Introduction
Osteoarthritis of the knee is a common degenerative
joint condition (1). According to
the Global Burden of Disease study, the global age-standardized
prevalence of knee OA is approximately 3.8% from 1990 to
2010(1). The primary manifestations
of osteoarthritis include the destruction of the cartilage and
sclerosis of the subchondral bone (2). However, although osteoarthritis is the
most common joint disease, the specific biological mechanisms
underlying its pathogenesis remain poorly understood (3).
Low expression levels of blood 25-hydroxyvitamin D
(vitamin D) have been revealed to be associated with the
progression of osteoarthritis, whereby vitamin D has been
discovered to protect against osteoarthritis (4). The vitamin D receptor is expressed on
the surface of chondrocytes, providing a basis for vitamin D action
on articular chondrocytes (5).
However, the specific mechanism through which vitamin D protects
articular chondrocytes from osteoarthritis remains unclear.
The Wingless-related integration site (Wnt)
signaling pathway component, β-catenin, stimulates bone
hypertrophy, matrix mineralization and matrix metalloproteinase
(MMP)-13 expression; the overexpression of β-catenin in
chondrocytes was demonstrated to strongly induce the expression of
matrix degrading enzymes (6). In
pathological conditions, the Wnt/β-catenin signaling pathway has
been indicated to activate cartilage matrix catabolism and destroy
articular cartilage (7).
The binding of vitamin D to the vitamin D receptor
was discovered to inhibit the Wnt/β-catenin signaling pathway
(8). The subsequent binding to
nuclear β-catenin promotes the translocation of β-catenin from the
nucleus, where it binds to an oligomeric casein kinase/adenomatous
polyposis coli/glycogen synthase kinase 3/β-axis complex, which
mediates β-catenin phosphorylation and accelerates β-catenin
hydrolysis (9). These events lead to
the reduction in β-catenin levels and the inhibition of the Wnt
signaling pathway (9). This may be
one of the mechanisms by which vitamin D protects articular
cartilage. Thus, the present study aimed to investigate whether
vitamin D affected chondrocyte fate through modulating the
Wnt/β-catenin signaling pathway.
Materials and methods
Chondrocyte isolation and culture
The present study was approved by the Animal Care
and Use Committee of Tianjin Union Medical Center and Affiliated
Zhongshan Hospital of Dalian University. A total of 10 female
Sprague Dawley Rats (age, 4 weeks; weight, 80 g) were obtained from
Charles River Laboratories, Inc. The rats were kept in a
clean-grade animal house at a temperature of 20±2˚C, a humidity of
60±5%, with 12 h light/dark cycles and free access to food and
water. Following anesthesia with pentobarbital sodium (30 mg/kg;
Shanghai Ziyuan Pharmaceutical Co., Ltd.), the articular cartilage
from the knee joint and femoral head tissue was removed and washed
three times with PBS, minced into small pieces and digested with
0.2% type II collagenase (Gibco; Thermo Fisher Scientific, Inc.) in
DMEM/F12 (Hyclone; GE Healthcare Life Sciences), supplemented with
100 U/l penicillin and 100 µg/ml streptomycin (Gibco; Thermo Fisher
Scientific, Inc.) at 37˚C for 6-8 h. The cell suspension was
centrifuged at 200 x g for 10 min at room temperature. Harvested
cells were subsequently cultured in DMEM/F12, supplemented with 10%
FBS (HyClone; Cytiva) at 37˚C and 5% CO2. The isolated
cells were used for the following experiments.
MTT assay
The cytotoxicity of vitamin D3 on rat chondrocytes
was determined using MTT assays. Briefly, 1x104
chondrocytes/well were cultured in 96-well plates with 10 nM
vitamin D3 (Beijing Solarbio Science & Technology Co., Ltd.)
for 72 h at 37˚C. MTT assays were performed according to the
manufacturer's protocol. Briefly, 20 µl MTT reagent (5 mg/ml;
Beijing Solarbio Science and Technology Co., Ltd.) was added to
each well and the plate was incubated at 37˚C for 4 h. The medium
was removed and 150 µl DMSO was subsequently added into each well.
The absorbance at 570 nm was measured using a microplate reader
(Bio-Rad Laboratories, Inc.). A single well contained chondrocytes
without vitamin D3 treatment (treatment control) and another well
did not contain cells (blank control).
In vitro cell treatment
Following 4-5 days in culture, the confluent
monolayers of chondrocytes in the culture plates were washed three
times with DMEM/F12, supplemented with 10% FBS (Hyclone; Cytiva)
and incubated overnight with serum-free DMEM at 37˚C. Serum-starved
chondrocytes were left untreated (control), treated with vitamin D3
(10 nM), treated with tumor necrosis factor (TNF)-α (10 ng/ml;
PeproTech, Inc.), treated with both TNF-α and PNU-74654 (10 µM;
Selleck Chemicals) or treated with both TNF-α (10 ng/ml) and
vitamin D3 (10 nM). Following 48 h of incubation at 37˚C with the
respective treatments, the chondrocytes were harvested for RNA and
protein isolation using reverse transcription-quantitative PCR
(RT-qPCR) and western blotting analysis, respectively.
RT-qPCR
RT-qPCR was performed according to a previously
described method (10). Briefly, a
confluent monolayer of rat chondrocytes was washed three times with
PBS and total RNA was extracted using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Total RNA was reverse transcribed into
cDNA using a PrimeScript RT reagent kit (Takara Bio, Inc.). The
thermocycling conditions were as follows: 37˚C for 15 min and 85˚C
for 5 sec. RT-qPCR was performed using a TB Green®
Premix Ex Taq™ II kit (Takara Bio, Inc.), according to the
manufacturer's protocols, on a StepOnePlus Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: Initial denaturation at
95˚C for 30 sec (stage 1), followed by 40 cycles of 95˚C for 5 sec
and 60˚C for 30 sec (stage 2). The primers pairs used to the qPCR
was presented in Table I. Expression
levels were quantified using the 2-ΔΔCq method (11) and normalized to GAPDH expression. All
experiments were carried out in triplicate.
 | Table IPrimers used for reverse
transcription-quantitative PCR. |
Table I
Primers used for reverse
transcription-quantitative PCR.
| Gene | Primer sequence
(5'-3') |
|---|
| GAPDH | F:
GGAATCCACTGGCGTCTTCA |
| | R:
GGTTCACGCCCATCACAAAC |
| Aggrecan | F:
TAAACCCGGTGTGAGAACCG |
| | R:
CCTGGGTGACAATCCAGTCC |
| Collagen II | F:
CCCCTGCAGTACATGCGG |
| | R:
CTCGACGTCATGCTGTCTCAAG |
| MMP-3 | F:
GCATTGGCTGAGTGAAAGAGAC |
| | R:
ATGATGAACGATGGACAGATGA |
| MMP-13 | F:
CAGTTGACAGGCTCCGAGAA |
| | R:
CGTGTGCCAGAAGACCAGAA |
| ADAMTS-4 | F:
GCCAGCAACCGAGGTCCCATA |
| | R:
CCACCACCAGTGTCTCCACGAAT |
| ADAMTS-5 | F:
GACAAGAGTCTGGAJGGTGAGCAA |
| | R:
GCTGCATCGTAGTGCTCCTCAT |
Western blotting
Rat chondrocytes were washed with ice-cold PBS and
total protein was extracted using RIPA lysis buffer (Beyotime
Institute of Biotechnology) for 20 min on ice. The cell lysates
were scraped into 1.5 ml microcentrifuge tubes using cell scrapers
and centrifuged for 10 min at 12,000 x g at 4˚C. The supernatants
were collected as the cytoplasmic extract, whilst the pellets were
resuspended in RIPA lysate buffer (Beyotime Institute of
Biotechnology) and kept on ice for 30 min. The cell lysates were
subsequently centrifuged for 10 min at 12,000 x g at 4˚C and the
supernatants were collected as nuclear extracts. Total protein in
each sample was quantified using the bicinchoninic acid (BCA) assay
method using an enhanced BCA Protein Assay kit (Beyotime Institute
of Technology). The protein samples were denatured at 95˚C for 5
min and 30 µg protein/lane was separated by 10% SDS-PAGE. The
separated proteins were transferred onto a PVDF membrane (EMD
Millipore) and incubated for 1 h with 5% non-fat milk at room
temperature. The membranes were washed with TBS-tween (TBST, 0.5%
Tween) and incubated with the following primary antibodies at 4˚C
overnight, The antibodies used were as follows: Anti-aggrecan
(1:1,000; cat. no. 13880-1-AP; Wuhan Sanying Biotechnology),
anti-collagen II (1:500; cat. no. 15943-1-AP; Wuhan Sanying
Biotechnology), anti-MMP-3 (1:1,000; cat. no. 17873-1-AP; Wuhan
Sanying Biotechnology), anti-MMP-13 (1:1000, cat. no. 18165-1-AP,
Wuhan Sanying Biotechnology), anti-A disintegrin and
metalloproteinase with thrombospondin motifs (ADAMTS)-4 (1:1,000;
cat. no. bs-4191R; BIOSS), anti-ADAMTS-5 (1:1,000; cat. no.
bs-3573R; BIOSS), anti-β-catenin (1:1,000; cat. no. 17565-1-AP;
Wuhan Sanying Biotechnology), anti-Wnt-3a (1:1,000; cat. no.
bs-23277R; BIOSS), anti-β-actin (1:1,000; cat. no. 20536-1-AP;
Wuhan Sanying Biotechnology) and anti-Lamin B1 (1:1,000; cat. no.
12987-1-AP; Wuhan Sanying Biotechnology). Following the primary
antibody incubation, the membranes were washed with TBST and
incubated with a horseradish peroxidase-conjugated goat anti-rabbit
IgG secondary antibody (1:5,000; cat. no. SA00001-2; Wuhan Sanying
Biotechnology) at room temperature for 1 h. Protein bands were
visualized using an enhanced chemiluminescence (Thermo Fisher
Scientific, Inc.) with a ChemiDoc XRS+ (Bio-Rad Laboratories, Inc.)
and quantified by Image Plus pro software (version 6.0; Media
Cybernetics, Inc.).
Statistical analysis
All experiments were performed in triplicate.
Statistical analysis was performed using GraphPad Prism software
(version 8.02; GraphPad Software, Inc.) and data are presented as
the mean ± standard deviation. Statistical differences between two
groups were determined using a Student's t-test, whereas
statistical differences between multiple groups were performed
using a one-way ANOVA, followed by a Tukey's test or a Tamhane's T2
test for data with a homogenous or non-homogenous variance,
respectively. P<0.05 was considered to indicate a statistically
significant difference.
Results
Viability of chondrocytes following
vitamin D3 treatment
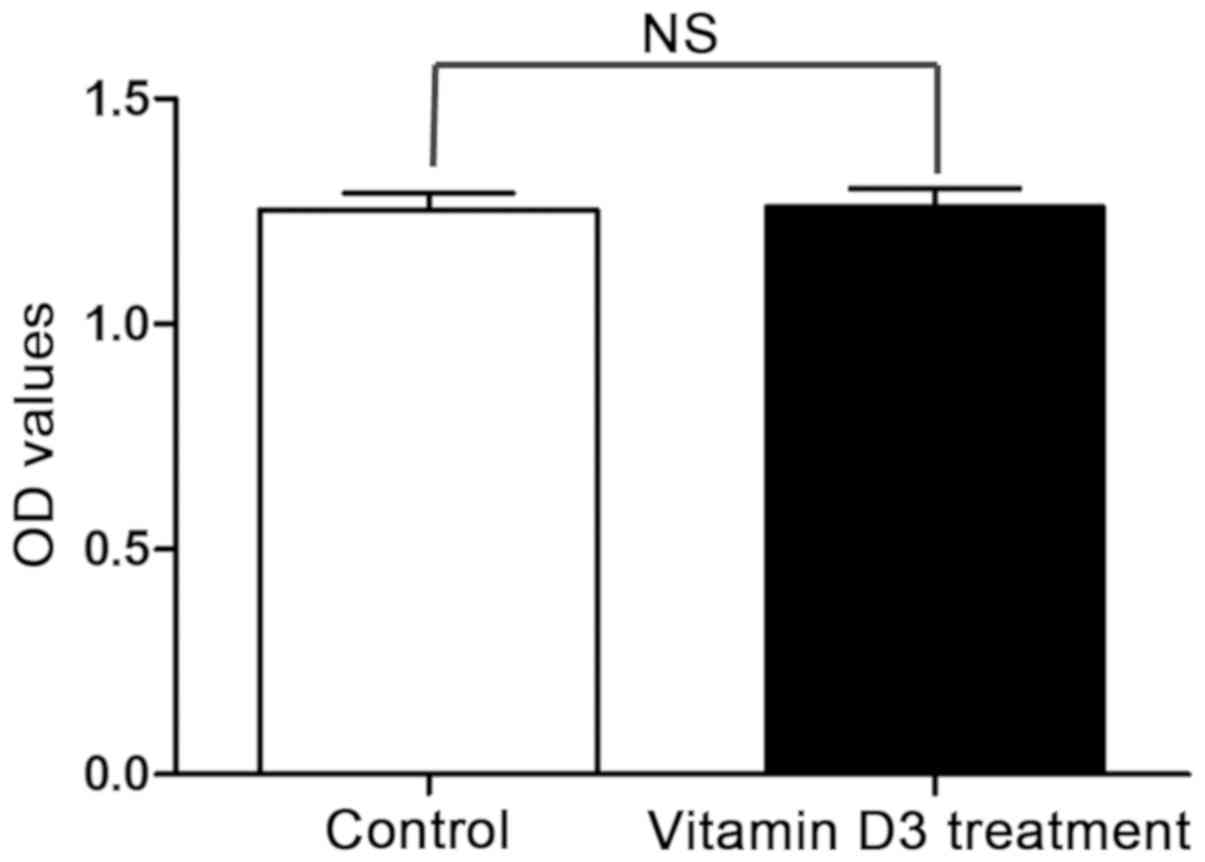
The MTT assay results demonstrated that there was no
significant difference in the viability of chondrocytes treated
with vitamin D3 compared with the control chondrocytes, indicating
that there was no significant cytotoxic effect of treating rat
chondrocytes with 10 nM vitamin D3 for 72 h (Fig. 1).
Effects of vitamin D3 treatment on
aggrecan and COL2A1 expression levels in rat chondrocytes
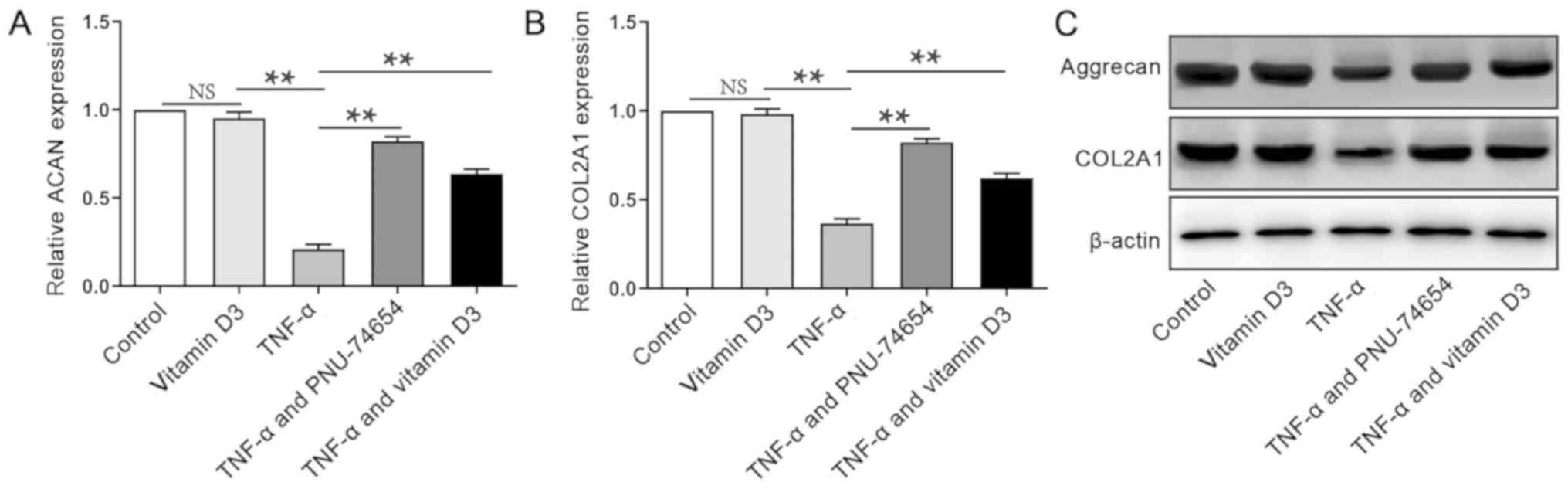
Articular cartilage is mainly composed of collagen
II and proteoglycan, of which aggregated proteoglycan is the most
abundant (12). In the present
study, RT-qPCR was used to determine the expression levels of
collagen II and the proteoglycan, aggrecan. The mRNA expression
levels of gene encoding aggrecan (ACAN) and gene encoding collagen
II (COL2A1) following TNF-α treatment were significantly reduced
(Fig. 2A and B) compared with negative controls. However,
the combined treatment with the Wnt/β-catenin signaling pathway
inhibitor PNU-74654 and TNF-α significantly increased the ACAN and
COL2A1 mRNA expression levels in the chondrocytes compared with the
chondrocytes in the TNF-α group. Similarly, the combined treatment
of vitamin D3 and TNF-α also significantly increased ACAN and
COL2A1 mRNA expression levels in the chondrocytes compared with
TNF-α treatment alone. However, combined treatment of vitamin D3
and TNF-α exhibited lower ACAN and COL2A1 gene expression compared
with co-treatment of PNU-74654 and TNF-α (Fig. 2A and B). Western blotting analysis also revealed
that the protein expression levels of aggrecan and COL2A1 followed
the same trend as the RT-qPCR results (Fig. 2C).
Effects of vitamin D3 treatment on
TNF-α-induced rat chondrocytes
To determine the regulatory effects of vitamin D3 on
MMP-3 and MMP-13 expression levels, the activation of these two
metalloproteinases by vitamin D3 in rat chondrocytes was
investigated. TNF-α treatment significantly increased the
expression levels of MMP-3 and MMP-13 mRNA in rat chondrocytes
compared with the control and vitamin D3 group (Fig. 3A and B). Notably, co-treatment of PNU-74654 and
TNF-α significantly inhibited the TNF-α-induced increases in MMP-3
and MMP-13 expression levels. In addition, the co-treatment of
vitamin D3 and TNF-α significantly reduced the increased expression
levels of MMP-3 and MMP-13 induced by TNF-α (Fig. 3A and B). Furthermore, vitamin D3 and TNF-α
treatment group had higher MMP-3 and MMP-13 gene expression levels
compared with the PNU-74654 and TNF-α treatment group. The western
blot data for MMP-3 and MMP-13 protein expression levels revealed
similar results to the RT-qPCR analysis (Fig. 3E). The expression levels of ADAMTS-4
and ADAMTS-5 were also significantly increased in chondrocytes
treated with TNF-α (10 ng/ml) compared with the chondrocytes in the
control and vitamin D3 treatment groups (Fig. 3C and D). However, the TNF-α-induced increases in
ADAMTS-4 and ADAMTS-5 expression levels were effectively attenuated
by the co-treatment with both PNU-74654 or vitamin D3. Similarly,
vitamin D3 and TNF-α treatment group had higher ADAMTS-4 and
ADAMTS-5 gene expression level than PNU-74654 and TNF-α treatment
group (Fig. 3C-E).
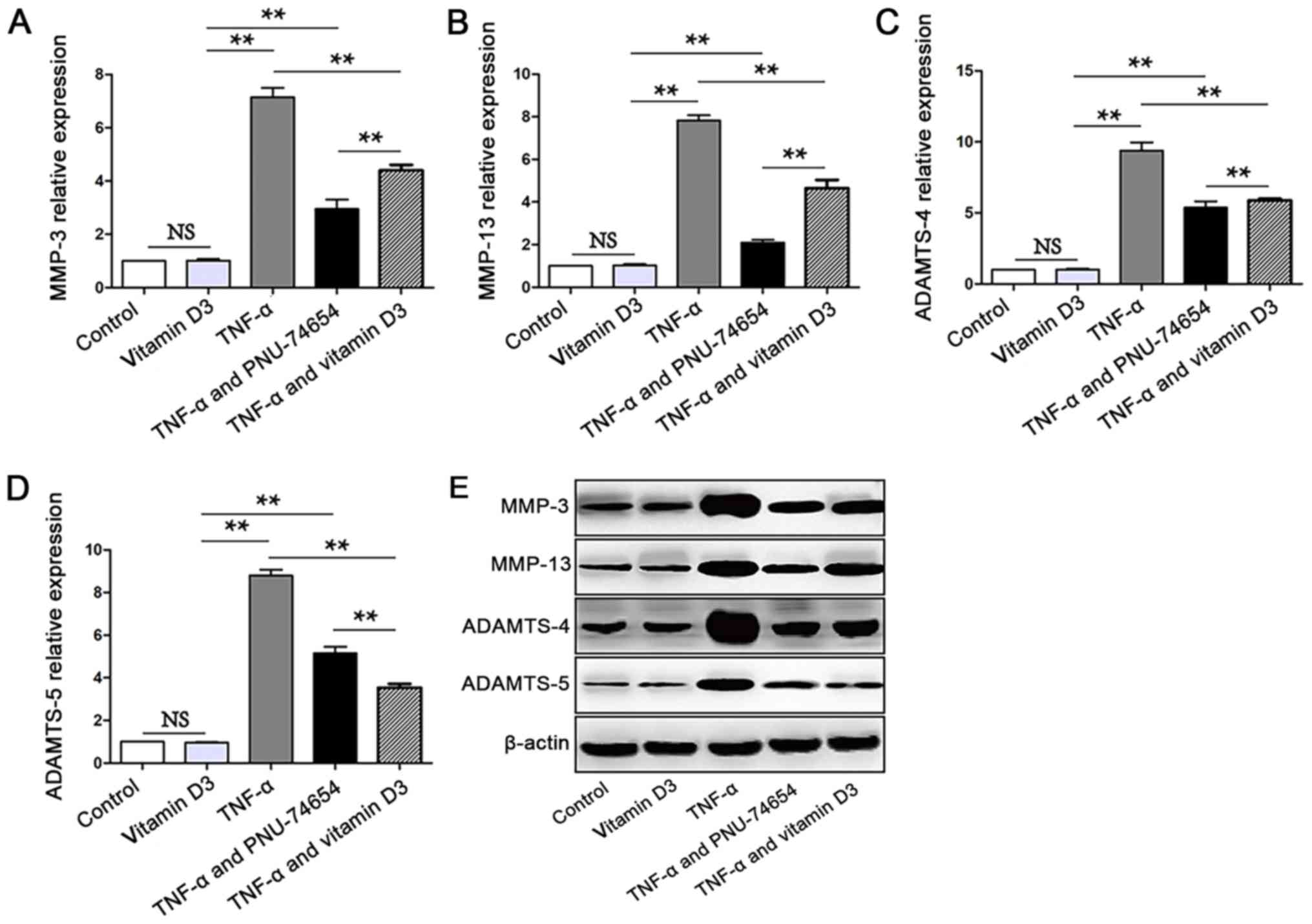 | Figure 3Vitamin D3 treatment attenuates the
increased expression levels of MMP-3, MMP-13, ADAMTS-4 and ADAMTS-5
induced by TNF-α. Reverse transcription-quantitative PCR was used
to analyze the expression levels of (A) MMP-3, (B) MMP-13, (C)
ADAMTS-4 and (D) ADAMTS-5. TNF-α treatment significantly increased
the mRNA expression levels of MMP-3, MMP-13, ADAMTS-4 and ADAMTS-5,
while vitamin D3 and PNU-74654 co-treatment significantly inhibited
the expression levels of these mRNAs in chondrocytes induced with
TNF-α. (E) Western blotting was used to determine the protein
expression levels of MMP-3, MMP-13, ADAMTS-4 and ADAMTS-5. TNF-α
treatment increased MMP-3, MMP-13, ADAMTS-4 and ADAMTS-5 expression
at the protein level. Vitamin D3 and PNU-74654 inhibited the
expression of MMP-3, MMP-13, ADAMTS-4 and ADAMTS-5 induced by TNF-α
at the protein level. **P<0.01. MMP, matrix
metalloproteinase; ADAMTS, A disintegrin and metalloproteinase with
thrombospondin motifs; TNF-α, tumor necrosis factor-α; NS,
non-significant. |
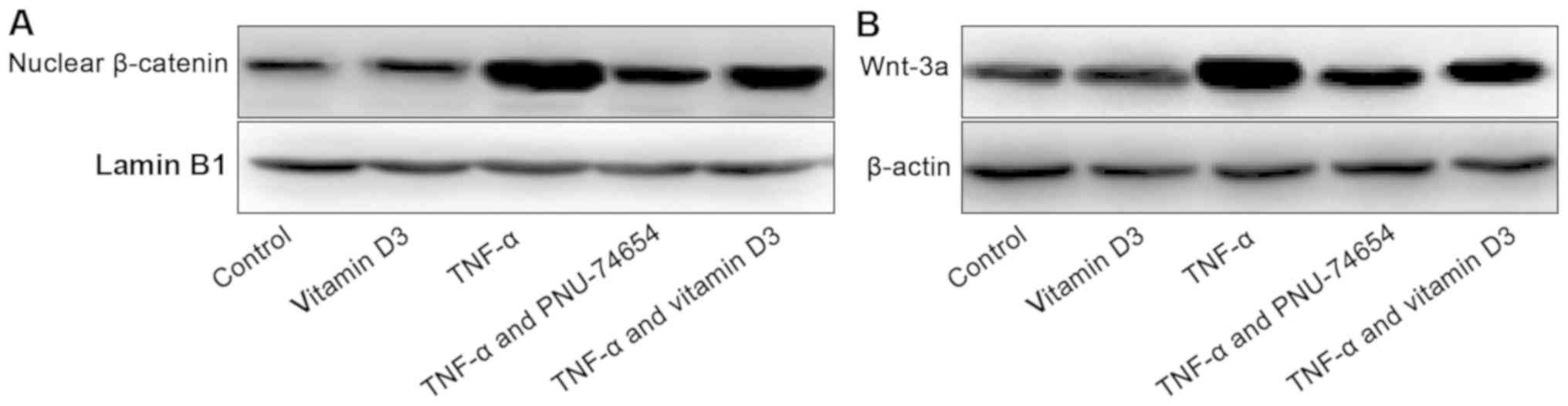
Effects of vitamin D3 treatment on the
Wnt/β-catenin signaling pathway
TNF-α treatment increased Wnt-3a and nuclear
β-catenin expression levels compared with control or vitamin D3
group, which suggested that the Wnt/β-catenin signaling pathway was
activated (Fig. 4A and B). Conversely, the co-treatment with
vitamin D3 or PNU-74654 revealed a reduction in both Wnt-3a and
nuclear β-catenin expression levels. And, vitamin D3 and TNF-α
treatment group have weaker inhibitory effect on Wnt-3a and nuclear
β-catenin than PNU-74654 and TNF-α treatment group. (Fig. 4A and B).
Discussion
It was previously established that vitamin D3
delayed the progression of knee osteoarthritis, although there is
controversy surrounding the specific mechanisms involved (13). The characteristic gradual
decomposition of extracellular matrix (ECM) in OA and the
subsequent degradation of articular cartilage (14) have been demonstrated to be mediated
by interleukin-1β (15), TNF-α
(16) and other factors (17).
Articular cartilage is a special type of connective
tissue; the highly organized ECM consists of major macromolecules,
including the proteoglycan aggrecan, the most abundant proteoglycan
found in the cartilage and COL2A1(18). In the present study, TNF-α treatment
decreased the levels of aggrecan and COL2A1 in the rat
chondrocytes, whereas the expression levels of aggrecan and COL2A1
II were increased in chondrocytes co-treated with TNF-α and vitamin
D3.
Aggrecanases belong to the ADAMTS family and the
most effective aggrecanases associated with joint diseases are
ADAMTS-4(19) and ADAMTS-5(20). Therefore, the ability of vitamin D3
to regulate the expression levels of ADAMTS-4 and ADAMTS-5 may be
of therapeutic value. The current study revealed that the
expression levels of ADAMTS-4 and ADAMTS-5 were significantly
increased in the rat chondrocytes induced by TNF-α, whereas this
increase was subsequently inhibited by the co-treatment with
vitamin D3.
Collagen degradation mainly occurs through the
action of MMPs and increased expression levels of MMPs have been
previously closely associated with the progression of OA (21,22).
Among the various MMPs, MMP-13 is the main enzyme involved in
cartilage erosion in OA because of its proteolytic activity over
COL2A1, the main component of cartilage ECM (23,24).
Therefore, inhibiting the expression levels of MMP-13 may lead to
cartilage protection and therefore have therapeutic value in the
treatment of OA (25,26). MMP-3 has also been discovered to be
closely associated with the progression of OA and increased MMP-3
expression levels have been observed to promote collagen
degradation in the cartilage matrix (27). In a previous study, the abnormal
expression levels of MMP-3 were related to the pathogenesis of OA
(28). Thus, the enzymes released by
chondrocytes in response to certain stimuli are closely associated
with the progression of cartilage degradation in OA. The results of
the present study demonstrated that the expression levels of MMP-3
and MMP-13 in chondrocytes stimulated by TNF-α were significantly
reduced following co-treatment with vitamin D3 compared with the
chondrocytes treated with TNF-α alone. Therefore, it was
hypothesized that vitamin D3 served a protective role in the
cartilage by blocking the expression and activation of MMPs and
ADAMTs, thus blocking the development and progression of OA.
The Wnt/β-catenin signaling pathway is an important
pathway involved in numerous biological processes, including cell
proliferation, migration and differentiation, cartilage homeostasis
(29) and joint remodeling (30). In a previous study, the inhibition of
the Wnt/β-catenin signaling pathway protected against cartilage
degradation in OA (31). The
upregulation of β-catenin in degraded cartilage indicated that the
stimulation of the Wnt signaling pathway led to cartilage loss
(32). In addition, in animal models
of OA, β-catenin was found to be overexpressed in articular
cartilage (33). In the present
study, TNF-α treatment increased the expression levels of Wnt-3a
and nuclear β-catenin in the chondrocytes. Notably, the
Wnt/β-catenin pathway inhibitor PNU-74654 inhibited the expression
levels of Wnt-3a and nuclear β-catenin, inhibited the TNF-α-induced
decreases in ACAN and COL2A expression levels and TNF-α-induced
increases in the expression levels of MMPs and ADAMTs in
chondrocytes. These findings indicated that TNF-α may activate the
Wnt/β-catenin signaling pathway in chondrocytes. The co-treatment
with vitamin D3 also inhibited the TNF-α-induced expression levels
of Wnt-3a and nuclear β-catenin, the TNF-α-induced reduced
expression levels of ACAN and COL2A1, and the TNF-α-induced
increased expression levels of the MMPs and ADAMTs in chondrocytes.
These results suggested that the protective effect of vitamin D3 on
chondrocytes may be at least partially mediated through the
inhibition of the Wnt/β-catenin signaling pathway. Other signaling
pathways are also thought to be involved in the regulation of MMPs
and ADAMTs in chondrocytes, thus further studies are required to
determine the mechanisms by which vitamin D3 regulates MMPs and
ADAMTs.
In conclusion, the results of the present study
provided evidence to suggest that vitamin D3 may inhibit the
expression levels of MMP-3, MMP-13, ADAMTS-4 and ADAMTS-5 through
inhibiting the Wnt/β-catenin signaling pathway. These data
suggested that vitamin D3 may have therapeutic value in the
prevention and treatment of OA.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Foundation of
Tianjin Union Medical Centre (grant no. 2017YJ013).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MT designed the experiments. TY and WS performed the
experiments. YD, YS, YR and WH analyzed data. All authors
contributed to the writing of the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
and Use Committee of Tianjin Union Medical Center and Affiliated
Zhongshan Hospital of Dalian University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bortoluzzi A, Furini F and Scirè CA:
Osteoarthritis and its management - Epidemiology, nutritional
aspects and environmental factors. Autoimmun Rev. 17:1097–1104.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kanemitsu M, Nakasa T, Shirakawa Y,
Ishikawa M, Miyaki S and Adachi N: Role of vasoactive intestinal
peptide in the progression of osteoarthritis through bone sclerosis
and angiogenesis i n subchondral bone. J Orthop Sci: Jan 9, 2020
(Epub ahead of print).
|
|
3
|
Majeed MH, Sherazi SAA, Bacon D and Bajwa
ZH: Pharmacological treatment of pain in osteoarthritis: A
descriptive review. Curr Rheumatol Rep. 20(88)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cao Y, Winzenberg T, Nguo K, Lin J, Jones
G and Ding C: Association between serum levels of 25-hydroxyvitamin
D and osteoarthritis: A systematic review. Rheumatology (Oxford).
52:1323–1334. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tetlow LC and Woolley DE: Expression of
vitamin D receptors and matrix metalloproteinases in osteoarthritic
cartilage and human articular chondrocytes in vitro. Osteoarthritis
Cartilage. 9:423–431. 2001.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sun X, Huang H, Pan X, Li S, Xie Z, Ma Y,
Hu B, Wang J, Chen Z and Shi P: EGR1 promotes the cartilage
degeneration and hypertrophy by activating the Krüppel-like factor
5 and β-catenin signaling. Biochim Biophys Acta Mol Basis Dis.
1865:2490–2503. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
De Santis M, Di Matteo B, Chisari E,
Cincinelli G, Angele P, Lattermann C, Filardo G, Vitale ND, Selmi C
and Kon E: The role of Wnt pathway in the pathogenesis of OA and
its potential therapeutic implications in the field of regenerative
medicine. BioMed Res Int. 2018(7402947)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gröschel C, Aggarwal A, Tennakoon S,
Höbaus J, Prinz-Wohlgenannt M, Marian B, Heffeter P, Berger W and
Kállay E: Effect of 1,25-dihydroxyvitamin D3 on the Wnt pathway in
non-malignant colonic cells. J Steroid Biochem Mol Biol. 155 (Pt
B):224–230. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nusse R and Clevers H: Wnt/β-Catenin
Signaling, Disease, and Emerging Therapeutic Modalities. Cell.
169:985–999. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhou B, Chen D, Xu H and Zhang X:
Proliferation of rabbit chondrocyte and inhibition of IL-1β-induced
apoptosis through MEK/ERK signaling by statins. In Vitro Cell Dev
Biol Anim. 53:124–131. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Δ Δ C(T)) method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
McCarty EC: Articular Cartilage: The
Search for the Holy Grail of Treatment and Restoration. Clin Sports
Med. 36:xv–xvi. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Castillo EC, Hernandez-Cueto MA,
Vega-Lopez MA, Lavalle C, Kouri JB and Ortiz-Navarrete V: Effects
of vitamin D supplementation during the induction and progression
of osteoarthritis in a rat model. Evid Based Complement Alternat
Med. 2012(156563)2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hunt MA, Charlton JM and Esculier JF:
Osteoarthritis year in review 2019: Mechanics. Osteoarthritis
Cartilage. 28:267–274. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zeng YF, Wang R, Bian Y, Chen WS and Peng
L: Catalpol attenuates IL-1β induced matrix catabolism, apoptosis
and inflammation in rat chondrocytes and inhibits cartilage
degeneration. Med Sci Monit. 25:6649–6659. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhao Y, Li Y, Qu R, Chen X, Wang W, Qiu C,
Liu B, Pan X, Liu L, Vasilev K, et al: Cortistatin binds to TNF-α
receptors and protects against osteoarthritis. EBioMedicine.
41:556–570. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Urban H and Little CB: The role of fat and
inflammation in the pathogenesis and management of osteoarthritis.
Rheumatology (Oxford). 57 (Suppl 4):iv10–iv21. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tian J, Rui YJ, Xu YJ and Zhang SA: J. T:
miR-143-3p regulates early cartilage differentiation of BMSCs and
promotes cartilage damage repair through targeting BMPR2. Eur Rev
Med Pharmacol Sci. 22:8814–8821. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chijiiwa M, Mochizuki S, Kimura T, Abe H,
Tanaka Y, Fujii Y, Shimizu H, Enomoto H, Toyama Y and Okada Y: CCN1
(Cyr61) is overexpressed in human osteoarthritic cartilage and
inhibits ADAMTS-4 (aggrecanase 1) activity. Arthritis Rheumatol.
67:1557–1567. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rogerson FM, Last K, Golub SB, Gauci SJ,
Stanton H, Bell KM and Fosang AJ: ADAMTS-9 in Mouse Cartilage Has
Aggrecanase Activity That Is Distinct from ADAMTS-4 and ADAMTS-5.
Int J Mol Sci. 20(573)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Seidl CI and Murphy CL: CI: Dual and
Opposing Regulation of MMP1 and MMP13 by Both Arms of miR-675 in
Human Articular Chondrocytes. Cell Physiol Biochem. 53:172–185.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Malemud CJ: Inhibition of MMPs and
ADAM/ADAMTS. Biochem Pharmacol. 165:33–40. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yu HT, Gu CZ and Chen JQ: MiR-9
facilitates cartilage regeneration of osteoarthritis in rabbits
through regulating Notch signaling pathway. Eur Rev Med Pharmacol
Sci. 23:5051–5058. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mazur CM, Woo JJ, Yee CS, Fields AJ,
Acevedo C, Bailey KN, Kaya S, Fowler TW, Lotz JC, Dang A, et al:
Osteocyte dysfunction promotes osteoarthritis through
MMP13-dependent suppression of subchondral bone homeostasis. Bone
Res. 7(34)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shu CC, Flannery CR, Little CB and Melrose
J: Catabolism of fibromodulin in developmental rudiment and
pathologic articular cartilage demonstrates novel roles for MMP-13
and ADAMTS-4 in C-terminal processing of SLRPs. Int J Mol Sci.
20(579)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tang LP, Ding JB, Liu ZH and Zhou GJ: LP:
LncRNA TUG1 promotes osteoarthritis-induced degradation of
chondrocyte extracellular matrix via miR-195/MMP-13 axis. Eur Rev
Med Pharmacol Sci. 22:8574–8581. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
van Geffen EW, van Caam APM, Schreurs W,
van de Loo FA, van Lent PLEM, Koenders MI, Thudium CS, Bay-Jensen
AC, Blaney Davidson EN and van der Kraan PM: IL-37 diminishes
proteoglycan loss in human OA cartilage: Donor-specific link
between IL-37 and MMP-3. Osteoarthritis Cartilage. 27:148–157.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Guo L, Hao R, Tian F, An N and Wang K:
Interferon regulatory factor 5 (IRF5) regulates the expression of
matrix metalloproteinase-3 (MMP-3) in human chondrocytes. Int
Immunopharmacol. 55:231–236. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Miyamoto K, Ohkawara B, Ito M, Masuda A,
Hirakawa A, Sakai T, Hiraiwa H, Hamada T, Ishiguro N and Ohno K:
Fluoxetine ameliorates cartilage degradation in osteoarthritis by
inhibiting Wnt/β-catenin signaling. PLoS One.
12(e0184388)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ma L, Wu J and Jin QH: The association
between parathyroid hormone 1-34 and the Wnt/β-catenin signaling
pathway in a rat model of osteoarthritis. Mol Med Rep.
16:8799–8807. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sun Y, Wang F, Sun X, Wang X, Zhang L and
Li Y: CX3CR1 regulates osteoarthrosis chondrocyte proliferation and
apoptosis via Wnt/β-catenin signaling. Biomed Pharmacother.
96:1317–1323. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yuasa T, Otani T, Koike T, Iwamoto M and
Enomoto-Iwamoto M: Wnt/beta-catenin signaling stimulates matrix
catabolic genes and activity in articular chondrocytes: Its
possible role in joint degeneration. Lab Invest. 88:264–274.
2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li WJ, Tang LP, Xiong Y, Chen WP, Zhou XD,
Ding QH and Wu LD: A possible mechanism in DHEA-mediated protection
against osteoarthritis. Steroids. 89:20–26. 2014.PubMed/NCBI View Article : Google Scholar
|