Introduction
Meibomian gland dysfunction (MGD) is a clinical
condition that is commonly encountered by ophthalmologists
(1). The worldwide incidence varies
from 3.5% to as high as 70%, depending on the geographical region
(2-5).
Affected patients usually present with symptoms of ocular burning,
irritation, red and watery eyes, as well as variation of visual
acuity (1). The underlying pathology
involves blockage of the terminal ducts of Meibomian glands with or
without qualitative/quantitative changes in their glandular
secretion (1,6). MGD is characterized by acinous atrophy,
hyperkeratinisation of the ductal epithelium and increased
viscosity of the meibum. This leads to reduced glandular secretion
and encourages growth of commensal bacteria. Lipase secreted by
these bacteria brings about changes in the composition and melting
point of meibum lipids, which further inhibits glandular output.
The reduced secretion of lipids thereby leads to evaporative dry
eye, hyperosmolarity and tear film instability (1,4,7,8).
Management of MGD is still empirical and studies
have evaluated a wide range of interventions with limited benefits.
These include conservative measures such as hot fomentation and lid
hygiene, as well as the use of steroidal or non-steroidal
anti-inflammatory agents and antibiotics (7-9).
Novel techniques, including meibomian gland probing (10,11),
intranasal neurostimulation (12),
intense pulsed light (IPL) (13-15)
and application of electronic heating devices (16), have also been evaluated, but data on
their efficacy are limited. Research is still ongoing to identify
promising treatment strategies for MGD that are not only
efficacious but also have a reasonable safety profile and are
acceptable for the patients (15-17).
IPL therapy is not new to the medical field, as it
has been widely used in dermatology for the management of a range
of conditions, including hypertrichosis, hemangiomas, venous
malformations and port-wine stains (18,19). IPL
works on the principle of applying a high-intensity non-laser light
source that produces wavelengths ranging from 1,500 to 1,200 nm
(19). On application to the skin,
the oxyhemoglobin in the red blood cells absorbs the light,
resulting in the generation of heat, which activates the
coagulation process leading to thrombosis of blood vessels
(17,19). The rationale that supports IPL as a
potential treatment strategy for MGD is that it has predominant
effects to accelerate thrombosis of abnormal blood vessels,
liquefaction of meibum leading to its improved secretion, reduced
growth and proliferation of pathogenic bacteria and reduction in
epithelial turnover (18). Apart
from these major effects, the procedure also initiates regional
photomodulatory effects, activates the fibroblasts and leads to
enhancement of collagen synthesis (18). A combination of these mechanisms
leads to improvements of the disease condition.
In the past decade, a number of studies have
investigated the efficacy of IPL in the management of MGD (20-31).
However, these standalone studies vary in terms of study design and
methodology. For routine use of IPL therapy in clinical practice,
there is a need for high-quality evidence. To the best of our
knowledge, there has been no previous study attempting to generate
high-level evidence for IPL therapy in MGD in the form of a
systematic review and meta-analysis. Therefore, the purpose of the
present study was to systematically review the literature and
perform a meta-analysis to assess the efficacy of IPL therapy in
the management of MGD.
Materials and methods
Search strategy
A thorough and systematic search was performed in
the EMBASE, PubMed, Cochrane Central, MEDLINE and Google Scholar
databases. Randomized controlled trials (RCTs) evaluating the
efficacy of IPL in the management of MGD were identified. Relevant
studies published since the year 1950 were eligible for inclusion
in the meta-analysis. The search was confined until July 2019. A
systematic search strategy was developed using the key terms
‘Meibomian gland disease’, ‘intense pulse light’ and ‘dry eye
disease’. There were no limitations regarding the language and only
studies on human subjects were eligible to be included. The
bibliographies of eligible articles were reviewed to identify
additional studies of relevance to this meta-analysis. The
Preferred Reporting Items for Systematic review and Meta-Analysis
guidelines were followed during the conduct of this review
(32).
Selection criteria and methods
A total of two authors (ST and HD) independently
reviewed citations and selected studies. After initial screening of
titles and abstracts and removal of duplicates, the full-text
articles of potentially suitable studies were reviewed. Any
discrepancies pertaining to the inclusion of studies were resolved
through discussion among the authors of the present study.
Inclusion criteria
For a study to be included in the meta-analysis, it
was required to be an RCT comparing the effect of IPL therapy for
MGD with a control. The study was required to include a population
of only MGD with pre-defined diagnostic criteria and to evaluate at
least one of the following outcome measures: Standard Patient
Evaluation of Eye Dryness (SPEED) score, tear break-up time (TBUT)
and Non-Invasive Tear Break-Up Time (NIBUT) score.
Exclusion criteria
Studies were excluded if they did not provide
extractable data on effect sizes (such as mean and standard
deviations for the score change) for relevant outcomes and if they
were non-randomized studies such as case reports, observational
studies (case-control, cohort studies) or review articles. Studies
including patients with a history of any ocular surgery and/or
those that used contact lenses, as well as patients who were not
followed up at future time-points, were also excluded.
Data extraction
Extraction of relevant data from included studies
was performed by two authors (HD and XH) independently using a
structured data extraction sheet. The following data were extracted
from the eligible studies: Surname of first author, year of
publication, country, study design, number of MGD participants
treated by IPL and placebo, mean age of patients in the IPL and
placebo group, sex distribution, type of intervention and
comparator, scale parameters measured (including SPEED and NIBUT),
IPL intensity, IPL machine used and follow-up duration.
Quality assessment
The methodological assessment was performed by two
authors (HD and XH) independently and any disagreements were
resolved by discussion among all of the authors. The Cochrane
Collaboration risk of bias assessment tool for RCTs was used for
quality assessment of the included trials (33). Studies were rated as low, high or
unclear risk of bias for: Random sequence generation, allocation
concealment, blinding of participants and personnel, blinding of
outcome assessment, incomplete outcome data, selective reporting
and other biases.
Statistical analysis
Statistical analysis was performed using Review
Manager [RevMan, version 5.3; Nordic Cochrane Centre (Cochrane
Collaboration)]. Pooled estimates for the continuous outcome
measures were reported as the standard mean difference (SMD) with
95% CI. The SMD was generated using the mean and standard deviation
(SD) reported in the individual RCTs included in the meta-analysis.
P<0.05 was considered to indicate statistical significance.
Heterogeneity was examined using Tau and Higgins' I2 and
it governed whether a fixed-effects model (I2 value
≤50%) or a random-effects model (I2 value >50%) was
used. Begg's funnel plot was used to visually inspect the presence
or absence of publication bias.
Results
Identification of relevant
studies
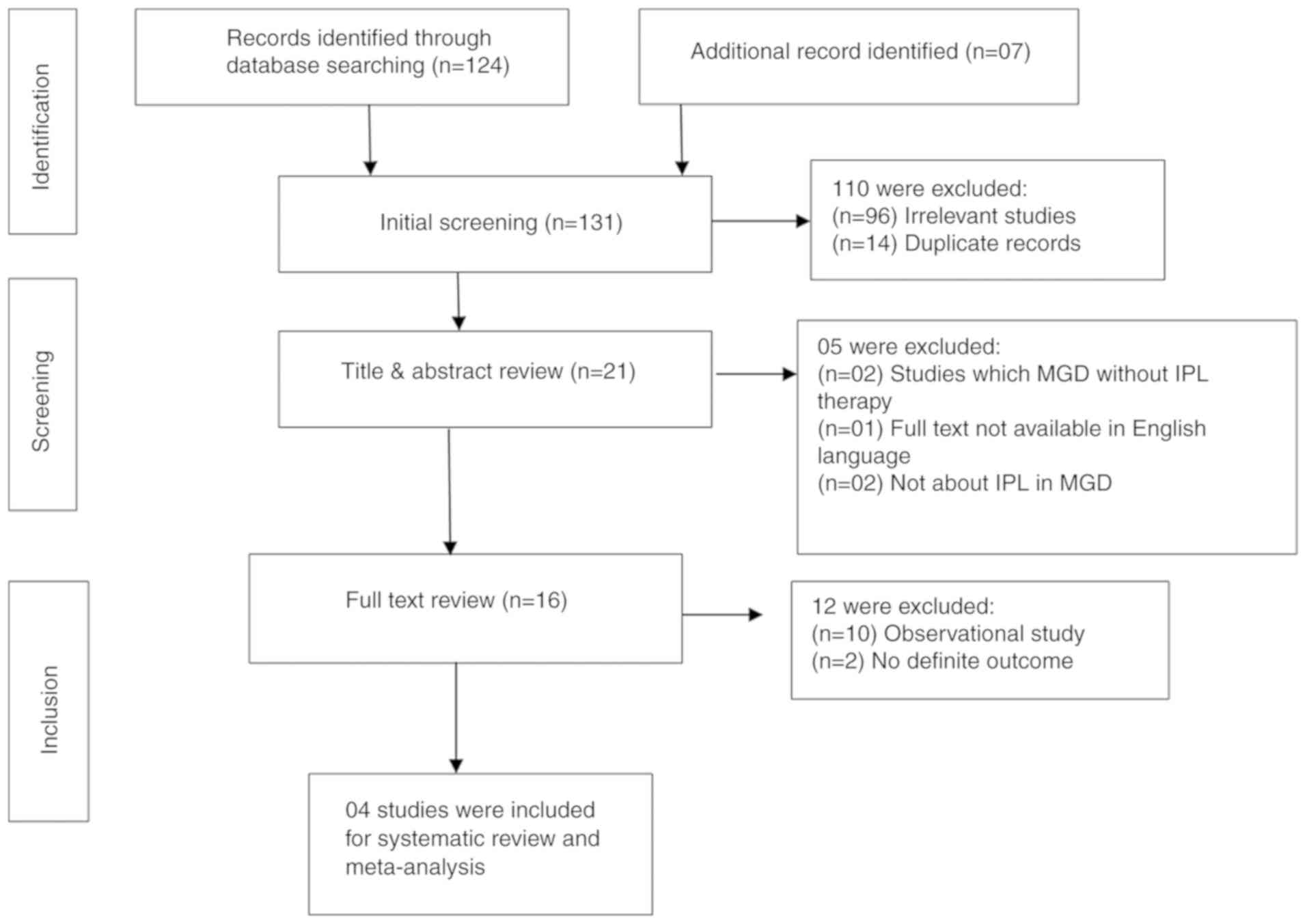
Fig. 1 presents a
flowchart of the literature search and indicates the process
through which the selection of articles was made for inclusion in
the present review. Using the pre-defined search strategy, a total
of 131 published articles were identified. Out of these, only 4
RCTs were finally included in the review. The reasons for exclusion
of studies were as follows: Not relevant to the topic (n=98),
duplicate records (n=14), studies with MGD but without IPL therapy
(n=2), observational studies (n=10), and full text not available in
English language (n=1).
Characteristics of the studies
The primary characteristics of the studies included
in the present systematic review and meta-analysis are listed in
Table I. Overall, four RCTs were
included in the meta-analysis, including 122 subjects with MGD and
120 control subjects. All of the included studies had obtained
approval from their institutional ethical committee and followed
the Declaration of Helsinki guidelines for research on human
subjects. The total sample size in the included studies ranged from
20 to 44 participants. In all studies included, data for only two
commonly used scales, i.e. SPEED and NIBUT score, were reported.
These scores were reported as the mean with SD; they were utilized
in the pooling of studies and subsequent calculation of effect
sizes with 95% CI. The IPL intensity ranged from 9 to 17
J/cm2. The follow-up ranged from 45 days to 9 months
(Table I).
 | Table IPrimary characteristics of the
published studies included in the present meta-analysis. |
Table I
Primary characteristics of the
published studies included in the present meta-analysis.
| | Sample size | Age (years), mean ±
standard deviation (range) | Sex (M/F) | |
|---|
| First author
(year) | Country | Study design | Duration of
inclusion | Diagnostic criteria
of MGD | IPL | Control | IPL | Control | IPL | Control | Intervention | Comparator | Measured
parameters | IPL intensity
(J/cm2), range | IPL machine
used | Follow-up
duration | (Refs.) |
|---|
| Craig (2015) | New Zealand | RCT | Not specified | Diagnosis based on
the ‘International Workshop on Meibomian Gland Dysfunction: Report
of the diagnosis subcommittee' (6) | 28 | 28 | 45±15 (22-73) | 45±15 (22-73) | 8/20 | 8/20 | IPL | Control eye | SPEED, LLG, NIBUT,
TER, TMH, VAS | 9-13 | E>Eye, (E-SWIN,
France) | 45 days | (20) |
| Rong (2018) | China | RCT | January2016-April
2017 | MGYSS of lower
eyelid of no more than 12, SPEED questionnaire score of at least 6
in both eyes | 28 | 28 | 42.17±17.62
(24-78) | 42.17±17.62
(24-78) | 10/18 | 10/18 | IPL | Control eye | MGYSS, TBUT, SPEED,
CFS | 14-16 | M22 IPL system
(Lumenis Ltd.) | 9 months | (25) |
| Rong (2018) | China | RCT | March-July
2016 | Obstruction of
gland orifices observed under slit lamp examination, MGYSS of lower
eyelid of no more than 12, SPEED questionnaire score of at least 6
in both eyes | 44 | 44 | 46.3±16.9
(23-86) | 46.3±16.9
(23-86) | 12/32 | 12/32 | IPL | Control eye | MGYSS, TBUT, SPEED,
CFS | 14-16 | M22 IPL system
(Lumenis Ltd.) | 12 wks | (24) |
| Arita (2019) | Japan | RCT | May 2016-August
2017 | Presence of ocular
symptoms, plugged gland orifices, vascularity and irregularity of
lid margins, and reduced meibum expression | 22 | 20 | 61.0±18.0
(23-81) | 61.9±12.2
(39-78) | 9/13 | 8/12 | IPL | Control eye | SPEED, NIBUT, BUT,
LLG, LLT, CFS score, Meibum grade & Meiboscore | 11-14 | M22 IPL system
(Lumenis Ltd.) | 32 wks | (22) |
Methodological quality
The quality of the individual studies is presented
in Table II. Adequate method of
randomization and allocation concealment were described in three
trials (20,24,25).
Blinding of participants and outcome assessment was employed by
three studies (20,24,25).
Attrition bias was high in one study (25). Only two studies were prospectively
registered (24,25).
 | Table IIQuality assessment of the studies
included in the meta-analysis. |
Table II
Quality assessment of the studies
included in the meta-analysis.
| Study | Random sequence
generation | Allocation
concealment | Blinding of
participants and personnel | Blinding of outcome
assessment | Incomplete outcome
data | Selective
reporting | Other bias |
|---|
| Craig et al
(20), 2015 | Low risk | Low risk | Low risk | Low risk | Low risk | Unclear risk | Low risk |
| Rong et al
(25), 2018 | Low risk | Low risk | Low risk | Low risk | High risk | Low risk | Low risk |
| Rong et al
(24) 2018 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Arita et al
(22), 2019 | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Unclear risk | Unclear risk |
Meta-analysis
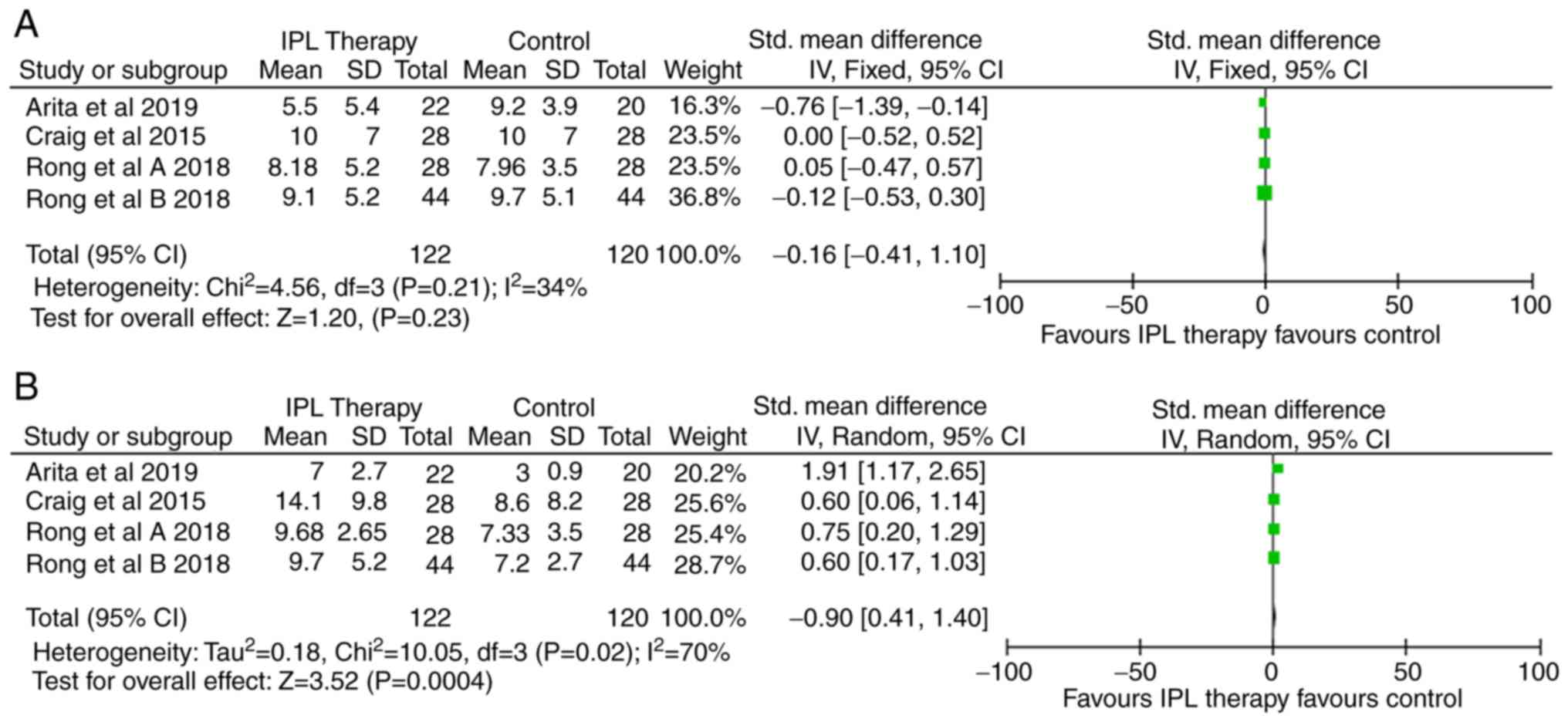
Data were synthesized from four RCTs employing IPL
therapy for the treatment of MGD, including 122 subjects in the IPL
arm and 120 subjects in the control arm. The results indicated a
non-significant reduction of MGD symptoms after IPL therapy based
on the SPEED score (SMD, -0.16; 95% CI, -0.41 to 0.10; Fig. 2A). However, there was a statistically
significant increase in the NIBUT score with IPL therapy compared
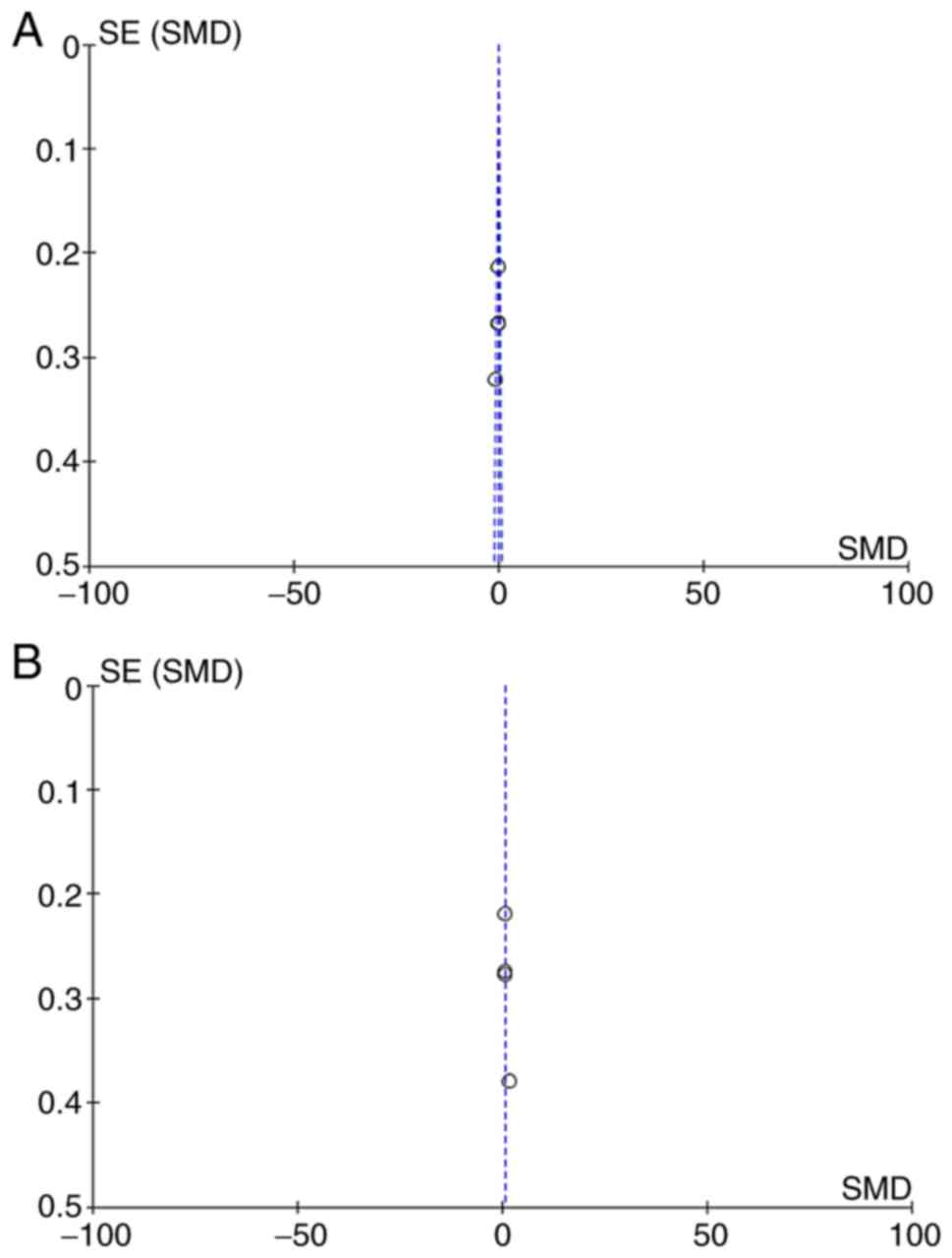
to the control arm (SMD, 0.90; 95% CI, 0.40 to 1.40; Fig. 2B). Begg's funnel plots were used to
assess potential publication bias of the included studies. The
shapes of the funnel plots suggested absence of publication bias
(Fig. 3A and B).
Salient features of the included
studies
The study by Craig et al (20) from 2015 was a prospective
placebo-controlled trial comprised of MGD patients. The study
indicated a substantial improvement in NIBUT, lipid layer grading
and tear film quality, as well as a decline in the SPEED score
provided by IPL treatment. The key strength of the study was that
only one eye of the participant was subjected to IPL treatment,
while the other was used as a control; this allowed for direct
comparison between the treatment group and the control group
without the requirement for documenting and adjusting for
confounders. However, the study also had various limitations.
First, the authors did not provide a comprehensive description of
the inclusion criteria and the participant selection method.
Furthermore, the study did not provide a comprehensive description
of the participant characteristics, which curtailed the external
validity of the results of the study. In addition, the study
indicated that the results obtained were cumulative in nature and
therefore, since the data collection was stopped at only day 45,
the full benefit of the treatment may not have been realized. As
another limitation, the lack of results in terms of SPEED scores
may have resulted from the short duration of the study and a
certain benefit may have been identified with a longer duration.
Finally, the control eye was exposed to light but had a white light
blocker to protect it. A more effective method would likely have
been to block all light or use no light at all, as it is possible
that a certain amount of light may have passed through the blocker.
Besides these issues, the authors did not perform any power
calculations to support that the sample size was adequate.
The study by Rong et al (25) from 2018 reported a significant
improvement of Meibomian gland function in the eyes with
experimental treatment compared to the control eyes in the same
patients, that received sham IPL treatment. The intervention and
control eyes received Meibomian gland expression (MGX) and
artificial tears. The study demonstrated that IPL treatment applied
directly on the eyelids provided sustained relief to patients with
MGD for at least 6 months by improving the secretion function of
the Meibomian gland, increasing TBUT and improving ocular symptoms.
In the eye receiving IPL, the Meibomian gland yielding secretion
score (MGYSS) of the upper as well as the lower eyelid
significantly improved at 1, 3 and 6 months after the treatment.
The study had a high loss to follow-up rate. Out of the 44
participants initially enrolled in the trial, only 28 completed the
assessments (i.e. 63.6%). This high attrition rate may have
affected the representativeness of the study sample.
The study by Rong et al (24) from 2018 documented that IPL treatment
on the eyelids combined with MGX provided sustained relief for at
least 6 months to MGD patients by improving Meibomian gland
secretion function, increasing TBUT and improving symptoms. The
MGYSS and of the upper and lower eyelid significantly improved in
the eyes subjected to IPL treatment at 1, 3 and 6 months after
therapy. The study revealed significantly greater improvements in
Meibomian gland secretory function in the lower eyelids as compared
with those in the upper eyelids. There were certain limitations to
this study that require mentioning. The majority of study
participants were females, which may affect the representativeness
and external validity of the results. Furthermore, the treatment
outcomes may have been influenced due to the study investigators
having chosen a relatively fixed treatment energy of 14-16
J/cm2.
In 2019, Arita et al (22) reported on a prospective multicentre
randomized trial demonstrating that a series of IPL-MGX treatment
sessions significantly improved the symptoms and signs of
refractory MGD as compared with MGX alone. The outcomes of the
study pertained to Meibomian glands and the lipid layer of the tear
film and these parameters were measured at each of the treatment
sessions (n=8 in total) prior to the start of treatment and
thereafter, and further, for up to 11 weeks after the final
treatment. A limitation of the study was insufficient power due to
the low number of enrolled patients. Although not a limitation
per se, the study did not attempt to present the mechanisms
underlying the effectiveness of IPL-MGX treatment.
Discussion
The present study is novel as it is, to the best of
our knowledge, the first meta-analysis that performed a data
synthesis of available evidence on the efficacy of IPL therapy in
the management of MGD. However, the results of this meta-analysis
are inconclusive regarding the benefit of IPL therapy in
alleviating the symptoms of MGD. The outcomes primarily considered
in the included studies were the NIBUT and SPEED scores. No
significant differences in SPEED scores between the two study
groups were obtained, but there was a statistically significant
difference in the NIBUT scores favoring treatment with IPL
therapy.
The observations derived from previous studies
indicate that the mechanism of action of IPL may have a
neurological basis, as flash application to temporal and lower
eyelid regions has been indicated to stimulate branches of
parasympathetic nerve that initiates normal activity of the
Meibomian gland. Furthermore, by causing thrombosis of abnormal
telangiectatic blood vessels, decreasing the levels of inflammatory
markers and curbing the proliferation of an abnormal bacterial
flora around the eyelids, IPL therapy appears to alleviate the
symptoms of MGD. Published studies, mostly observational in nature,
evaluating the effectiveness of IPL in the treatment of MGD have
reported improvements in the oil flow score (34), tear film osmolarity (17), redness and vascularity (34,35),
Meibomian gland expression (26,35),
Meibum viscosity and secretion quality (26,34,35),
corneal fluorescein staining (17,35), lid
margin edema (28) and conjunctival
injection (26,35).
The present analysis was based on two important
outcome measures (SPEED and NIBUT). The SPEED questionnaire is an
easy to use, repeatable, valid and subjective tool to quantify the
symptoms of a patient with dry eye disease (36). Other objective techniques of dry eye
assessment included TBUT and NIBUT (37). TBUT is an invasive method requiring
instillation of fluorescein solution in the eye, which disturbs the
tear film equilibrium, causing increased evaporation and tear film
destabilization. Given the reduced sensitivity and specificity of
TBUT, non-invasive methods of tear film assessment, including
NIBUT, have been developed, which do not require instillation of
fluorescein. NIBUT is considered to be more precise in assessing
tear film stability compared with TBUT (37). In the present analysis, no
significant difference in the SPEED scores between the IPL and
control group was obtained. This may be attributed to the lack of
significant differences in SPEED scores in three of the four
studies included in the present review. Only the trial by Arita
et al (22) reported
significantly reduced SPEED scores in the IPL therapy group. The
lack of a statistically significant difference in SPEED scores in
the present analysis may be attributed to use of artificial
teardrops in the control group of certain trials (24,25),
which may have influenced this subjective outcome. However, the
present results demonstrated a significant increase in the NIBUT
values in the IPL group as compared with those in the control
group, suggesting the potential benefit of IPL therapy in the
management of MGD. Reduced NIBUT scores are characteristic for
patients with MGD and the increase in NIBUT values confirms the
role of IPL in improving tear film stability.
The strengths of the present review include a lack
of publication bias, confirming the validity of the results. The
present results provide important clues on which future research
may be based. Continued efforts to perform research studies,
particularly RCTs, are necessary to further establish the efficacy
of IPL therapy as a treatment option in the management of MGD.
The present meta-analysis also has certain
limitations. The participants in the included studies were
predominantly female adults and therefore, the results are likely
to relate to this population, thereby limiting the external
validity. Secondly, the present meta-analysis was limited to only
NIBUT and SPEED scores and other outcome measures such as MGYSS and
corneal fluorescein staining could not be analyzed. Finally, the
limited number of studies with small sample size included in the
present analysis limited the possibility to draw strong
conclusions.
In conclusion, the results of the present study did
not provide conclusive evidence for the benefit of IPL therapy in
the management of MGD. The present analysis indicates that IPL
therapy may result in an improvement of objective NIBUT scores but
has no significant effect on subjective SPEED scores. Given the
limited number of studies performed to date, there is a requirement
for more well-designed prospective RCTs with a larger sample size
to provide further evidence on the efficacy of IPL therapy.
Acknowledgements
Not applicable.
Funding
This study was supported by the Beijing Talents Fund
(grant no. 2015000021467G176).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL designed the study. ST, HD and XH were involved
in the literature search and data interpretation. ST was
responsible for data analysis. SL prepared the manuscript. XH
edited the manuscript. All authors read and approved the final
manuscript.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bron AJ, Benjamin L and Snibson GR:
Meibomian gland disease. Classification and grading of lid changes.
Eye (Lond). 5:395–411. 1991.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lekhanont K, Rojanaporn D, Chuck RS and
Vongthongsri A: Prevalence of dry eye in Bangkok, Thailand. Cornea.
25:1162–1167. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lin PY, Tsai SY, Cheng CY, Liu JH, Chou P
and Hsu WM: Prevalence of dry eye among an elderly Chinese
population in Taiwan: The Shihpai Eye Study. Ophthalmology.
110:1096–1101. 2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Uchino M, Dogru M, Yagi Y, Goto E, Tomita
M, Kon T, Saiki M, Matsumoto Y, Uchino Y, Yokoi N, et al: The
features of dry eye disease in a Japanese elderly population. Optom
Vis Sci. 83:797–802. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jie Y, Xu L, Wu YY and Jonas JB:
Prevalence of dry eye among adult Chinese in the Beijing Eye Study.
Eye (Lond). 23:688–693. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tomlinson A, Bron AJ, Korb DR, Amano S,
Paugh JR, Pearce EI, Yee R, Yokoi N, Arita R and Dogru M: The
international workshop on meibomian gland dysfunction: Report of
the diagnosis subcommittee. Invest Ophthalmol Vis Sci.
52:2006–2049. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dougherty JM, McCulley JP, Silvany RE and
Meyer DR: The role of tetracycline in chronic blepharitis.
Inhibition of lipase production in staphylococci. Invest Ophthalmol
Vis Sci. 32:2970–2975. 1991.PubMed/NCBI
|
|
8
|
Tabbara KF, al-Kharashi SA, al-Mansouri
SM, al-Omar OM, Cooper H, el-Asrar AM and Foulds G: Ocular levels
of azithromycin. Arch Ophthalmol. 116:1625–1628. 1998.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Schultz C: Safety and efficacy of
cyclosporine in the treatment of chronic dry eye. Ophthalmol Eye
Dis. 6:37–42. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ma X and Lu Y: Efficacy of intraductal
meibomian gland probing on tear function in patients with
obstructive meibomian gland dysfunction. Cornea. 35:725–730.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sik Sarman Z, Cucen B, Yuksel N, Cengiz A
and Caglar Y: Effectiveness of intraductal meibomian gland probing
for obstructive meibomian gland dysfunction. Cornea. 35:721–724.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gumus K, Schuetzle KL and Pflugfelder SC:
Randomized controlled crossover trial comparing the impact of sham
or intranasal tear neurostimulation on conjunctival goblet cell
degranulation. Am J Ophthalmol. 177:159–168. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Raulin C, Greve B and Grema H: IPL
technology: A review. Lasers Surg Med. 32:78–87. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Babilas P, Schreml S, Szeimies R-M and
Landthaler M: Intense pulsed light (IPL): A review. Lasers Surg
Med. 42:93–104. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Schuh A, Priglinger S and Messmer EM:
Intense pulsed light (IPL) as a therapeutic option for Meibomian
gland dysfunction. Ophthalmologe. 116:982–988. 2019.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
16
|
Brinton M, Kossler AL, Patel ZM, Loudin J,
Franke M, Ta CN and Palanker D: Enhanced tearing by electrical
stimulation of the anterior ethmoid nerve. Invest Ophthalmol Vis
Sci. 58:2341–2348. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Toyos R, McGill W and Briscoe D: Intense
pulsed light treatment for dry eye disease due to meibomian gland
dysfunction; a 3-year retrospective study. Photomed Laser Surg.
33:41–46. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dell SJ, Gaster RN, Barbarino SC and
Cunningham DN: Prospective evaluation of intense pulsed light and
meibomian gland expression efficacy on relieving signs and symptoms
of dry eye disease due to meibomian gland dysfunction. Clin
Ophthalmol. 11:817–827. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Goldberg DJ: Current trends in intense
pulsed light. J Clin Aesthet Dermatol. 5:45–53. 2012.PubMed/NCBI
|
|
20
|
Craig JP, Chen YH and Turnbull PR:
Prospective trial of intense pulsed light for the treatment of
meibomian gland dysfunction. Invest Ophthalmol Vis Sci.
56:1965–1970. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Arita R, Mizoguchi T, Fukuoka S and
Morishige N: Multicenter study of intense pulsed light therapy for
patients with refractory meibomian gland dysfunction. Cornea.
37:1566–1571. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Arita R, Mizoguchi T, Fukuoka S and
Morishige N: Therapeutic efficacy of intense pulsed light in
patients with refractory meibomian gland dysfunction. Ocul Surf.
17:104–110. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yin Y, Liu N, Gong L and Song N: Changes
in the meibomian gland after exposure to intense pulsed light in
meibomian gland dysfunction (MGD) patients. Curr Eye Res.
43:308–313. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rong B, Tang Y, Tu P, Liu R, Qiao J, Song
W, Toyos R and Yan X: Intense pulsed light applied directly on
eyelids combined with meibomian gland expression to treat meibomian
gland dysfunction. Photomed Laser Surg. 36:326–332. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Rong B, Tang Y, Liu R, Tu P, Qiao J, Song
W and Yan X: Long-term effects of intense pulsed light combined
with meibomian gland expression in the treatment of meibomian gland
dysfunction. Photomed Laser Surg. 36:562–567. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jiang X, Lv H, Song H, Zhang M, Liu Y, Hu
X, Li X and Wang W: Evaluation of the safety and effectiveness of
intense pulsed light in the treatment of meibomian gland
dysfunction. J Ophthalmol. 2016(1910694)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gupta PK, Vora GK, Matossian C, Kim M and
Stinnett S: Outcomes of intense pulsed light therapy for treatment
of evaporative dry eye disease. Can J Ophthalmol. 51:249–253.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Vegunta S, Patel D and Shen JF:
Combination therapy of intense pulsed light therapy and meibomian
gland expression (IPL/MGX) can improve dry eye symptoms and
meibomian gland function in patients with refractory dry eye: A
retrospective analysis. Cornea. 35:318–322. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Karaca EE, Evren Kemer Ö and Özek D:
Intense regulated pulse light for the meibomian gland dysfunction.
Eur J Ophthalmol. 30:289–292. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Seo KY, Kang SM, Ha DY, Chin HS and Jung
JW: Long-term effects of intense pulsed light treatment on the
ocular surface in patients with rosacea-associated meibomian gland
dysfunction. Cont Lens Anterior Eye. 41:430–435. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Toyos R, Toyos M, Willcox J, Mulliniks H
and Hoover J: Evaluation of the safety and efficacy of intense
pulsed light treatment with meibomian gland expression of the upper
eyelids for dry eye disease. Photobiomodul Photomed Laser Surg.
37:527–531. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Moher D, Shamseer L, Clarke M, Ghersi D,
Liberati A, Petticrew M, Shekelle P and Stewart LA: PRISMA-P Group.
Preferred reporting items for systematic review and meta-analysis
protocols (PRISMA-P) 2015 statement. Syst Rev. 4(1)2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Higgins J and Green S: Cochrane Handbook
for systemic reviews of interventions. Version 5.1: The Cochrane
Collaboration, 2011. https://handbook-5-1.cochrane.org.
Accessed July 1, 2019.
|
|
34
|
Vora GK and Gupta PK: Intense pulsed light
therapy for the treatment of evaporative dry eye disease. Curr Opin
Ophthalmol. 26:314–318. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Albietz JM and Schmid KL: Intense pulsed
light treatment and meibomian gland expression for moderate to
advanced meibomian gland dysfunction. Clin Exp Optom. 101:23–33.
2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ngo W, SItu P, Keir N, Korb D, Blackie C
and Simpson T: Psychometric properties and validation of the
standard patient evaluation of eye dryness questionnaire. Cornea.
32:1204–1210. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Vidas Pauk S, Petriček I, Jukić T,
Popović-Suić S, Tomić M, Kalauz M, Jandroković S and Masnec S:
Noninvasive tear film break-up time assessment using handheld lipid
layer examination instrument. Acta Clin Croat. 58:63–71.
2019.PubMed/NCBI View Article : Google Scholar
|