Introduction
Von Hippel-Lindau (VHL) disease (1) is a rare autosomal dominant inherited
disease that predisposes the affected individual to various benign
or malignant tumors with an incidence rate of 1 in every
36,000-50,000 worldwide in 2018 (2-4).
Currently, >40 lesions for VHL disease have been primarily
observed in the central nervous system (CNS) and in 14 different
organs, including hemangioblastomas (HB; 44-72% of cases), renal
cell carcinoma (RCC; 25-45% of cases), pheochromocytoma
(PHEO)/paragangliomas (PGL; 10-30% of cases), endolymphatic sac
tumors (ELSTs; 6-15% of cases), renal cysts (60% of cases),
pancreatic neuroendocrine tumors (PNETs; 5-10% of cases),
pancreatic serous cystadenoma/cysts (72-90% of cases), and
papillary cystadenoma (PC) of the epididymis and the broad ligament
of the uterus (2-15).
VHL disease is primarily caused by inactivation of the VHL
tumor-suppressive protein. The VHL gene (OMIM, 608537) is located
on human chromosome 3p25.3 and encodes the VHL protein (pVHL),
which forms a complex with elongation factor and is critical for
pVHL to function as an E3 ligase (16). VHL germline mutations result in the
dysfunction of E3 ligase and the accumulation of hypoxia-inducible
factor-α (HIF-α; VHL-Elongin-HIF-α complex), leading to decreased
ubiquitylation and proteasomal degradation of HIFs, namely HIF-1α
and HIF-2α. Elevated levels of HIFs subsequently regulate
overactivation of the downstream pathways in which vascular
endothelial growth factor (VEGF), platelet-derived growth factor-β
(PDGF-β) and transforming growth factor-α (TGF-α) are involved,
which accelerates tumorigenesis (14). The present study analyzed the
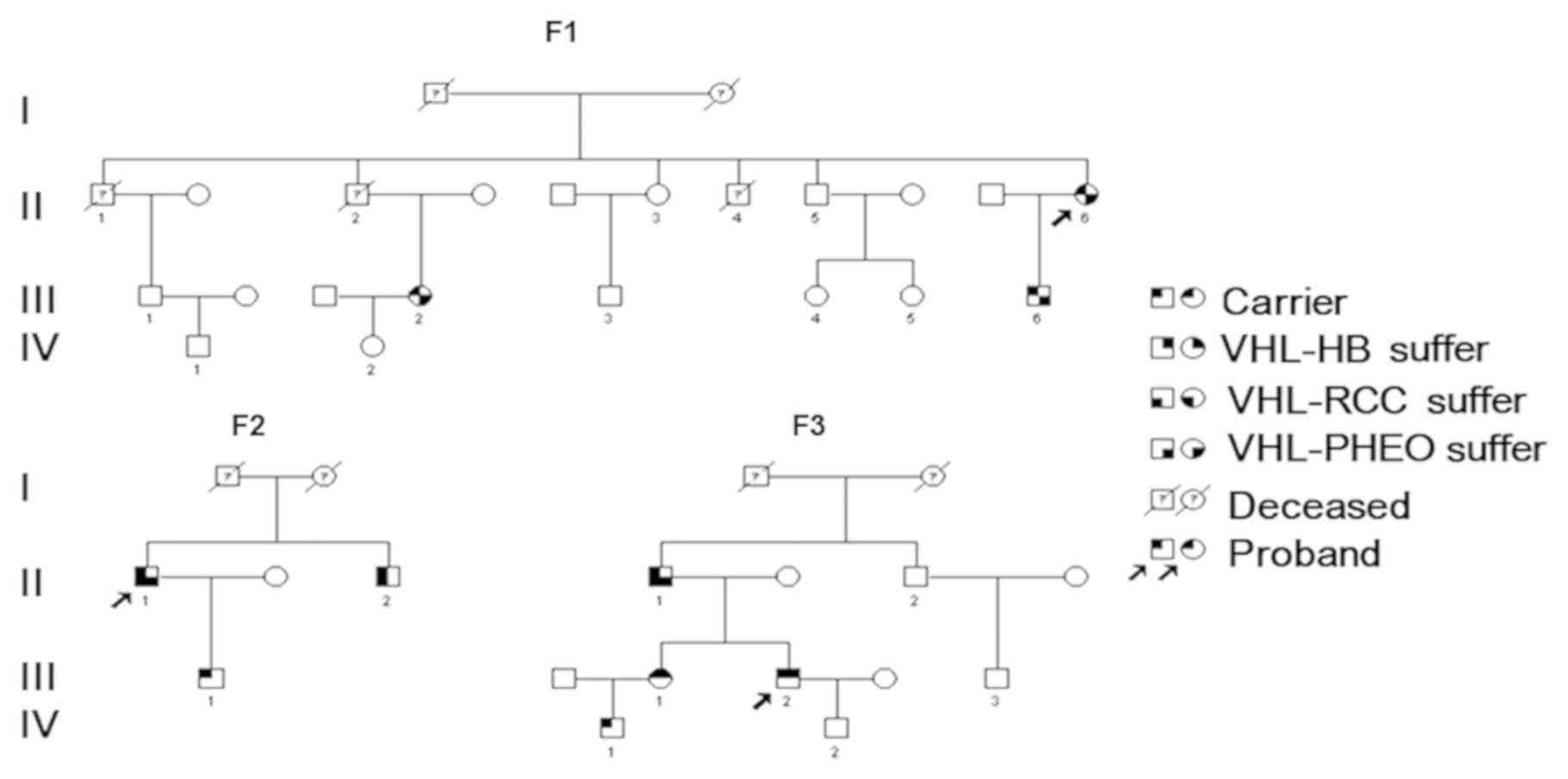
clinical data of 9 patients from 3 families with VHL disease. The
mutations in the VHL gene of patients and their family members were
determined.
Materials and methods
Patients
Between May 1985 and October 2017, three Han
pedigrees with VHL disease were recruited separately and followed
up by Lishui People's Hospital (Lishui, China), the Second
Affiliated Hospital of Zhejiang University School of Medicine
(Hangzhou, China) and the First People's Hospital of Wenling City
(Wenling, China), respectively. The patients underwent collection
of their medical history, family survey (pedigree analysis) and a
detailed physical examination and associated auxiliary examination
by a specialist (GL, ZZ and HZ, respectively) from each of the
three hospitals aforementioned in a blinded manner. There were 21
members in the pedigrees, including 15 males and 6 females, aged
9-66 years (Fig. 1 and Table I). The present study was approved by
the Ethics Committee of Lishui People's Hospital and written
informed consent was obtained from all patients.
 | Table IClinical phenotype and genotype
characteristics of patients with VHL syndrome in 3 families. |
Table I
Clinical phenotype and genotype
characteristics of patients with VHL syndrome in 3 families.
| Patients | Sex | Age at diagnosis
(years) | VHL gene
mutation | CNS-HB tumor size
(cm) | RCC size (cm) | PHEO size (cm) | Operative type | Pathological
diagnosis | Final
diagnosis | VHL type |
|---|
| F1-II6 | Female | 18 | p.R161Q | - | - | Right,
5.5x4.5x2.8 | Right PHEO
resection | Right PHEO | Right PHEO | VHL-IIB |
| F1-III2 | Male | 9 (right)/17
(left) | p.R161Q | - | - | Left, 7.0x5.5x3.4;
right, 5.6x3.8x3.0 | Left and right PHEO
resection | Bilateral PHEO
(metachronous) | Bilateral PHEO
(metachronous) | VHL-IIB |
| F1-III6 | Female | 28 | p.R161Q | - | - | Left, 6.0x4.7x3.5;
right, 3.5x2.8x2.5 | Bilateral PHEO
resection | Bilateral PHEO
(synchronous) | Bilateral PHEO
(synchronous) | VHL-IIB |
| F2-II1 | Male | 24 | p.N78S | - | Left, 1.0x1.0x0.8;
right, 1.0x1.0x1.0 | Right,
4.1x3.4x3.9 | Right PHEO
resection + right RCC resection + resection of bilateral epididymal
cyst | Right PHEO + right
RCC + bilateral epididymal cyst | Right PHEO + right
PHEOa + bilateral
epididymal cust + multiple pancreatic cysts | VHL-IIB |
| F2-II2 | Male | 27 | p.N78S | - | Right,
4.0x3.0x2.5 | - | Right radical
nephrectomy | Right RCC | Right
PHEOa + multiple cysts
of kidney and pancreas + bilateral epididymal nodules | VHL-IIB |
| F2-III1 | Female | 5 | p.N78S | - | - | - | - | - | VHL gene mutation
carrier | VHL-IIB |
| F3-II1 | Male | 66 | p.R167Q | - | Left, 1.0x1.0x0.8;
right, 1.0x1.0x0.9 | Right,
4.5x3.5x2.6 | Right PHEO
resection + right RCC resection | Right PHEO + right
RCC | Right PHEO +
bilateral RCCa +
multiple cysts of kidney and pancreas + PNETs | VHL-IIB |
| F3-III1 | Female | 45 | p.R167Q | 3.2x2.1x1.0 | - | - | Cerebellar HB
resection | HB | Cerebellar HB | VHL-IIIB |
| F3-III2 | Male | 34 | p.R167Q | Right 2.1x1.0x0.9;
right, 0.6x0.5x0.5 | - | - | Gamma knife
radiosurgery for CNS-HB | - | Multiple
CNS-HBb | VHL-IIIB |
| F3-IV1 | Male | 21 | p.R167Q | - | - | - | - | - | VHL variant
carrier | VHL-IIIB |
Detection of VHL gene mutation
A total of 5 ml peripheral blood (EDTA
anticoagulant) was collected from each of the 21 family members
from the 3 families, including the 3 probands (F1-II6, F2-II1 and
F3-III2). Extraction of genomic DNA from peripheral blood
leukocytes was performed using a QIAamp Blood kit (Qiagen GmbH)
according to the manufacturer's protocol. The primer sequences were
designed based on the VHL gene sequence found in GeneBank using the
Primer-Blast online tool (https://omictools.com/primer-blast-tool) and
synthesized by Sangon Biotech Co., Ltd. The primer sequences were
forward, 5'-ACCGGTGTGGCTCTTTAACA-3' and reverse,
5'-TCCTGTACTTACCACAACAACCTT-3'. The 20 µl solution used for the PCR
amplification consisted of 17 µl KAPA2G Robust HotStart ReadyMix
(cat. no. KK5701; Beijing Huaruikang Technology Co., Ltd.), 1 µl
each of the aforementioned upstream and downstream primers (10 µM)
and 1 µl of genomic DNA (200 ng) from both the patients and family
members. PCR was performed as follows: Pre-denaturation at 94˚C for
5 min, followed by 30 cycles of denaturation at 94˚C for 30 sec,
annealing at 65˚C for 30 sec, 72˚C for 1 min; and extension at 72˚C
for 5 min. The 2 µl PCR amplified product was used for subsequent
electrophoresis with 1% agarose gel for identification and
purification. The gel was stained with ethidium bromide (cat. no.
E7637; Sigma-Aldrich; Merck KGaA) for visualization. The purified
product was sent to Shanghai Xiang Yin Biotechnology Co., Ltd. for
sequencing with an ABI 3730XL sequencer. All mutations were
confirmed by bi-directional sequencing.
Imaging examination and detection of
biochemical indexes
The brain, adrenal glands, kidney, and pancreas were
imaged using ultrasound, CT or MRI scans and fundus screening was
performed in patients with VHL mutations. Serological tests,
including the measurement of catecholamine and/or urine
vanillicmandelic acid for 24 h, were performed using an AU400
Automated Chemistry Analyzer (Olympus Corporation).
Follow-up
All patients were followed up by telephone or
hospital visits for 5 years. The follow-up interval was every 2
months in the first year, then every 6 months in the second year
and once every year thereafter. During follow-up, general
conditions, including blood pressure, blood biochemical indices,
recurrence or metastasis of tumor were assessed for every
patient.
Results
Family 1 (F1) investigation and
general clinical material
Proband (F1-II6) was female and aged 50 years. The
patient was admitted to Lishui People's Hospital (Lishui, China) in
May 1985 due to paroxysmal headache, palpitation and hyperhidrosis
that had occurred for 3 months. The results of physical
examinations revealed that patient heart rate was 110 bpm and blood
pressure was 210/120 mmHg. Ultrasound and CT results revealed a
mass sized 5.5x4.5x2.8 cm on the right side of the adrenal gland
and it was considered as right PHEO. Retinal hemangioblastoma was
excluded by an ophthalmologist. After blood pressure and volume
expansion were adjusted with phenylbenzylamine hydrochloride
tablets, right adrenal tumor resection was performed under general
anesthesia. The postoperative pathology results confirmed that the
patient suffered from right PHEO.
The proband's son (F1-III6) was 25 years old and was
admitted to Lishui People's Hospital (Lishui, China) in March 2002
due to paroxysmal headache and hypertension (140-160/100-110 mmHg).
The results of the physical examination revealed that his levels of
serum catecholamine were increased, including dopamine (88.64 ng/l;
normal reference value, <100 ng/l), adrenaline (106.23 ng/l;
normal reference value <600 ng/l) and norepinephrine (827.15
ng/l; normal reference value <100 ng/l). Ultrasound and CT
results revealed a mass sized 5.6x3.8x3.0 cm on the right side of
the adrenal gland. After blood pressure and volume expansion were
adjusted with phenylbenzylamine hydrochloride tablets, right
adrenal tumor laparoscopic resection was performed under general
anesthesia. The postoperative pathology results confirmed that the
patient suffered from right PHEO. In 2010, this patient suffered
from paroxysmal hypertension and headache for a second time and his
levels of serum catecholamine were also increased, including
dopamine (600.36 ng/l), adrenaline (297.94 ng/l) and norepinephrine
(1,093.84 ng/l). The ultrasound and CT results revealed a mass
sized 7.0x5.5x3.4 cm on the left side of the adrenal gland. After
blood pressure and volume expansion were adjusted with
phenylbenzylamine hydrochloride tablets, left adrenal tumor
resection was performed under general anesthesia. The postoperative
pathology results confirmed that the patient suffered from left
PHEO. The final diagnosis of this patient was bilateral PHEO.
The proband's niece (F1-III2) was 38 years old and
was admitted to Lishui People's Hospital in October 2002 due to
persistent headache and dizziness that had lasted for 3 days. The
results of physical examination demonstrated that the patient's
blood pressure was 160/100 mmHg and their serum catecholamine
levels were increased. Ultrasound/CT revealed a bilateral mass on
the adrenal gland. The left mass was 6.0x4.7x3.5 cm in size and the
right mass was 3.5x2.8x2.5 cm in size. After blood pressure and
volume expansion were adjusted with phenylbenzylamine hydrochloride
tablets, bilateral adrenal tumor laparoscopic resection was
performed under general anesthesia. The postoperative pathology
results confirmed that the patient suffered from bilateral PHEO.
The father of this patient (F1-II2, the proband's brother) died
from hypertensive intracerebral hemorrhage 26 years ago, aged 38
years.
Family 2 (F2) investigation and
general clinical material
The proband (F2-II1) of F2 was male and aged 33
years. The patient was admitted to the Second Affiliated Hospital
of Zhejiang University School of Medicine (Hangzhou, China) in
September 2009 due to paroxysmal headache, dizziness and
palpitation with hyperhidrosis. Physical examination results
revealed that the patients' heart rate was 100 bpm and blood
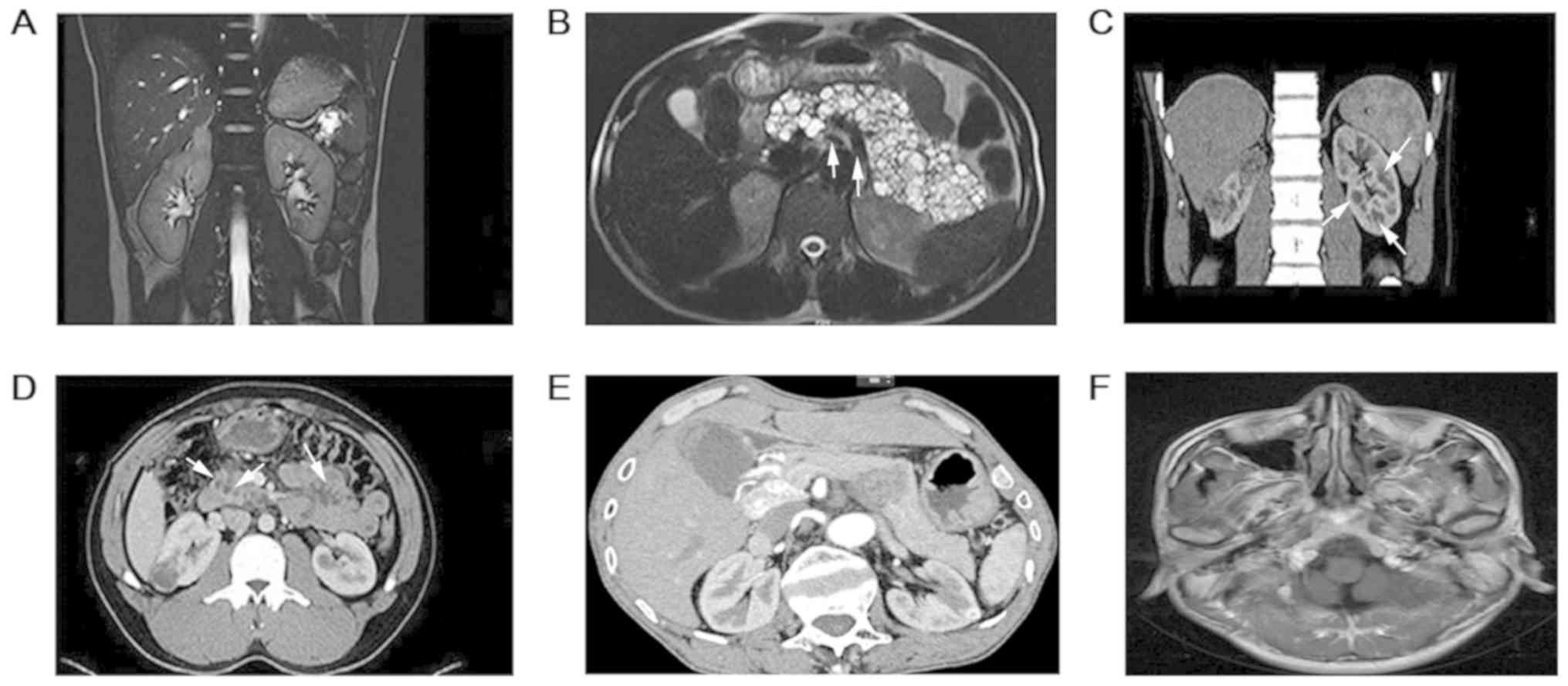
pressure was 105/78 mmHg. MRI results revealed a mass sized
4.1x3.4x3.9 cm at the right adrenal gland and multiple renal tumors
(the largest diameter of the tumors, 1.0x1.0x1.0 cm on the left;
1.0x1.0x0.8 cm on the right) and multiple cysts in the pancreas
(Fig. 2A and B). An ophthalmologist (from the Second
Affiliated Hospital of Zhejiang University School of Medicine)
excluded the existence of retinal hemangioblastoma preoperatively.
The concentration of urine vanillicmandelic acid at 24 h was 138.3
µmol/24 h (normal reference value, <33 µmol/d). Combined with
clinical manifestations, the patient was considered as right PHEO,
with double renal masses and multiple pancreatic cysts. After the
patient's blood pressure and volume expansion was adjusted with
phenylbenzylamine hydrochloride tablets, resection of the right
adrenal tumor and the upper pole of the right kidney were performed
under general anesthesia. The postoperative pathology results
confirmed that the patient had right PHEO and right RCC (Fuhrman II
grade) (17). In April 2012, the
patient underwent bilateral epididymal nodule excision due to the
presence of multiple nodules in the bilateral epididymal head.
Postoperative pathology results confirmed that the patient suffered
from papillary cystadenoma of the bilateral epididymal head.
The proband's younger brother (F2-II2) was aged 32
years. In 2012, the patient presented with multiple masses in both
kidneys (left, 1.0x1.0x1.0 cm; right, 4.0x3.5x2.6 cm), multiple
cysts in both kidneys, and pancreas and epididymal nodules
(Fig. 2C and D). The patient underwent laparoscopic right
radical nephrectomy under general anesthesia. The postoperative
pathology results confirmed that the patient suffered from multiple
RCC in the right kidney (Fuhrman II grade). IFN and IL-2 were used
for postoperative treatment.
Family 3 (F3) investigation and
general clinical material
Proband (F3-III2) was male and aged 39 years. In
April 2013, the patient was admitted to the First People's Hospital
of Wenling City (Wenling, China) having suffered with dizziness and
headaches for 1 month. A cranial MRI examination revealed that
there was a mass sized 2.1x1.0x0.9 cm at the right margin of the
sellar region and a mass sized 0.6x0.5x0.5 cm at the cerebellar
vermis. The masses were markedly enhanced with clear margins. The
patient was considered to suffer from CNS-HB. An ophthalmologist
(The First People's Hospital of Wenling City) excluded retinal
hemangioblastoma. As the patient refused surgical treatment,
cranial gamma knife radiotherapy (peripheral dose 5 Gy, central
dose 10 Gy) was performed 6 times with a 5 Gy dose at the
peripheral and 10 Gy dose at the central positions. CNS-HB of the
F3-III2 was stable after 5 years of follow-up.
The proband's elder sister was aged 45 years
(F3-III1). In May 2015, the patient was admitted to the First
People's Hospital of Wenling City due to dizziness lasting 3
months. A brain MRI revealed that there was a mass sized
3.2x2.1x1.0 cm at the vermis of the cerebellum, with clear margins.
The mass was excised via surgery. The postoperative pathology
results confirmed that the patient suffered from cerebellar
hemangioblastoma.
The proband's father (F3-II1) was aged 66 years. The
patient was hospitalized in the First People's Hospital of Wenling
City presenting with cough and expectoration with low fever for 2
months in February 2014. Physical examination results revealed that
the patient's temperature was 37.8˚C and his blood pressure
fluctuated between 104-123/66-96 mmHg. CT examination results
revealed that the patient suffered from chronic obstructive
pulmonary disease with pulmonary infection. Ultrasound and CT
results revealed a mass sized 4.5x3.5x2.6 cm on the right side of
the adrenal gland, multiple solid lesions of both kidneys (left,
1.0x1.0x0.8 cm; right, 1.0x1.0x0.9 cm) with multiple small cysts
and pancreatic head lesions (2.6x2.0x2.0 cm). Following treatment
of a lung infection and the adjustment of blood pressure and volume
expansion with phenylbenzylamine hydrochloride tablets, resection
of the right adrenal tumor and right kidney tumor were performed
under general anesthesia. The postoperative pathology results
revealed that the patient suffered from right PHEO and right RCC
(Fuhrman II grade).
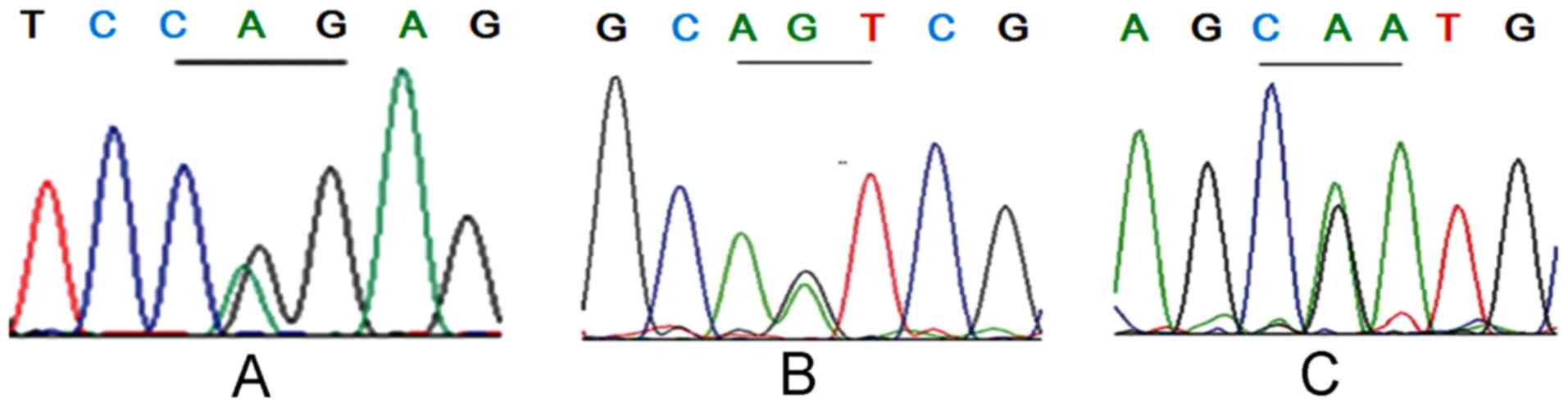
VHL gene detection results
Of the 22 patients from 3 VHL families, 10 had three
VHL germline missense mutations within coding regions, respectively
(Fig. 3). In the F1 family, 3 cases
harbored the p.R161Q (c.482G>A) mutation at exon 3 (Fig. 3A); 3 cases in the F2 family harbored
the p.N78S (c.233A>G) mutation at exon 1 (Fig. 3B); and 4 cases in the F4 family
harbored the p.R167Q (c.500G>A) mutation at exon 3 (Fig. 3C). Apart from 8 patients with the VHL
clinical phenotype who had been surgically and pathologically
diagnosed, 2 new cases (F2-III1 and F3-IV2) were revealed to be
asymptomatic carriers of VHL gene mutations. The remaining 12
family members had no VHL gene mutations and no clinical
manifestations or abnormalities following imaging and serological
tests associated with VHL disease. The clinical phenotype and
genotype characteristics are presented in Table I.
Discussion
VHL disease, also known as VHL syndrome, is
characterized by a variety of benign, malignant tumors and multiple
organ cysts (18). VHL patients
usually develop clinical symptoms after reaching 20 years of age,
with 90-100% penetrance of clinical symptoms between 65 and 70
years old (19). Retinal and
cerebellar HB are typically the most common and earliest presenting
forms of VHL disease (4,7,9). VHL
syndrome can be divided into two types: Type I and type II. Type I
syndrome has CNS-HB and/or RCC among other tumors (except PHEO) and
can also be divided into type IA (high-risk RCC) and type IB
(without RCC). The patients with type II must have PHEO and can be
divided into type IIA (includes PHEO but not RCC), type IIB (RCC +
PHEO) and type IIC (only PHEO) (2,3,14). The clinical diagnosis of this disease
should be based on VHL symptoms, heredity and family factors. If a
patient possesses one of the following three conditions, they may
be diagnosed with VHL disease: i) At least two positions where
CNS-HB exists; ii) CNS-HB at one position and one other organ tumor
(RCC, PHEO/PGL, PNETs or ELSTs); iii) at least one visceral tumor
(RCC, PHEO/PGL, PNETs or ELSTs) associated with the VHL gene
mutation at the pathogenic germline level, or a parent has been
diagnosed with VHL (3). Despite the
high heterogeneity of clinical phenotypes, VHL gene mutations could
be detected at the germline level in almost all patients with VHL
disease (2). The patients in the
three families included in the present study were diagnosed as VHL
type II. The patients in F2 and F3 were VHL type IIB and the
patients in F1 were VHL type IIC (Table
I).
The VHL gene contains three exons and encodes a
polysaccharide anchored membrane protein containing 213 amino acids
(20). The N-terminal region of the
protein contains α and β domains (20). The α domain binds to the elongation
factor C/B to form a complex and the β domain binds to the HIF-α
gene (21). pVHL downregulates the
expression of HIF-α, activates the ubiquitination of epidermal
growth factor receptor (EGFR), regulates glucose
metabolism-associated genes [glucose transporter 1 (GLUT1) and
posphofructokinase 1], growth factors (TGF, PDGF and VEGF) and the
cascade reaction of nerve growth factor/JunB/Egl nine homolog
3-associated apoptotic pathway, ultimately leading to the formation
of benign and malignant multiple organ diseases (21). Emerging evidence has indicated that
HIF-1α serves an important role during clear cell (cc)RCC and HB
development (21). Previously,
almost 400 VHL gene mutants have been revealed to cause VHL disease
(22). The majority of mutations are
missense (27-38%), nonsense (13-27%), large fragment deletions
(9-20%), microdeletions (10%), truncation and rearrangement (25%)
mutations (22). Splicing site
mutations are rare. Of all the mutations, ~20% of the patients
exhibited new mutations (de novo) or their parents are
chimeras (22). VHL type I disease
is caused primarily by a large fragment deletion of the VHL gene,
including C3orf10 gene inactivation, truncation and missense
mutations, which lead to the loss of pVHL function or structural
changes in the protein (3). VHL type
II disease associated with PHEO is mostly caused by missense
mutations (78-96%), which often lead to partial functional defects
of the pVHL. The most frequent mutations of VHL type II disease are
p.R167W and p.R167Q mutations at position 167 of exon 3 in the VHL
gene (2,3). Notably, although the stabilization of
HIF-α is closely associated with the occurrence of RCC, both IIA
and IIB antimutagenesis can inactivate VHL function, which in turn
inhibits the regulation of HIF-α activation. However, only patients
in stage IIB exhibit ccRCC (23).
Currently, certain hypotheses suggest that the occurrence of ccRCC
is associated with the expression of HIF-2α (20,21).
However, IIA mutations do not form enough HIF-2α to form HB,
despite producing the appropriate quantity of HIF-α. This explains
why HB is the most common clinical manifestation of VHL (21). Liu et al (15) demonstrated that patients with
non-HIF-α binding sites demonstrated an improved survival rate when
compared with those that exhibited HIF-α binding sites and
truncated mutations (15). All
patients in the three families of the present study were VHL type
II with missense mutations in the VHL gene and the patients of the
F3 family harbored p.R167Q (α) mutations accompanied with CNS-HB.
No CNS-HB was observed in the F1 (p.R161Q, α) and F2 (p.N78S, β)
families, which differed from the clinical phenotype of previously
reported pedigrees (7,8). The presence of different clinical
phenotypes or disease progression in different or identical
pedigrees or members indicated that there may be other pathogenic
or modifier factors, and/or secondary mutation attacks at the
somatic cell level in addition to genetic factors (3). This also indicated that individualized
treatment should be adopted for heterogeneous patients of different
or the same pedigrees.
The typical genotype-phenotype correlation dictates
that VHL missense mutations are responsible for type II disease
with a high risk of PHEO and that truncating mutations are
responsible for type I disease with a low risk of PHEO (24). Deletion of all or part of the VHL
gene as well as the nearby gene BRK1 (also known as HSPC300 and
C3orf10) leads to retinal HB and CHB with a low risk of RCC, which
is sometimes called type IB VHL disease (25). The results of the present study
supported the notion that missense mutations tend result in PHEO.
In addition, mutations at different sites are associated with
diverse risks of VHL-associated lesions (22). Patients with VHL that present with
missense mutations in the HIF-α binding site (HM) are associated
with a lower risk of PHEO and higher risks of CHB and pancreatic
tumors or cysts, while missense mutations located at sites other
than that of the HIF-α binding site (nHM) are associated with a
higher risk of PHEO, which results in better survival time
(24). Furthermore, patients with
truncating mutations are more likely to develop RCC than those with
HM (26). In contrast to the present
study, it has been indicated that p.N78S (HM)-associated PHEO
alone, p.R161Q (nHM)-associated RCC/PHEO and p.R167Q
(nHM)-associated CNS/RCC/PHEO exhibit diverse phenotypes (16). Of note, the pathogenesis of CHB and
RCC, upregulated HIF-α expression and consequent overexpression of
VEGF and other HIF-associated genes are the primary causative
factors of tumor progression (14).
In PHEO, HIF-α (particularly HIF-2α) dysregulation results in the
overexpression of various HIF-inducible genes, including GLUT1 and
VEGF, which themselves serve important roles in tumor development
(27). However, the development of
PHEO appears to be HIF-independent. In patients with the typical
IIC phenotype, as exhibited by the F1 family of the current study,
mutant pVHL is able to degrade HIFs, and it has been hypothesized
that mutations associated with PHEO may induce gain of function
through an intact but altered pVHL (28). In addition, Gossage et al
(14) reported that mutations in the
elongin C binding domain of pVHL are associated with PHEO. These
binding sites are implicated in the p53-mediated apoptosis of
sympathetic neuronal precursor cells, which then go on to form PHEO
(14). It is speculated that such
large phenotypic variation may result from numerous other factors
that are potentially environmental and may affect the phenotypes
induced by specific mutations (7).
The current clinical treatment strategy of VHL
disease focuses primarily on the treatment of VHL
disease-associated CNS-HB, RCC, PHEO and PNETs (19). The average age of CNS-HB at diagnosis
is 33 years (range, 7-78 years) (8,19). For
CNS-HB, 90% presented in multiple forms and the majority occurred
in the cerebellum (45-50%) and spinal cord (40-45%) (8,20).
Complete excision of CNS-HB based on microsurgical treatment was
rare (<1%) with recurrence of the tumor (8,20). Mild
nerve defects usually recovered within 2 weeks to 6 months after
excision (9). Stereotactic
radiotherapy is a potential treatment option for patients who
cannot tolerate surgery, or where resection of CNS-HB was
difficult. The 2- and 15-year remission rates were 91 and 51%,
respectively (10,11). A previous study also demonstrated
that a gamma ray single dose of 20 Gy at the peripheral position
and a 40 Gy dose at the central position could effectively treat
CNS-HB (12). Consistently, the
F3-III2 in this study was only treated by cranial gamma knife
radiotherapy and was stable after 5 years of follow-up. These
results indicate that radiotherapy may be an alternative treatment
method for patients with CNS-HB who refuse to undergo surgical
excision, but the long-term effectiveness requires continued
observation. Retinal HB can occur in ~50% of patients with VHL
(10). The mean age at diagnosis is
25 years (range, 1-68 years) and the disease is characterized by
multiple and bilateral tumors, with sizes varying from <1 to
several optic discs in diameter (29). In general, small lesions can be
treated with greater success and fewer complications compared with
those that are larger (30). The
majority of peripheral retinal HB cases can be treated with laser
photocoagulation (small peripheral HB), cryotherapy (large HB) or
photodynamic therapy (31). However,
these treatments cannot be used when the tumor is near the optic
nerve, in which case the therapeutic approach is only surveillance,
resulting in a high risk of damage to the optic nerve (32). The average age at diagnosis of
VHL-RCC is 39 years (range, 13-70 years) (13,20).
VHL-RCC is characterized by simultaneous or heterogeneous bilateral
tumors that are multifocal and low grade with a slow progression
(13,20). The metastasis rate of VHL-RCC is very
low when tumors are <3 cm and physical therapy, such as waiting
for observation or radiofrequency ablation, may be used in clinical
treatment (13,22). Nephron-sparing surgery should be
performed if the tumor is >3 cm, which should effectively
decrease renal insufficiency despite the high recurrence rate of
localized neoplasms (13).
In the present study, the diagnostic ages of 3
patients with VHL-RCC (F2-II1, F2-II2 and F3-II1) were 33, 32 and
66 years, respectively. F2-II2 underwent radical resection due to
multiple and large tumors of the right kidney. The other two cases
underwent unilateral single tumor enucleation (similar to biopsy
for definite pathology), all of which were Fuhrman grade II and RCC
was relatively stable after follow-up. The average age at diagnosis
of VHL-PHEO was 27 years (range, 2.75-58.00 years) (33,34). Of
the VHL-PHEO cases, 90% of tumors were located in the adrenal
gland, and 20-50% of VHL-PHEO were bilateral (33). Malignant VHL-PHEO was rare (1-5%)
(33). VHL-PHEO can be asymptomatic
at an early stage and often secretes large quantities of
norepinephrine, which differs from MEN2-PHEO, where large
quantities of adrenaline are secreted (2,33-35). PHEO excision or
partial adrenalectomy decreases the risk of or avoids adrenal
cortical insufficiency or crisis (36). It is worth noting that, 50% of
patients developed a second PHEO within 30 years after initial
diagnosis (2,36,37). In
the present study, the average diagnostic age of 5 patients with
VHL-PHEO was 34.6 years. There were 3 patients (F1-II6, F2-II1 and
F3-II1) with unilateral PHEO and two patients (F1-III2 and F1-III6)
with bilateral PHEO, including one patient (F1-III2) with
first-time diagnosis of unilateral PHEO and contralateral PHEO
occurring 8 years after surgery. Patients received PHEO resection,
after which adrenal function was normal. A previous study
determined that the average diagnostic age of PNETs was 35 years
(range, 10-75 years). PNETs were asymptomatic and grew slowly. Of
the PNETs, 80-93% were <3.0 cm. The phenomena of tumor >3.0
cm, doubling time <500 days and codon 161 and 167 mutations at
exon 3 were potentially malignant signs (3,38,39). In
the present study, one patient (F3-II1, 66 years old) with PNETs
required close monitoring for the possibility of malignancy,
although no definite signs or symptoms were identified via clinical
imaging. VHL-associated renal and pancreatic cysts and
epididymis/broad ligament cystadenoma of the uterus is usually
asymptomatic and often not treated clinically. The average
diagnostic age of ELSTs is 22 years (range, 12-50 years). Of the
ELSTs recorded in a previous study, 30% were bilateral and the
majority were benign. Clinical symptoms of ELSTs include hearing
loss (84-100%), tinnitus (73-77%), dizziness (62-68%) and facial
paralysis (8%). Surgical resection of the tumor is the primary
treatment method for ELSTs (2,3,9). In the present study, 10 patients with
VHL disease were not associated with ELSTs and it was speculated
that the phenomenon may be associated with the small sample size of
the family and patients. In addition, two asymptomatic VHL gene
carriers (F2-III1, 5 years of age, p.N78S; F3-IV2, 21 years of age,
p.R167Q) were identified via gene testing. Early diagnosis and
genetic counseling, regular cancer screening and surveillance are
therefore helpful for disease management and for the improvement of
prognosis (40).
In conclusion, VHL is a complex disease that can be
easily misdiagnosed. Future studies should aim to improve the
understanding and attention to molecular diagnosis and management
based on VHL gene detection, which may be helpful for early
diagnosis and standardization of treatment, improvement of patient
prognosis and quality of life, and for the decrease in clinical
risk of VHL disease.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ designed the study. GL collected the clinical
data, performed the experiments and wrote the manuscript. YZ and ZZ
collected clinical data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Lishui People's Hospital, The Second Affiliated
Hospital of Zhejiang University School of Medicine and the First
People's Hospital of Wenling City. Written informed consent was
obtained from all participants.
Patient consent for publication
Patients provided their written consent for the
publication of these images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Online Mendelian Inheritance in Man (OMIM)
193300. BioPortal: Nov 18, 2019 (https://www.uniprot.org/database/DB-0062).
|
|
2
|
Ganeshan D, Menias CO, Pickhardt PJ,
Sandrasegaran K, Lubner MG, Ramalingam P and Bhalla S: Tumors in
von Hippel-Lindau syndrome: From head to toe-comprehensive
state-of-the-art review. Radiographics. 38:849–866. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chittiboina P and Lonser RR: von
Hippel-Lindau disease. In: Handbook of Clinical Neurology.
Elsevier, pp139-156, 2015.
|
|
4
|
Aronoff L, Malkin D, van Engelen K,
Gallinger B, Wasserman J, Kim RH, Villani A, Meyn MS and Druker H:
Evidence for genetic anticipation in vonHippel-Lindau syndrome. J
Med Genet. 55:395–402. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kruizinga RC, Sluiter WJ, de Vries EG,
Zonnenberg BA, Lips CJ, van der Horst-Schrivers AN, Walenkamp AM
and Links TP: Calculating optimal surveillance for detection of von
Hippel-Lindau-related manifestations. Endocr Relat Cancer.
21:63–71. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Palmer LS and Linehan WM: Editorial
comment. J Urol. 183:2351. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Iida K, Okimura Y, Takahashi K, Inomata S,
Iguchi G, Kaji H and Chihara K: A variety of phenotype with R161Q
germline mutation of the von Hippel-Lindau tumor suppressor gene in
Japanese kindred. Int J Mol Med. 13:401–404. 2004.PubMed/NCBI
|
|
8
|
Gong K, Zhang N, Zhang K and Na Y: The
relationship of erythropoietin overexpression with von
Hippel-Lindau tumour suppressor gene mutations between
hypoxia-inducible factor-1α and -2α in sporadic clear cell renal
carcinoma. Int J Mol Med. 26:907–912. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
David Dornbos 3III, Kim HJ, Butman JA and
Lonser RR: Review of the neurological implications of von
Hippel-Lindau disease. JAMA Neurol. 75:620–627. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Asthagiri AR, Mehta GU, Zach L, Li X,
Butman JA, Camphausen KA and Lonser RR: Prospective evaluation of
radiosurgery for hemangioblastomas in von Hippel-Lindau disease.
Neuro Oncol. 12:80–86. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lonser RR, Butman JA, Huntoon K, Asthagiri
AR, Wu T, Bakhtian KD, Chew EY, Zhuang Z, Linehan WM and Oldfield
EH: Prospective natural history study of central nervous system
hemangioblastomas in von Hippel-Lindau disease. J Neurosurg.
120:1055–1062. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nambu S, Otani R, Higuchi F, Uzuka T,
Matsuda H, Kim P and Ueki K: Histology of hemangioblastoma treated
with stereotactic radiosurgery confirms its effectiveness. J Clin
Neurosci. 51:43–45. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kim E and Zschiedrich S: Renal cell
carcinoma in von Hippel-Lindau disease-from tumor genetics to novel
therapeutic strategies. Front Pediatr. 6(16)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gossage L, Eisen T and Maher ER: VHL, the
story of a tumour suppressor gene. Nat Rev Cancer. 15:55–64.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Liu SJ, Wang JY, Peng SH, Li T, Ning XH,
Hong BA, Liu JY, Wu PJ, Zhou BW, Zhou JC, et al: Genotype and
phenotype correlation in von Hippel-Lindau disease based on
alteration of the HIF-α binding site in VHL protein. Genetics in
Medicine Official Journal of the American College of Medical
Genetics, 2018.
|
|
16
|
Liu Q, Yuan G, Tong D, Liu G, Yi Y, Zhang
J, Zhang Y, Wang LA, Wang L, Zhang D, et al: Novel
genotype-phenotype correlations in five Chinese families with Von
Hippel-Lindau disease. Endocr Connect. 7:870–878. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Smith ZL, Pietzak EJ, Meise CK, Van
Arsdalen K, Wein AJ, Malkowicz SB and Guzzo TJ: Simplification of
the Fuhrman grading system for renal cell carcinoma. Can J Urol.
22:8069–8073. 2015.PubMed/NCBI
|
|
18
|
Ben-Skowronek I and Kozaczuk S: Von
Hippel-Lindau Syndrome. Horm Res Paediatr. 84:145–152.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chou A, Toon C, Pickett J and Gill AJ: von
Hippel-Lindau syndrome. Front Horm Res. 41:30–49. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Arjumand W and Sultana S: Role of VHL gene
mutation in human renal cell carcinoma. Tumour Biol. 33:9–16.
2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tarade D and Ohh M: The HIF and other
quandaries in VHL disease. Oncogene. 37:139–147. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ordóñez-Navadijo Á, Fuertes-Yebra E,
Acosta-Iborra B, Balsa E, Elorza A, Aragonés J and Landazuri MO:
Mutant versions of von Hippel-Lindau (VHL) can protect HIF1α from
SART1-mediated degradation in clear-cell renal cell carcinoma.
Oncogene. 35:587–594. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Clifford SC, Cockman ME, Smallwood AC,
Mole DR, Woodward ER, Maxwell PH, Ratcliffe PJ and Maher ER:
Contrasting effects on HIF-1alpha regulation by disease-causing
pVHL mutations correlate with patterns of tumourigenesis in von
Hippel-Lindau disease. Hum Mol Genet. 10:1029–1038. 2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shuin T, Ashida S, Yao M and Kanno H: Von
Hippel-Lindau disease. Nihon Rinsho. 58:1448–1454. 2000.PubMed/NCBI(In Japanese).
|
|
25
|
Nordstrom-O'Brien M, van der Luijt RB, van
Rooijen E, van den Ouweland AM, Majoor-Krakauer DF, Lolkema MP, van
Brussel A, Voest EE and Giles RH: Genetic analysis of von
Hippel-Lindau disease. Hum Mutat. 31:521–537. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liu SJ, Wang JY, Peng SH, Li T, Ning XH,
Hong BA, Liu JY, Wu PJ, Zhou BW, Zhou JC, et al: Genotype and
phenotype correlation in von Hippel-Lindau disease based on
alteration of the HIF-alpha binding site in VHL protein. Genet Med.
20:1266–1273. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Young RM and Simon MC: Untuning the tumor
metabolic machine: HIF-α: pro- and antitumorigenic? Nat Med.
18:1024–1025. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Hoffman MA, Ohh M, Yang H, Klco JM, Ivan M
and Kaelin WG Jr: von Hippel-Lindau protein mutants linked to type
2C VHL disease preserve the ability to downregulate HIF. Hum Mol
Genet. 10:1019–1027. 2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Niemelä M, Lemeta S, Sainio M, Rauma S,
Pukkala E, Kere J, Böhling T, Laatikainen L, Jääskeläinen J and
Summanen P: Hemangioblastomas of the retina: Impact of von
Hippel-Lindau disease. Invest Ophthalmol Vis Sci. 41:1909–1915.
2000.PubMed/NCBI
|
|
30
|
González Escobar AB, Morillo Sánchez MJ
and García-Campos JM: Von Hippel-Lindau disease: Family study. Arch
Soc Esp Oftalmol. 87:368–372. 2012.PubMed/NCBI View Article : Google Scholar : (In Spanish).
|
|
31
|
Richard S, Gardie B, Couvé S and Gad S:
Von Hippel-Lindau: How a rare disease illuminates cancer biology.
Semin Cancer Biol. 23:26–37. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lefevre A, Mathis T, Denis P and Kodjikian
L: Retinal hemangioblastoma: Treatment strategy and long-term
follow-up in a retrospective cohort. J Fr Ophtalmol. 41:164–169.
2018.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
33
|
Woodward ER and Maher ER: Von
Hippel-Lindau disease and endocrine tumour susceptibility. Endocr
Relat Cancer. 13:415–425. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sovinz P, Urban C, Uhrig S, Stepan V,
Lackner H, Schwinger W, Benesch M, Moser A, Spuller E and Speicher
MR: Pheochromocytoma in a 2.75-year-old-girl with a germline von
Hippel-Lindau mutation Q164R. Am J Med Genet A. 152A:1752–1755.
2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Colvin A, Saltzman AF, Walker J, Bruny J
and Cost NG: Metastatic pheochromocytoma in an asymptomatic
12-Year-Old with von Hippel-Lindau disease. Urology. 119:140–142.
2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Petr EJ and Else T: Genetic predisposition
to endocrine tumors: diagnosis, surveillance and challenges in
care. Semin Oncol. 43:582–590. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lenders JW, Duh Q-Y, Eisenhofer G,
Gimenez-Roqueplo AP, Grebe SK, Murad MH, Naruse M, Pacak K and
Young WF Jr: Endocrine Society. Pheochromocytoma and paraganglioma:
An endocrine society clinical practice guideline. J Clin Endocrinol
Metab. 99:1915–1942. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tirosh A, Sadowski SM, Linehan WM, Libutti
SK, Patel D, Nilubol N and Kebebew E: Association of VHL genotype
with pancreatic neuroendocrine tumor phenotype in patients with von
Hippel-Lindau disease. JAMA Oncol. 4:124–126. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Krauss T, Ferrara AM, Links TP, Wellner U,
Bancos I, Kvachenyuk A, Villar Gómez de Las Heras K, Yukina MY,
Petrov R, Bullivant G, et al: Preventive medicine of von
Hippel-Lindau disease-associated pancreatic neuroendocrine tumors.
Endocr Relat Cancer. 25:783–793. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wang J-Y, Peng S-H, Li T, Ning XH, Liu SJ,
Hong BA, Liu JY, Wu PJ, Zhou BW, Zhou JC, et al: Risk factors for
survival in patients with von Hippel-Lindau disease. J Med Genet.
55:322–328. 2018.PubMed/NCBI View Article : Google Scholar
|