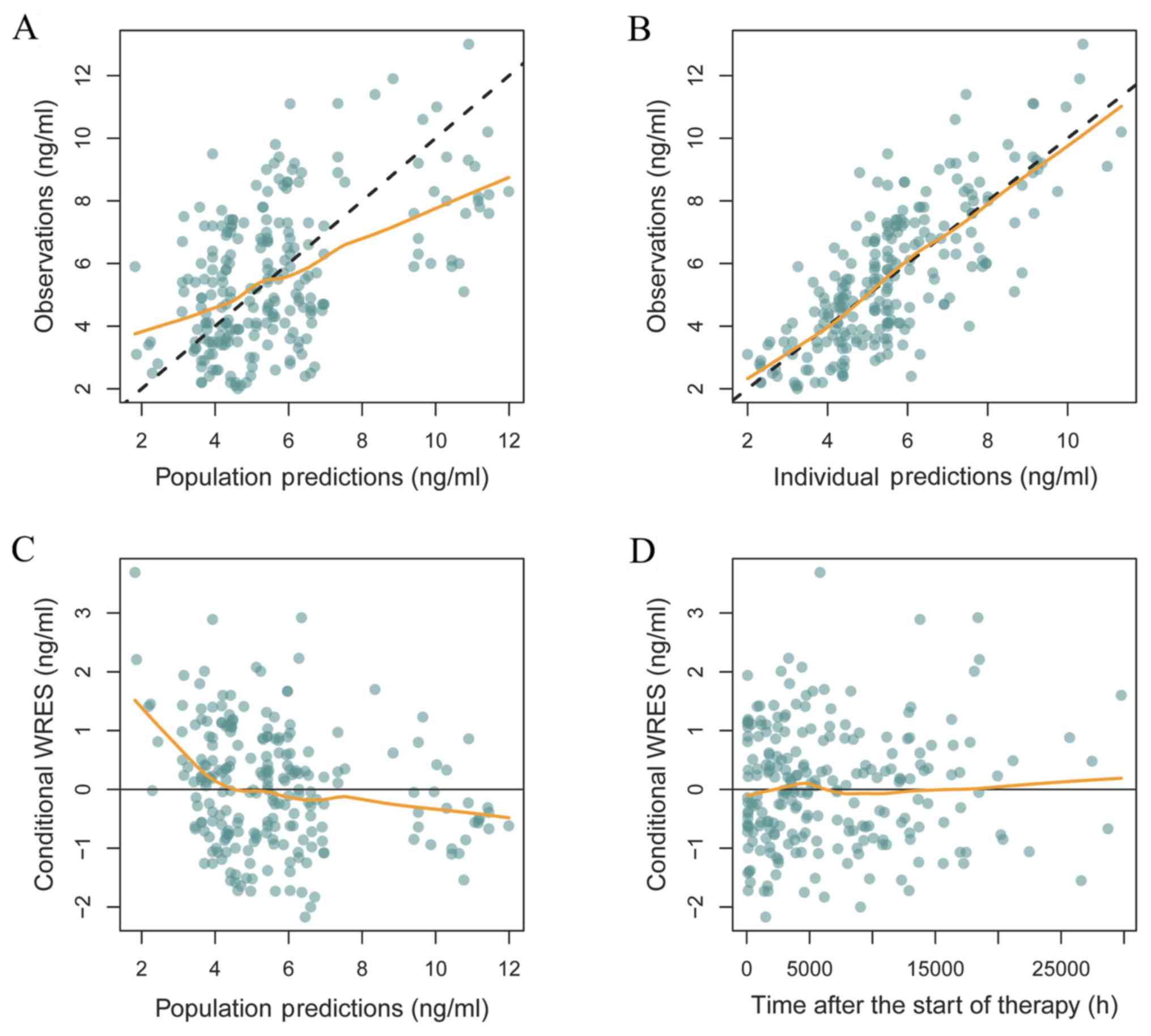
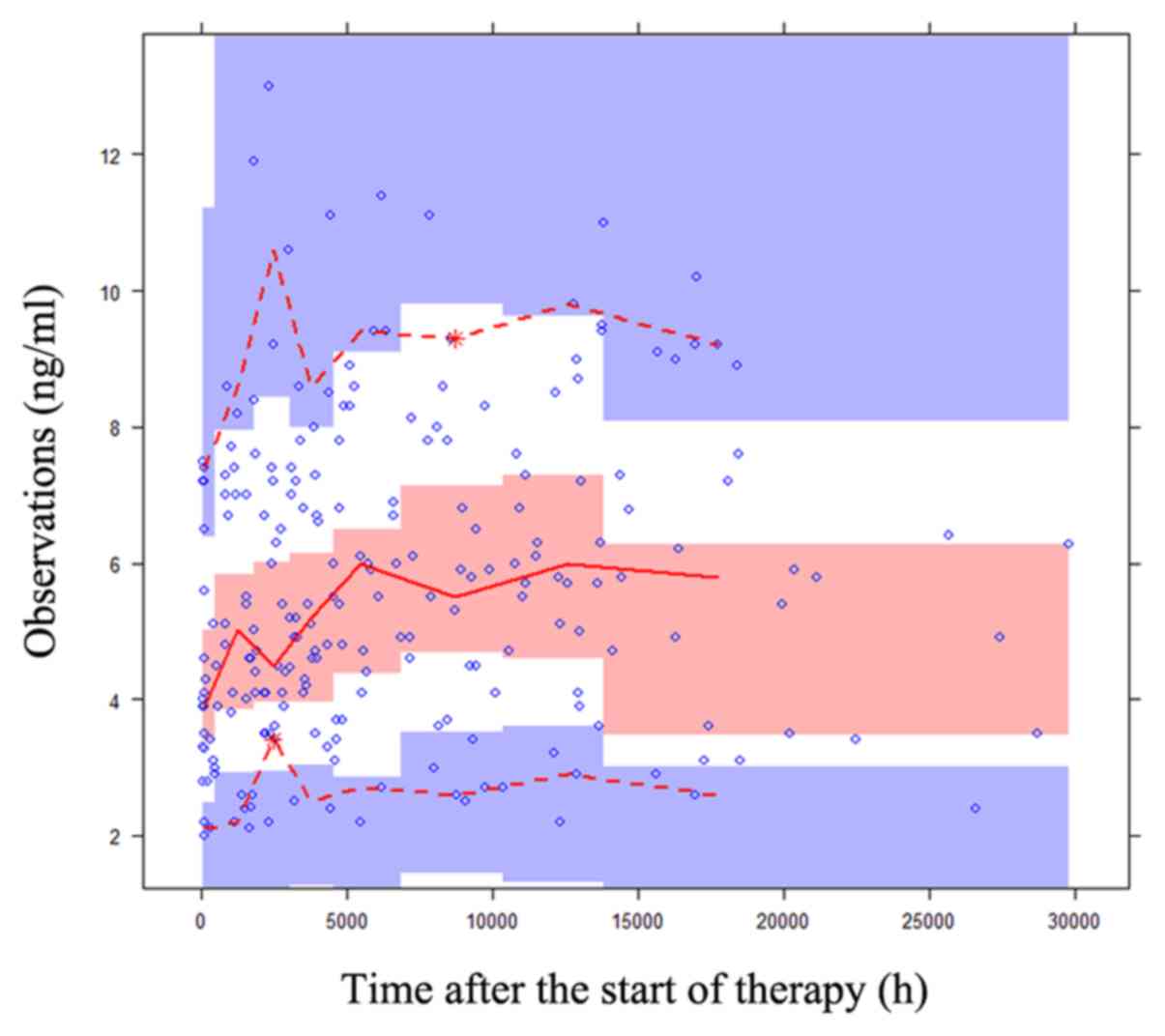
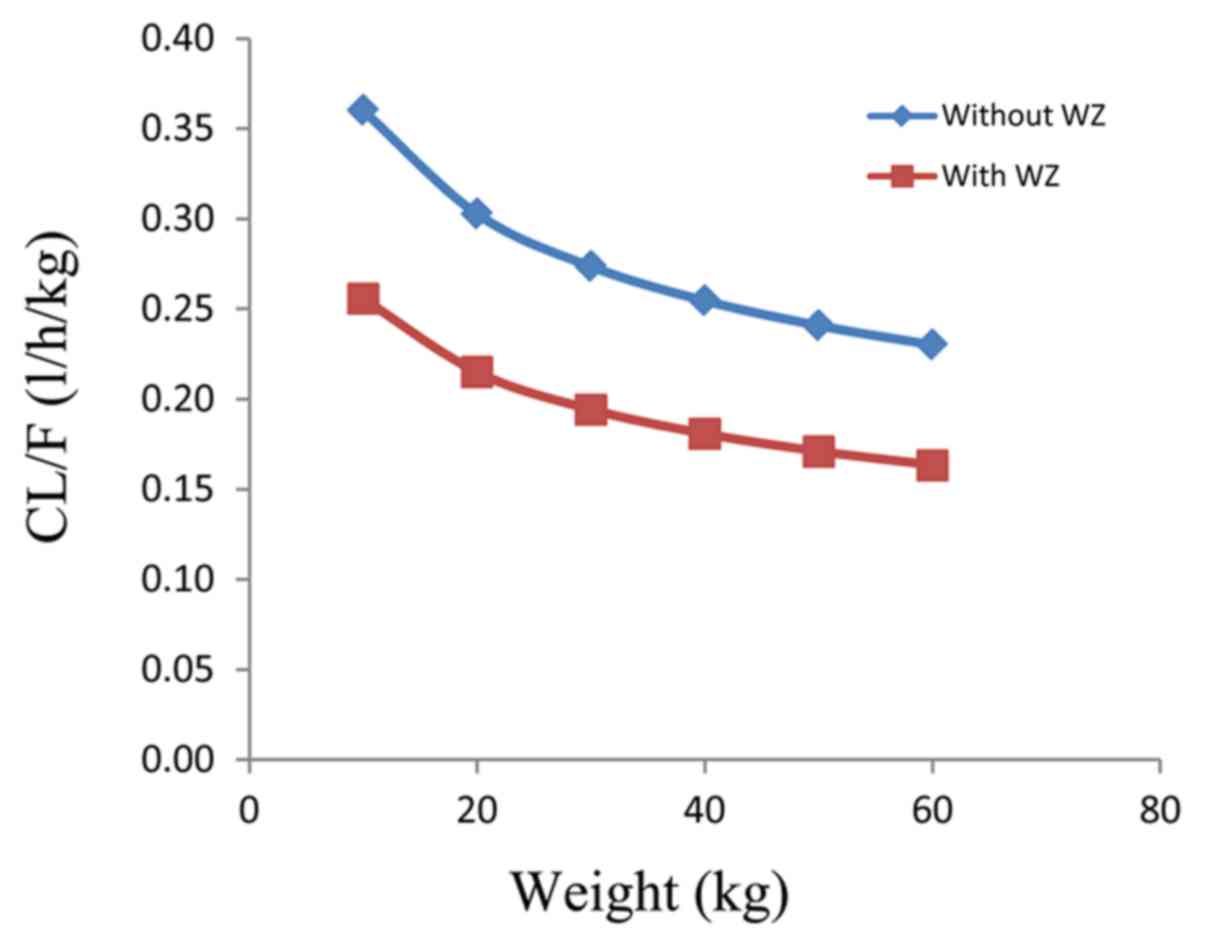
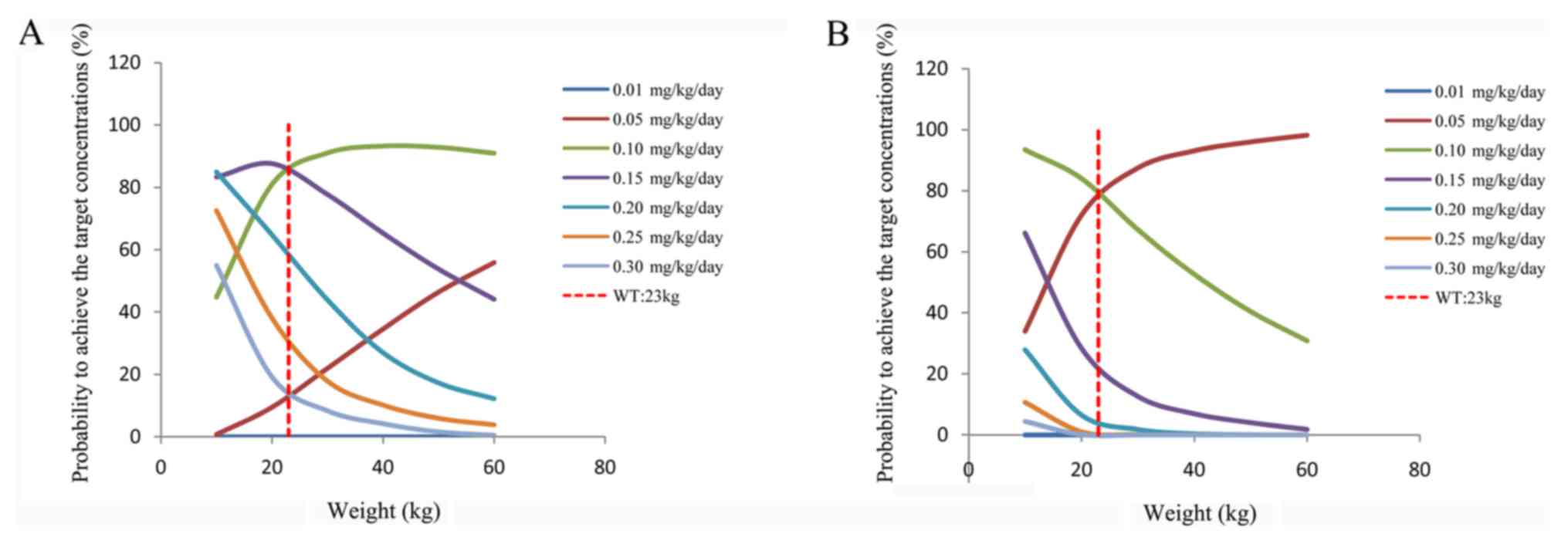
|
1
|
Papadimitraki ED and Isenberg DA:
Childhood- and adult-onset lupus: An update of similarities and
differences. Expert Rev Clin Immunol. 5:391–403. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Takeuchi T, Tsuzaka K, Abe T, Yoshimoto K,
Shiraishi K, Kameda H and Amano K: T cell abnormalities in systemic
lupus erythematosus. Autoimmunity. 38:339–346. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rahman A and Isenberg DA: Systemic lupus
erythematosus. N Engl J Med. 358:929–939. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gurevitz SL, Snyder JA, Wessel EK, Frey J
and Williamson BA: Systemic lupus erythematosus: A review of the
disease and treatment options. Consult Pharm. 28:110–121.
2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
D'Cruz DP, Khamashta MA and Hughes GR:
Systemic lupus erythematosus. Lancet. 369:587–596. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Huang JL, Yeh KW, Yao TC, Huang YL, Chung
HT, Ou LS, Lee WI and Chen LC: Pediatric lupus in Asia. Lupus.
19:1414–1418. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tanaka H, Joh K and Imaizumi T: Treatment
of pediatric-onset lupus nephritis: A proposal of optimal therapy.
Clin Exp Nephrol. 21:755–763. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Takei S, Maeno N, Shigemori M, Imanaka H,
Mori H, Nerome Y, Kanekura S, Takezaki T, Hokonohara M, Miyata K
and Fujikawa S: Clinical features of Japanese children and
adolescents with systemic lupus erythematosus: Results of 1980-1994
survey. Acta Paediatr Jpn. 39:250–256. 1997.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bertsias GK, Tektonidou M, Amoura Z,
Aringer M, Bajema I, Berden JH, Boletis J, Cervera R, Dörner T,
Doria A, et al: Joint European league against rheumatism and
European renal association-European dialysis and transplant
association (EULAR/ERA-EDTA) recommendations for the management of
adult and paediatric lupus nephritis. Ann Rheum Dis. 71:1771–1782.
2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhou T, Lin S, Yang S and Lin W: Efficacy
and safety of tacrolimus in induction therapy of patients with
lupus nephritis. Drug Des Devel Ther. 13:857–869. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Vadcharavivad S, Praisuwan S,
Techawathanawanna N, Treyaprasert W and Avihingsanon Y: Population
pharmacokinetics of tacrolimus in Thai kidney transplant patients:
Comparison with similar data from other populations. J Clin Pharm
Ther. 41:310–328. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang D, Chen X, Xu H and Li Z: Population
pharmacokinetics and dosing regimen optimization of tacrolimus in
Chinese pediatric hematopoietic stem cell transplantation patients.
Xenobiotica. 50:178–185. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang DD, Lu JM, Li Q and Li ZP: Population
pharmacokinetics of tacrolimus in paediatric systemic lupus
erythematosus based on real-world study. J Clin Pharm Ther.
43:476–483. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen X, Wang D, Xu H and Li Z: Initial
dose optimization of tacrolimus for children with systemic lupus
erythematosus based on the CYP3A5 polymorphism and coadministration
with Wuzhi capsule. J Clin Pharm Ther. 45:309–317. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mizuno T, Fukuda T, Christians U,
Perentesis JP, Fouladi M and Vinks AA: Population pharmacokinetics
of temsirolimus and sirolimus in children with recurrent solid
tumours: A report from the Children's Oncology Group. Br J Clin
Pharmacol. 83:1097–1107. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yang JW, Liao SS, Zhu LQ, Zhao Y, Zhang Y,
Sun XY, Rao W, Qu W, Li WZ and Sun LY: Population pharmacokinetic
analysis of tacrolimus early after Chinese pediatric liver
transplantation. Int J Clin Pharmacol Ther. 53:75–83.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Lindbom L, Pihlgren P and Jonsson EN:
PsN-Toolkit-a collection of computer intensive statistical methods
for non-linear mixed effect modeling using NONMEM. Comput Methods
Programs Biomed. 79:241–257. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jusko WJ, Piekoszewski W, Klintmalm GB,
Shaefer MS, Hebert MF, Piergies AA, Lee CC, Schechter P and Mekki
QA: Pharmacokinetics of tacrolimus in liver transplant patients.
Clin Pharmacol Ther. 57:281–290. 1995.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Anderson BJ and Holford NH:
Mechanism-based concepts of size and maturity in pharmacokinetics.
Annu Rev Pharmacol Toxicol. 48:303–332. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ho S, Clipstone N, Timmermann L, Northrop
J, Graef I, Fiorentino D, Nourse J and Crabtree GR: The mechanism
of action of cyclosporin A and FK506. Clin Immunol Immunopathol.
80:S40–S45. 1996.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang DD, Chen X and Li ZP: Tacrolimus
ameliorates proteinuria in Chinese pediatric lupus nephritis
patients. Int J Clin Exp Med. 12:10931–10937. 2019.
|
|
22
|
Andreu F, Colom H, Grinyo JM, Torras J,
Cruzado JM and Lloberas N: Development of a population PK model of
tacrolimus for adaptive dosage control in stable kidney transplant
patients. Ther Drug Monit. 37:246–255. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Benkali K, Rostaing L, Premaud A, Woillard
JB, Saint-Marcoux F, Urien S, Kamar N, Marquet P and Rousseau A:
Population pharmacokinetics and Bayesian estimation of tacrolimus
exposure in renal transplant recipients on a new once-daily
formulation. Clin Pharmacokinet. 49:683–692. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bergmann TK, Hennig S, Barraclough KA,
Isbel NM and Staatz CE: Population pharmacokinetics of tacrolimus
in adult kidney transplant patients: Impact of CYP3A5 genotype on
starting dose. Ther Drug Monit. 36:62–70. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Han N, Ha S, Yun HY, Kim MG, Min SI, Ha J,
Lee JI, Oh JM and Kim IW: Population
pharmacokinetic-pharmacogenetic model of tacrolimus in the early
period after kidney transplantation. Basic Clin Pharmacol Toxicol.
114:400–406. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhao W, Elie V, Roussey G, Brochard K,
Niaudet P, Leroy V, Loirat C, Cochat P, Cloarec S, André JL, et al:
Population pharmacokinetics and pharmacogenetics of tacrolimus in
de novo pediatric kidney transplant recipients. Clin Pharmacol
Ther. 86:609–618. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zuo XC, Ng CM, Barrett JS, Luo AJ, Zhang
BK, Deng CH, Xi LY, Cheng K, Ming YZ, Yang GP, et al: Effects of
CYP3A4 and CYP3A5 polymorphisms on tacrolimus pharmacokinetics in
Chinese adult renal transplant recipients: A population
pharmacokinetic analysis. Pharmacogenet Genomics. 23:251–261.
2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lu YX, Su QH, Wu KH, Ren YP, Li L, Zhou TY
and Lu W: A population pharmacokinetic study of tacrolimus in
healthy Chinese volunteers and liver transplant patients. Acta
Pharmacol Sin. 36:281–288. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Musuamba FT, Guy-Viterbo V, Reding R,
Verbeeck RK and Wallemacq P: Population pharmacokinetic analysis of
tacrolimus early after pediatric liver transplantation. Ther Drug
Monit. 36:54–61. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wallin JE, Bergstrand M, Wilczek HE,
Nydert PS, Karlsson MO and Staatz CE: Population pharmacokinetics
of tacrolimus in pediatric liver transplantation: Early
posttransplantation clearance. Ther Drug Monit. 33:663–672.
2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang XQ, Wang ZW, Fan JW, Li YP, Jiao Z,
Gao JW, Peng ZH and Liu GL: The impact of sulfonylureas on
tacrolimus apparent clearance revealed by a population
pharmacokinetics analysis in Chinese adult liver-transplant
patients. Ther Drug Monit. 34:126–133. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhu L, Yang J, Zhang Y, Jing Y, Zhang Y
and Li G: Effects of CYP3A5 genotypes, ABCB1 C3435T and G2677T/A
polymorphism on pharmacokinetics of Tacrolimus in Chinese adult
liver transplant patients. Xenobiotica. 45:840–846. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang DD, Chen X, Fu M, Zheng QS, Xu H and
Li ZP: Model extrapolation to a real-world dataset: Evaluation of
tacrolimus population pharmacokinetics and drug interaction in
pediatric liver transplantation patients. Xenobiotica. 50:371–379.
2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang DD, Chen X and Li ZP: Wuzhi capsule
and haemoglobin influence tacrolimus elimination in paediatric
kidney transplantation patients in a population pharmacokinetics
analysis: A retrospective study. J Clin Pharm Ther. 44:611–617.
2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fay JW, Nash RA, Wingard JR, Przepiorka D,
Collins RH, Anasetti C, Devine SM, Pineiro LA, Storb RF, Aro RM, et
al: FK 506-based immunosuppression for prevention of graft versus
host disease after unrelated donor marrow transplantation.
Transplant Proc. 27(1374)1995.PubMed/NCBI
|
|
36
|
Fay JW, Wingard JR, Antin JH, Collins RH,
Piñeiro LA, Blazar BR, Saral R, Bierer BE, Przepiorka D,
Fitzsimmons WE, et al: FK506 (Tacrolimus) monotherapy for
prevention of graft-versus-host disease after histocompatible
sibling allogenic bone marrow transplantation. Blood. 87:3514–3519.
1996.PubMed/NCBI
|
|
37
|
Nash RA, Etzioni R, Storb R, Furlong T,
Gooley T, Anasetti C, Appelbaum FR, Doney K, Martin P, Slattery J,
et al: Tacrolimus (FK506) alone or in combination with methotrexate
or methylprednisolone for the prevention of acute graft-versus-host
disease after marrow transplantation from HLA-matched siblings: A
single-center study. Blood. 85:3746–3753. 1995.PubMed/NCBI
|
|
38
|
Nash RA, Piñeiro LA, Storb R, Deeg HJ,
Fitzsimmons WE, Furlong T, Hansen JA, Gooley T, Maher RM, Martin P,
et al: FK506 in combination with methotrexate for the prevention of
graft-versus-host disease after marrow transplantation from matched
unrelated donors. Blood. 88:3634–3641. 1996.PubMed/NCBI
|
|
39
|
Przepiorka D, Ippoliti C, Khouri I, Woo M,
Mehra R, Le Bherz D, Giralt S, Gajewski J, Fischer H, Fritsche H,
et al: Tacrolimus and minidose methotrexate for prevention of acute
graft-versus-host disease after matched unrelated donor marrow
transplantation. Blood. 88:4383–4389. 1996.PubMed/NCBI
|
|
40
|
Uberti JP, Silver SM, Adams PT, Jacobson
P, Scalzo A and Ratanatharathorn V: Tacrolimus and methotrexate for
the prophylaxis of acute graft-versus-host disease in allogeneic
bone marrow transplantation in patients with hematologic
malignancies. Bone Marrow Transplant. 19:1233–1238. 1997.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Nash RA, Antin JH, Karanes C, Fay JW,
Avalos BR, Yeager AM, Przepiorka D, Davies S, Petersen FB, Bartels
P, et al: Phase 3 study comparing methotrexate and tacrolimus with
methotrexate and cyclosporine for prophylaxis of acute
graft-versus-host disease after marrow transplantation from
unrelated donors. Blood. 96:2062–2068. 2000.PubMed/NCBI
|
|
42
|
Wang D, Chen X, Xu H and Li Z: Population
pharmacokinetics and dosing regimen optimisation of tacrolimus in
Chinese pediatric hematopoietic stem cell transplantation patients.
Xenobiotica. 50:178–185. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Monchaud C, de Winter BC, Knoop C, Estenne
M, Reynaud-Gaubert M, Pison C, Stern M, Kessler R, Guillemain R,
Marquet P and Rousseau A: Population pharmacokinetic modelling and
design of a Bayesian estimator for therapeutic drug monitoring of
tacrolimus in lung transplantation. Clin Pharmacokinet. 51:175–186.
2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Han Y, Zhou H, Cai J, Huang J, Zhang J,
Shi SJ, Liu YN and Zhang Y: Prediction of tacrolimus dosage in the
early period after heart transplantation: A population
pharmacokinetic approach. Pharmacogenomics. 20:21–35.
2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Chandrasekhara PK, Jayachandran NV, Thomas
J, Agrawal S and Narsimulu G: Successful treatment of pyoderma
gangrenosum associated with juvenile idiopathic arthritis with a
combination of topical tacrolimus and oral prednisolone. Clin
Rheumatol. 28:489–490. 2009.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Shimizu M, Ueno K, Ishikawa S, Tokuhisa Y,
Inoue N and Yachie A: Treatment of refractory polyarticular
juvenile idiopathic arthritis with tacrolimus. Rheumatology
(Oxford). 53:2120–2122. 2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Tanaka H, Tsugawa K, Suzuki K, Oki ES,
Nonaka K, Kimura S and Ito E: Treatment of difficult cases of
systemic-onset juvenile idiopathic arthritis with tacrolimus. Eur J
Pediatr. 166:1053–1055. 2007.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wang D, Chen X and Li Z: Treatment of
patients with systemic-onset juvenile idiopathic arthritis with
tacrolimus. Exp Ther Med. 17:2305–2309. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Choudhry S, Bagga A, Hari P, Sharma S,
Kalaivani M and Dinda A: Efficacy and safety of tacrolimus versus
cyclosporine in children with steroid-resistant nephrotic syndrome:
A randomized controlled trial. Am J Kidney Dis. 53:760–769.
2009.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Gulati A, Sinha A, Gupta A, Kanitkar M,
Sreenivas V, Sharma J, Mantan M, Agarwal I, Dinda AK, Hari P and
Bagga A: Treatment with tacrolimus and prednisolone is preferable
to intravenous cyclophosphamide as the initial therapy for children
with steroid-resistant nephrotic syndrome. Kidney Int.
82:1130–1135. 2012.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Gulati S, Prasad N, Sharma RK, Kumar A,
Gupta A and Baburaj VP: Tacrolimus: A new therapy for
steroid-resistant nephrotic syndrome in children. Nephrol Dial
Transplant. 23:910–913. 2008.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Loeffler K, Gowrishankar M and Yiu V:
Tacrolimus therapy in pediatric patients with treatment-resistant
nephrotic syndrome. Pediatr Nephrol. 19:281–287. 2004.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Roberti I and Vyas S: Long-term outcome of
children with steroid-resistant nephrotic syndrome treated with
tacrolimus. Pediatr Nephrol. 25:1117–1124. 2010.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Yang EM, Lee ST, Choi HJ, Cho HY, Lee JH,
Kang HG, Park YS, Cheong HI and Ha IS: Tacrolimus for children with
refractory nephrotic syndrome: A one-year prospective, multicenter,
and open-label study of Tacrobell(R), a generic formula. World J
Pediatr. 12:60–65. 2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Wang D, Lu J, Li Q and Li Z: Population
pharmacokinetics of tacrolimus in pediatric refractory nephrotic
syndrome and a summary of other pediatric disease models. Exp Ther
Med. 17:4023–4031. 2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Duddridge M and Powell RJ: Treatment of
severe and difficult cases of systemic lupus erythematosus with
tacrolimus. A report of three cases. Ann Rheum Dis. 56:690–692.
1997.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Miyasaka N, Kawai S and Hashimoto H:
Efficacy and safety of tacrolimus for lupus nephritis: A
placebo-controlled double-blind multicenter study. Mod Rheumatol.
19:606–615. 2009.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Watanabe H, Yamanaka R, Sada KE, Zeggar S,
Katsuyama E, Katsuyama T, Narazaki MT, Tatebe NT, Sugiyama K,
Watanabe KS, et al: The efficacy of add-on tacrolimus for minor
flare in patients with systemic lupus erythematosus: A
retrospective study. Lupus. 25:54–60. 2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Yoon KH: Efficacy and cytokine modulating
effects of tacrolimus in systemic lupus erythematosus: A review. J
Biomed Biotechnol. 2010(686480)2010.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Szeto CC, Kwan BC, Lai FM, Tam LS, Li EK,
Chow KM, Gang W and Li PK: Tacrolimus for the treatment of systemic
lupus erythematosus with pure class V nephritis. Rheumatology
(Oxford). 47:1678–1681. 2008.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Kusunoki Y, Tanaka N, Kaneko K, Yamamoto
T, Endo H and Kawai S: Tacrolimus therapy for systemic lupus
erythematosus without renal involvement: A preliminary
retrospective study. Mod Rheumatol. 19:616–621. 2009.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Alsuwaida A: Successful management of
systemic lupus erythematosus nephritis flare-up during pregnancy
with tacrolimus. Mod Rheumatol. 21:73–75. 2011.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Li H, Zhang X and Chen J: Successful
treatment of steroid-refractory systemic lupus
erythematosus-associated protein-losing enteropathy using
combination therapy with tacrolimus and steroid. Lupus.
20:1109–1111. 2011.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Kaieda S, Kobayashi T, Moroki M, Honda S,
Yuge K, Kawano H, Mitsuyama K, Sata M, Ida H, Hoshino T and Fukuda
T: Successful treatment of rectal ulcers in a patient with systemic
lupus erythematosus using corticosteroids and tacrolimus. Mod
Rheumatol. 24:357–360. 2014.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Politt D, Heintz B, Floege J and Mertens
PR: Tacrolimus-(FK 506) based immunosuppression in severe systemic
lupus erythematosus. Clin Nephrol. 62:49–53. 2004.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Zhang Z, Yang C, Zhang L, Yi Q and Hao Z:
Efficacy and safety of tacrolimus in myasthenia gravis: A
systematic review and meta-analysis. Ann Indian Acad Neurol.
20:341–347. 2017.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Wang L, Zhang S, Xi J, Li W, Zhou L, Lu J,
Lu J, Zhang T and Zhao C: Efficacy and safety of tacrolimus for
myasthenia gravis: A systematic review and meta-analysis. J Neurol.
264:2191–2200. 2017.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Komaki Y, Komaki F, Ido A and Sakuraba A:
Efficacy and safety of tacrolimus therapy for active ulcerative
colitis; A systematic review and meta-analysis. J Crohns Colitis.
10:484–494. 2016.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Matsuoka K, Saito E, Fujii T, Takenaka K,
Kimura M, Nagahori M, Ohtsuka K and Watanabe M: Tacrolimus for the
treatment of ulcerative colitis. Intest Res. 13:219–226.
2015.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Hanouneh M, Ritchie MM, Ascha M, Ascha MS,
Chedid A, Sanguankeo A, Zein NN and Hanouneh IA: A review of the
utility of tacrolimus in the management of adults with autoimmune
hepatitis. Scand J Gastroenterol. 54:76–80. 2019.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Scott LJ, McKeage K, Keam SJ and Plosker
GL: Tacrolimus: A further update of its use in the management of
organ transplantation. Drugs. 63:1247–1297. 2003.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Naesens M, Kuypers DR and Sarwal M:
Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol.
4:481–508. 2009.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Entani C, Izumino K, Iida H, Fujita M,
Asaka M, Takata M and Sasayama S: Effect of a novel
immunosuppressant, FK506, on spontaneous lupus nephritis in
MRL/MpJ-lpr/lpr mice. Nephron. 64:471–475. 1993.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Jusko WJ, Thomson AW, Fung J, McMaster P,
Wong SH, Zylber-Katz E, Christians U, Winkler M, Fitzsimmons WE,
Lieberman R, et al: Consensus document: Therapeutic monitoring of
tacrolimus (FK-506). Ther Drug Monit. 17:606–614. 1995.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Venkataramanan R, Swaminathan A, Prasad T,
Jain A, Zuckerman S, Warty V, McMichael J, Lever J, Burckart G and
Starzl T: Clinical pharmacokinetics of tacrolimus. Clin
Pharmacokinet. 29:404–430. 1995.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Zheng QS and Li LJ: Pharmacometrics: A
quantitative tool of pharmacological research. Acta Pharmacol Sin.
33:1337–1338. 2012.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Chen CY, Zhou Y, Cui YM, Yang T, Zhao X
and Wu Y: Population pharmacokinetics and dose simulation of
oxcarbazepine in Chinese paediatric patients with epilepsy. J Clin
Pharm Ther. 44:300–311. 2019.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Chen Y, Wu D, Dong M, Zhu Y, Lu J, Li X,
Chen C and Li Z: Population pharmacokinetics of vancomycin and
AUC-guided dosing in Chinese neonates and young infants. Eur J Clin
Pharmacol. 74:921–930. 2018.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Zheng Y, Liu SP, Xu BP, Shi ZR, Wang K,
Yang JB, Huang X, Tang BH, Chen XK, Shi HY, et al: Population
pharmacokinetics and dosing optimization of azithromycin in
children with community-acquired pneumonia. Antimicrob Agents
Chemother. 62(e00686-18)2018.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Wang DD, Ye QF, Chen X, Xu H and Li ZP:
Population pharmacokinetics and initial dosing regimen optimization
of cyclosporin in pediatric hemophagocytic lymphohistiocytosis
patients. Xenobiotica. 50:435–441. 2020.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Lu T, Zhu X, Xu S, Zhao M, Huang X, Wang Z
and Zhao L: Dosage optimization based on population pharmacokinetic
analysis of tacrolimus in chinese patients with nephrotic syndrome.
Pharm Res. 36(45)2019.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Wang X, Han Y, Chen C, Ma L, Xiao H, Zhou
Y, Cui Y, Wang F, Su B, Yao Y and Ding J: Population
pharmacokinetics and dosage optimization of tacrolimus in pediatric
patients with nephrotic syndrome. Int J Clin Pharmacol Ther.
57:125–134. 2019.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Wei H, Tao X, Di P, Yang Y, Li J, Qian X,
Feng J and Chen W: Effects of traditional chinese medicine Wuzhi
capsule on pharmacokinetics of tacrolimus in rats. Drug Metab
Dispos. 41:1398–1403. 2013.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Xin HW, Li Q, Wu XC, He Y, Yu AR, Xiong L
and Xiong Y: Effects of Schisandra sphenanthera extract on the
blood concentration of tacrolimus in renal transplant recipients.
Eur J Clin Pharmacol. 67:1309–1311. 2011.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Xin HW, Wu XC, Li Q, Yu AR, Zhu M, Shen Y,
Su D and Xiong L: Effects of Schisandra sphenanthera extract on the
pharmacokinetics of tacrolimus in healthy volunteers. Br J Clin
Pharmacol. 64:469–475. 2007.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Qin XL, Bi HC, Wang CX, Li JL, Wang XD,
Liu LS, Chen X and Huang M: Study of the effect of Wuzhi tablet
(Schisandra sphenanthera extract) on tacrolimus tissue distribution
in rat by liquid chromatography tandem mass spectrometry method.
Biomed Chromatogr. 24:399–405. 2010.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Qin XL, Bi HC, Wang XD, Li JL, Wang Y, Xue
XP, Chen X, Wang CX, Xu le J, Wang YT and Huang M: Mechanistic
understanding of the different effects of Wuzhi Tablet (Schisandra
sphenanthera extract) on the absorption and first-pass intestinal
and hepatic metabolism of Tacrolimus (FK506). Int J Pharm.
389:114–121. 2010.PubMed/NCBI View Article : Google Scholar
|