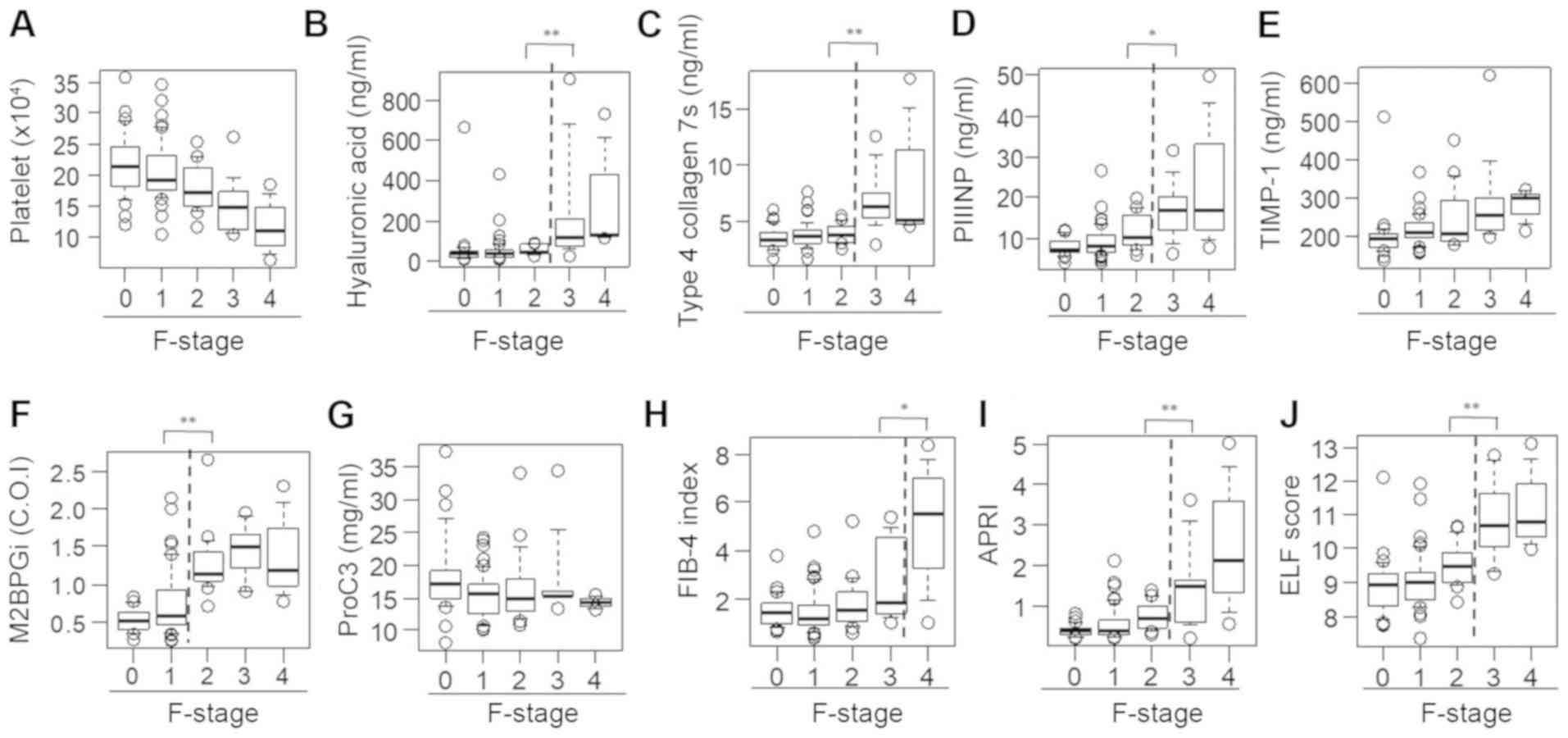 | Figure 2Serum levels of fibrosis biomarkers
for staging liver fibrosis in patients with chronic hepatitis B. A
box plot of each fibrosis marker according to the fibrosis stage is
presented. (A) Platelet count, (B) hyaluronan acid, (C) 7S domain
of type 4, (D) PIIINP, (E) TIMP-1, (F) M2BPGi, (G) Pro-C3, (H)
FIB-4 index, (I) APRI and (J) ELF score. Data are presented as mean
± standard deviation. *P<0.05 and
**P<0.01 as indicated. M2BPGi, Mac-2
binding protein glycosylation isomer; PIIINP, procollagen type III
N-terminal peptide; APRI, aspartate aminotransferase-to-platelet
ratio index; ELF, enhanced liver fibrosis; FIB-4 index, fibrosis
index based on four factors; TIMP-1, tissue inhibitor of
metalloproteinases 1; Pro-C3, N-terminal type III collagen
propeptide; F, fibrosis. |
Diagnostic performances of serum
fibrosis biomarkers for identifying significant liver fibrosis in
patients with CHB
The diagnostic sensitivity, specificity, PPV, NPV
and accuracy of the PLT count, HA level, 7S collagen level, PIIINP
level, TIMP-1 level, M2BPGi level, Pro-C3 level, FIB-4 index, APRI
and ELF score for the differentiation of ≥F2 fibrosis in patients
with CHB are shown in Table III.
The AUCs of these markers for the accurate diagnosis of significant
liver fibrosis (≥F2) were 0.757, 0.776, 0.739, 0.778, 0.713, 0.902,
0.452, 0.676, 0.812 and 0.816, respectively (Table III). These findings indicated that
the serum M2BPGi level was most accurately identifying significant
liver fibrosis when compared with other non-invasive fibrosis
biomarkers in patients with CHB.
 | Table IIIDiagnostic accuracy of serum fibrosis
markers for significant fibrosis in patients with chronic hepatitis
B. |
Table III
Diagnostic accuracy of serum fibrosis
markers for significant fibrosis in patients with chronic hepatitis
B.
| Biomarker | AUC | 95% CI | Cut-off | Sensitivity (95%
CI) | Specificity (95%
CI) | PPV (95% CI) | NPV (95% CI) | Accuracy (95%
CI) |
|---|
| Platelet count | 0.757 | 0.641-0.872 | 17.5 | 70.4
(49.8-86.2) | 76.8
(65.1-86.1) | 54.3
(36.6-71.2) | 86.9
(75.8-94.2) | 75.0
(65.1-83.3) |
| Hyalorinic
acid | 0.776 | 0.675-0.877 | 61.08 | 63.0
(42.4-80.6) | 84.1
(73.3-91.8) | 60.7
(40.6-78.5) | 85.3
(74.6-92.7) | 78.1
(68.5-85.9) |
| Type 4 collagen
7S | 0.739 | 0.621-0.856 | 4.6 | 59.3
(38.8-77.6) | 83.6
(72.5-91.5) | 59.3
(38.8-77.6) | 83.6
(72.5-91.5) | 76.6
(66.7-84.7) |
| PIIINP | 0.778 | 0.668-0.888 | 15.0 | 48.1
(28.7-68.1) | 97.1
(89.9-99.6) | 86.7
(59.5-98.3) | 82.7
(72.7-90.2) | 83.3
(74.4-90.2) |
| TIMP-1 | 0.713 | 0.591-0.833 | 264.3 | 44.4
(25.5-64.7) | 94.2
(85.8-98.4) | 75.0
(47.6-92.7) | 81.2
(71.0-89.1) | 80.2
(70.8-87.6) |
| M2BPGi | 0.902 | 0.841-0.962 | 0.890 | 92.6
(75.7-99.1) | 82.4
(71.2-90.5) | 67.6
(50.2-82.0) | 96.6
(88.1-99.6) | 85.3
(76.5-91.7) |
| Pro-C3 | 0.452 | 0.325-0.579 | 10.781 | 100 (81.7-100) | 11.6
(5.1-21.6) | 30.7
(21.3-41.4) | 100 (51.8-100) | 36.5
(26.9-46.9) |
| FIB-4 | 0.676 | 0.555-0.798 | 1.264 | 77.8
(57.7-91.4) | 50.7
(38.4-63.0) | 38.2
(25.4-52.3) | 85.4
(70.8-94.4) | 58.3
(47.8-68.3) |
| APRI | 0.812 | 0.709-0.914 | 0.534 | 81.5
(61.9-93.7) | 75.4
(63.5-84.9) | 56.4
(39.6-72.2) | 91.2
(80.7-97.1) | 77.1
(67.4-85.0) |
| ELF score | 0.816 | 0.723-0.909 | 9.250 | 77.8
(75.7-91.4) | 73.9
(61.9-83.7) | 53.8
(37.2-69.9) | 89.5
(78.5-96.0) | 75.0
(65.1-83.3) |
Diagnostic performance of M2BPGi for
identifying the different stages of liver fibrosis in patients with
CHB
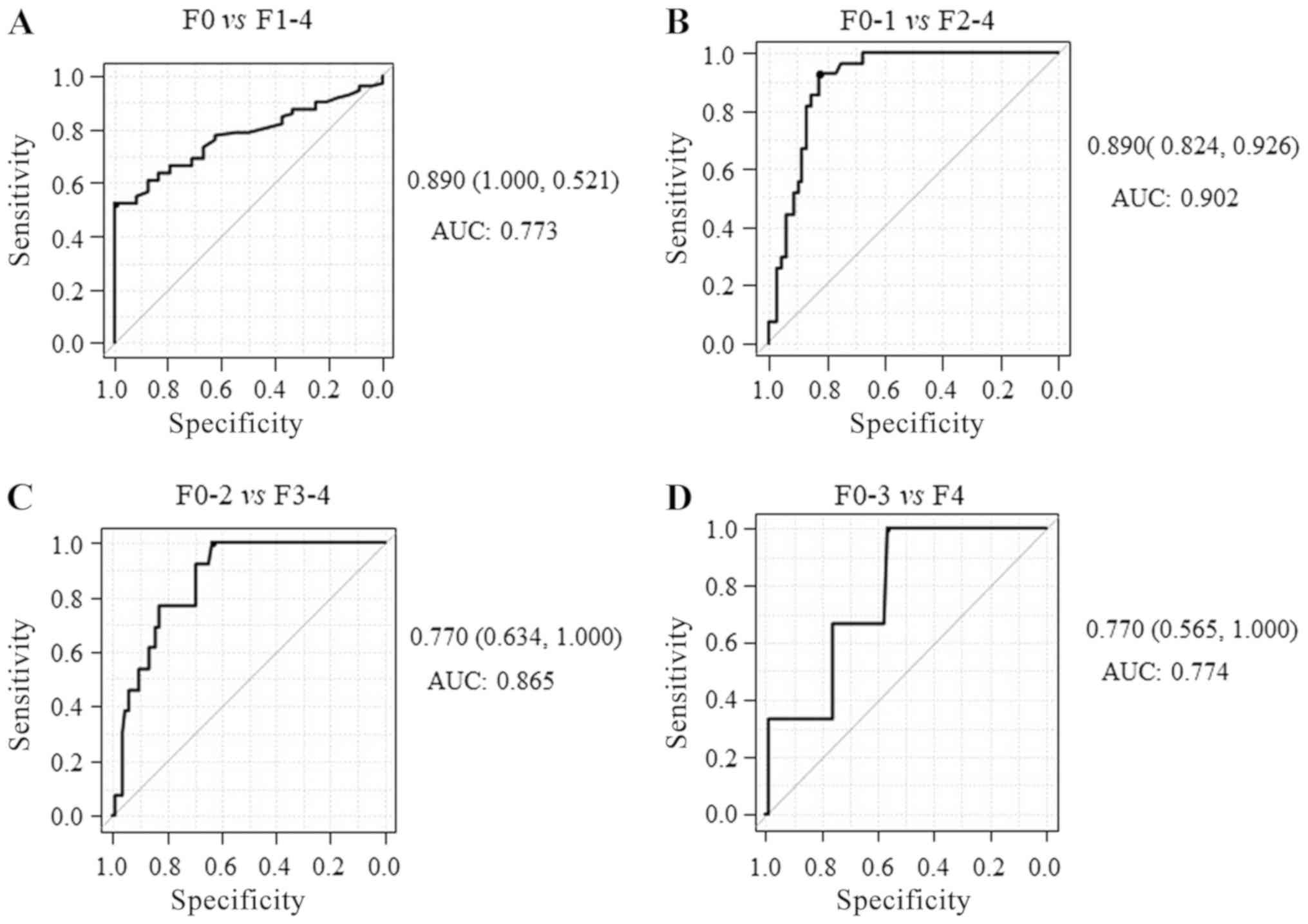
The AUCs for identifying mild fibrosis (F1-4),
significant fibrosis (F2-4), severe (F3-4) and advanced
fibrosis/cirrhosis (F4) were 0.773 (sensitivity, 52.1%;
specificity, 100%; Fig. 3A) 0.902
(sensitivity, 92.6%; specificity, 82.4%; Fig. 3B), 0.865 (sensitivity, 100%;
specificity, 63.4%; Fig. 3C) and
0.774 (sensitivity, 100%; specificity, 56.5%; Fig. 3D), respectively, indicating that
M2BPGi has higher diagnostic accuracy for significant fibrosis than
mild/severe fibrosis or cirrhosis. Together, these results
suggested that serum M2BPGi had the best performance for
identifying significant liver fibrosis in patients with CHB. The
diagnostic accuracy of serum M2BPGi level was compared to that of
other fibrosis markers including the PLT count; HA, 7S collagen,
PIIINP, TIMP-1 and Pro-C3 levels; FIB-4 index; APRI; and ELF score.
Significant differences were observed in the AUCs between M2BPGi
level and PLT count, HA level, 7S collagen level, PIIINP level,
TIMP-1 level, Pro-C3 level and FIB-4 index (P<0.05, P<0.05,
P<0.01, P<0.05, P<0.01, P<0.001 and P<0.001,
respectively) and no significant difference was found between
M2BPGi level and APRI and ELF score (P=0.093 and P=0.072,
respectively) in patients with CHB (Table SI).
Association of the M2BPGi level with
the fibrosis stage in terms of histological necroinflammatory
activity
The ALT level was significantly higher in patients
with significant liver necroinflammation (n=23) compared with those
without significant necroinflammation (n=73; P<0.01; Table SII). A significant correlation was
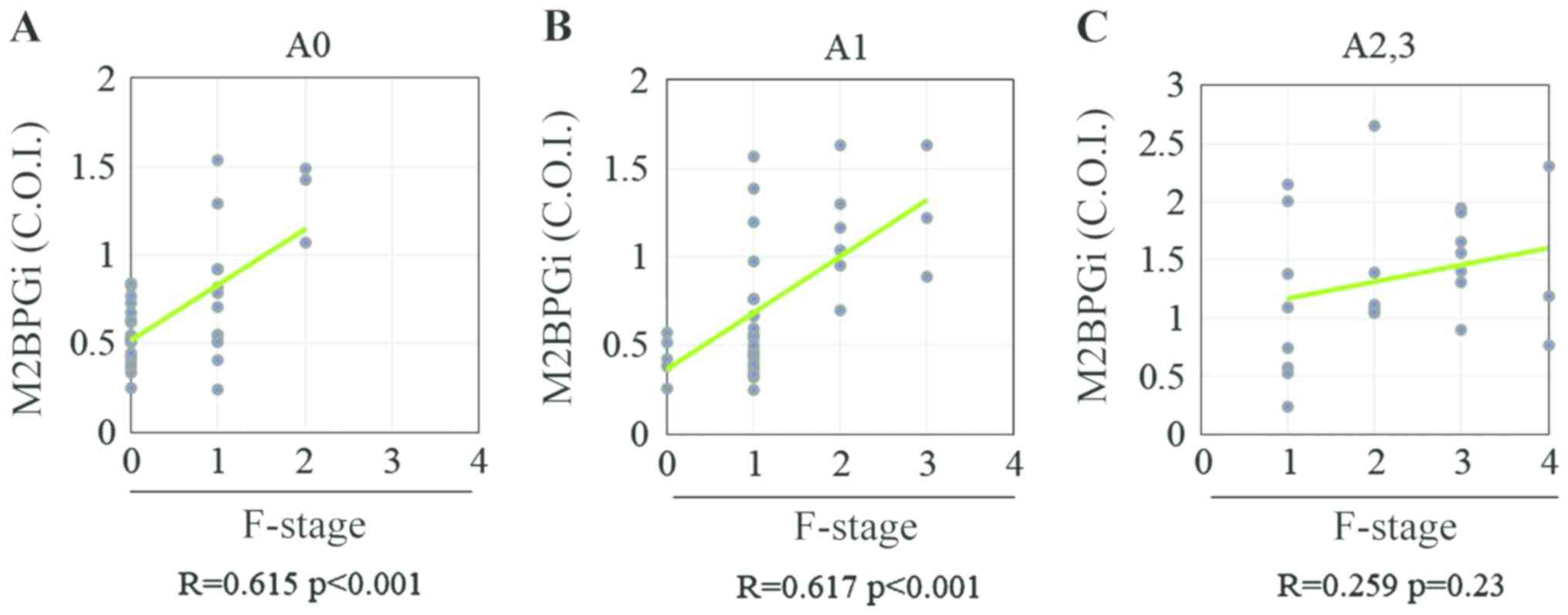
observed between the M2BPGi level and fibrosis stage in patients
with CHB without significant liver necroinflammation (A0-1;
Fig. 4A and B), whereas no significant correlation was
observed between these two variables in those with significant
liver necroinflammation (≥A2; Fig.
4C). These findings indicated that significant liver
necroinflammation might affect the M2BPGi level in patients with
CHB. Among patients with CHB without significant liver
necroinflammation, Spearman's rank correlation coefficients between
the fibrosis stage and PLT count, HA level, 7S collagen level,
PIIINP level, TIMP-1 level, M2BPGi level, Pro-C3 level, FIB-4
index, APRI and ELF score were -0.31, 0.04, 0.21, 0.20, 0.15, 0.59,
-0.10, 0.19, 0.28 and 0.26, respectively (Fig. 5A-J). The PLT count and M2BPGi level
were the only variables associated with the liver fibrosis stage in
patients with CHB without significant liver necroinflammation
(Table IV).
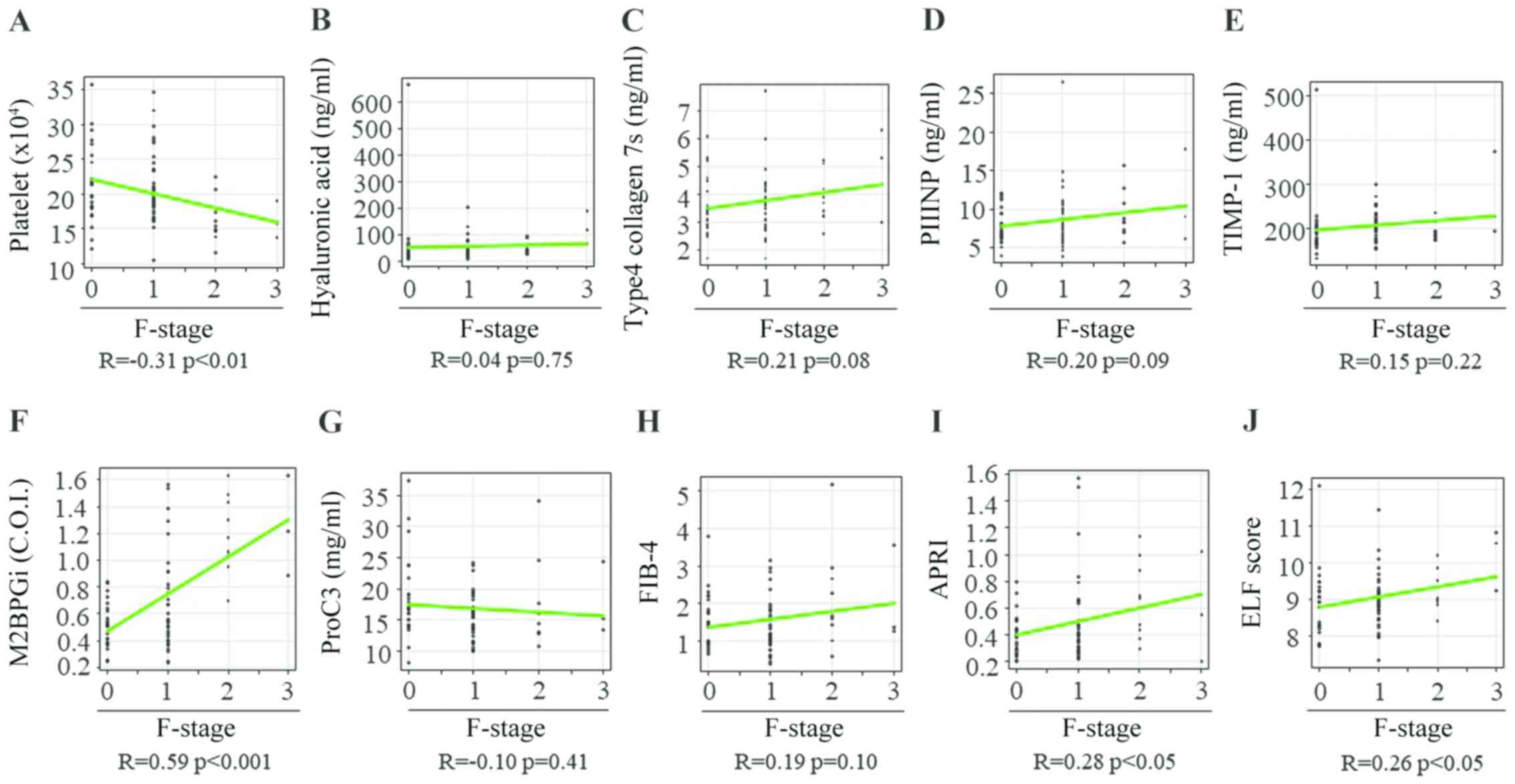 | Figure 5Correlation between 10 liver fibrosis
biomarkers and fibrosis stage in patients with chronic hepatitis B
without significant liver necroinflammation. (A) Platelet count,
(B) hyaluronan acid, (C) 7S domain of type 4, (D) PIIINP; (E)
TIMP-1, (F) M2BPGi, (G) Pro-C3, (H) FIB-4 index, (I) APRI and (J)
ELF score. PIIINP, procollagen type III N-terminal peptide; TIMP-1,
tissue inhibitor of metalloproteinases 1; M2BPGi, Mac-2 binding
protein glycosylation isomer; Pro-C3, N-terminal type III collagen
propeptide; FIB-4 index, fibrosis index based on four factors;
APRI, aspartate aminotransferase-to-platelet ratio index; ELF,
enhanced liver fibrosis; F, fibrosis. |
 | Table IVBaseline characteristics of patients
with chronic hepatitis B without significant liver inflammation
stratified according to liver fibrosis stages. |
Table IV
Baseline characteristics of patients
with chronic hepatitis B without significant liver inflammation
stratified according to liver fibrosis stages.
| Variable (reference
range) | F0 (n=25) | F1 (n=36) | F2 (n=9) | F3 (n=3) | Overall
P-value |
|---|
| Male/female | 11/14 | 19/17 | 3/6 | 2/1 | NS |
| Age (years) | 53.7±2.4 | 50.0±2.3 | 51.2±4.6 | 55.9±8.0 | NS |
| Platelet count
(104/µl) (10.4-37.9) | 21.6±1.1 |
21.0±0.8b | 16.4±1.1 | 16.4±1.2 | 0.03 |
| AST (IU/l)
(10-40) | 26.1±2.6 | 31.3±3.4 | 31.9±3.4 | 27.2±7.6 | NS |
| ALT (IU/l)
(5-45) | 22.0±2.4 | 38.3±6.0 | 30.0±5.0 | 22.7±6.2 | NS |
| Serum albumin
(g/dl) (3.7-5.5) | 4.2±0.1 | 4.2±0.1 | 4.0±0.1 | 4.4±0.2 | NS |
| Total Bilirubin
(mg/dl) (0.3-1.2) | 0.8±0.0 | 0.8±0.1 | 0.9±0.1 | 1.0±0.2 | NS |
| HBV DNA (Log
copies/ml) | 3.5±0.4 | 4.5±0.4 | 5.5±0.6 | 3.4±1.4 | NS |
| HBsAg (IU/ml)
(<0.05) | 12,128±5,088 | 41,852±2,863 | 10,580±8,840 | 1,602±520 | NS |
| Hyaluronic acid
(ng/ml) (<50.0) | 61.7±25.8 | 45.0±6.1 | 47.4±7.7 |
123.9±30.0c | NS |
| Type 4 collagen 7S
(ng/ml) (<6.0) | 3.6±0.2 | 3.8±0.1 | 3.8±0.3 |
4.9±0.8c | NS |
| PIIINP (ng/ml)
(3.6-9.52) | 7.9±0.5 | 8.7±7.3 | 9.4±1.0 | 11.0±2.9 | NS |
| TIMP-1 (ng/ml) | 198.6±13.8 | 211.1±5.7 |
193.5±6.5c | 264.4±45.0 | NS |
| M2BPGi (COI)
(<1.00) | 0.53±0.32 |
0.68±0.6b | 1.20±0.1 | 1.25±0.17 | <0.01 |
| ProC3 (ng/ml) | 18.5±1.3 | 15.8±0.6 | 17.4±2.3 | 17.7±2.8 | NS |
| FIB-4 | 1.52±0.14 | 1.38±0.12 | 2.15±0.43 | 2.07±0.61 | NS |
| APRI | 0.43±0.05 | 0.50±0.05 | 0.66±0.09 | 0.59±0.20 | NS |
| ELF score | 8.91±0.18 |
8.99±0.12c | 9.21±0.17 |
10.20±0.40d | NS |
Levels of serum fibrosis biomarkers
according to the degree of liver fibrosis in patients with CHB
without significant liver necroinflammation
Significant differences were observed between
patients with F1 fibrosis and those with F2 fibrosis with regard to
the PLT count (F1, 21.0±5.0 vs. F2, 16.4±3.2x104/µl;
P<0.01; Fig. 6A) and M2BPGi level
(F1, 0.68±0.34 vs. F2, 1.20±0.28 COI; P<0.01; Fig. 6B). There were significant differences
between patients with F2 fibrosis and those with F3 fibrosis with
regard to the TIMP-1 level (F2, 193.5±19.5 vs. F3, 264.4±77.7
ng/ml; P<0.01; Fig. 6C), ELF
score (F2, 9.21±0.52 vs. F3, 10.20±0.69; P<0.05; Fig. 6D) and HA level (F2, 47.4±23.1 vs. F3,
123.9±51.9 ng/ml; P<0.01; Fig.
6E). However, no differences were observed among the fibrosis
groups with regard to the 7S collagen level, PIIINP level, Pro-C3
level, FIB-4 index and APRI (Fig.
6F-J).
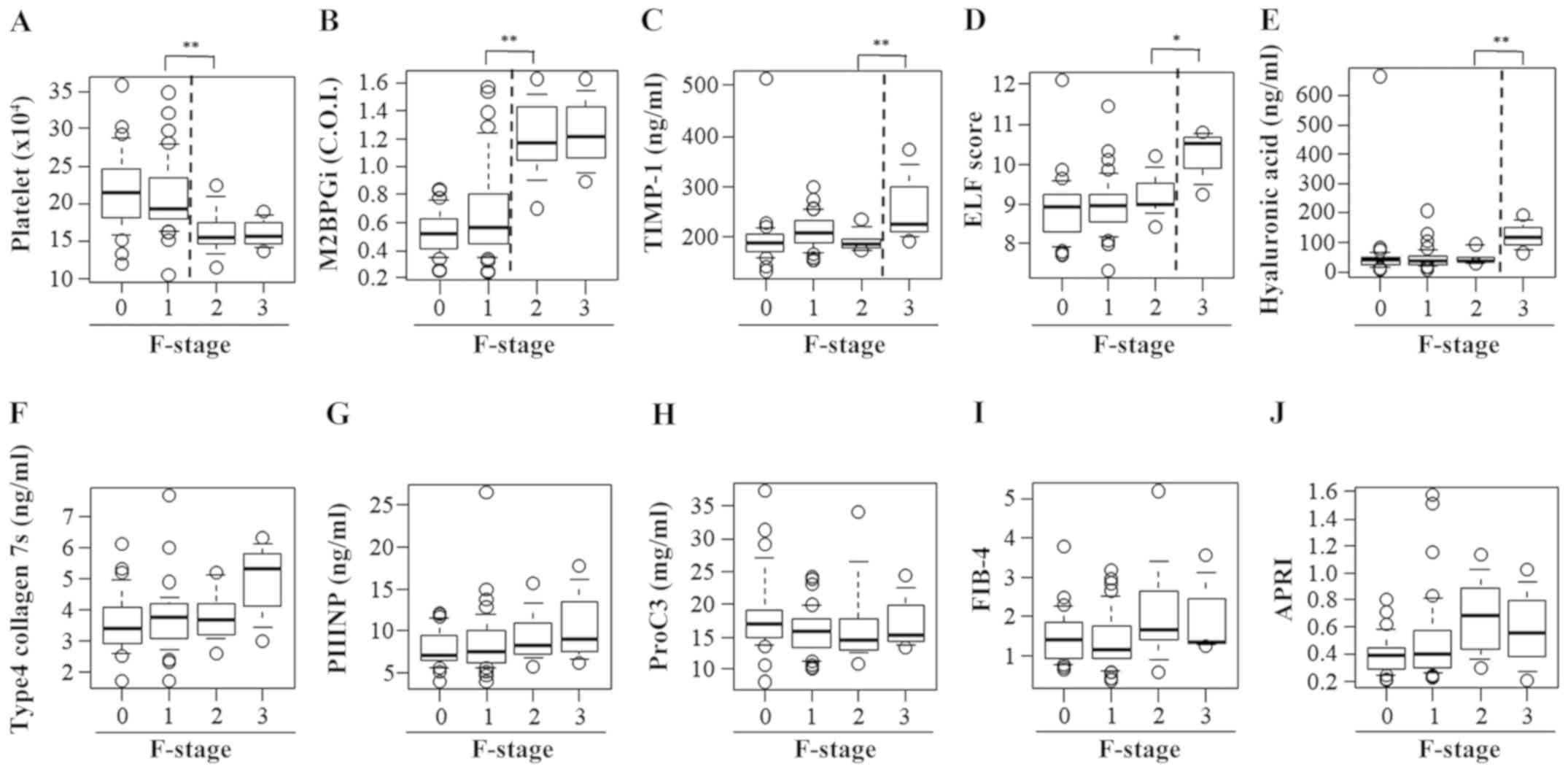 | Figure 6Serum levels of fibrosis biomarkers
for staging liver fibrosis in patients with chronic hepatitis B
without significant liver necroinflammation. A box plot of each
fibrosis marker according to the fibrosis stage is presented. (A)
Platelet count, (B) M2BPGi, (C) TIMP-1, (D) ELF score, (E)
hyaluronan acid, (F) 7S domain of type 4 collagen, (G) PIIINP, (H)
Pro-C3, (I) FIB-4 index and (J) APRI. Data are presented as mean ±
standard deviation. *P<0.05 and
**P<0.01 as indicated. M2BPGi, Mac-2
binding protein glycosylation isomer; TIMP-1, tissue inhibitor of
metalloproteinases 1; ELF, enhanced liver fibrosis; PIIINP,
procollagen type III N-terminal peptide; Pro-C3, N-terminal type
III collagen propeptide; FIB-4 index, fibrosis index based on four
factors; APRI, aspartate aminotransferase-to-platelet ratio index;
F, fibrosis. |
Diagnostic performances of serum
fibrosis biomarkers for identifying significant liver fibrosis in
patients with CHB without significant liver necroinflammation
The optimal cut-off value and diagnostic performance
of each serum fibrosis biomarker for identifying significant liver
fibrosis in patients with CHB without significant liver
necroinflammation are presented in Table
V. The AUCs of the PLT count, HA level, 7S collagen level,
PIIINP level, TIMP-1 level, M2BPGi level, Pro-C3 level, FIB-4
index, APRI and ELF score for the correct diagnosis of significant
liver fibrosis were 0.807, 0.678, 0.603, 0.632, 0.501, 0.921,
0.458, 0.678, 0.708 and 0.688, respectively. These findings
indicated that the serum M2BPGi level more accurately identified
significant liver fibrosis when compared with other non-invasive
fibrosis biomarkers in patients with CHB without significant liver
necroinflammation.
 | Table VDiagnostic accuracy of serum fibrosis
markers for significant fibrosis in patients with chronic hepatitis
B without significant liver inflammation. |
Table V
Diagnostic accuracy of serum fibrosis
markers for significant fibrosis in patients with chronic hepatitis
B without significant liver inflammation.
| Biomarker | AUC | 95% CI | Cut-off | Sensitivity (95%
CI) | Specificity (95%
CI) | PPV (95% CI) | NPV (95% CI) | Accuracy (95%
CI) |
|---|
| Platelet | 0.807 | 0.670-0.947 | 16.2 | 66.7
(34.9-90.1) | 88.5
(77.8-95.3) | 53.3
(16.6-78.7) | 93.1
(83.3-98.1) | 84.9
(74.6-92.2) |
| Hyaluronic
acid | 0.678 | 0.522-0.833 | 26.6 | 100 (64.0-100) | 37.7
(25.6-51.0) | 24.0
(13.1-38.2) | 100 (78.9-100) | 47.9
(36.1-60.0) |
| Type 4 collagen
7S | 0.603 | 0.416-0.791 | 5.1 | 33.3
(9.9-65.1) | 91.8
(81.9-97.3) | 44.4
(13.7-78.8) | 87.5
(76.8-94.4) | 82.2
(71.5-90.2) |
| PIIINP | 0.632 | 0.454-0.810 | 8.27 | 66.7
(34.9-90.1) | 65.6
(52.3-77.3) | 27.6
(12.7-47.2) | 90.9
(78.3-97.5) | 65.8
(53.7-76.5) |
| TIMP-1 | 0.501 | 0.329-0.674 | 174.7 | 100 (64.0-100) | 24.6
(14.5-37.3) | 20.7
(11.2-33.4) | 100 (69.8-100) | 37.0
(26.0-49.1) |
| M2BPGi | 0.921 | 0.856-0.986 | 0.890 | 91.7
(61.5-99.8) | 86.7
(75.4-94.1) | 57.9
(33.5-79.7) | 98.1
(89.9-100) | 87.5
(77.6-94.1) |
| Pro-C3 | 0.458 | 0251-0.664 | 24.35 | 25.0
(5.5-57.2) | 95.1
(86.3-99.0) | 50.0
(11.8-88.2) | 86.6
(76.0-93.7) | 83.6
(73.0-91.2) |
| FIB-4 | 0.678 | 0.500-0.855 | 1.264 | 83.3
(51.6-97.9) | 50.8
(37.7-63.9) | 25.0
(12.7-41.2) | 93.9
(79.8-93.3) | 56.2
(44.1-67.8) |
| APRI | 0.708 | 0.524-0.892 | 0.438 | 75.0
(42.8-94.5) | 65.6
(52.3-77.3) | 30.0
(14.7-49.4) | 93.0
(80.9-98.5) | 67.1
(55.1-77.7) |
| ELF score | 0.688 | 0.530-0.846 | 8.87 | 91.7
(61.5-99.8) | 44.3
(31.5-57.6) | 24.4
(12.9-39.5) | 96.4
(81.7-99.9) | 52.1
(40.0-63.9) |
Diagnostic performance of serum M2BPGi
for identifying the different stages of liver fibrosis in patients
with CHB without significant liver necroinflammation
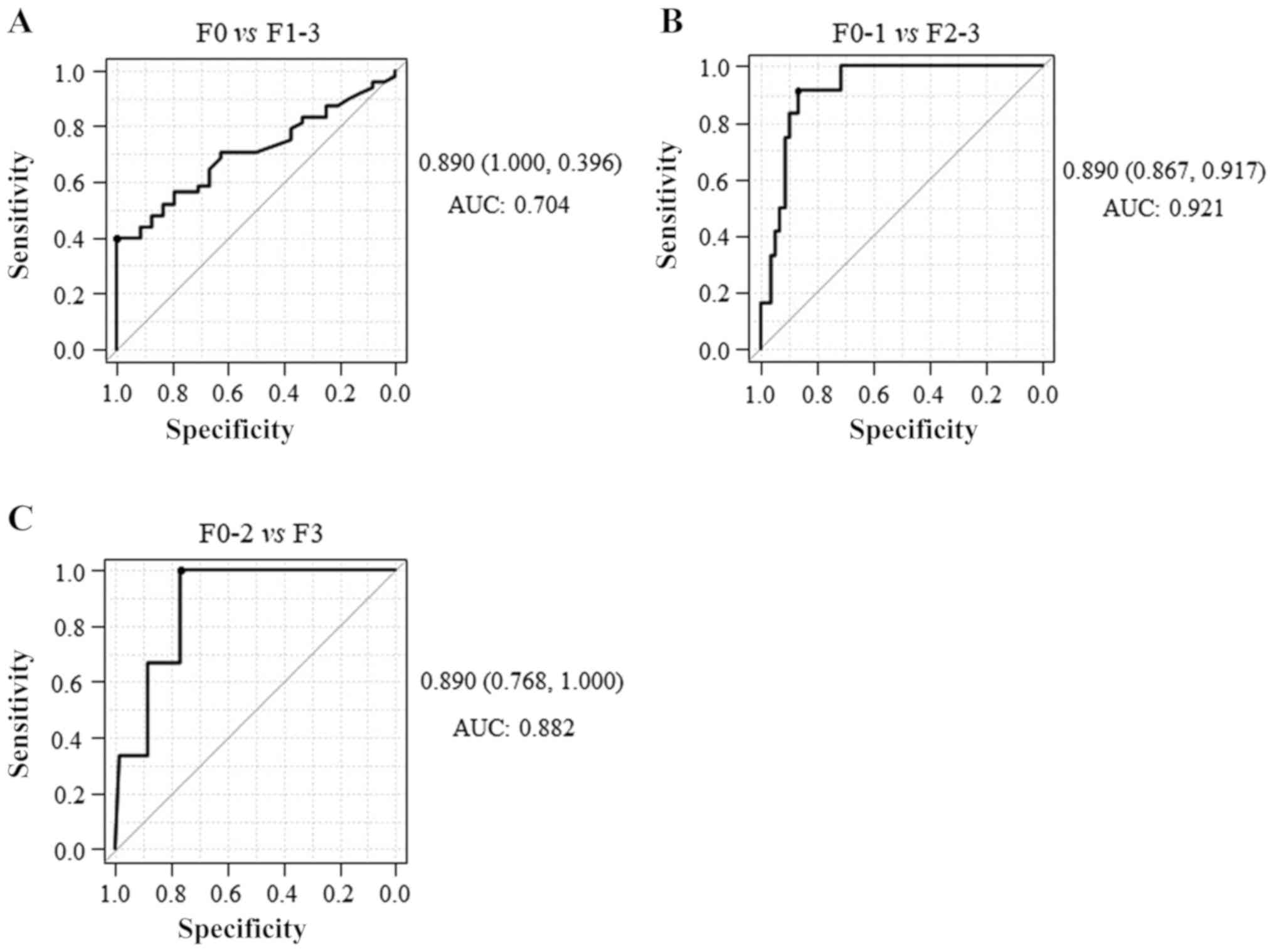
The AUCs of the M2BPGi level for identifying F1-3,
F2-3 and F3 were 0.704, 0921 and 0.882, respectively (Fig. 7), indicating that M2BPGi exhibited a
higher diagnostic accuracy for significant fibrosis than mild or
severe fibrosis. These results suggested that serum M2BPGi had the
best performance for identifying significant liver fibrosis in
patients with CHB who had significant liver fibrosis but did not
have significant liver necroinflammation. In addition, significant
differences were identified in the AUCs between M2BPGi level and HA
level, 7S collagen level, PIIINP level, TIMP-1 level, Pro-C3 level,
FIB-4 index, APRI and ELF score (P<0.01, P<0.05, P<0.01,
P<0.001, P<0.001, P<0.05, P<0.05 and P<0.05,
respectively) but not between M2BPGi level and PLT count in
patients with CHB without significant liver necroinflammation
(Table SI).
Discussion
Liver fibrosis staging plays an important role in
the selection of patients with CHB for antiviral therapy and liver
biopsy is a reference tool for the decision to start therapy.
However, biopsy has limitations, such as high cost, invasiveness,
bleeding complications and sampling variability. The serum
biomarkers of liver fibrosis are considered to have limited
diagnostic utility (22). There is
no established biomarker for patients with CHB who require
antiviral therapy. To the best of the authors' knowledge, the
present study is the first to report that the serum M2BPGi level is
a useful marker for identifying liver histological findings in
patients with CHB without significant necroinflammation and in need
of antiviral therapy.
M2BPGi is a glycosylated secretory protein
synthesized by activated hepatic stellate cells (Ac-HSCs) and is
emerging as a serum marker for liver fibrosis (26). M2BPGi serves as a juxtacrine
messenger between Ac-HSCs and Kupffer cells during progression of
liver fibrosis (27). Bekki et
al (28) demonstrated that
M2BPGi is exclusively produced in Ac-HSCs and plays an important
role in the progression of liver fibrosis. M2BPGi has been recently
developed as a novel serum biomarker that is strongly correlated
with liver fibrosis in patients with CHC (29). Several studies have identified that
M2BPGi can serve as a serum fibrosis marker in patients with CHB
(30-33),
although M2BPGi levels vary for the same fibrosis stage between
patients with CHB and CHC (31).
This may be partly explained by the fact that the generative nodule
size and fibrous septum thickness (composed of collagen fibrils)
substantially differ between patients with CHB and CHC (34). These findings support the potential
role of M2BPGi as a surrogate biomarker that reflects hepatic
stellate cell function (28).
M2BPGi levels have been found to rapidly decrease
with reduced hepatic inflammation during direct-acting antiviral
therapy for HCV infection in patients with CHC (27,35). In
agreement with the current findings, serum M2BPGi levels were
significantly higher in patients with CHB with significant liver
necroinflammation than in those without significant liver
necroinflammation (36). All serum
fibrosis biomarkers, except the Pro-C3 level, were correlated with
the fibrosis stage in all patients with CHB, whereas the PLT count
and M2BPGi level were exclusively associated with the fibrosis
stage in patients with CHB without significant liver
necroinflammation. In addition, in a previous study, the M2BPGi
level was found to be correlated with the serum C-X-C motif
chemokine 10 level, which is closely related to the migration of
inflammatory cells to the local focus in the liver (30). These results further supported the
hypothesis that fibrosis markers are substantially affected by
liver inflammation. However, Miyaki et al (32) and Liu et al (33) reported that M2BPGi levels reflect
fibrosis progression and are not affected by inflammation or ALT
fluctuations in treatment-naïve patients with CHB. The reasons for
the different results between the studies remain unclear. However,
one possible explanation is the difference in the percentage of
patients with cirrhosis between the studies, which warrants further
investigation.
The present study found that the M2BPGi level had
the highest diagnostic performance for identifying significant
liver fibrosis in patients with CHB without significant
necroinflammation. Recently, the ELF score and M2BPGi level
demonstrated comparable diagnostic performances for identifying
significant liver fibrosis in patients with CHB (32). A recent report by Jekarl et al
(20) demonstrated that the PLT
count, ELF score and M2BPGi level accurately identified significant
liver fibrosis in treatment-naïve patients with CHB. Serum M2BPGi
has been shown to have a good performance for diagnosing severe
fibrosis in patients with CHB treated with nucleoside analogs
(33). Mak et al (37) demonstrated that M2BPGi was
significantly correlated with severe fibrosis and cirrhosis in
patients with CHB treated with nucleoside analogs. The difference
in the ability of serum M2BPGi to identify the liver fibrosis stage
between the studies might be attributed to the patient distribution
according to the fibrosis stage at inclusion and the administration
of nucleoside analogs that might reduce hepatic fibrosis, in
addition to the influence of hepatic inflammation. The current
results confirm that M2BPGi is a novel non-invasive diagnostic
biomarker that can identify treatment-naïve patients with CHB in
need of treatment.
The present study has several limitations. First,
this was a retrospective study. Second, liver biopsy has the
drawback of being prone to sampling errors in fibrosis staging and
inflammation grading, potentially leading to bias. Third, the
sample size (especially the number of patients with cirrhosis) was
small for analysis. It is difficult to obtain a representative
liver biopsy specimen from patients with cirrhosis whose platelet
counts are lower than 5x104/µl. Thus, further research
with a large number of patients is required to validate the use of
serum M2BPGi level in the detection of significant fibrosis in
patients with CHB.
In conclusion, serum M2BPGi level is a useful marker
for identifying liver histological findings in patients with CHB
without significant necroinflammation in need of antiviral therapy,
although M2BPGi level not only identifies the status of liver
fibrosis but also reflects liver necroinflammation (27).
Supplementary Material
Baseline characteristics of patients
with chronic hepatitis B stratified according to different grades
of necroinflammation.
Baseline characteristics of patients
with chronic hepatitis B stratified according to different grades
of necroinflammation.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YT, KKa, HT, KN, SSat, SSai, YS, KKi, NS, HK, KM,
RN, TA and AM performed data analysis. AM supervised all
statistical analyses performed in this study. HY and TN made
substantial contributions to the conception and design of the study
and analysis and interpretation of the data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent for the use of resected
tissue was obtained from all patients and the study protocol was
approved by the Ethics Committee of Nara Medical University
(approval no. 1077).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Okura Y, Namisaki T, Moriya K, Kitade M,
Takeda K, Kaji K, Noguchi R, Nishimura N, Seki K, Kawaratani H, et
al: Combined treatment with dipeptidyl peptidase-4 inhibitor
(sitagliptin) and angiotensin-II type 1 receptor blocker (losartan)
suppresses progression in a non-diabetic rat model of
steatohepatitis. Hepatol Res. 47:1317–1328. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Saleh HA and Abu-Rashed AH: Liver biopsy
remains the gold standard for evaluation of chronic hepatitis and
fibrosis. J Gastrointestin Liver Dis. 16:425–426. 2007.PubMed/NCBI
|
|
3
|
Fujinaga Y, Namisaki T, Moriya K, Kitade
M, Kawaratani H, Shimozato N, Kaji K, Takaya H, Sawada Y, Seki K,
et al: Identification of clinical risk factors for histological
progression of primary biliary cholangitis. Hepatol Res.
49:1015–1025. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Venkatesh SK, Yin M, Takahashi N, Glockner
JF, Talwalkar JA and Ehman RL: Non-invasive detection of liver
fibrosis: MR imaging features vs. MR elastography. Abdom Imaging.
40:766–775. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mattos AZ and Mattos AA: Transient
elastography vs. aspartate aminotransferase to platelet ratio index
in hepatitis C: A meta-analysis. Ann Hepatol. 16:349–357.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yasui Y, Abe T, Kurosaki M, Matsunaga K,
Higuchi M, Tamaki N, Watakabe K, Okada M, Wang W, Shimizu T, et al:
Non-invasive liver fibrosis assessment correlates with collagen and
elastic fiber quantity in patients with hepatitis C virus
infection. Hepatol Res. 49:33–41. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Regev A, Berho M, Jeffers LJ, Milikowski
C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR and Schiff ER:
Sampling error and intraobserver variation in liver biopsy in
patients with chronic HCV infection. Am J Gastroenterol.
97:2614–2618. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Baranova A, Lal P, Birerdinc A and
Younossi ZM: Non-invasive markers for hepatic fibrosis. BMC
Gastroenterol. 11(91)2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Parikh P, Ryan JD and Tsochatzis EA:
Fibrosis assessment in patients with chronic hepatitis B virus
(HBV) infection. Ann Transl Med. 5(40)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Terrault NA, Lok ASF, McMahon BJ, Chang
KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH and Wong JB: Update
on prevention, diagnosis, and treatment of chronic hepatitis B:
AASLD 2018 hepatitis B guidance. Hepatology. 67:1560–1599.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Drafting Committee for Hepatitis
Management Guidelines and the Japan Society of Hepatology. JSH
Guidelines for the management of hepatitis B virus infection.
Hepatol Res. 44 (Suppl 1):S1–S58. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kim BK, Kim DY, Park JY, Ahn SH, Chon CY,
Kim JK, Paik YH, Lee KS, Park YN and Han KH: Validation of FIB-4
and comparison with other simple noninvasive indices for predicting
liver fibrosis and cirrhosis in hepatitis B virus-infected
patients. Liver Int. 30:546–553. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nishikawa H, Takata R, Enomoto H, Yoh K,
Kishino K, Shimono Y, Iwata Y, Hasegawa K, Nakano C, Nishimura T,
et al: Proposal of a predictive model for advanced fibrosis
containing Wisteria floribunda agglutinin-positive Mac-2-binding
protein in chronic hepatitis C. Hepatol Res. 47:E74–E84.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hansen JF, Juul Nielsen M, Nyström K,
Leeming DJ, Lagging M, Norkrans G, Brehm Christensen P and Karsdal
M: PRO-C3: A new and more precise collagen marker for liver
fibrosis in patients with chronic hepatitis C. Scand J
Gastroenterol. 53:83–87. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Luo Y, Oseini A, Gagnon R, Charles ED,
Sidik K, Vincent R, Collen R, Idowu M, Contos MJ, Mirshahi F, et
al: An Evaluation of the collagen fragments related to fibrogenesis
and fibrolysis in nonalcoholic steatohepatitis. Sci Rep.
8(12414)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yoshiji H, Noguchi R and Fukui H: Combined
effect of an ACE inhibitor, perindopril, and interferon on liver
fibrosis markers in patients with chronic hepatitis C. J
Gastroenterol. 40:215–216. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Casals G, Fernández-Varo G, Melgar-Lesmes
P, Marfà S, Reichenbach V, Morales-Ruiz M and Jiménez W: Factors
involved in extracellular matrix turnover in human derived
cardiomyocytes. Cell Physiol Biochem. 32:1125–1136. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Saw S, Zhao H, Tan P, Saw B and Sethi S:
Evaluation of the automated ADVIA centaur® XP syphilis
assay for serological testing. Diagn Microbiol Infect Dis. 88:7–11.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hogemann B, Voss B, Pott G, Rauterberg J
and Gerlach U: 7 S collagen: A method for the measurement of serum
concentrations in man. Clin Chim Acta. 144:1–10. 1984.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jekarl DW, Choi H, Lee S, Kwon JH, Lee SW,
Yu H, Kim M, Kim Y, Sung PS and Yoon SK: Diagnosis of liver
fibrosis with wisteria floribunda agglutinin-positive Mac-2 binding
protein (WFA-M2BP) among chronic hepatitis B patients. Ann Lab Med.
38:348–354. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nielsen MJ, Nedergaard AF, Sun S, Veidal
SS, Larsen L, Zheng Q, Suetta C, Henriksen K, Christiansen C,
Karsdal MA and Leeming DJ: The neo-epitope specific PRO-C3 ELISA
measures true formation of type III collagen associated with liver
and muscle parameters. Am J Transl Res. 5:303–315. 2013.PubMed/NCBI
|
|
22
|
Noguchi R, Kaji K, Namisaki T, Moriya K,
Kitade M, Takeda K, Kawaratani H, Okura Y, Aihara Y, Furukawa M, et
al: Serum angiotensin-converting enzyme level for evaluating
significant fibrosis in chronic hepatitis B. World J Gastroenterol.
23:6705–6714. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Treatment with ursodeoxycholic acid in
clinical hepatology. Proceedings of a workshop. Goteborg, Sweden,
3-4 February 1994. Scand J Gastroenterol Suppl. 204:1–72.
1994.PubMed/NCBI
|
|
24
|
Intraobserver and interobserver variations
in liver biopsy interpretation in patients with chronic hepatitis
C. The French METAVIR Cooperative Study Group. Hepatology 20:
15-20, 1994.
|
|
25
|
Rossi E, Adams LA, Bulsara M and Jeffrey
GP: Assessing liver fibrosis with serum marker models. Clin Biochem
Rev. 28:3–10. 2007.PubMed/NCBI
|
|
26
|
Yamada N and Mizuta K: Advanced assessment
of serum Mac-2 binding protein glycosylation isomer in patients
with biliary atresia. J Gastroenterol. 54:204–205. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shirabe K, Bekki Y, Gantumur D, Araki K,
Ishii N, Kuno A, Narimatsu H and Mizokami M: Mac-2 binding protein
glycan isomer (M2BPGi) is a new serum biomarker for assessing liver
fibrosis: More than a biomarker of liver fibrosis. J Gastroenterol.
53:819–826. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bekki Y, Yoshizumi T, Shimoda S, Itoh S,
Harimoto N, Ikegami T, Kuno A, Narimatsu H, Shirabe K and Maehara
Y: Hepatic stellate cells secreting WFA+ -M2BP: Its role
in biological interactions with Kupffer cells. J Gastroenterol
Hepatol. 32:1387–1393. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Matsuura K, Aizawa N, Enomoto H,
Nishiguchi S, Toyoda H, Kumada T, Iio E, Ito K, Ogawa S, Isogawa M,
et al: Circulating let-7 levels in serum correlate with the
severity of hepatic fibrosis in chronic hepatitis C. Open Forum
Infect Dis. 5(ofy268)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ishii A, Nishikawa H, Enomoto H, Iwata Y,
Kishino K, Shimono Y, Hasegawa K, Nakano C, Takata R, Nishimura T,
et al: Clinical implications of serum Wisteria floribunda
agglutinin-positive Mac-2-binding protein in treatment-naive
chronic hepatitis B. Hepatol Res. 47:204–215. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nishikawa H, Enomoto H, Iwata Y, Kishino
K, Shimono Y, Hasegawa K, Nakano C, Takata R, Nishimura T, Yoh K,
et al: Serum Wisteria floribunda agglutinin-positive Mac-2-binding
protein for patients with chronic hepatitis B and C: A comparative
study. J Viral Hepat. 23:977–984. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Miyaki E, Imamura M, Hiraga N, Murakami E,
Kawaoka T, Tsuge M, et al: Daclatasvir and asunaprevir treatment
improves liver function parameters and reduces liver fibrosis
markers in chronic hepatitis C patients. Hepatol Res.
2016;46(8):758-64.
|
|
33
|
Liu J, Hu HH, Lee MH, Korenaga M, Jen CL,
Batrla-Utermann R, et al: Serum Levels of M2BPGi as Short-Term
Predictors of Hepatocellular Carcinoma in Untreated Chronic
Hepatitis B Patients. Sci Rep. 2017;7(1):14352.
|
|
34
|
Nishimura T, Iijima H, Nishikawa H, Kondo
R, Yano H, Kage M, Aoki T, Nakano C, Yuri Y, Ishii N, et al: Liver
fibrosis markers as assessed by ultrasound elastography and serum
samples: A large comparative study in hepatitis virus B and C liver
diseases. Hepatol Res. 49:721–730. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yasui Y, Kurosaki M, Komiyama Y, Takada H,
Tamaki N, Watakabe K, Okada M, Wang W, Shimizu T, Kubota Y, et al:
Wisteria floribunda agglutinin-positive Mac-2 binding protein
predicts early occurrence of hepatocellular carcinoma after
sustained virologic response by direct-acting antivirals for
hepatitis C virus. Hepatol Res. 48:1131–1139. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nakamura M, Kanda T, Jiang X, Haga Y,
Takahashi K, Wu S, Yasui S, Nakamoto S and Yokosuka O: Serum
microRNA-122 and Wisteria floribunda agglutinin-positive Mac-2
binding protein are useful tools for liquid biopsy of the patients
with hepatitis B virus and advanced liver fibrosis. PLoS One.
12(e0177302)2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Mak LY, Wong DK, Cheung KS, Seto WK, Lai
CL and Yuen MF: Role of serum M2BPGi levels on diagnosing
significant liver fibrosis and cirrhosis in treated patients with
chronic hepatitis B virus infection. Clin Transl Gastroenterol.
9(163)2018.PubMed/NCBI View Article : Google Scholar
|