Introduction
Cerebral apoplexy is a serious brain injury disease
caused by obstruction of blood circulation in the brain, and its
incidence and mortality are much higher than those of other brain
injury diseases, which threatens the quality of life and health of
many patients; according to statistics, the annual incidence of
stroke in China is approximately 116-219 per 100,000 people
(1,2). In addition, 85% of acute stroke
belonged to acute ischemic stroke (AIS) (3). AIS has become the leading cause of
permanent disability in adults and the second most common cause of
dementia, and its mortality rate ranks third in the world (4). According to epidemiological statistics
in the United States, 610,000 new cases occur every year, and the
incidence is still increasing year by year. The incidence of stroke
has increased by 12% in low and middle income countries over the
past 30 years, and the people suffering from stroke have become
younger (5-7).
According to the condition of the patient, the corresponding
treatment plan can be provided, but Zhong et al (8) reported on 2,944 patients in 22
hospitals in Suzhou and found that 3.7% of the patients died
directly during in-hospital treatment. In addition, it was reported
that 20% of AIS patients have cardiemphraxis and 76% of AIS
patients have obvious autonomic nervous dysfunction (9,10). Zhong
et al (11) results on
253,680 patients show that after treatment, the patients were
admitted back to hospital because of infection, coronary artery
disease and recurrent stroke, and the readmission rates for 30 days
and 1 year were 17.4 and 42.5% respectively.
AIS causes strong inflammatory reaction, so some
inflammatory indexes are closely related to AIS process (12). The neutrophil-to-lymphocyte ratio
(NLR) is an inflammatory predictor widely used in the diagnosis of
cancer (13). Some studies have also
found that NLR can be used to predict the risk of cardiovascular
and cerebrovascular diseases, and to predict the early clinical
results of AIS (14,15). Interleukin (IL-33) is a member of the
IL-1 family and binds to its receptor ST2 to prevent hypertrophy
and fibrosis in the myocardium (16). The low level of serum IL-33 is
associated with the large infarct volume and greater stroke
severity of the AIS patient, and IL-33 can be used as a biomarker
for diagnosis and for predicting the prognosis (17).
In order to prevent obstruction, patients generally
need to use aspirin that can inhibit platelet aggregation (18). Atorvastatin is a drug with
lipid-regulating effect, and is used for treating cardiovascular
and cerebrovascular diseases such as hypercholesterolemia and
coronary heart disease. Recent studies have reported that
atorvastatin can also play a beneficial role in cerebral
circulation and cerebral parenchyma during ischemic stroke and
reperfusion, which can protect the nerves of patients with AIS. The
levels of tumor necrosis factor-α, interleukin (IL)-6 and vascular
cell adhesion molecule-1 in patient's plasma were significantly
decreased by taking atorvastatin (19). The study of Pignatelli et al
(20) shows that atorvastatin can
rapidly reduce oxidative stress and platelet activation by directly
inhibiting platelet NOx2, and finally inhibiting platelet
isoprostol and thrombus A2. Aspirin has been used to treat AIS
patients with anti-blocking therapy, but the specific efficacy of
aspirin combined with atorvastatin and its effect on NLR and IL-33
were not clear.
Therefore, we used atorvastatin combined with
aspirin in the treatment of AIS patients and observe its clinical
efficacy and the effect on NLR and IL-33, so as to provide evidence
and direction for clinical treatment.
Patients and methods
General patient data
This is a retrospective study. Altogether 108
patients with AIS treated in Luoyang Central Hospital Affiliated to
Zhengzhou University (Luoyang, China) from April 2016 to October
2017 were selected as the subject. The patients were divided into
groups according to their medical records as archived by the
Luoyang Central Hospital. The 56 patients in the observation group
were treated with atorvastatin combined with aspirin, and included
37 males and 19 females, with an average age of 51.63±9.41 years.
Further 52 patients were treated with aspirin alone as the control
group in this study, including 40 males and 12 females, with an
average age of 52.46±10.54 years. This study was approved by the
Medical Ethics Committee of Luoyang Central Hospital Affiliated to
Zhengzhou University, and all the patients were informed and signed
the informed consent form.
Inclusion and exclusion criteria
Inclusion criteria: the patient was diagnosed with
AIS by imaging and pathology; the diagnostic criteria were in line
with the guidelines issued by the Stroke Committee of the American
Heart Association in 2013(21); all
the patients were admitted to hospital within 6 h of onset;
patients with complete clinical data; patients could be followed up
by telephone.
Exclusion criteria: patients with severe liver and
renal insufficiency; patients with other malignant tumors; patients
with serious cardiovascular and cerebrovascular diseases; patients
with severe inflammation; pregnant or lactating women.
Instruments and kits
Blood analyzer (SYSMEX, XS-800i) and its matching
reagent were used for detection of blood routine indexes. IL-33
enzyme-linked immunosorbent assay (ELISA) detection kit (Shanghai
Enzyme-linked Biotechnology Co., Ltd., ml058087), aspirin (Shandong
Xinhua Pharmaceutical Co., Ltd., SFDA approval no. H37020354),
atorvastatin (Pfizer, SFDA approval no. H20051407).
Therapeutic regimen
Both groups were treated with routine treatment such
as anti-infection and prevention of stress ulcer after admission.
The control group was given 100 mg of oral aspirin once a day on
the basis of the routine treatment. The observation group was
treated with 10 mg of oral atorvastatin once a day on the basis of
the treatment of the control group.
Sample collection
Aseptic venous blood (8 ml) was collected at 7:00
a.m. the next day after admission; 3 ml was added to the
anticoagulant tube, and 5 ml was added to the coagulation tube. The
NLR expression in venous blood of anticoagulant tube was detected
by blood routine examination. The venous blood in the coagulation
tube was centrifuged immediately at 3,000 x g at 4˚C for 10 min.
The serum was then separated and placed in a refrigerator at
-80˚C.
ELISA detection method
IL-33 was detected by ELISA. The sample was diluted
with sample diluent at 1:1, and then 50 µl of the diluted sample
was added to the reaction well. Then, 50 µl of diluted standard
material or 50 µl of sample to be tested was added into reaction
well or blank well. Immediately 50 µl of biotin labeled antibody
was added. The well was covered with the membrane plate and the
sample was gently shaken and mixed and incubated at 37˚C for 1 h.
Then the liquid in the well was discarded and each reaction well
was filled with washing fluid. Then shaken for 30 sec, discarding
the washing fluid, and dried with absorbent paper. This operation
was repeated 3 times. Then 80 µl of affinity enzyme-HRP was added
to each well. The sample was gently shaken and mixed and incubated
at 37˚C for 30 min. The liquid in the well was discarded and each
reaction well was filled with washing fluid. Then shaken for 30
sec, discarding the washing fluid, and dried with absorbent paper.
This stage was also repeated 3 times. Then 50 µl of substrate A and
50 µl of B were added to each well. The sample was gently shaken
and mixed and incubated at 37˚C for 10 min avoid light. The enzyme
standard plate was taken out and 50 µl of terminating solution was
added quickly. The results were determined immediately after the
termination solution was added. The optical density value (OD
value) of each well was detected at the wavelength of 450 nm.
Follow-up
A total of 108 patients or family members were
followed up by telephone and interview bimonthly. The follow-up
time was 1 year.
Observation index
Main observation index: the NLR and IL-33 levels in
observation group and control group before and after treatment were
compared; the scores of National Institutes of Health Stroke scale
(NIHSS) before and after treatment were compared; the Modified
Rankin Scale (MRS) was used for evaluation of curative effect
(score equal to or less than 2 points is effective in the
treatment), total effective rate of treatment, and survival rate of
patients 1 year after treatment.
Secondary observation index: clinical data of the
two groups of patients, total length of hospitalization, the
occurrence of complications.
Statistical methods
SPSS20.0 (SPSS) medical statistical analysis
software was used to statistically analyze the collected data, and
GraphPad Prism 7 (GraphPad) to plot figures. Enumeration data
utilization rate (%) was detected by Chi-square test and
represented by χ2. Fisher's test was used when the
number of samples was ≥40, and the theoretical frequency was <1.
The measurement data were represented by mean ± standard deviation
(mean ±SD), and all measurement data were in accordance with normal
distribution. Independent sample t-test was used for comparison
between the two groups. Intra-group comparison used pairing sample
t-test and was represented by t. Grade data were analyzed using
rank sum test, and Pearson's analysis was used to analyze the
correlation between NLR, IL-33 and NIHSS score. K-M survival was
used for analysis of 1 year survival of patients. The log rank test
was used for analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
General data of the two groups
The clinical data of the two groups were collected
and compared, the results showed that there was no significant
difference in sex, age, BMI, previous medical history
(hypertension, diabetes, hyperlipidemia), smoking history, alcohol
abuse history, residence, platelet count, blood glucose, urea
nitrogen, creatine, cardiac troponin T (cTnT), cardiac troponin I
(cTnI), total cholesterol, glycerin trilaurate, infarct location
and infarct size between the observation group and the control
group (P>0.05) (Table I).
 | Table IClinical data of patients [n (%), mean
± SD]. |
Table I
Clinical data of patients [n (%), mean
± SD].
| Factors | Observation group
(n=56) | Control group
(n=52) | t/χ2/Z
value | P-value |
|---|
| Sex | | | 1.552 | 0.213 |
|
Male | 37 (66.07) | 40 (76.92) | | |
|
Female | 19 (33.93) | 12 (23.08) | | |
| Age (years) | 51.63±9.41 | 52.46±10.54 | 0.432 | 0.666 |
| BMI
(kg/m2) | 23.65±1.82 | 24.04±1.97 | 1.069 | 0.287 |
| Previous medical
history |
|
Hypertension | 19 (33.93) | 21 (40.38) | 0.482 | 0.488 |
|
Diabetes | 13 (23.21) | 10 (19.23) | 0.255 | 0.613 |
|
Hyperlipidemia | 8 (14.29) | 7 (13.46) | 0.015 | 0.902 |
| Smoking history | | | 0.097 | 0.755 |
|
Yes | 21 (37.50) | 18 (34.62) | | |
|
No | 35 (62.50) | 34 (65.38) | | |
| Alcohol abuse
history | | | 0.186 | 0.667 |
|
Yes | 9 (16.07) | 10 (19.23) | | |
|
No | 47 (83.93) | 42 (80.77) | | |
| Residence | | | 1.055 | 0.304 |
|
Urban | 43 (76.79) | 44 (84.62) | | |
|
Rural | 13 (23.21) | 8 (15.38) | | |
| Platelet count
(x109/l) | 152.93±54.41 | 147.23±51.62 | 0.558 | 0.578 |
| Blood glucose
(mmol/l) | 6.67±2.21 | 6.52±1.86 | 0.380 | 0.705 |
| Urea nitrogen
(mmol/l) | 6.17±3.72 | 6.29±3.81 | 0.166 | 0.869 |
| Creatine
(µmol/l) | 75.86±13.74 | 77.29±15.31 | 0.512 | 0.610 |
| cTnT (µg/l) | 5.32±2.47 | 5.36±2.51 | 0.269 | 0.789 |
| cTnI (µg/l) | 8.43±1.66 | 8.29±1.54 | 0.453 | 0.651 |
| Total cholesterol
(mmol/l) | 6.58±0.87 | 6.54±0.83 | 0.244 | 0.808 |
| Glycerin trilaurate
(mmol/l) | 3.32±0.74 | 3.47±0.81 | 1.006 | 0.317 |
| Infarct
location | | | 0.537 | 0.999 |
|
Frontal
lobe | 9 (16.07) | 9 (17.31) | | |
|
Temporal
lobe | 7 (12.50) | 7 (13.46) | | |
|
Parietal
lobe | 6 (10.71) | 5 (9.62) | | |
|
Occipital
lobe | 6 (10.71) | 7 (13.46) | | |
|
Basal
ganglion | 8 (14.29) | 7 (13.46) | | |
|
Thalamus | 9 (16.07) | 7 (13.46) | | |
|
Cerebellum | 8 (14.29) | 8 (15.38) | | |
|
Brainstem | 3 (5.36) | 2 (3.85) | | |
| Infarct size | | | 0.248 | 0.804 |
|
Lacunar
infarction | 32 (57.14) | 28 (53.85) | | |
|
Medium
infarction | 16 (28.57) | 17 (32.69) | | |
|
Massive
infarction | 8 (14.29) | 7 (13.46) | | |
Comparison of NLR and IL-33 level
between the two groups before and after treatment
The NLR in the two groups before and after treatment
was compared, but there was no difference between the observation
group (6.58±2.14) and the control group (6.64±2.20) before
treatment. The NLR of the observation group (2.74±0.16) and the
control group (3.07±0.22) after treatment was significantly lower
than that before treatment (P<0.05). The NLR of the observation
group after treatment was significantly lower than that of the
control group (P<0.05), and the difference was significantly
higher than that of the control group (P<0.05). The level of
IL-33 in the two groups before and after treatment was compared.
There was no difference between the observation group (54.28±18.24
ng/l) and the control group (52.47±16.46 ng/l) before treatment.
After treatment, The level of IL-33 in the two groups was
significantly higher than that before treatment (P<0.05). The
level of IL-33 in the observation group (68.86±15.46 ng/l) was
significantly higher than that in the control group (59.27±18.74
ng/l) after treatment (P<0.05), and the difference was
significantly higher than that in the control group (P<0.05)
(Fig. 1).
 | Figure 1NLR ratio and IL-33 level before and
after treatment in the two groups. (A) There was no significant
difference in NLR between the two groups before treatment (t=0.192,
P=0.849). After treatment, the NLR of the control group was
significantly lower than that before treatment (t=14.689,
P<0.001); NLR was significantly lower in the observation group
than before treatment (t=15.401, P<0.001), and the NLR in the
observation group was significantly lower than that in the control
group (t=8.961, P<0.001). (B) There was no significant
difference in IL-33 between the two groups before treatment
(t=0.540, P=0.590). After treatment, IL-33 was significantly higher
in the control group than before treatment (t=2.440, P=0.020);
IL-33 was significantly higher than before treatment (t=4.336,
P<0.001), and IL-33 was significantly higher in the observation
group than in the control group (t=2.910, P=0.004).
*P<0.05, **P<0.01,
***P<0.001. NLR, neutrophils to lymphocytes ratio;
IL-33, interleukin-33. |
Comparison of NIHSS score between the
two groups before and after treatment
The NIHSS scores of the two groups before and after
treatment were compared, and there was no difference between the
two groups (P>0.05). The NIHSS scores in both groups after
treatment were significantly lower than those before treatment
(P<0.05). The NIHSS score in the observation group was
significantly lower than that in the control group (P<0.05), and
the difference was significantly higher than that in the control
group (P<0.05) (Table II).
 | Table IINIHSS scores of patients in both
groups before and after treatment (mean ± SD). |
Table II
NIHSS scores of patients in both
groups before and after treatment (mean ± SD).
| Treatment | Observation group
(n=56) | Control group
(n=52) | t value | P-value |
|---|
| Before
treatment | 11.36±3.74 | 11.41±3.78 | 0.234 | 0.816 |
| After
treatment |
6.75±1.28a |
7.86±2.34a | 3.088 | 0.003 |
| Difference | 4.62±1.35 | 3.53±1.16 | 4.483 | <0.001 |
Correlation between NLR, IL-33 and
NIHSS score
The relationship between NLR, IL-33 and NIHSS scores
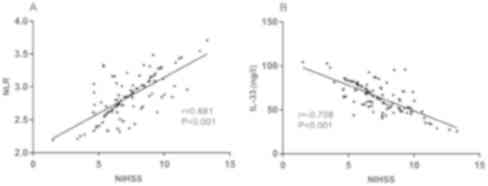
was analyzed by Pearson's correlation analysis. NLR was positively
correlated with NIHSS score, and IL-33 was negatively correlated
with NIHSS score (Fig. 2).
Complications in both groups
By comparing the complications between the two
groups, it was found that there was no significant difference in
gastrointestinal bleeding, pulmonary infection and epilepsia
between the observation group and the control group (P>0.05),
but the heart rate and brain edema in the observation group were
significantly lower than those in the control group (P<0.05)
(Table III).
 | Table IIIComplications of the two groups [n
(%)]. |
Table III
Complications of the two groups [n
(%)].
| Complication | Observation group
(n=56) | Control group
(n=52) | χ2
value | P-value |
|---|
| Gastrointestinal
bleeding | 2 (3.57) | 3 (5.77) | 0.295 | 0.587 |
| Heart rate
disorder | 7 (12.50) | 15 (28.85) | 4.441 | 0.035 |
| Pulmonary
infection | 4 (7.14) | 6 (11.54) | 0.620 | 0.431 |
| Epilepsia | 4 (7.14) | 8 (15.38) | 1.854 | 0.173 |
| Brain edema | 6 (10.71) | 13 (25.00) | 3.796 | 0.051 |
Evaluation of curative effect in the
two groups
By comparing the curative effect evaluation of the
two groups, it was found that the total effective rate of the
patients in the observation group was much higher than that in the
control group (P<0.05) (Table
IV).
 | Table IVEvaluation of the curative effect of
the two groups of patients [n (%)]. |
Table IV
Evaluation of the curative effect of
the two groups of patients [n (%)].
| MRS | Observation group
(n=56) | Control group
(n=52) | χ2
value | P-value |
|---|
| 0 No symptoms | 19 (33.93) | 12 (23.08) | 1.552 | 0.213 |
| 1 Symptomatic, no
significant disability | 18 (32.14) | 11 (21.15) | 1.658 | 0.198 |
| 2 Slight
disability | 9 (16.07) | 8 (15.38) | 0.010 | 0.922 |
| 3 Moderate
disability | 6 (10.71) | 9 (17.31) | 0.980 | 0.322 |
| 4 Moderately severe
disability | 2 (3.57) | 7 (13.45) | 3.453 | 0.063 |
| 5 Severe
disability | 2 (3.57) | 5 (9.62) | 1.625 | 0.202 |
| 6 Dead | 0 (0.00) | 0 (0.00) | | >0.999 |
| Total effective
treatment | 46 (82.14) | 31 (59.62) | 6.686 | 0.010 |
Comparison of the total
hospitalization time between the two groups
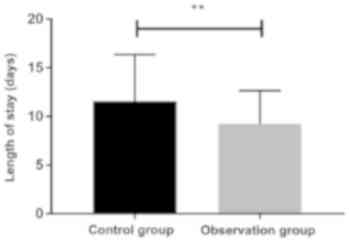
By comparing the total hospitalization time of the
two groups, it was found that the total hospitalization time of the
patients in the observation group (9.24±3.42 days) was
significantly shorter than that in the control group (11.57±4.78
days) (P<0.05) (Fig. 3).
One year survival rate of the two
groups
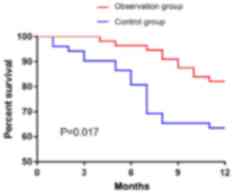
According to the statistics of one year survival of
the two groups, 108 patients or family members were followed up; 0
patients were lost; 29 patients died; 79 survived at one year, and
the survival rate was 73.15%. In the observation group, 10 cases
died, 46 cases survived, the survival rate was 82.14%. In the
control group, 19 cases died, 33 cases survived, the survival rate
was 63.46%. By drawing the one year survival rate of the two
groups, it was found that the one year survival rate of the
patients in the observation group was much better than that in the
control group (P=0.017) (Fig.
4).
Discussion
In this study, we found that the NLR of AIS patients
treated with atorvastatin combined with aspirin and those treated
with aspirin alone were significantly lower than those before
treatment. We speculate that with the development of therapeutic
efficacy, the inflammation of patients is reduced, so NLR, an
inflammatory marker, also decreases. In the study of Hao et
al (22), interventional therapy
combined with routine drug therapy and single drug therapy reduced
the loss of nerve function in patients with ischemic
cerebrovascular disease, and NLR decreased significantly with the
treatment and improvement of patients. The NLR in the observation
group was significantly lower than that in the control group after
treatment, which may suggest that the combination therapy is better
than aspirin alone to reduce inflammation. The level of IL-33 in
both groups was significantly higher than that in the control group
after treatment. In the study of Yang et al (23), it is mentioned that the infusion of
IL-33 into rats with transient middle cerebral artery occlusion can
reduce the area of cerebral infarction, while IL-33 enhances the
expression of IL-10, anti-inflammatory and tissue repair M2 genes
in primary microglia, enhances the survival of neurons, and play a
neuroprotective role. Panahi et al (24) also report that IL-33 converts
microglia from inflammatory M1 to anti-inflammatory and tissue
repair M2 phenotypes to reduce brain injury caused by ischemic
stroke. Therefore, we speculate that after treatment, the level of
IL-33 increases to protect and repair the nerve, and the patient's
anti-inflammation and repair function are enhanced, so that the
condition can be improved. After treatment, the level of IL-33 in
the observation group was significantly higher than that in the
control group, suggesting that the patients treated with combined
medication may have more improvement and stronger ability of
anti-inflammation and tissue repair than those treated with aspirin
alone. This result is also similar to the results of Galun et
al (25), finding that stroke
patients with smaller cerebral infarction had higher serum IL-33,
and mild stroke patients had higher serum IL-33 than severe stroke
patients.
The specific pathogenesis of AIS is not clear, but
inflammation and thrombosis are currently considered to be the key
factors in the pathogenesis of ischemic stroke (17,26,27).
Platelet activation and coagulation thrombosis play an important
role in the pathological and physiological process of recurrent
ischemic vascular events in stroke patients (28). Aspirin is a conventional antiplatelet
drug, but in recent years, it was reported that platelet
aggregation in patients with AIS treated with statins combined with
aspirin is significantly lower than that in patients without
treatment, and the incidence of neurological deterioration was less
than that of patients without treatment (29). Therefore, we studied the efficacy of
atorvastatin combined with aspirin compared with aspirin alone.
The NIHSS scores of the two groups before and after
treatment were compared. NIHSS score can be used to assess stroke
severity (30). It was found that
the NIHSS score of the two groups after treatment was significantly
lower than that before treatment, and the score of the observation
group was significantly lower than that of the control group, which
indicated that the condition of the patients was improved after
drug treatment, and the improvement degree of the patients in the
observation group was better than that in the control group.
Pearson's correlation analysis was used to detect the correlation
between NLR and IL-33 and NIHSS scores. NLR was positively
correlated with NIHSS score, and IL-33 was negatively correlated
with NIHSS score. Then, the complications between the two groups
was compared. There was no significant difference in
gastrointestinal bleeding, pulmonary infection and epilepsy between
the observation group and the control group. The number of heart
rate disorders and brain edema and the total number of
complications in the observation group were significantly lower
than those in the control group. The curative effect of the two
groups was evaluated by MRS score, and it was found that the total
effective rate of the patients in the observation group was
significantly higher than that in the control group, while the
total hospitalization time in the observation group was
significantly lower than that in the control group. Finally, the
1-year survival rate of all patients was studied, and it was found
that the 1-year survival rate of all patients was 73.13%; that of
the observation group was 82.14%, and that of the control group was
63.46%. The 1-year survival rate of the observation group was
significantly higher than that of the control group. This suggests
that atorvastatin combined with aspirin can improve the survival of
patients.
Collectively, atorvastatin calcium combined with
aspirin has a better effective rate in the treatment of acute
ischemic stroke than aspirin alone. The combination can reduce the
NLR, increase the expression level of IL-33 in serum, reduce the
occurrence of complications and hospitalization time, and increase
the survival rate of patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL and XR were responsible for ELISA. LZ analyzed
and interpreted the patient data. XR helped with statistical
analysis. WL wrote the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Medical Ethics
Committee of Luoyang Central Hospital Affiliated to Zhengzhou
University (Luoyang, China). Signed informed consents were obtained
from the patients and/or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang J, Hu Z, Yang S, Liu C, Yang H, Wang
D and Guo F: Inflammatory cytokines and cells are potential markers
for patients with cerebral apoplexy in intensive care unit. Exp
Ther Med. 16:1014–1020. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Suwanwela NC and Poungvarin N: Asap; Asian
Stroke Advisory Panel. Stroke burden and stroke care system in
Asia. Neurol India. 64 (Suppl):S46–S51. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Haq S, Mathur M, Singh J, Kaur N, Sibia RS
and Badhan R: Colour Doppler evaluation of extracranial carotid
artery in patients presenting with acute ischemic stroke and
correlation with various risk factors. J Clin Diagn Res.
11:TC01–TC05. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Goldstein LB, Bushnell CD, Adams RJ, Appel
LJ, Braun LT, Chaturvedi S, Creager MA, Culebras A, Eckel RH, Hart
RG, et al: American Heart Association Stroke Council; Council on
Cardiovascular Nursing; Council on Epidemiology and Prevention;
Council for High Blood Pressure Research; Council on Peripheral
Vascular Disease, and Interdisciplinary Council on Quality of Care
and Outcomes Research: Guidelines for the primary prevention of
stroke: A guideline for healthcare professionals from the American
Heart Association/American Stroke Association. Stroke. 42:517–584.
2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bonardo P, Pantiu F, Chertcoff A, León
Cejas L, Pacha S, Uribe Roca C, Ernst G, Fernández Pardal M and
Reisin R: Blood pressure evolution in young patients with acute
ischemic stroke: A new model for understanding the natural course
of spontaneous hypertension? Int J Neurosci. 128:140–145.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jardim PC, Souza WK, Lopes RD, Brandão AA,
Malachias MV, Gomes MM, Moreno Júnior H, Barbosa EC and Póvoa RM: I
RBH - First Brazilian Hypertension Registry. Arq Bras Cardiol.
107:93–98. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Owaki A, Inaguma D, Aoyama I, Inaba S,
Koide S, Ito E, Takahashi K, Hayashi H, Hasegawa M and Yuzawa Y:
AICOPP group. Serum phosphate level at initiation of dialysis is
associated with all-cause mortality: A multicenter prospective
cohort study. Ren Fail. 40:475–482. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhong C, You S, Chen J, Zhai G, Du H, Luo
Y, Dong X, Cao Y, Liu CF and Zhang Y: Serum alkaline phosphatase,
phosphate, and in-hospital mortality in acute ischemic stroke
patients. J Stroke Cerebrovasc Dis. 27:257–266. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wrigley P, Khoury J, Eckerle B, Alwell K,
Moomaw CJ, Woo D, Flaherty ML, De Los Rios la Rosa F, Mackey J,
Adeoye O, et al: Prevalence of positive troponin and echocardiogram
findings and association with mortality in acute ischemic stroke.
Stroke. 48:1226–1232. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xiong L, Tian G, Leung H, Soo YOY, Chen X,
Ip VHL, Mok VCT, Chu WCW, Wong KS and Leung TWH: Autonomic
dysfunction predicts clinical outcomes after acute ischemic stroke:
A prospective observational study. Stroke. 49:215–218.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhong W, Geng N, Wang P, Li Z and Cao L:
Prevalence, causes and risk factors of hospital readmissions after
acute stroke and transient ischemic attack: A systematic review and
meta-analysis. Neurol Sci. 37:1195–1202. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Luo WJ and Zhang WF: The relationship of
blood cell-associated inflammatory indices and diabetic
retinopathy: A Meta-analysis and systematic review. Int J
Ophthalmol. 12:312–323. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lian L, Xia YY, Zhou C, Shen XM, Li XL,
Han SG, Zheng Y, Mao ZQ, Gong FR, Wu MY, et al: Application of
platelet/lymphocyte and neutrophil/lymphocyte ratios in early
diagnosis and prognostic prediction in patients with resectable
gastric cancer. Cancer Biomark. 15:899–907. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wu M, Zhou L, Zhu D, Lai T, Chen Z and
Shen H: Hematological indices as simple, inexpensive and practical
severity markers of obstructive sleep apnea syndrome: A
meta-analysis. J Thorac Dis. 10:6509–6521. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yu S, Arima H, Bertmar C, Clarke S, Herkes
G and Krause M: Neutrophil to lymphocyte ratio and early clinical
outcomes in patients with acute ischemic stroke. J Neurol Sci.
387:115–118. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen WY, Hong J, Gannon J, Kakkar R and
Lee RT: Myocardial pressure overload induces systemic inflammation
through endothelial cell IL-33. Proc Natl Acad Sci USA.
112:7249–7254. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Qian L, Yuanshao L, Wensi H, Yulei Z,
Xiaoli C, Brian W, Wanli Z, Zhengyi C, Jie X, Wenhui Z, et al:
Serum IL-33 is a novel diagnostic and prognostic biomarker in acute
ischemic stroke. Aging Dis. 7:614–622. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kim JT, Park MS, Choi KH, Cho KH, Kim BJ,
Han MK, Park TH, Park SS, Lee KB, Lee BC, et al: Different
antiplatelet strategies in patients with new ischemic stroke while
taking aspirin. Stroke. 47:128–134. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yilmaz AB, Gokhan S, Sener A and Erel O:
Analysis of Neutrophil/Lymphocyte ratio and Thiol/Disulfide
homeostasis parameters in patients admitted to the emergency
department with ischemic stroke. Pak J Med Sci. 34:1418–1423.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pignatelli P, Carnevale R, Pastori D,
Cangemi R, Napoleone L, Bartimoccia S, Nocella C, Basili S and
Violi F: Immediate antioxidant and antiplatelet effect of
atorvastatin via inhibition of Nox2. Circulation. 126:92–103.
2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jauch EC, Saver JL, Adams HP Jr, Bruno A,
Connors JJ, Demaerschalk BM, Khatri P, McMullan PW Jr, Qureshi AI,
Rosenfield K, et al: American Heart Association Stroke Council;
Council on Cardiovascular Nursing; Council on Peripheral Vascular
Disease; Council on Clinical Cardiology: Guidelines for the early
management of patients with acute ischemic stroke: A guideline for
healthcare professionals from the American Heart
Association/American Stroke Association. Stroke. 44:870–947.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hao Y, Qi Z, Ding Y, Yu X, Pang L and Zhao
T: Effect of interventional therapy on IL-1β, IL-6, and
neutrophil-lymphocyte ratio (NLR) levels and outcomes in patients
with ischemic cerebrovascular disease. Med Sci Monit. 25:610–617.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang Y, Liu H, Zhang H, Ye Q, Wang J, Yang
B, Mao L, Zhu W, Leak RK, Xiao B, et al: ST2/IL-33-dependent
microglial response limits acute ischemic brain injury. J Neurosci.
37:4692–4704. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Panahi M, Papanikolaou A, Torabi A, Zhang
JG, Khan H, Vazir A, Hasham MG, Cleland JGF, Rosenthal NA, Harding
SE, et al: Immunomodulatory interventions in myocardial infarction
and heart failure: A systematic review of clinical trials and
meta-analysis of IL-1 inhibition. Cardiovasc Res. 114:1445–1461.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Galun D, Bogdanovic A, Djokic Kovac J,
Bulajic P, Loncar Z and Zuvela M: Preoperative
neutrophil-to-lymphocyte ratio as a prognostic predictor after
curative-intent surgery for hepatocellular carcinoma: Experience
from a developing country. Cancer Manag Res. 10:977–988.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Dhanesha N, Prakash P, Doddapattar P,
Khanna I, Pollpeter MJ, Nayak MK, Staber JM and Chauhan AK:
Endothelial cell-derived von Willebrand factor is the major
determinant that mediates von Willebrand factor-dependent acute
ischemic stroke by promoting postischemic thrombo-inflammation.
Arterioscler Thromb Vasc Biol. 36:1829–1837. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Vaz HA, Guimaraes RB and Dutra O:
Challenges in high-sensitive troponin assay interpretation for
intensive therapy. Rev Bras Ter Intensiva. 31:93–105.
2019.PubMed/NCBI View Article : Google Scholar : (In Portuguese).
|
|
28
|
Zhang J, Zhang J, Sun H, Ming T, Liu X,
Cong Y, Li F and Li Z: Association between platelet function and
recurrent ischemic vascular events after TIA and minor stroke. Int
J Clin Pharmacol Ther. 55:789–797. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Yi X, Han Z, Wang C, Zhou Q and Lin J:
Statin and aspirin pretreatment are associated with lower
neurological deterioration and platelet activity in patients with
acute ischemic stroke. J Stroke Cerebrovasc Dis. 26:352–359.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Furlanis G, Ajčević M, Stragapede L,
Lugnan C, Ridolfi M, Caruso P, Naccarato M, Ukmar M and Manganotti
P: Ischemic volume and neurological deficit: Correlation of
computed tomography perfusion with the National Institutes of
Health Stroke Scale Score in acute ischemic stroke. J Stroke
Cerebrovasc Dis. 27:2200–2207. 2018.PubMed/NCBI View Article : Google Scholar
|