Introduction
Endometrial cancer (EC) is the most frequently
occurring malignant gynecological cancer (1). Globally, it accounts for approximately
5% of total cancer cases with a mortality rate of over 2% in women.
EC can be grossly divided into two groups. The majority of cases of
EC are type I, estrogen and progesterone receptor-positive cancers
with chronic estrogen exposure and insufficient opposing
progesterone exposure (2,3). In contrast, poorly differentiated type
II ECs are primarily uterine serous carcinomas and are correlated
with poor prognoses (4,5). Although numerous patients suffering
from EC in a low-grade or early-stage can have a good prognosis,
the prognosis of metastatic or late-stage EC is still poor
(6,7). Therefore, valid therapies for advanced
EC are needed.
Metastasis is an essential process in the
progression of cancer and can be divided into two steps. First, the
cancer cells translocate to a distant organ. The translocated cells
then transform into a metastasis at the organ (8,9). In EC,
the metastatic process relies on alterations in the extracellular
matrix (ECM) and cell-cell adhesion (10,11). A
potential signaling event in the metastasis of EC is the
overexpression of focal adhesion kinase (FAK) (12). FAK is known for its essential role in
integrin signaling and is overexpressed in a number of human
tumors. It is also essential in a variety of cell processes such as
growth, survival, invasion and migration (13-15).
Furthermore, it has been shown that the migration of cancer cells
can be blocked after inhibiting activation of the FAK signaling
pathway (16,17).
It has been reported that collagen triple helix
repeat containing 1 (CTHRC1) plays an important role in vascular
remodeling, morphogenesis and bone formation (18-20).
Furthermore, recent studies have shown that CTHRC1 can be detected
in human solid cancers, such as lung, breast, thyroid, pancreatic
and ovarian cancers, and could reduce collagen matrix deposition
(21-25).
Although it has been reported that CTHRC1 is overexpressed in a
number of human solid cancers, the molecular mechanism of CTHRC1
action in EC cells is unclear. It has been reported that CTHRC1 may
promote the migration of pancreatic cancer cells via activation of
the FAK signaling pathway (26). A
previous study indicated that CTHRC1 is overexpressed in EC
(27). However, the detailed
mechanisms of CTHRC1 in EC remain unknown. Therefore, the goal of
the current research was to explore the role of CTHRC1 in EC.
To the best of our knowledge, the present study is
the first to indicate that CTHRC1 may promote the migration of EC
cells. CTHRC1 was seen to be highly expressed in EC tissues. In
addition, overexpression of CTHRC1 promoted the migration of EC
cells in vitro. Recombinant CTHRC1 protein also promoted
migration of EC cells. The results of the present study indicated
that CTHRC1 mediated the migration of EC cells via the FAK
signaling pathway.
Materials and methods
Ethics
The present study was approved by the Human
Investigation Ethics Committee of the Shanghai First Maternity and
Infant Hospital (Shanghai, China). Samples were collected from
patients after receiving their written informed consent.
CTHRC1 expression and Kaplan-Meier
survival curves analysis in data from The Cancer Genome Atlas
(TCGA) database
Chandrashekar et al (28) reported an analysis platform on
(http://ualcan.path.uab.edu/analysis.html) using the
TCGA database. 546 EC and 35 normal tissue specimens were analyzed
on the platform, where the mRNA expression levels of CTHRC1 were
then compared between normal and EC groups from the TCGA database
on the website, as previously described (28). The significance threshold was set at
P<0.05 following t-test. Kaplan-Meier survival curve analysis of
162 high CTHRC1 expression and 216 low CTHRC1 expression EC
patients was performed using OncoLnc (http://www.oncolnc.org) (29). To separate patients into high/low
expression groups, the 30% upper percentile and 40% lower
percentile, as calculated using ROC, were used as the cut-off. The
significance threshold was set at P<0.05 following a Mantel-Cox
test.
Immunohistochemistry (IHC)
A total of 18 EC and 11 adjacent normal endometrial
tissues, which were excised 2 cm from the tumor tissues, were
obtained from female Chinese patients (Set 1; age range, 50-65
years; mean age, 59±2.77 years) who had undergone surgical
treatment between January 2017 and December 2019 at the Shanghai
First Maternity and Infant Hospital (Shanghai, China). No patient
had undergone endocrine therapy, radiotherapy or chemotherapy prior
to surgery. Endometrial tissues were sliced into 5-µm thick
sections and embedded in paraffin. Next, sections were mounted on
poly-L-lysine-coated slides and then deparaffinized in xylene and
dehydrated with gradient ethanol. Antigen retrieval was performed
in 10 mM citrate buffer (pH 6.0) for 10 min. Endogenous peroxidase
activity was blocked with 3% hydrogen peroxide for 10 min at room
temperature. Following blocking with 1% horse serum albumin
(Beijing Solarbio Science & Technology Co., Ltd.) for 20 min at
room temperature, the sections were incubated with rabbit
polyclonal antibody against CTHRC1 (cat. no. ab85739; 1:100; Abcam)
overnight at 4˚C and then incubated with rabbit polyclonal
secondary antibody (cat. no. ab205718; 1:20,000; Abcam) at 37˚C for
30 min. The sections were stained with DAB for 5 min and re-stained
with hematoxylin for 2 min both at room temperature. The percentage
of positive tumor cells was graded as follows: 0, none; 1, 1-25%;
2, 26-50%; 3, 51-75%; and 4, 76-100%. Immunostaining intensity was
rated as follows: 0, none; 1, weak; 2, moderate; and 3, intense.
Specimens were considered immunopositive when ≥1% of the tumor
cells had clear evidence of immunostaining. An IHC score was
calculated for each specimen using the ImageJ software (version
1.8.0; National Institutes of Health), in which the percent
positive rating was multiplied by the intensity rating. Each
component of the tumor was scored independently by four
investigators who were blinded to the tissue origin and the results
were summed.
ELISA
Blood samples were obtained from a different set of
39 patients with EC and 12 healthy females (Set 2; age range, 40-65
years; mean age, 54±6.46 years) who underwent surgical treatment
between January and December 2018 at the Shanghai First Maternity
and Infant Hospital. None of the patients had undergone
radiotherapy, endocrine therapy or chemotherapy before surgery. The
healthy individuals were visiting the hospital for a health
examination. The blood was obtained from the elbow vein and was
centrifuged for 10 min at 851xg at 4˚C without anticoagulant,
following which the serum samples were collected. The serum was
frozen for storage at -80˚C. The expression level of CTHRC1 was
measured using a CTHRC1 ELISA kit (cat. no. EK13502; Signalway
Antibody LLC), following the manufacturer's instructions.
Cell culture and treatment
Human EC cell lines Ishikawa and ECC1 were acquired
from the Cell Bank of Shanghai Institute of Cell Biology, Chinese
Academy of Sciences. ECC1 cells were authenticated by STR profiling
and the locations of the cell samples were matched with the STR
data of ECC-1 cells found in the databases of the American Type
Culture Collection, Japanese Collection of Research Bioresources
Cell Bank and Riken BioResource Research Center. Ishikawa and ECC1
cells were cultured in Dulbecco's modified Eagle's Medium/Nutrient
Mixture F12 (DMEM/F12; Biological Industries) and RPMI-1640 medium
(1640; Biological Industries), respectively, each supplemented with
100 U/ml penicillin/streptomycin (Gibco; Thermo Fisher Scientific,
Inc.) and 10% fetal bovine serum (FBS; Biological Industries). The
cells were cultured at 37˚C in a 5% CO2 humidified
incubator. The Parental Cells group was cultured as aforementioned
without any treatment for use to show the basal expression level of
CTHRC1 via western blotting. Ishikawa and ECC1 cells cultured in
complete medium treated with PBS or recombinant protein CTHRC1 (100
µg/ml; Abcam), CTHRC1 with the FAK signaling inhibitor defactinib
(1 µM; cat. no. HY-12289; MedChemExpress) or recombinant protein
CTHRC1 (100 µg/ml) with the FAK signaling inhibitor Y15 (1 µM; cat.
no. HY-12444; MedChemExpress) were termed Control, +CTHRC1, +CTHRC1
+Defactinib or +CTHRC1 +Y15. All drug treatments were performed at
37˚C. Both cell lines were tested negative for mycoplasma.
Cell transfection
To overexpress human CTHRC1 in the Ishikawa and ECC1
EC cell lines, the coding sequence (5'-ATGCGACCCCAGGGCCCCGC-3') of
CTHRC1 was cloned into a lentiviral vector with
Ubi-MCS-3FLAG-SV40-puromycin using the Champion™ pET160 Gateway™
Expression Kit with Lumio™ Technology according to manufacturer's
protocols (Invitrogen; Thermo Fisher Scientific, Inc.). The Gateway
cloning protocols were followed. Both cell lines were infected with
non-target or CTHRC1-specific lentiviral particles in 12-well
plates with polybrene (6 mg/ml; Beijing Solarbio Science &
Technology Co., Ltd.). Puromycin (1 µg/ml) was added to the cells
to generate stable CTHRC1-overexpressing clones. The experimental
groups were termed negative control (NC) and CTHRC1 overexpressing
(CTHRC1-OE).
Protein extraction and western
blotting
To explore protein expression,
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology) with a 1% dilution of the protease inhibitor
phenylmethanesulfonyl fluoride (Beyotime Institute of
Biotechnology) were used to lyse the parental cells, NC, CTHRC1-OE,
control, +CTHRC1, +CTHRC1+Defactinib and +CTHRC1+Y15 cells. The
concentration of extracted protein was determined using a BCA
protein assay kit (Thermo Fisher Scientific, Inc.). The protein
samples were mixed with loading buffer and then boiled for 10 min.
A total of 20 µg protein were loaded into each lane of an 10%
SDS-PAGE gel for protein separation and then transferred to
polyvinylidene fluoride membranes. The membranes were blocked with
5% bovine serum albumin (Sangon Biotech Co., Ltd.) for 1 h at room
temperature and incubated with antibodies against CTHRC1 (cat. no.
ab85739; 1:1,000; Abcam), Phosphorylated (p)-FAKTyr397
(p-FAKTyr397; cat. no. 8556; 1:1,000; Cell Signaling
Technology, Inc.) or Total-FAK (T-FAK; cat. no. 3285; 1:1,000; Cell
Signaling Technology, Inc.) overnight at 4˚C. The membranes were
incubated with peroxidase-linked secondary anti-rabbit antibody
(cat. no. 7074; 1:2,000; Cell Signaling Technology, Inc.) for 1 h
at room temperature to detect the bound primary antibodies, and the
blotted proteins were visualized using Immobilon™ Western
Chemiluminescent HRP Substrate (EMD Millipore). The intensity of
the protein bands was quantified using ImageJ software (version
1.8.0; National Institutes of Health). The relative expression of
target proteins was described as a ratio relative to the expression
of GAPDH and statistical data were based on at least three
experimental replicates.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Fresh EC and adjacent non-tumor tissues (14 of each)
were acquired from female patients (Set 3; age range, 40-65 years;
mean age, 53±6.21 years) who underwent surgical treatment between
January 2016 and December 2018 at the Shanghai First Maternity and
Infant Hospital. The patients of Set 3 were different from Set 1
and Set 2. None of the patients had undergone radiotherapy,
endocrine therapy, or chemotherapy before surgery.
TRIzol® reagent (Thermo Fisher Scientific, Inc.) was
used to extract the total RNA. RT-qPCR was performed as previously
described (30). One Step TB
Green® PrimeScript™ RT-qPCR kit (Takara Bio, Inc.) was
used for RT-qPCR assays with the CFX96 Real Time-PCR Detection
System (Bio-Rad Laboratories, Inc.). RT-qPCR was performed under
the following thermocycling conditions: 30 sec at 95˚C followed by
40 cycles of 5 sec at 95˚C, 20 sec at 60˚C and 10 sec at 72˚C; 1
min at 95˚C, 30 sec at 60˚C; and 30 sec at 95˚C. Primer sequences
were as follows: CTHRC1 forward, 5'-AGGGCTGCTTTTAACTCTGGT-3' and
reverse, 5'-AGGGCTGCTTTTAACTCTGGT-3'; β-actin forward,
5'-AGCCTCGCCTTTGCCGAT-3' and reverse, 5'-CTTCTGACCCATGCCCACC-3'.
All experiments were performed at least three times. The relative
mRNA expression level was measured using the 2-ΔΔCq
method (31).
Cell proliferation assay
Cell proliferation was assessed by Cell Counting
Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc.).
Ishikawa and ECC1 cells were transfected first as aforementioned
and then seeded into 96-well plates (4,000 cells/well). For CTHRC1
treatment, Ishikawa and ECC1 cells were seeded into a 96-well plate
(3,000 cells/well) and treated with either PBS (Control) or the
recombinant protein CTHRC1 (100 µg/ml; +CTHRC1) in complete medium.
After 0, 24 and 48 h, 10 µl of CCK-8 solution was added to each
well, followed by further incubation of the plates at 37˚C for 4 h.
Subsequently, a microplate reader (Molecular Devices, LLC) was used
to measure the ultraviolet absorbance of all samples at 450 nm. All
experiments were performed at least three times.
Cell migration and invasion
assays
For the migration assay, Ishikawa and ECC1 cells
were transfected first as aforementioned, then harvested and
re-suspended in serum-free DMEM/F12 and RPMI-1640 medium
(4x105 cells/ml), respectively, and then were placed
into the upper Transwell® chamber (8x104
cells/well; pore size, 8 µm; Corning, Inc.). For CTHRC1 treatment,
Ishikawa and ECC1 cells were harvested, re-suspended and then
placed into the upper Transwell® chambers. PBS (Control)
or recombinant protein CTHRC1 (100 µg/ml; +CTHRC1) or recombinant
protein CTHRC1 (100 µg/ml) with defactinib (1 µM;
+CTHRC1+Defactinib) or recombinant protein CTHRC1 (100 µg/ml) with
Y15 (1 µM; +CTHRC1+Y15) were added to both the upper and bottom
chambers. For invasion assay, 50 µl Matrigel matrix (1:5;
pre-diluted with serum-free medium; incubated at 4˚C for
pre-coating liquid and at 37˚C for 30 min for gelation; BD
Biosciences) was put onto the upper chamber and 2x105
transfected Ishikawa and ECC1 cells were seeded into the Matrigel
matrix, which was pre-coated at 37˚C, 2 h later. For CTHRC1
treatment, Ishikawa and ECC1 cells were seeded into the Matrigel
matrix as aforementioned and treated with either Control or
+CTHRC1. After that, 800 µl DMEM/F12 and RPMI-1640 medium
supplemented with 20% FBS were added into the lower chamber. After
16 h (migration assay) or 24 h incubation (invasion assay with
Matrigel pre-coating) at 37˚C, cells were stained with Calcein-AM
(0.2 µg/ml; cat. no. C3100MP; Invitrogen; Thermo Fisher Scientific,
Inc.) for 30 min at 37˚C. Leica DMi8 inverted fluorescent
microscope (magnification, x200; Leica Microsystems GmbH) was used
to observe cell migration and invasion. The number of cells that
had migrated or invaded was counted using MetaMorph®
Microscopy Automation and Image Analysis Software (version 2.5;
Molecular Devices, LLC). Five fields of view were imaged per
chamber. Each migration and invasion assay was repeated three times
on different days with different batches of cells. All experiments
were performed at least three times.
Wound healing assay
Ishikawa and ECC1 cells were transfected first as
aforementioned and seeded into 6-well plates at a concentration of
1x106 cells/well until they reached ≥90% confluence. The
monolayer was scratched with a sterile 200 µl pipette tip and then
cell debris was removed using PBS three times, followed by
incubation in serum-free medium. For CTHRC1 treatment, Ishikawa and
ECC1 cells were seeded as aforementioned and treated with PBS
(Control) or recombinant protein CTHRC1 (100 µg/ml; +CTHRC1) or
recombinant protein CTHRC1 (100 µg/ml) with defactinib (1 µM;
+CTHRC1+Defactinib) or recombinant protein CTHRC1 (100 µg/ml) with
Y15 (1 µM; +CTHRC1+Y15) in complete medium until they reached ≥90%
confluence. The cells were incubated in serum-free medium treated
with Control or +CTHRC1 or +CTHRC1+Defactinib or +CTHRC1+Y15 after
scratching. The margin of the wound was detected using a Leica DMi8
inverted light microscope (magnification, x50; Leica Microsystems
GmbH) at 0 and 24 h. In total, four fields of view were imaged for
every well. All experiments were repeated at least three times.
Cell immunofluorescence
Ishikawa and ECC1 cells were transfected first as
aforementioned, following which they were seeded into 35 mm
confocal dishes and cultured in complete medium overnight. For
CTHRC1 treatment, Ishikawa and ECC1 cells were seeded as above and
cultured in complete medium treated with PBS (Control) or
recombinant protein CTHRC1 (100 µg/ml; +CTHRC1) overnight. Cells
were then fixed with 4% paraformaldehyde for 10 min at room
temperature, permeabilized with 0.1% Triton X-100 for 10 min at
room temperature and blocked with 5% goat serum (Beijing Solarbio
Science & Technology Co., Ltd.) for 30 min at room temperature
before incubation with antibody against vinculin (cat. no.
ab129002; 1:100; Abcam) at 4˚C overnight. The cells were then
incubated with Alexa Fluor® 555 secondary antibody (cat.
no. P0179; 1:1,000; Beyotime Institute of Biotechnology) for 1 h at
room temperature and then phalloidin-FITC (cat. no. C1033; 1:100;
Beyotime Institute of Biotechnology) for 30 min at room
temperature. Cells were counterstained with DAPI (cat. no. C1002;
1:500; Beyotime Institute of Biotechnology) for 2 min at room
temperature before being analyzed using a confocal microscope
(magnification, x100; Leica TCS SP8; Leica Microsystems GmbH).
Statistical analysis
SPSS version 23.0 (IBM Corp.) was used for
statistical analysis and graphs were edited using GraphPad Prism
version 6.0 (GraphPad Software, Inc.). Measurement data were
analyzed using unpaired or paired Student's t-test, or one-way
analysis of variance, as appropriate. Multiple comparisons with
equal variances assumed were performed by Tukey's post hoc test.
Each experiment was performed in triplicate. All data are presented
as the mean ± SD. Differences were considered statistically
significant at P<0.05.
Results
CTHRC1 is expressed in EC tissues
To verify CTHRC1 expression patterns, IHC analysis
was performed on samples from EC (n=18) and normal endometrium
(n=11) tissues. IHC results showed stronger CTHRC1 staining in the
representative EC tissues than in the normal endometrial tissues
(P<0.001; Fig. 1A). To explore
the expression of CTHRC1 in EC, the relative RNA expression levels
of CTHRC1 were compared in adjacent normal tissues (n=14) and EC
tissues (n=14) using RT-qPCR. CTHRC1 expression was significantly
increased in EC tissues compared with normal tissues (P<0.05;
Fig. 1B). ELISA was performed to
determine the concentration of secreted CTHRC1 in plasma. CTHRC1
release was significantly increased in the EC group (n=39) compared
to the normal group (n=12) (P<0.05; Fig. 1C). The results indicated that the
expression levels of CTHRC1 in EC tissues were also significantly
increased compared to normal tissues (P<0.001; Fig. 1D). Furthermore, patients where EC
samples had high CTHRC1 expression had worse survival rates than
patients where EC samples had low CTHRC1 expression (P<0.05;
log-rank; Fig. 1E). Taken together,
these results indicated that CTHRC1 was significantly increased in
EC.
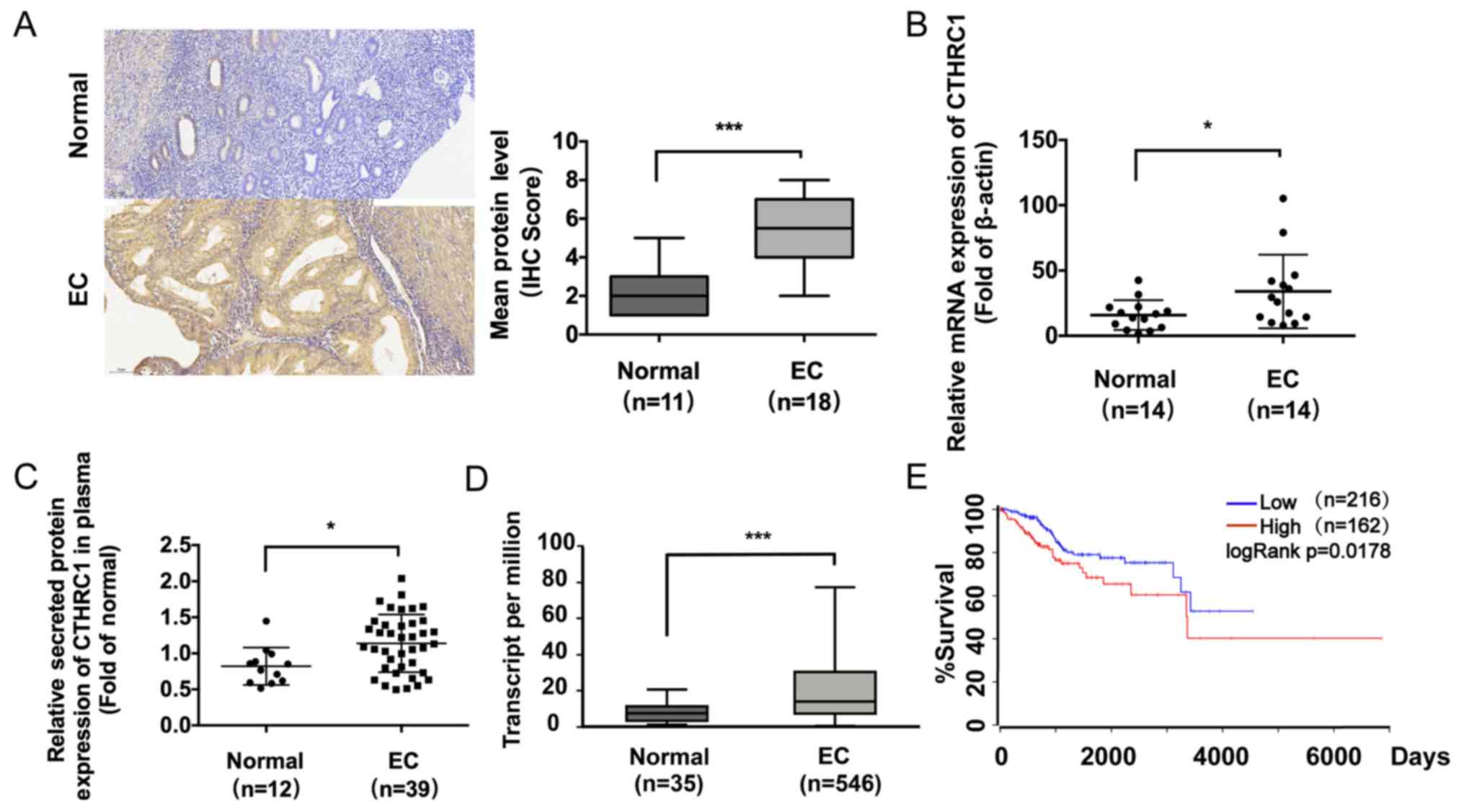 | Figure 1CTHRC1 is commonly upregulated in EC.
(A) Immunohistochemical analysis of CTHRC1 expression in adjacent
normal endometrium (n=11) and EC (n=18) tissues. Magnification,
x200. No or weak expression of CTHRC1 was observed in normal
endometrium tissues. Strong cell membrane and cytoplasmic
expression of CTHRC1 was observed in most EC tissues. (B) Relative
RNA expression levels of CTHRC1 in EC tissues (n=14) and adjacent
normal tissues (n=14) were assessed by reverse
transcription-quantitative PCR analysis. (C) ELISA analysis of
levels of secreted CTHRC1 in the plasma of EC patients or normal
females (EC group, n=39; normal group, n=12). (D) Relative mRNA
expression of CTHRC1 in the normal and EC groups in TCGA Uterine
Corpus Endometrial Cancer database (EC tissues, n=546; normal
tissues, n=35). (E) Kaplan-Meier curves of survival for EC patients
with high or low CTHRC1 expression (high CTHRC1 expression, n=162;
low CTHRC1 expression, n=216). Experiments were repeated in
triplicate. Data are presented as the mean ± SD.
*P<0.05, ***P<0.001. CTHRC1, collagen
triple helix repeat containing 1; EC, endometrial cancer; TCGA, The
Cancer Genome Atlas. |
Overexpression of CTHRC1 enhances the
migration of EC cells in vitro
To explore the effect of CTHRC1 on EC, CTHRC1 was
overexpressed in Ishikawa and ECC1 cells. CTHRC1 expression was
analyzed by western blotting (Fig.
2A). CCK-8 assay was performed to investigate the proliferative
ability of Ishikawa and ECC1 cells. There was no significant
difference in proliferation between the NC cells and CTHRC1-OE
cells in both Ishikawa and ECC1 cell lines (Fig. 2B). To explore the role of CTHRC1 on
the migration of Ishikawa and ECC1 cells, Transwell migration
assays were performed. As shown in Fig.
2C, compared with cells transfected with the empty vector,
migration was significantly increased in the CTHRC1-OE cells. In
addition, the relationship between CTHRC1 and cell migration was
investigated by wound healing assays. The results demonstrated that
cells in the CTHRC1-OE group showed more rapid wound closure
ability compared with the NC group (Fig.
2D). Transwell invasion assays revealed that invasion was
significantly increased in the CTHRC1-OE cells compared to the NC
cells (Fig. 2E). Cells were stained
for vinculin, a focal adhesion protein, and with phalloidin, a
cytoskeletal protein dye, to display the focal adhesion and
cytoactin remodeling. Confocal immunofluorescence staining revealed
weaker vinculin and stronger phalloidin staining in CTHRC1-OE cells
compared with the NC (Fig. 2F).
These results indicated that overexpression of CTHRC1 could promote
the migration of EC cells. However, there was no significant
influence on cell proliferation in vitro.
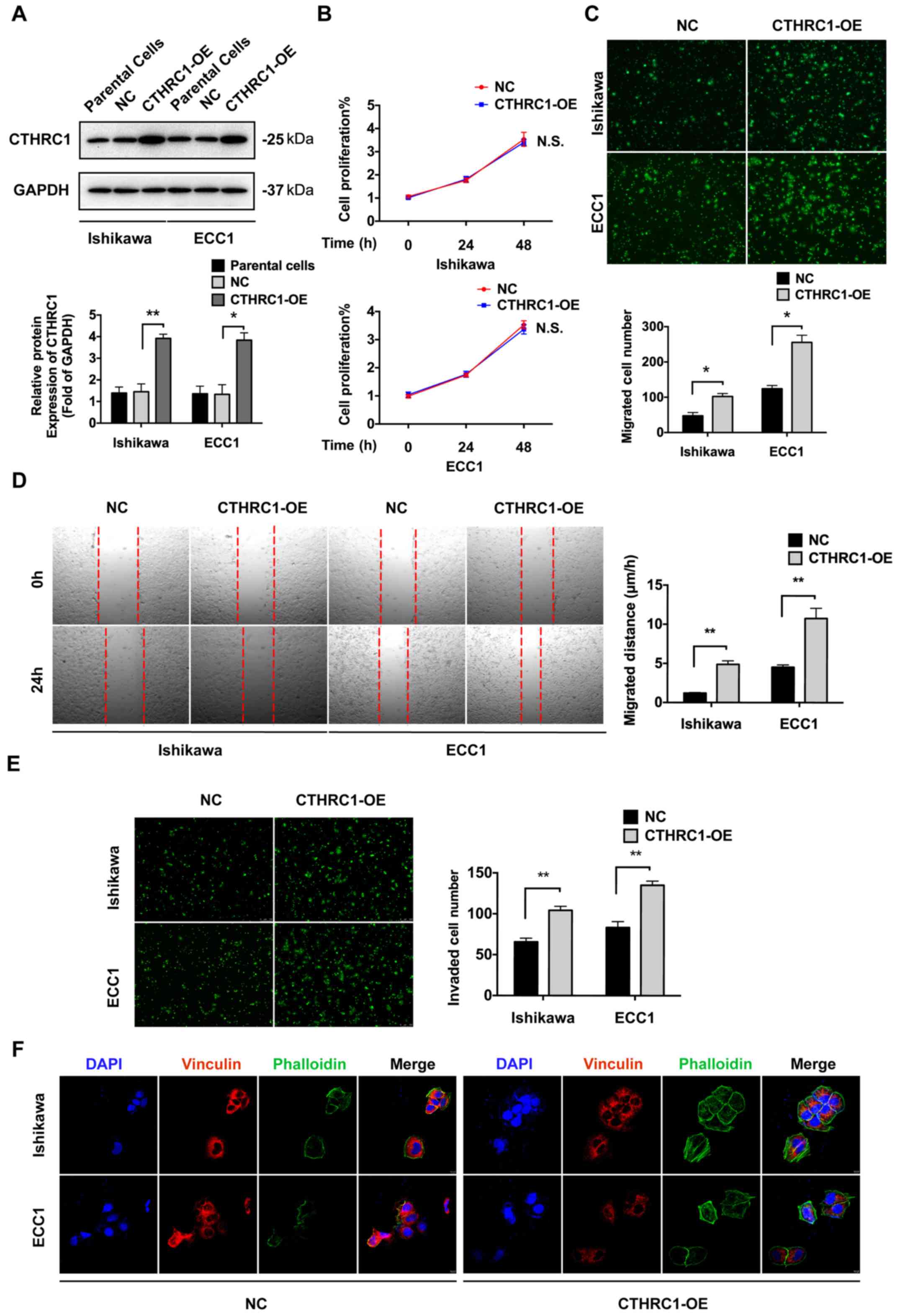 | Figure 2Overexpression of CTHRC1 promotes the
migration of EC cells in vitro. (A) Western blot analysis of
the expression levels of CTHRC1 in Ishikawa and ECC1 cells. (B)
Proliferation of Ishikawa and ECC1 cells with CTHRC1-OE was
confirmed by CCK-8 assays. (C) Migration of Ishikawa and ECC1 cells
with CTHRC1-OE confirmed by Transwell assays. Magnification, x200.
(D) Migration of Ishikawa and ECC1 cells with CTHRC1-OE confirmed
by wound healing assays. Magnification, x50. (E) Invasion of
Ishikawa and ECC1 cells with CTHRC1-OE confirmed by Transwell
assays with Matrigel. Magnification, x200). (F) Focal adhesion and
actin remodeling were analyzed by immunofluorescence.
Magnification, x100. Vinculin was marked by red fluorescence and
phalloidin, an actin stain, was marked by green fluorescence, while
the nucleus was stained with DAPI (blue). The results were
determined in triplicate. Data are presented as the mean ± SD.
*P<0.05, **P<0.01. CTHRC1, Collagen
triple helix repeat containing 1; EC, endometrial cancer; CCK-8,
Cell Counting Kit-8; OE, overexpression. |
Recombinant CTHRC1 promotes EC cell
migration in vitro
Ishikawa and ECC1 cells were treated with
recombinant CTHRC1 protein to investigate the effect of CTHRC1 on
EC. CCK-8 assays were performed to investigate the proliferation
ability of Ishikawa and ECC1 cells. There was no significant
difference in proliferation after treatment with CTHRC1 protein in
either Ishikawa or ECC1 cells (Fig.
3A). To investigate the effect of CTHRC1 on Ishikawa and ECC1
migration, Transwell migration assays were performed. Following
CTHRC1 treatment, migration was significantly enhanced in both ECC1
and Ishikawa cells (Fig. 3B).
Furthermore, wound healing assays were performed to investigate the
migration of EC cells. Cells in the +CTHRC1 group showed more rapid
wound closure ability compared with that in the Control group
(Fig. 3C). Transwell invasion assays
showed that invasion was significantly increased in +CTHRC1
compared with Control (Fig. 3D).
Confocal immunofluorescence staining revealed weaker vinculin and
stronger phalloidin staining in +CTHRC1 than that in Control
(Fig. 3E). These results indicated
that CTHRC1 treatment could enhance the migration of EC cells in
vitro.
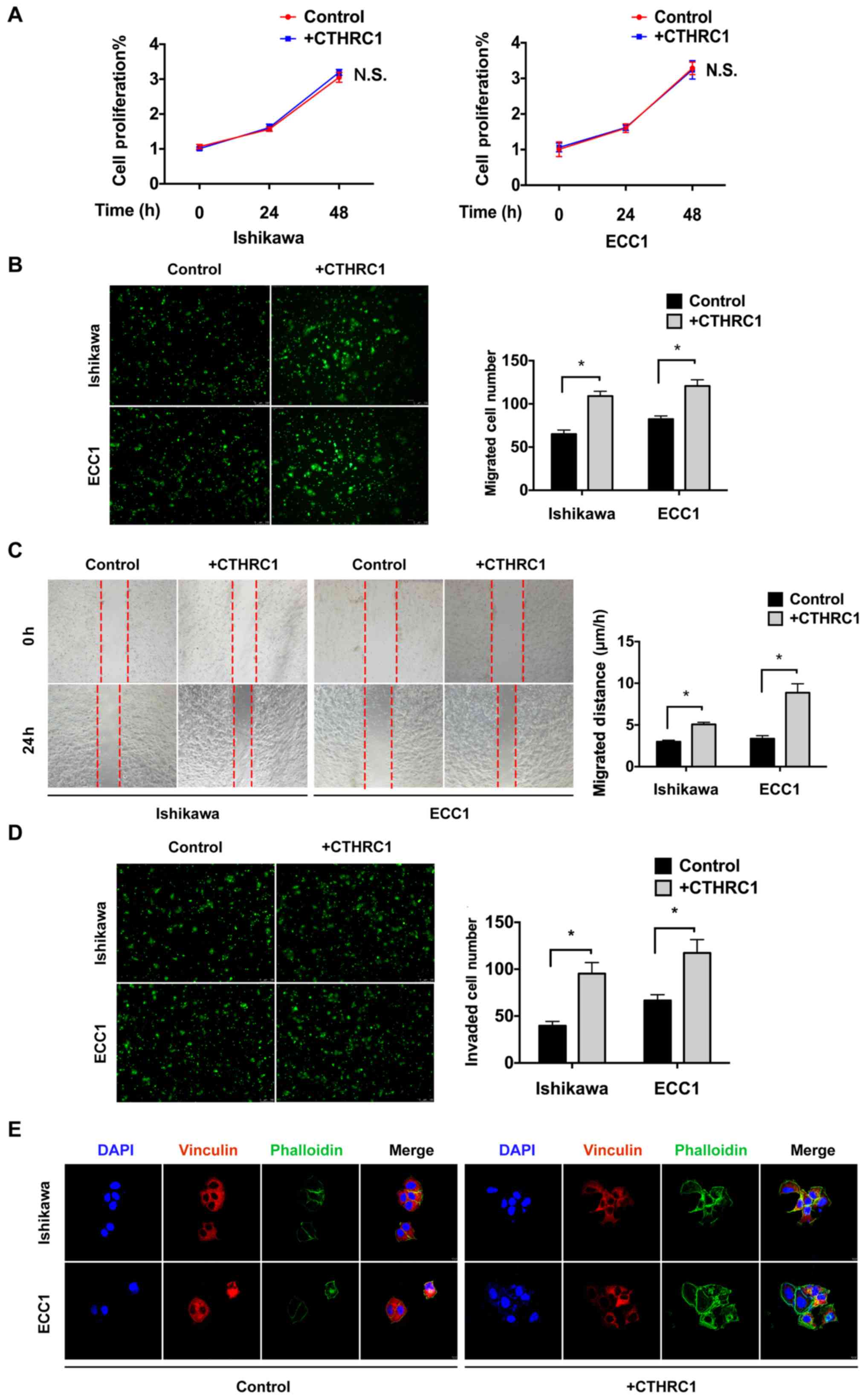 | Figure 3Recombinant CTHRC1-mediated promotion
of EC cell migration in vitro. (A) Proliferation of +CTHRC1
Ishikawa and ECC1 cells confirmed by CCK-8 assays. (B) Migration of
Ishikawa and ECC1 cells with +CTHRC1 confirmed by Transwell assays.
Magnification, x200. (C) Migration of Ishikawa and ECC1 cells with
+CTHRC1 confirmed by wound healing assays. Magnification, x50. (D)
Invasion of Ishikawa and ECC1 cells with +CTHRC1 confirmed by
Transwell assays with Matrigel. Magnification, x200. (E) Focal
adhesion and cyto-actin remodeling were analyzed by
immunofluorescence. Magnification, x100. Vinculin was marked by red
fluorescence and phalloidin was marked by green fluorescence, while
the nucleus was stained with DAPI (blue). The experiments were
performed in triplicate, data are presented as the mean ± SD.
*P<0.05. CTHRC1, Collagen triple helix repeat
containing 1; EC, endometrial cancer; CCK-8, cell counting kit-8;
+CTHRC1, cells with recombinant CTHRC1 protein treatment. |
CTHRC1 mediates the migration of EC
cells via the FAK signaling pathway
To explore the molecular mechanisms underlying
CTHRC1 activity, the signaling pathways responsible for
CTHRC1-mediated cell migration were investigated. P-FAK and T-FAK
are both important factors in the FAK signaling pathway. The
protein levels of P-FAK and T-FAK were examined by western blot
analysis. Overexpression of CTHRC1 led to significantly increased
expression of P-FAK compared to the NC cells (Fig. 4A). In addition, treatment with
recombinant protein CTHRC1 also led to increased expression of
P-FAK, however treatments on the +CTHRC1 cells with two FAK
signaling inhibitors, defactinib and Y15, respectively, led to
decreased expression of P-FAK compared to the +CTHRC1 group
(Fig. 4B). Transwell migration
assays and wound healing assays revealed that the two inhibitors of
the FAK signaling pathway led to reduced cell migration compared
with the +CTHRC1 group (Fig. 4C and
D). These results suggested that
CTHRC1 mediated the migration of EC cells via the FAK signaling
pathway (Fig. 4E).
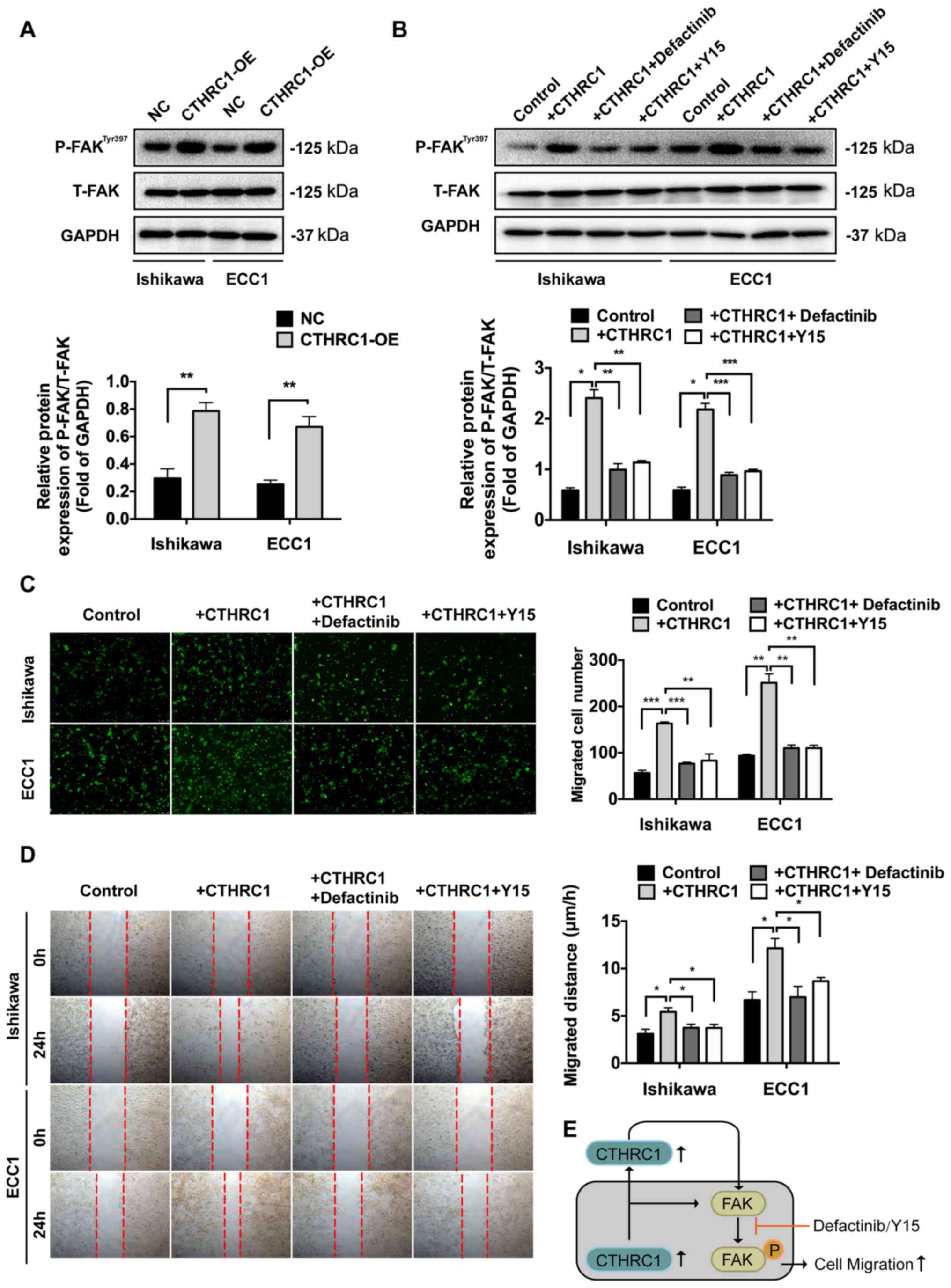 | Figure 4CTHRC1 mediates the migration of EC
cells via the FAK signaling pathway. (A) Western blot analysis of
P-FAKTyr397 and T-FAK in cells with CTHRC1-OE. (B)
Western blot analysis of P-FAKTyr397 and T-FAK in the
+CTHRC1 group or +CTHRC1 cells with defactinib or Y15. Migration
confirmed by (C) Transwell assays, magnification, x200; and (D)
wound healing assays. Magnification, x50. (E) The high expression
of CTHRC1 in EC cells and plasma promoted EC cell migration by
activating the FAK signaling pathway. The FAK signaling inhibitors,
defactinib and Y15, could reverse the enhanced migration mediated
by CTHRC1. The experiments were performed in triplicate. Data are
presented as the mean ± SD. *P<0.05,
**P<0.01, ***P<0.001. CTHRC1, collagen
triple helix repeat containing 1; EC, endometrial cancer; FAK,
focal adhesion kinase; P-FAK, phospho-FAK; T-FAK, Total-FAK; OE,
overexpression. |
Discussion
EC is one of the most common cancers in women. In
addition, the incidence and mortality rates of EC are expected to
rise both in developed and developing countries with the increasing
aging population and prevalence of obesity (32). Treatment for metastatic EC is
ineffective and metastasis is the major reason for relapse and
death, since there is no effective therapy for this condition
(33). Therefore, identifying the
molecular mechanisms of the peritoneal metastatic process is
crucial for developing novel preventive strategies. CTHRC1 has been
reported to be involved in bone formation, vascular remodeling and
morphogenesis (18-20).
Previous studies indicated that CTHRC1 was overexpressed in human
pancreatic cancer tissues, hepatocellular carcinoma, colorectal
cancer and gastrointestinal stromal tumors (34-37).
Overexpression of CTHRC1 is reported during tumor invasion and
metastasis (27). where it can
promote migration by activating FAK signaling in pancreatic cancer
(16). However, the role of CTHRC1
in EC is still unknown. The results of the present study suggest
that CTHRC1 is highly expressed in EC and can promote the migration
of EC cells in vitro. The present study also indicated that
CTHRC1 mediates the migration of EC cells via the FAK signaling
pathway.
Expression levels of CTHRC1 were compared between EC
tissues and normal tissues and it was observed that CTHRC1 levels
were significantly higher in EC tissues than in normal and
precancerous tissues. Meanwhile, secreted CTHRC1 in plasma was
significantly increased in EC patients compared with controls. Data
from the TCGA database were consistent with these findings and
indicated that high CTHRC1 expression predicted a poor prognosis in
EC. To investigate the role of CTHRC1 in EC, CTHRC1 was
overexpressed in Ishikawa and ECC1 cells, two standard cellular
models of EC. The effects of CTHRC1 were examined via Transwell
migration assays, wound healing assays and Transwell invasion
assays with Matrigel. The results indicated that CTHRC1 could
promote cell migration in vitro. However, CCK-8 assays
showed that there was no significant effect of CTHRC1 on cell
proliferation. It has been shown that as carcinomas arising from
epithelial tissues progress to higher pathological grades of
malignancy, reflected in local invasion and distant metastasis, the
associated cancer cells typically develop alterations in their
shape as well as in their attachment to other cells and to the ECM
(38). Expression of genes encoding
cell-cell and cell-ECM adhesion molecules is demonstrably altered
in certain highly aggressive carcinomas, with those favoring
cytostasis typically being downregulated (39). To investigate the focal adhesion and
cytoactin remodeling of the CTHRC1-OE group, immunofluorescence was
performed to stain for vinculin, a focal adhesion protein, and for
filamentous actin, using cytoskeletal protein dye phalloidin. The
results indicated downregulated focal adhesion protein and an
altered cytoskeleton. Furthermore, the effect of CTHRC1 was
demonstrated via CTHRC1 protein treatment. CTHRC1 protein could
promote migration in vitro, but had no effect on cell
proliferation. Immunofluorescence showed analogous changes in the
+CTHRC1 group. Evidence to date supports the notion that CTHRC1
integrates multiple aggressive signaling pathways (40,41). The
FAK signaling pathway is known for its essential role in integrin
signaling and overexpressed in many human tumors. It is also
important in a variety of cellular functions such as growth,
survival, invasion and migration (13-15).
Furthermore, in previous studies, it was shown that migration of
cancer cells was decreased after inhibiting the activation of FAK
signaling (16,17). Overexpression of FAK has a potential
role in the initiation of metastatic signaling events (12). To further demonstrate the underlying
molecular mechanism of CTHRC1 in EC migration, the FAK signaling
pathway was investigated. Overexpression or treatment with CTHRC1
could promote an increase in the levels of P-FAK. Furthermore, the
two FAK signaling inhibitors, defactinib and Y15, reversed the
enhanced migration and the increased P-FAK levels mediated by
CTHRC1, respectively, indicating that CTHRC1 mediated the migration
of EC cells via the FAK signaling pathway. Therefore, the present
findings are the first to suggest a role for CTHRC1 in affecting
the migration of EC cells through the FAK signaling pathway.
In conclusion, the results of the present study
indicated that CTHRC1 was expressed at a higher level in EC tissues
than in normal tissues. Additionally, they suggested that CTHRC1
may promote the migration of EC cells in vitro via the FAK
signaling pathway. Therefore, the CTHRC1/FAK signaling pathway may
be a potential therapeutic target for the treatment of EC.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81672574) and the
Outstanding Youth Projects of Shanghai Municipal Commission of
Health and Family Planning (grant no. 2018YQ23).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request. The datasets generated and/or analyzed during the current
study are available in the TCGA repository and the results shown
here are in whole or part based upon data generated by the
following website: https://ualcan.path.uab.edu/analysis.html.
Authors' contributions
XH and YB performed all experiments and wrote the
manuscript. XWe, MW and YL were responsible for data processing,
figure processing and statistical analysis. XWa designed the study,
approved the final version to be published and agreed to be
accountable for all aspects of the work. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This research was approved by the Human
Investigation Ethics Committee of the Shanghai First Maternity and
Infant Hospital. Samples were collected from patients after
receiving their written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu Y, Patel L, Mills GB, Lu KH, Sood AK,
Ding L, Kucherlapati R, Mardis ER, Levine DA, Shmulevich I, et al:
Clinical significance of CTNNB1 mutation and Wnt pathway activation
in endometrioid endometrial carcinoma. J Natl Cancer Inst.
106(106)2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Amant F, Moerman P, Neven P, Timmerman D,
Van Limbergen E and Vergote I: Endometrial cancer. Lancet.
366:491–505. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Baum M, Budzar AU, Cuzick J, Forbes J,
Houghton JH, Klijn JG and Sahmoud T: ATAC Trialists' Group.
Anastrozole alone or in combination with tamoxifen versus tamoxifen
alone for adjuvant treatment of postmenopausal women with early
breast cancer: First results of the ATAC randomised trial. Lancet.
359:2131–2139. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sasaki M, Dharia A, Oh BR, Tanaka Y,
Fujimoto S and Dahiya R: Progesterone receptor B gene inactivation
and CpG hypermethylation in human uterine endometrial cancer.
Cancer Res. 61:97–102. 2001.PubMed/NCBI
|
|
5
|
Daikoku T, Hirota Y, Tranguch S, Joshi AR,
DeMayo FJ, Lydon JP, Ellenson LH and Dey SK: Conditional loss of
uterine Pten unfailingly and rapidly induces endometrial cancer in
mice. Cancer Res. 68:5619–5627. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Colombo N, Preti E, Landoni F, Carinelli
S, Colombo A, Marini C and Sessa C: ESMO Guidelines Working Group.
Endometrial cancer: ESMO Clinical Practice Guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 24 (Suppl
6):vi33–38. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yoney A, Yildirim C, Isikli L and Unsal M:
Prognostic factors and treatment outcomes in patients with operated
endometrial cancer: Analysis of 674 patients at a single
institution. J BUON. 16:64–73. 2011.PubMed/NCBI
|
|
8
|
Su JL, Yang PC, Shih JY, Yang CY, Wei LH,
Hsieh CY, Chou CH, Jeng YM, Wang MY, Chang KJ, et al: The
VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells.
Cancer Cell. 9:209–223. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Holst F, Werner HMJ, Mjøs S, Hoivik EA,
Kusonmano K, Wik E, Berg A, Birkeland E, Gibson WJ, Halle MK, et
al: PIK3CA amplification associates with aggressive phenotype but
not markers of AKT-mTOR signaling in endometrial carcinoma. Clin
Cancer Res. 25:334–345. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kodama J, Hasengaowa Kusumoto T, Seki N,
Matsuo T, Ojima Y, Nakamura K, Hongo A and Hiramatsu Y: Prognostic
significance of stromal versican expression in human endometrial
cancer. Ann Oncol. 18:269–274. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lin Q, Chen H, Zhang M, Xiong H and Jiang
Q: Knocking down FAM83B inhibits endometrial cancer cell
proliferation and metastasis by silencing the PI3K/AKT/mTOR
pathway. Biomed Pharmacother. 115(108939)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Alowayed N, Salker MS, Zeng N, Singh Y and
Lang F: LEFTY2 controls migration of human endometrial cancer cells
via focal adhesion kinase activity (FAK) and miRNA-200a. Cell
Physiol Biochem. 39:815–826. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nishita M, Enomoto M, Yamagata K and
Minami Y: Cell/tissue-tropic functions of Wnt5a signaling in normal
and cancer cells. Trends Cell Biol. 20:346–354. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang J, Wen T, Li Z, Che X, Gong L, Yang
X, Zhang J, Tang H, He L, Qu X, et al: MicroRNA-1224 inhibits tumor
metastasis in intestinal-type gastric cancer by directly targeting
FAK. Front Oncol. 9(222)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Balsas P, Palomero J, Eguileor A,
Rodríguez ML, Vegliante MC, Planas-Rigol E, Sureda-Gómez M, Cid MC,
Campo E and Amador V: SOX11 promotes tumor protective
microenvironment interactions through CXCR4 and FAK regulation in
mantle cell lymphoma. Blood. 130:501–513. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jiang H, Liu X, Knolhoff BL, Hegde S, Lee
KB, Jiang H, Fields RC, Pachter JA, Lim KH and DeNardo DG:
Development of resistance to FAK inhibition in pancreatic cancer is
linked to stromal depletion. Gut. 69:122–132. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yamaura T, Kasaoka T, Iijima N, Kimura M
and Hatakeyama S: Evaluation of therapeutic effects of FAK
inhibition in murine models of atherosclerosis. BMC Res Notes.
12(200)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Takeshita S, Fumoto T, Matsuoka K, Park
KA, Aburatani H, Kato S, Ito M and Ikeda K: Osteoclast-secreted
CTHRC1 in the coupling of bone resorption to formation. J Clin
Invest. 123:3914–3924. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Pyagay P, Heroult M, Wang Q, Lehnert W,
Belden J, Liaw L, Friesel RE and Lindner V: Collagen triple helix
repeat containing 1, a novel secreted protein in injured and
diseased arteries, inhibits collagen expression and promotes cell
migration. Circ Res. 96:261–268. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yang XM, You HY, Li Q, Ma H, Wang YH,
Zhang YL, Zhu L, Nie HZ, Qin WX, Zhang ZG, et al: CTHRC1 promotes
human colorectal cancer cell proliferation and invasiveness by
activating Wnt/PCP signaling. Int J Clin Exp Pathol. 8:12793–12801.
2015.PubMed/NCBI
|
|
21
|
He W, Zhang H, Wang Y, Zhou Y, Luo Y, Cui
Y, Jiang N, Jiang W, Wang H, Xu D, et al: CTHRC1 induces non-small
cell lung cancer (NSCLC) invasion through upregulating MMP-7/MMP-9.
BMC Cancer. 18(400)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tang L, Dai DL, Su M, Martinka M, Li G and
Zhou Y: Aberrant expression of collagen triple helix repeat
containing 1 in human solid cancers. Clin Cancer Res. 12:3716–3722.
2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Guo B, Yan H, Li L, Yin K, Ji F and Zhang
S: Collagen triple helix repeat containing 1 (CTHRC1) activates
Integrin β3/FAK signaling and promotes metastasis in ovarian
cancer. J Ovarian Res. 10(69)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tang Z, Wang Y, Liu X and Liu Q: An
immunohistochemical study of CTHRC1, Vimentin, E-cadherin
expression in papillary thyroid carcinoma. Lin Chung Er Bi Yan Hou
Tou Jing Wai Ke Za Zhi. 32:595–598. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
25
|
Park EH, Kim S, Jo JY, Kim SJ, Hwang Y,
Kim JM, Song SY, Lee DK and Koh SS: Collagen triple helix repeat
containing-1 promotes pancreatic cancer progression by regulating
migration and adhesion of tumor cells. Carcinogenesis. 34:694–702.
2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lee J, Song J, Kwon ES, Jo S, Kang MK, Kim
YJ, Hwang Y, Bae H, Kang TH, Chang S, et al: CTHRC1 promotes
angiogenesis by recruiting Tie2-expressing monocytes to pancreatic
tumors. Exp Mol Med. 48(e261)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li LY, Yin KM, Bai YH, Zhang ZG, Di W and
Zhang S: CTHRC1 promotes M2-like macrophage recruitment and
myometrial invasion in endometrial carcinoma by integrin-Akt
signaling pathway. Clin Exp Metastasis. 36:351–363. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chandrashekar DS, Bashel B, Balasubramanya
SA, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BV and Varambally
S: UALCAN: A portal for facilitating tumor subgroup gene expression
and survival analyses. Neoplasia. 19:649–658. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Anaya J: OncoLnc: Linking TCGA survival
data to mRNAs, miRNAs, and lncRNAs. PeerJ Comput Sci.
2(e67)2016.
|
|
30
|
Chen G, Wang D, Zhao X, Cao J, Zhao Y,
Wang F, Bai J, Luo D and Li L: miR-155-5p modulates malignant
behaviors of hepatocellular carcinoma by directly targeting CTHRC1
and indirectly regulating GSK-3β-involved Wnt/β-catenin signaling.
Cancer Cell Int. 17(118)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Saed L, Varse F, Baradaran HR, Moradi Y,
Khateri S, Friberg E, Khazaei Z, Gharahjeh S, Tehrani S,
Sioofy-Khojine AB, et al: The effect of diabetes on the risk of
endometrial Cancer: An updated a systematic review and
meta-analysis. BMC Cancer. 19(527)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Nomura H, Aoki D, Michimae H, Mizuno M,
Nakai H, Arai M, Sasagawa M, Ushijima K, Sugiyama T, Saito M, et
al: Japanese Gynecologic Oncology Group: Effect of taxane plus
platinum regimens vs doxorubicin plus cisplatin as adjuvant
chemotherapy for endometrial cancer at a high risk of progression:
A Randomized Clinical Trial. JAMA Oncol. 5:833–840. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Durmus T, LeClair RJ, Park KS, Terzic A,
Yoon JK and Lindner V: Expression analysis of the novel gene
collagen triple helix repeat containing-1 (Cthrc1). Gene Expr
Patterns. 6:935–940. 2006.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lau EY, Lo J, Cheng BY, Ma MK, Lee JM, Ng
JK, Chai S, Lin CH, Tsang SY, Ma S, et al: Cancer-associated
fibroblasts regulate tumor-initiating cell plasticity in
hepatocellular carcinoma through c-Met/FRA1/HEY1 signaling. Cell
Rep. 15:1175–1189. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tan F, Liu F, Liu H, Hu Y, Liu D and Li G:
CTHRC1 is associated with peritoneal carcinomatosis in colorectal
cancer: A new predictor for prognosis. Med Oncol.
30(473)2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ma MZ, Zhuang C, Yang XM, Zhang ZZ, Ma H,
Zhang WM, You H, Qin W, Gu J, Yang S, et al: CTHRC1 acts as a
prognostic factor and promotes invasiveness of gastrointestinal
stromal tumors by activating Wnt/PCP-Rho signaling. Neoplasia.
16:265–278, 278.e1-278.e13. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Berx G and van Roy F: Involvement of
members of the cadherin superfamily in cancer. Cold Spring Harb
Perspect Biol. 1(a003129)2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Cavallaro U and Christofori G: Cell
adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev
Cancer. 4:118–132. 2004.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wang P, Wang YC, Chen XY, Shen ZY, Cao H,
Zhang YJ, Yu J, Zhu JD, Lu YY and Fang JY: CTHRC1 is upregulated by
promoter demethylation and transforming growth factor-β1 and may be
associated with metastasis in human gastric cancer. Cancer Sci.
103:1327–1333. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Liu G, Sengupta PK, Jamal B, Yang HY,
Bouchie MP, Lindner V, Varelas X and Kukuruzinska MA:
N-glycosylation induces the CTHRC1 protein and drives oral cancer
cell migration. J Biol Chem. 288:20217–20227. 2013.PubMed/NCBI View Article : Google Scholar
|


















