Introduction
Fatigue is a complex state of psychophysiology.
Although the study of fatigue has been carried out since the early
20th century, there is still no accurate definition and
there are differences in the classification of fatigue. Fatigue is
generally divided into physical and mental fatigue.
Fatigue may lead to loss of attention and mood
disorders, and reduce work efficiency (1). Some of the higher-risk, stressful
occupations, such as pilots, drivers, police and medical staff, are
prone to these symptoms. Mental and physical fatigue is one of the
major public health problems worldwide. The annual global injury
caused by fatigue accounts for 21.7% of occupational injuries
(2) and the deaths caused by fatigue
driving account for 57% of traffic deaths; especially the accidents
of coach on highway caused by fatigue are >7 times the ordinary
accidents (3). In China, ~600,000
individuals die each year due to fatigue accidents (4).
In China, the clinical doctors in the Emergency
Department not only work continuously for a long time, but also
work with great intensity; they often do not get enough rest to
relieve fatigue and are prone to occupational fatigue. Occupational
fatigue not only damages their health, but also threatens the
medical level and quality of care for patients. Since the World
Health Organization listed fatigue as one of the main factors that
threaten human health in the 21st century, fatigue has
gradually attracted widespread attention in the research field
(5-9).
Therefore, the identification of markers of fatigue and the timely
monitoring of vulnerable individuals can greatly prevent injuries
to themselves and prevent harm to other individuals due to
occupational fatigue.
The methods of assessing fatigue can be divided into
subjective and objective assessments. Due to the non-specificity
and subjectivity of fatigue, the earliest research on fatigue is
almost subjective (mostly fatigue scale); however, subjective
assessment cannot avoid the inherent low inefficiency and
reliability. For a deeper understanding and more accurate
measurement of fatigue, intervention of physiological and
biochemical indicators is essential.
Physiological indicators of fatigue are mainly
electrophysiological indicators, such as electroencephalogram
(EEG), electrooculogram, electrocardiogram and event-related
potentials, with EEG being the most studied one (10). It is generally believed that the α
wave is the basic rhythm of normal human brain wave. When the
cerebral cortex is excited, β waves appear. Appearance of θ waves
is generally a manifestation of nervous system inhibition. As early
as 1993, Brookhuis and de Waard (11) used the brain energy parameter (θ+α)/β
to assess driver status and found that the parameter showed an
upward trend with the increase of working time and fatigue state.
Hypothalamic-pituitary-adrenal (HPA) is an important part of the
neuroendocrine system, which mainly maintains homeostasis and
response to stress. Activation of HPA is an adaptive protective
response to external stress. When the body is under stress,
hypothalamus promotes corticotrophin to release hormone secretion,
activates adrenal cortex function and elevates the hormone level of
cortisol in serum. Therefore, some scholars proposed that EEG is
the most reliable indicator of clinical psychological fatigue
(12). However, in practical terms,
the measurement of EEG requires expensive equipment and the
procedure is quite complicated. Therefore, the research on EEG is
almost entirely limited to laboratory research. At the same time,
the collection of biochemical indicators involves invasive
techniques. Therefore, as a tool to quickly evaluate fatigue, the
biochemical indicators are not suitable in a number of occasions.
With the advancement of society and the continuous improvement of
cultural living standards, there is an increasing demand for
medical examinations and diagnostic methods that are non-invasive,
simple and rapid. Therefore, in recent years, saliva specimens have
received widespread attention from researchers. Compared with serum
samples, saliva collection is safe and convenient, non-invasive,
and free of the risk of transmission of blood-borne diseases. For
patients, saliva collection is painless and easy to accept. Saliva
has the advantage of being able to be sampled in real time compared
with urine specimens. Saliva detection research has attracted great
interest and achieved some initial results. Our preliminary
experiments (13) showed that
salivary components under finite fatigue conditions can obtain good
peptide spectrum recognition in the range of 2,000-15,000 Da during
time-of-flight mass spectrometry detection, and it was shown that
the detection of multiple peptides and fatigue presented certain
correlations. Through a series of statistical analyses, the
following fatigue-related proteins were identified: Ig κ chain V-I
region Lay, growth factor receptor-bound protein 2, ADP-ribosyl
cyclase/cyclic ADP-ribose hydrolase 2, lipocalin-1, mitochondrial
malate dehydrogenase, heat shock cognate 71 kDa protein, Ig κ chain
V-III region SIE, Golgi membrane protein 1, cystatin-A, mucin-19,
heat shock protein β-1, Ig γ-3 chain C region, Serpin B13, Rab GDP
dissociation inhibitor β, Annexin A1, afamin, protein
disulfide-isomerase A3, Ig κ chain V-III region HAH, transmembrane
protease serine 11D, nucleoside diphosphate kinase B, bactericidal
permeability-increasing protein, ubiquitin-like modifier-activating
enzyme 1, macrophage-capping protein, myeloid-derived growth
factor, pancreatic α-amylase, L-lactate dehydrogenase B chain,
mitochondrial peroxiredoxin-5, prominin-1, type II cytoskeletal
keratin 4, and salivary acidic proline-rich phosphoprotein
1/2(13).
Occupational fatigue is not a simple physical or
mental fatigue. The mechanism of occupational fatigue is very
complicated. It is all-embracing and there is no unified fatigue
theory. At the same time, due to the multiple factors of fatigue
and their mutual influence, the fatigue factor theory of single
factor is also unscientific. Numerous hypotheses about the
mechanism of fatigue have been proposed, such as the HPA axis
imbalance (14). Exercise fatigue
stress activates the HPA axis and the sympathetic nervous system,
leading to the activation of immune cells (mainly microglia and
astrocytes) in the brain and the secretion of a large number of
inflammatory factors [such as interleukin-1β (IL-1β) and tumor
necrosis factor α], resulting in neuroinflammation. IL-1β is a
molecular of IL-1 composed of 153 amino acids, and almost all
nucleated cells secrete IL-1β. IL-1β is one of the most potent
inflammatory mediators in the body and has a number of biological
functions. Under normal circumstances, IL-1β is at low levels in
the central nervous system and plays an immunomodulatory role, such
as promoting T-cell differentiation and B-cell proliferation. When
the body is under stress, the hypothalamus promotes the secretion
of corticotropin-releasing hormones, activates the adrenal function
and raises the serum cortisol levels. Cortisol is a neuroendocrine
hormone involved in the metabolism of sugar, fat and protein. It
has anti-inflammatory, anti-allergic, immunosuppressive effects,
activates the nervous system vitality, and regulates cardiovascular
system function. Cortisol affects sleep patterns, mood, cognition
and sensation through the blood-brain barrier. In the fatigue
state, small molecules, such as salivary gangliosides and cortisol,
can be changed regularly; however, it is difficult to establish a
convenient detection system.
Chromogranin A (CgA) is an acidic hydrophilic
secreted protein in the chromaffin-like particles of neuroendocrine
cells. CgA was originally discovered in the secretory granules of
chromaffin cells of the adrenal gland. In the adrenal medullary
chromaffin particles, which are co-secreted with catecholamines and
calcium, CgA can also reflect the function of the adrenal cortex to
some extent. Therefore, in the present study, it was speculated
that changes in the levels of IL-1β, cortisol and CgA in saliva are
associated with fatigue.
Doctors in the Emergency Department were selected as
research subjects as they have a long working time and labor
intensity, and are more prone to fatigue. Saliva detection is a
detection method that has emerged in recent years. It is
characterized by non-invasiveness, convenience, and less cost, and
has attracted the attention of scholars in disease diagnosis and
prognosis, drug detection, and physical and psychological stress
evaluation. In the present study, IL-1β, cortisol and CgA in saliva
were used as indicators to detect whether there were any changes in
the three indexes after work and recovery. The analysis of fatigue
markers in saliva provides reference for the establishment and
verification of the fatigue-saliva marker detection model and the
rapid detection of fatigue with saliva.
Subjects and methods
General information
From December 2015 to March 2016, a total of 56
emergency physicians were selected, according to specific inclusion
and exclusion criteria, from the Affiliated Hospital of Hebei
University of Engineering (Handan, China), including 28 males and
28 females, 30-49 years of age. The CRP and cholesterol levels of
subjects were within the normal range. In total, 47 pairs of
samples were successfully collected, including samples from 24
males and 23 females. Among the remaining 9 subjects, samples were
not successfully collected from 4 cases, due to insufficient saliva
secretion, and 5 were lost after work due to departmental rotation.
Of the 47 subjects, there were 4 subjects (3 males and 1 female)
without fatigue waves in the EEG after working for 18 h. Saliva was
collected before work (after full rest) and after work (≥24 h). The
study was approved by the Ethics Committee of the Affiliated
Hospital of Hebei University of Engineering and registered
(registration no. ChiC-TR-DCD-14005746). All subjects who
participated in this research signed an informed consent and had
complete clinical data.
Inclusion and exclusion criteria of
research subjects
Inclusion criteria: Healthy subjects who volunteered
to participate in the study and signed an informed consent; with no
oral diseases, no organic diseases or chronic fatigue symptoms; who
received no medications or dietary supplements in the past 3
months. Exclusion criteria: i) Subjects with sustained or recurrent
fatigue that lasted >6 months, with sore throat, swollen neck or
axillary lymph nodes, muscle pain, or multiple non-arthritic pain;
ii) with headache; iii) with sleep disorder; iv) with discomfort
that persisted >24 h after exertion; v) with hypoglycemia, heart
disease, or vi) subjects recently under diet to lose weight.
Collection and preservation of
saliva
Before sampling, the participants were asked to use
distilled water to wash their mouth in order to remove any food
residues (15). All subjects were
asked to sit vertically in front of the mirror for 5 min after
squatting. Eyes and mouth were kept open, the tongue kept touching
with palate, and saliva was let to flow down the lower lip. Fresh
saliva was stored in a 2-ml saliva collection tube. If the amount
of saliva was insufficient, saliva collection tube was used to
gently stimulate the lower lip to increase the secretion of saliva.
Doctors' saliva samples were collected in their spare time after
shift, and were marked every morning. Samples before work (before
night shift) were marked as ‘A’, samples after work (after night
shift) were marked as ‘B’, and samples after recovery (before next
day work) were marked as ‘C’, and they were marked in the form of
‘personnel number-fatigue status number’. All samples were sent to
the Laboratory of the Medical School of Hebei University of
Engineering and stored in a -70˚C refrigerator. The Emergency
Department was close to the Laboratory and the samples were
collected in winter with a temperature below 0˚C, thus no
insulation was used outside.
Indicator detection EEG data
collection
After a long period of work (≥18 h) and after
collecting saliva, doctors were subjected to EEG to detect the
presence of fatigue waves (16)
(slow δ- and θ-wave increase, and fast α- and β-wave decrease).
SOLAR-RTA and SOLAR-BFM brain function monitoring system (SOLAR
Electronic Technology Co., Ltd.) was used in video EEG monitoring
and EEG acquisition system. Real-time analysis of EEG function,
comprehensive analysis of brain function, and analysis of leading
volatility trend of brain function vital signs were carried out
(ISO9001 and ISO13485 international quality management system
certification). EEG was recorded for at least 30 min. Silver disc
electrodes were placed in conventional manner. Scalp record point
was 16. The silver plate electrode was used as the recording
electrode. The sampling rate was filtered, each record was not
<30 min, and each case was recorded at least once.
Biochemical indicator detection
Quantikine® HS Enzyme-linked
immunosorbent assay (ELISA) Human IL-1β/IL-1F2 Immunoassay (cat.
no. HSLB00D) and Parameter™ Cortisol Assay (cat. no.
KGE008B) (both from R&D Systems, Inc.) were used. Human CgA
ELISA kit was purchased from Shanghai Jianglai Biotechnology Co.,
Ltd. The detection of pH values was performed using RAPIDPoint 405
Blood Gas Analyzer produced by Siemens AG.
Quality control
Members of the research team were trained before the
clinical trial. Volunteers were informed in detail about the
clinical trial and signed an informed consent form. Volunteers were
numbered.
Detection methods
Saliva samples were taken from the -70˚C
refrigerator, thawed at room temperature, and centrifuged at 10,000
x g at 4˚C for 5 min. The levels of CgA, IL-1β, cortisol and pH in
pre-fatigue and post-fatigue samples were determined according to
the manufacturer's instructions.
Statistical analysis
Continuous variables were tested for normal
distribution. For the non-normally distributed data, Wilcoxon test
was used to detect whether the index difference between before and
after working for 18 h was significant in all subjects and the
subjects in the fatigue wave group. Spearman's correlation analysis
was performed on the differences in the levels of IL-1β, cortisol
and CgA before and after work to explore the correlation with the
appearance of fatigue waves in the EEG. ROC curve analysis was used
to analyze the predictive value of these three indicators for
fatigue. All analyses were performed using SPSS 17.0 software (IBM
Corp.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Levels of IL-1β, cortisol and CgA in
fatigue wave group and all research subjects
There was no significant difference in the content
of IL-1β or cortisol in the subjects of the fatigue wave group,
between before and after working for 18 h (P>0.05). However,
there was a statistically significant difference in the content of
CgA in the subjects of the fatigue wave group between before and
after working for 18 h (P<0.05; Table
I).
 | Table IComparison of three indicators before
and after 18 h of work in the fatigue wave group (n=43) [M
(QR)]. |
Table I
Comparison of three indicators before
and after 18 h of work in the fatigue wave group (n=43) [M
(QR)].
| Time | IL-1β | Cortisol | CgA |
|---|
| Before work | 25.816 (14.108,
75.763) | 5.815 (2.465,
13.785) | 274.596 (238.373,
289.7016) |
| After work | 30.942 (5.966,
66.528) | 7.64 (2.28,
17.49) | 254.523 (220.592,
286.965) |
| Z value | 0.181 | 0.193 | 2.174 |
| P-value | 0.856 | 0.847 | 0.030 |
There was no significant difference in the content
of IL-1β or cortisol in all subjects between before and after
working for 18 h (P>0.05). However, there was a significant
difference in the content of CgA in all subjects between before and
after working for 18 h (P<0.05; Table II).
 | Table IIComparison of three indicators before
and after 18 h of work in all research subjects (n=47) [M
(QR)]. |
Table II
Comparison of three indicators before
and after 18 h of work in all research subjects (n=47) [M
(QR)].
| Time | IL-1β | Cortisol | CgA |
|---|
| Before work | 24.9229 (14.1083,
72.5811) | 5.8150 (2.4650,
13.7850) | 269.17 (238.37,
289.70) |
| After work | 30.5769 (5.9282,
59.7377) | 7.6400 (2.2800,
17.6650) | 254.86 (220.59,
286.96) |
| Z value | 0.455 | 0.677 | 2.116 |
| P-value | 0.649 | 0.498 | 0.034 |
Correlation of IL-1β, cortisol and CgA
with fatigue waves in EEG
Spearman's correlation analysis was performed to
examine the correlation between the IL-1β difference of all
subjects before and after work and whether there were fatigue waves
in EEG. The results showed that the correlation coefficient was
r=-0.158, P=0.288. In addition, Spearman's correlation analysis on
the cortisol difference of all subjects before and after work and
whether there were fatigue waves in EEG showed that the correlation
coefficient was r=0.139, P=0.351; and for the CgA difference of all
subjects before and after work and whether there was fatigue waves
in EEG, the results showed that the correlation coefficient was
r=-0.135, P=0.364.
Diagnostic value of the difference of
IL-1β, cortisol and CgA content before and after work
The existence of fatigue waves in EEG was used as
the gold standard to analyze the diagnostic value of the difference
of IL-1β content before and after work. The results for IL-1β
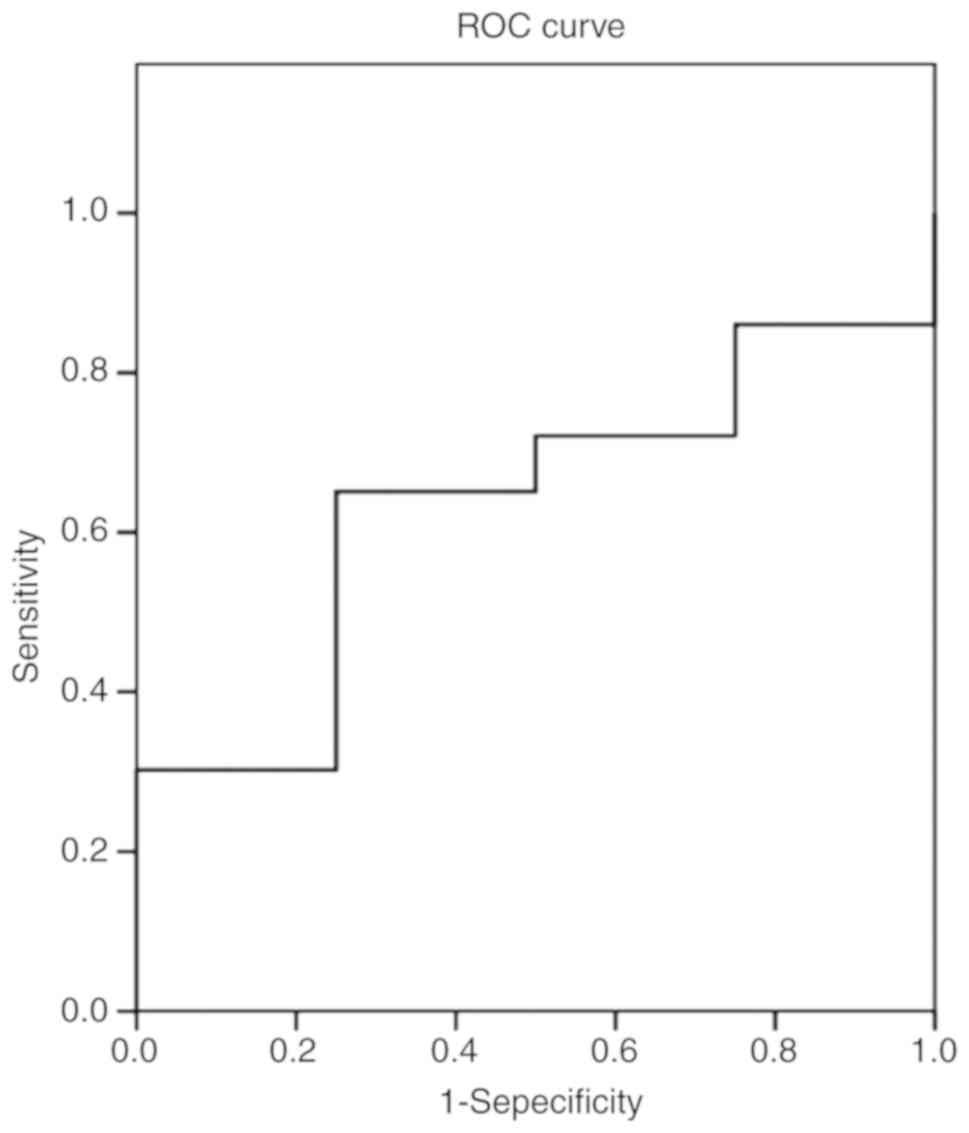
showed that the area under the ROC curve was 0.634 (Fig. 1), for cortisol the area under the ROC
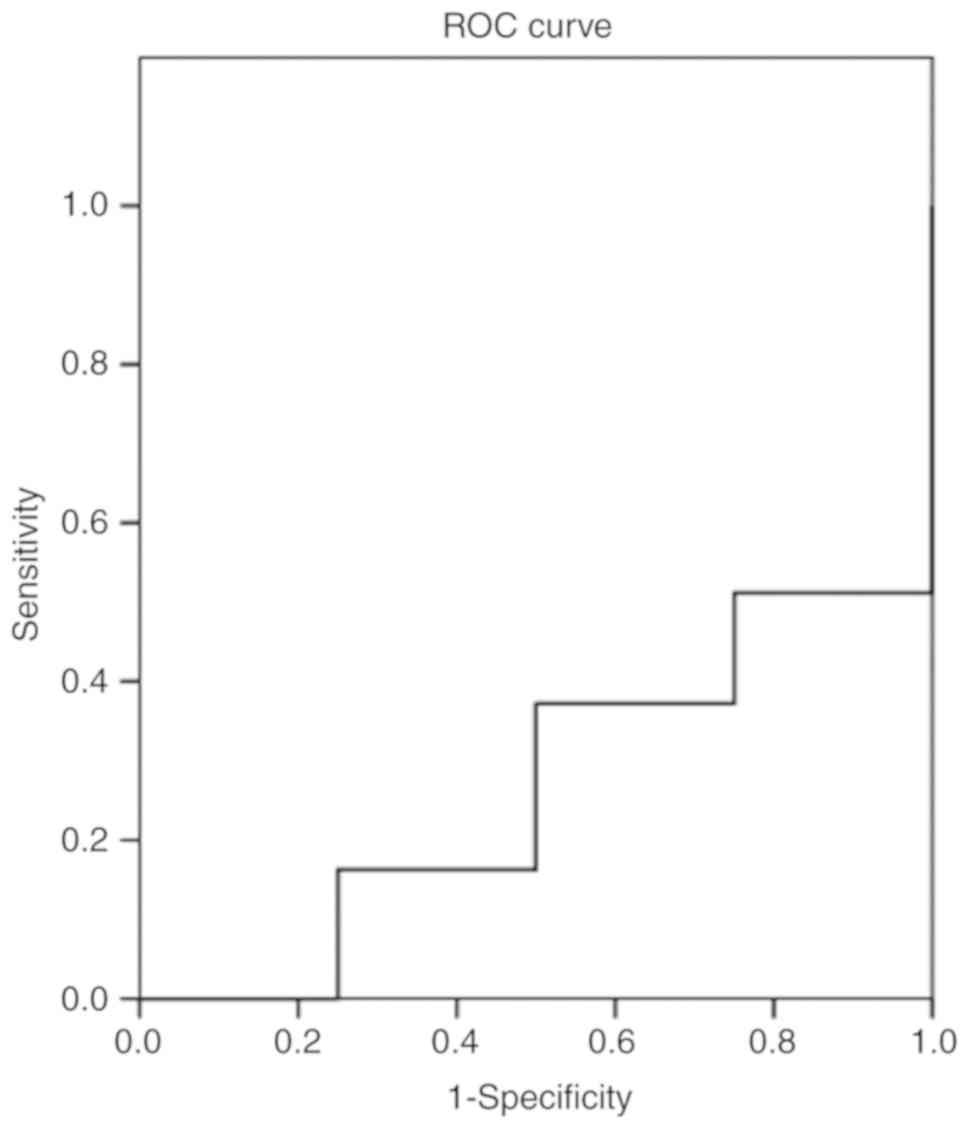
curve was 0.262 (Fig. 2), and for
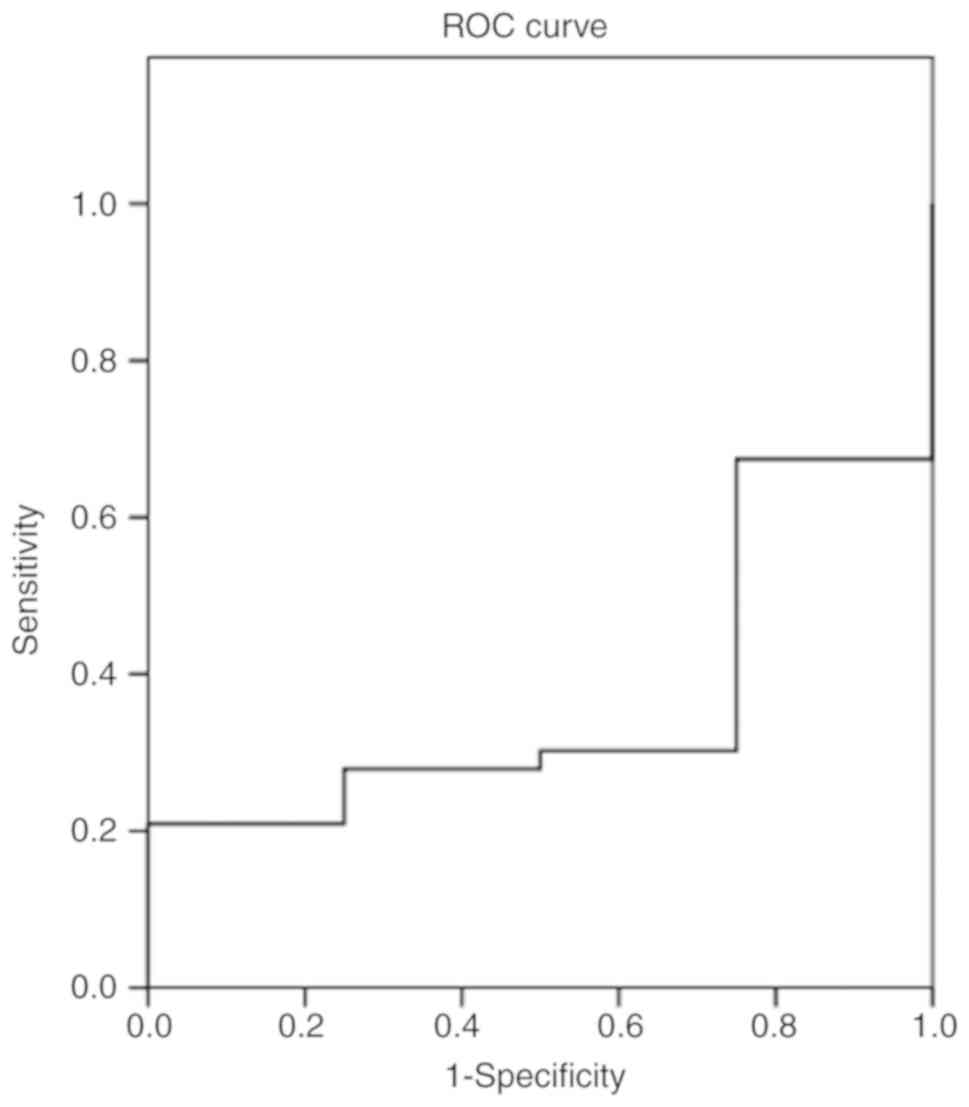
CgA the area under the ROC curve was 0.366 (Fig. 3).
Discussion
Recent neuropathological studies have shown that
glial cell-mediated neuroinflammation leads to a decline in
learning and memory (17-19).
IL-1β is a molecular form of IL-1 composed of 153 amino acids, and
almost all nucleated cells secrete IL-1β. IL-1β is one of the most
potent inflammatory mediators in the body and has a wide range of
biological functions. Under normal circumstances, IL-1β is at low
levels in the central nervous system and plays an immunomodulatory
role, such as promoting T-cell differentiation and B-cell
proliferation. When the body receives a stress response, IL-1β
levels rise and produce toxic effects. In 1993, a study on patients
with prostate cancer (20) showed
that the severe fatigue was always accompanied by high plasma IL-1β
levels during radiation therapy. Another study revealed that
excessive immune activation has a detrimental effect on learning
and memory, and IL-1β is a key mediator in this process (21). Baum et al (22) have also shown that after 12 weeks of
training, the synthesis of IL-1β and IL-6 in the plasma of
cross-country runners increased. These studies have shown that
exercise fatigue leads to an increase in inflammatory factors in
peripheral tissues. In addition, it has been reported that exercise
fatigue can directly lead to elevated levels of inflammatory
factors in the brain (23). Further
research has revealed that exercise-induced inflammatory responses
are first mediated by the HPA axis, and inflammatory factors can
also stimulate the HPA axis, causing elevated levels of
glucocorticoids, such as adrenal ketones, to affect inflammation
and immune responses (24,25). In the present study, there was no
significant difference in the IL-1β content of subjects in the
fatigue wave group before and after working for 18 h (P>0.05),
which is different from previous studies (20,22).
This may be because the present research focused on emergency
doctors and comprehensive fatigue, but not simple exercise fatigue
or cancer-induced fatigue. Moreover, previous studies have mainly
detected the level of IL-1β in plasma, whereas in the present study
the changes in the content of IL-1β in saliva were detected. There
was no significant difference in the content of IL-1β in all 47
subjects before and after working for 18 h (P>0.05). As there
were only 4 subjects in the study without fatigue waves in EEG, and
the number of these subjects was not sufficient to constitute a
control group, the comparison of the contents of IL-1β, cortisol
and CgA of all subjects before and after working for 18 h was just
a confirmation of the statistical results of 43 cases with fatigue
waves in the EEG.
Spearman's correlation analysis was performed on the
IL-1β difference of all subjects before and after work and whether
there was fatigue waves in EEG. The results showed that the
correlation coefficient was r=-0.158, P=0.288. The existence of
fatigue waves in EEG was used as the gold standard to analyze the
diagnostic value of the difference of IL-1β content before and
after work. The results showed that the area under the ROC curve
was 0.634. This may be due to the fact that in the presented study
whole saliva was collected, which was susceptible to the external
environment. In addition, the saliva samples in this study were
concentrated by high-speed centrifugation to effectively detect the
components, which may have affected the sensitivity and specificity
of the detection. Therefore, the saliva preparation method should
be improved in order to reduce the loss of fatigue markers in
saliva, to reflect objectively the content of fatigue markers in
saliva, and effectively detect the low content of fatigue markers,
leading to the identification of fatigue markers. In addition,
saliva samples can be pre-graded according to the molecular weight
of fatigue markers to improve the detection rate of fatigue
markers. These are the keys to our future study.
Cortisol is a neuroendocrine hormone that
participates in the body's metabolism of sugar, fat and protein,
and has anti-inflammatory, anti-allergic and immunosuppressive
effects. Cortisol can activate the nervous system vitality and
regulate the cardiovascular system function. Cortisol affects sleep
patterns, mood, cognition, and sensation through the blood-brain
barrier. Salivary cortisol can reflect the level of biologically
active free cortisol in the blood (26). Compared with blood tests, saliva
detection has the advantages of non-invasion, convenience, and high
re-sampling ability, and no sharp increase in cortisol content
caused by stress. Cortisol circadian rhythm can reflect the rhythm
characteristics of the HPA axis, and has become a common indicator
to study circadian rhythm (27,28).
However, when studying the role of HPA axis in Chronic Fatigue
Syndrome (CFS), the conclusions of different studies are not
consistent. Moorkens et al (29) collected samples from 22 cases of CFS
patients and 9 healthy controls, 5 times from 22:00 to 06:00 to
detect cortisol, and a peak in CFS patients was shown to decrease.
Hamilos et al (30) collected
blood samples 7 times in 24 h and the same results were obtained.
MacHale et al (31) collected
blood samples from 30 patients with CFS and 15 healthy subjects
during the day and night, and found no significant difference in
cortisol levels between CFS patients and healthy subjects.
Papanicolaou et al (32)
found no difference in 6 sampling tests within 24 h. Inconsistent
results were also obtained from saliva tests (33-36).
In the present study, there was no significant difference in the
cortisol content of 43 subjects in the fatigue wave group before
and after working for 18 h (P>0.05) and neither was any
significant difference in the cortisol content of all 47 subjects
before and after working for 18 h (P>0.05).
Spearman's correlation analysis was performed on the
cortisol difference of all subjects before and after work and
whether there was fatigue waves in EEG. The results revealed that
the correlation coefficient was r=0.139, P=0.351. Whether there
were fatigue waves in EEG was used as the gold standard to analyze
the diagnostic value of the difference of cortisol content before
and after work. The results showed that the area under the ROC
curve was 0.262. This may be because our research is
self-controlled, and there is no control group for non-fatigue.
Perhaps the emergency doctors need long-term night shift and
circadian rhythm is destructed, and total amount of cortisol in
saliva is different from non-fatigue subjects, which requires a
more in-depth research. Moreover, cortisol content in saliva
collected at regular intervals can only reflect cortisol level in a
short period of time, and cannot reflect the overall situation of
cortisol secretion during long-term occupational fatigue.
Multi-time sampling will complicate the sample collection process,
which will result in difficulty in sample collection, affect the
subject compliance, and cause errors in sampling time. Therefore,
the present study only sampled before and after fatigue. Schmidt
et al (37) examined salivary
cortisol levels in 265 breast cancer patients undergoing adjuvant
therapy, 5 times a day, and showed that physical fatigue was not
associated with morning cortisol level, cortisol response after
waking, or the slope of cortisol. In addition, emotional and
cognitive fatigues were not associated with any cortisol
parameters. The present study, revieled as well that the difference
in cortisol between before and after fatigue was not related to the
presence of fatigue waves in EEG, further confirming that the
cortisol content in saliva is of no value in the diagnosis of
occupational fatigue.
There is no report on the relationship between CgA
and fatigue. There was a statistically significant difference in
the content of CgA in the 43 subjects in the fatigue wave group
before and after working for 18 h (P<0.05). There was a
statistically significant difference in CgA content in all 47
subjects before and after working for 18 h (P<0.05), which
further proved our hypothesis.
Spearman's correlation analysis was also performed
on the CgA difference of all subjects before and after work and
whether there were fatigue waves in EEG. The results showed that
the correlation coefficient was r=-0.135, P=0.364. Whether there
was fatigue waves in EEG was used as the gold standard to analyze
the diagnostic value of the difference of CgA content before and
after work. The results showed that the area under the ROC curve
was 0.366. At the same time, as a protein molecule, chromophorin is
superior to cortisol in immunogenicity. Our study is a preliminary
study for the rapid detection of CgA as a biomarker of fatigue by
colloidal gold and other methods.
In this study, there was no control group and the
comparison of fatigue before and after work of subjects was
investigated. In addition, the relationship between blood and
salivary levels of IL-1β, cortisol and CgA were not analyzed. At
the same time, the number of samples tested was relatively small.
More subjects with different backgrounds should be enrolled to
develop a simple and quick detection method of fatigue.
Acknowledgements
Not applicable.
Funding
The study was supported by the National Natural
Science Foundation of China (project no. 81373095).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MD and CZ wrote the manuscript. MD and YX performed
ELISA. YL and XL collected and analyzed the general data of the
research subjects. CZ and XW were responsible for the detection of
the biochemical indicators. FL, YS and JW assisted with statistical
analysis. NX collected saliva samples. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Affiliated Hospital of Hebei University of Engineering (Handan,
China). All subjects who participated in this research signed an
informed consent and had complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lal SK and Craig A: Driver fatigue:
Electroencephalography and psychological assessment.
Psychophysiology. 39:313–321. 2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Arlinghaus A, Lombardi DA, Willetts JL,
Folkard S and Christiani DC: A structural equation modeling
approach to fatigue-related risk factors for occupational injury.
Am J Epidemiol. 176:597–607. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Philip P, Sagaspe P, Moore N, Taillard J,
Charles A, Guilleminault C and Bioulac B: Fatigue, sleep
restriction and driving performance. Accid Anal Prev. 37:473–478.
2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang G, Yau KK, Zhang X and Li Y: Traffic
accidents involving fatigue driving and their extent of casualties.
Accid Anal Prev. 87:34–42. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Janssen N and Nijhuis FJ: Associations
between positive changes in perceived work characteristics and
changes in fatigue. J Occup Environ Med. 46:866–875.
2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
de Croon EM, Blonk RW, de Zwart BC,
Frings-Dresen MH and Broersen JP: Job stress, fatigue, and job
dissatisfaction in Dutch lorry drivers: Towards an occupation
specific model of job demands and control. Occup Environ Med.
59:356–361. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bultmann U, Kant IJ, Schroer CA and Kasl
SV: The relationship between psychosocial work characteristics and
fatigue and psychological distress. Int Arch Occup Environ Health.
75:259–266. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
van der Ploeg E and Kleber RJ: Acute and
chronic job stressors among ambulance personnel: Predictors of
health symptoms. Occup Environ Med. 60 (Suppl 1):i40–i46.
2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dimsdale JE, Ancoli-Israel S, Elsmore TF
and Gruen W: Taking fatigue seriously: I. Variations in fatigue
sampled repeatedly in healthy controls. J Med Eng Technol.
27:218–222. 2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cockram MS, Murphy E, Ringrose S,
Wemelsfelder F, Miedema HM and Sandercock DA: Behavioural and
physiological measures following treadmill exercise as potential
indicators to evaluate fatigue in sheep. Animal. 6:1491–1502.
2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Brookhuis KA and de Waard D: The use of
psychophysiology to assess driver status. Ergonomics. 36:1099–1110.
1993.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shen K, Li X, PM Pullens W, Zheng H, Ong
CJ and V Wilder-Smith E: Key feature extraction for fatigue
identification using random forests. Conf Proc IEEE Eng Med Biol
Soc. 2:2044–2047. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xu YL, Gong YN, Xiao D, Zhao CX, Gao XH,
Peng XH, Xi AP, He LH, Lu LP, Ding M, et al: Discovery and
identification of fatigue-related biomarkers in human saliva. Eur
Rev Med Pharmacol Sci. 22:8519–8536. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bower JE, Ganz PA, Dickerson SS, Petersen
L, Aziz N and Fahey JL: Diurnal cortisol rhythm and fatigue in
breast cancer survivors. Psychoneuroendocrinology. 30:92–100.
2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nunes LA, Brenzikofer R and Macedo DV:
Reference intervals for saliva analytes collected by a standardized
method in a physically active population. Clin Biochem.
44:1440–1444. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lal SK, Craig A, Boord P, Kirkup L and
Nguyen H: Development of an algorithm for an EEG-based driver
fatigue countermeasure. J Safety Res. 34:321–328. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Srinivasan D, Yen JH, Joseph DJ and
Friedman W: Cell type-specific interleukin-1beta signaling in the
CNS. J Neurosci. 24:6482–6488. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang DS, Zurek AA, Lecker I, Yu J,
Abramian AM, Avramescu S, Davies PA, Moss SJ, Lu WY and Orser BA:
Memory deficits induced by inflammation are regulated by
α5-subunit-containing GABAA receptors. Cell Rep. 2:488–496.
2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Song C, Phillips AG, Leonard BE and
Horrobin DF: Ethyl-eicosapentaenoic acid ingestion prevents
corticosterone-mediated memory impairment induced by central
administration of interleukin-1beta in rats. Mol Psychiatry.
9:630–638. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Greenberg DB, Gray JL, Mannix CM,
Eisenthal S and Carey M: Treatment-related fatigue and serum
interleukin-1 levels in patients during external beam irradiation
for prostate cancer. J Pain Symptom Manage. 8:196–200.
1993.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rachal Pugh C, Fleshner M, Watkins LR,
Maier SF and Rudy JW: The immune system and memory consolidation: A
role for the cytokine IL-1beta. Neurosci Biobehav Rev. 25:29–41.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Baum M, Klöpping-Menke K,
Müller-Steinhardt M, Liesen H and Kirchner H: Increased
concentrations of interleukin 1-beta in whole blood cultures
supernatants after 12 weeks of moderate endurance exercise. Eur J
Appl Physiol Occup Physiol. 79:500–503. 1999.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pervaiz N and Hoffman-Goetz L: Immune cell
inflammatory cytokine responses differ between central and systemic
compartments in response to acute exercise in mice. Exerc Immunol
Rev. 18:142–157. 2012.PubMed/NCBI
|
|
24
|
Barriga C, Martin MI, Ortega E and
Rodriguez AB: Physiological concentrations of melatonin and
corticosterone in stress and their relationship with phagocytic
activity. J Neuroendocrinol. 14:691–695. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Besedovsky HO and Rey AD: Physiology of
psychoneuroimmunology: A personal view. Brain Behav Immun.
21:34–44. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Castro M, Elias PC, Martinelli CE Jr,
Antonini SR, Santiago L and Moreira AC: Salivary cortisol as a tool
for physiological studies and diagnostic strategies. Braz J Med
Biol Res. 33:1171–1175. 2000.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jerjes WK, Peters TJ, Taylor NF, Wood PJ,
Wessely S and Cleare AJ: Diurnal excretion of urinary cortisol,
cortisone, and cortisol metabolites in chronic fatigue syndrome. J
Psychosom Res. 60:145–153. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jerjes WK, Cleare AJ, Wessely S, Wood PJ
and Taylor NF: Diurnal patterns of salivary cortisol and cortisone
output in chronic fatigue syndrome. J Affect Disord. 87:299–304.
2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Moorkens G, Berwaerts J, Wynants H and Abs
R: Characterization of pituitary function with emphasis on GH
secretion in the chronic fatigue syndrome. Clin Endocrinol (Oxf).
53:99–106. 2000.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hamilos DL, Nutter D, Gershtenson J,
Redmond DP, Clementi JD, Schmaling KB, Make BJ and Jones JF: Core
body temperature is normal in chronic fatigue syndrome. Biol
Psychiatry. 43:293–302. 1998.PubMed/NCBI View Article : Google Scholar
|
|
31
|
MacHale SM, Cavanagh JT, Bennie J, Carroll
S, Goodwin GM and Lawrie SM: Diurnal variation of adrenocortical
activity in chronic fatigue syndrome. Neuropsychobiology.
38:213–217. 1998.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Papanicolaou DA, Amsterdam JD, Levine S,
McCann SM, Moore RC, Newbrand CH, Allen G, Nisenbaum R, Pfaff DW,
Tsokos GC, et al: Neuroendocrine aspects of chronic fatigue
syndrome. Neuroimmunomodulation. 11:65–74. 2004.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wood B, Wessely S, Papadopoulos A, Poon L
and Checkley S: Salivary cortisol profiles in chronic fatigue
syndrome. Neuropsychobiology. 37:1–4. 1998.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Strickland P, Morriss R, Wearden A and
Deakin B: A comparison of salivary cortisol in chronic fatigue
syndrome, community depression and healthy controls. J Affect
Disord. 47:191–194. 1998.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gaab J, Huster D, Peisen R, Engert V,
Schad T, Schurmeyer TH and Ehlert U: Low-dose dexamethasone
suppression test in chronic fatigue syndrome and health. Psychosom
Med. 64:311–318. 2002.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cleare AJ: The neuroendocrinology of
chronic fatigue syndrome. Endocr Rev. 24:236–252. 2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Schmidt ME, Semik J, Habermann N,
Wiskemann J, Ulrich CM and Steindorf K: Cancer-related fatigue
shows a stable association with diurnal cortisol dysregulation in
breast cancer patients. Brain Behav Immun. 52:98–105.
2016.PubMed/NCBI View Article : Google Scholar
|