Introduction
Hepatitis B virus (HBV), also known as hepatitis B
or second liver virus, is a very common infectious disease
(1) with a wide transmission method
and a high transmission speed, which can be transmitted through the
blood and other body fluids of the patients. Hepatitis B,
tuberculosis and acquired immune deficiency syndrome (AIDS) are
known as the ‘three major infectious diseases in the world’ at
present (2,3). According to statistics, there are more
than 400 million patients with hepatitis B worldwide, being 8 times
that of AIDS (4). Hepatitis B
infection has strong regional differences, the number of hepatitis
B patients in Southeast Asia and Africa is significantly higher
than that of other regions (5).
According to the relevant statistics, there are more than 90
million patients in China and other populous countries (6). With the development and popularization
of hepatitis B antibody vaccines, the infection rate of hepatitis B
has been effectively controlled, and its incidence is decreasing
year by year (7). However,
continuous studies have pointed out that the incidence of hepatitis
B has begun to rise again this year, and the reasons are pointing
to the effective duration of hepatitis B antibody vaccine (8-10).
Hepatitis B antibody vaccine is one of the necessary vaccines in
human body, but most patients have only been vaccinated at birth
and neglected its importance in their subsequent life, which leads
to the gradual disappearance of antibody vaccines after long-term
metabolism in the body (11).
Everyone is recommended to undergo a comprehensive physical
examination at least once in three years to prevent all possible
diseases (12). However, because the
medical concept has not been popularized, most patients ignore
their own health. Moreover, the incidence of hepatitis B has showed
an upward trend recently, and there is no relevant research on the
re-vaccination time of hepatitis B antibody vaccine at present.
Examination of the five HBV markers [hepatitis B
surface antigen (HBsAg), hepatitis B surface antibody (HBsAb),
hepatitis Be antigen (HBeAg), hepatitis Be antibody (HBeAb),
hepatitis B core antibody (HBcAb)] is the most convenient and
common method for detecting hepatitis B. However, there is still
lack of research support to determine the effective duration of
hepatitis B vaccine by the examination results. If the
re-vaccination time of hepatitis B vaccine can be predicted by
examination of the five HBV markers, which is highly feasible, the
clinical screening rate can be effectively improved and the
occurrence of hepatitis B can also be reduced. Therefore, the
hepatitis B antibodies of patients who underwent examination of the
five HBV markers in recent years were quantitatively analyzed in
this study to explore the optimal protective time and
re-vaccination time of hepatitis B antibody vaccine for human body,
and to provide reference and guidance for clinical practice.
Patients and methods
General information
A total of 3,243 patients examined by five HBV
markers in Women and Children's Health Care Hospital of Linyi
(Linyi, China) from January 2015 to December 2017 were selected as
the research subjects and analyzed retrospectively, including 1,782
males and 1,461 females with an age range of 16-97 years and an
average age of 26.83±42.37 years.
The study was approved by the Ethics Committee of
the Women and Children's Health Care Hospital of Linyi. Signed
informed consents were obtained from the patients and/or
guardians.
Inclusion and exclusion criteria
Inclusion criteria: Patients examined by five HBV
markers in Women and Children's Health Care Hospital of Linyi;
patients with complete case data; patients with hepatitis B
vaccination history before examination. Exclusion criteria:
Patients with liver neoplasms; patients with liver cirrhosis or
fatty liver; patients with cirrhosis and fatty liver; patients
smoking or with alcohol consumption within 24 h before the
examination; patients undergoing liver surgery; severe ICU
patients.
Methods
The time interval from the previous hepatitis B
vaccination was 3 to 16 years, and the average distance from the
inoculation was 5.42±2.97 years, therefore the average of 5 years
was taken as the basis of grouping. According to the previous time
of the hepatitis B antibody vaccination, subjects were divided into
three groups: Short-term group (<5 years, n=798); medium-term
group (>5 years - ≤10 years, n=1,242); long-term group (>10
years, n=1,203). Examination of the five HBV markers was carried
out by the laboratory of Women and Children's Health Care Hospital
of Linyi. Fasting venous blood (4 ml) was extracted, placed at room
temperature for 30 min, then centrifuged at 50 x g at 4˚C for 10
min to obtain the upper serum. Qualitative analysis of five HBV
markers was conducted by the enzyme-linked immunosorbent assay
(ELISA) (Shanghai Yiji Industries Co., Ltd., DA6471044) in strict
accordance with the instructions of the kit. If the results were
false positive, repeated experiments were carried out to determine
the accuracy. Quantitative analysis of five HBV markers was
conducted by chemiluminescence immunoassay (CLIA) after the
completion of qualitative analysis with MAGICL6800 automatic
chemiluminescence determinator (Getein Biotechnology Co., Ltd).
Observation indicators
Qualitative and quantitative detection results of
five HBV markers in three groups of patients. Qualitative results:
HBsAg-positive indicates the presence of HBV. HBsAb-positive
indicates successful vaccination of hepatitis B vaccine and the
existence of hepatitis B antibody. HBeAg-positive indicates that
HBV replicates actively in human body, and the blood is poisonous
and highly infectious. HBeAb-positive indicates that replication of
HBV has changed from active to relatively static. HBcAb-positive
indicates that the patient has been or is infected with HBV. The
most seriously infected patients in the three groups were selected
for a more in-depth geometric mean titer (GMT) analysis: The
ability of antibodies to neutralize antigens such as viruses or
toxins, the greater the numerical results, the stronger the ability
of viruses or toxins. MGT is widely used to evaluate the
concentration of viruses in the blood (13). Anti-HBs were detected by solid phase
radioimmunoassay.
Statistical analysis
The data were analyzed and processed by the SPSS24.0
(IBM Corp); the quantitative data such as quantitative results of
hepatitis B five markers were expressed as mean ± standard
deviation (SD), one-way ANOVA followed by LSD test was used for
comparison between multiple groups; counting data such as HBsAg
positive rates were expressed as rate, and Chi-square test was used
for comparison between groups. P<0.050 represents a
statistically significant difference.
Results
Clinical data comparison
There was no significant difference in age, body
weight, body mass index, sex, marital status, smoking and drinking
habit between the three groups (P>0.050), which indicated that
there was a comparability between the patients in the three groups
(Table I).
 | Table IClinical data comparison [mean ± SD, n
(%)]. |
Table I
Clinical data comparison [mean ± SD, n
(%)].
| Variables | Short-term group
(n=798) | Medium-term group
(n=1,242) | Long-term group
(n=1,203) | F value or
χ2 | P-value |
|---|
| Age (years) | | | | 2.519 | 0.081 |
| | 33.67±29.48 | 34.68±31.55 | 36.81±35.26 | | |
| Body weight (kg) | | | | 0.294 | 0.745 |
| | 64.92±20.36 | 65.16±19.77 | 64.53±21.14 | | |
| BMI | | | | 1.814 | 0.163 |
| | 27.37±5.94 | 27.15±6.08 | 26.86±5.95 | | |
| Sex | | | | 0.346 | 0.841 |
|
Male | 442 (55.39) | 687 (55.31) | 653 (54.28) | | |
|
Female | 356 (44.61) | 555 (44.69) | 550 (45.72) | | |
| Residency | | | | 0.865 | 0.649 |
|
Urban | 712 (89.22) | 1,123 (90.42) | 1,086 (90.27) | | |
|
Rural | 86 (10.78) | 119 (9.58) | 117 (9.73) | | |
| Smoking | | | | 0.364 | 0.834 |
|
Yes | 468 (58.65) | 712 (57.33) | 693 (57.61) | | |
|
No | 330 (41.35) | 530 (42.67) | 510 (42.39) | | |
| Drinking | | | | 0.052 | 0.974 |
|
Yes | 318 (39.85) | 497 (40.02) | 476 (39.57) | | |
|
No | 480 (60.15) | 745 (59.98) | 727 (60.43) | | |
Qualitative results of five HBV
markers
Comparison of the qualitative examination results of
five HBV markers in the three groups showed that there were
significant differences in each examination (P<0.001). The HBsAg
in the long-term and the medium-term group showed no significant
difference (P>0.001), but was significantly lower than that in
the short-term group (P<0.050). The results of HBsAb, HBeAg,
HBeAb, HBcAb detection showed that there was no significant
difference between short-term and medium-term group (P>0.050),
while the long-term group was lower than the other two groups
(P<0.001) (Table II). After
examination, 14 patients with HBsAg (+), HBeAg (+) and HBcAb (+) in
the long-term group, showed fatigue, dizziness, loss of appetite,
nausea, insomnia and occasionally suffered from distended pain,
blunt pain or stabbing pain in the right upper abdomen or right
back. Alanine aminotransferase, aspartate aminotransferase and
total bilirubin levels in liver function examination were higher
than those in normal controls, among which 3 cases were suspected
to be compensated cirrhosis.
 | Table IIQualitative results of hepatitis B
virus markers [n (%)]. |
Table II
Qualitative results of hepatitis B
virus markers [n (%)].
| Markers | Short-term group
(n=798) | Medium-term group
(n=1,242) | Long-term group
(n=1,203) | χ2 | P-value |
|---|
| HBsAg | | | | 46.34 | <0.001 |
|
+ | 22 (2.76) | 117 (9.42) | 134 (11.14) | | |
|
- | 776 (97.24) | 1,125
(90.58)a | 1,069
(88.86)a | | |
| HBsAb | | | | 66.692 | <0.001 |
|
+ | 784 (98.25) | 1,207 (97.18) | 1,114 (92.60) | | |
|
- | 14 (1.75) | 35 (2.82) | 89
(7.40)a,b | | |
| HBeAg | | | | 34.973 | <0.001 |
|
+ | 0 (0.00) | 5 (0.40) | 29 (2.41) | | |
|
- | 798 (100.00) | 1,237 (99.60) | 1,174
(97.59)a,b | | |
| HBeAb | | | | 34.563 | <0.001 |
|
+ | 3 (0.38) | 10 (0.81) | 40 (3.33) | | |
|
- | 795 (99.62) | 1,232 (99.19) | 1,163
(96.67)a,b | | |
| HBcAb | | | | 55.263 | <0.001 |
|
+ | 791 (99.12) | 1,224 (98.55) | 1,134 (94.26) | | |
|
- | 7 (0.88) | 18 (1.45) | 69
(5.74)a,b | | |
Quantitative results of five HBV
markers
The quantitative results of HBsAg, HBsAb, HBeAg,
HBeAb, HBcAb in the short-term group were as follows: 0.12±0.03
ng/ml, 332.51±45.68 mIU/ml, 0.07±0.08 PEIU/ml, 0.10±0.05 PEIU/ml,
and 0.29±0.16 PEIU/ml, respectively. Those in the medium-term group
were 0.28±0.17 ng/ml, 264.62±37.88 mIU/ml, 0.26±0.10 PEIU/ml,
0.15±0.02 PEIU/ml, 0.57±0.10 PEIU/ml, respectively, and those in
the long-term group were 0.48±0.26 ng/ml, 153.67±58.69 mIU/ml,
0.39±0.17 PEIU/ml, 0.17±0.14 PEIU/ml, 0.82±0.27 PEIU/ml,
respectively. There were statistical differences in the
quantitative results of each indicator between the three groups
(P<0.050). HBsAg, HBeAg, HBeAb, HBcAb in the long-term group
were significantly higher than those in the other two groups, while
those in the medium-term group were higher than those in the
short-term group (P<0.050). The HBsAb in the long-term group was
significantly lower than that in the other two groups, and that in
the medium-term group was lower than that in the short-term group
(P<0.050) (Fig. 1).
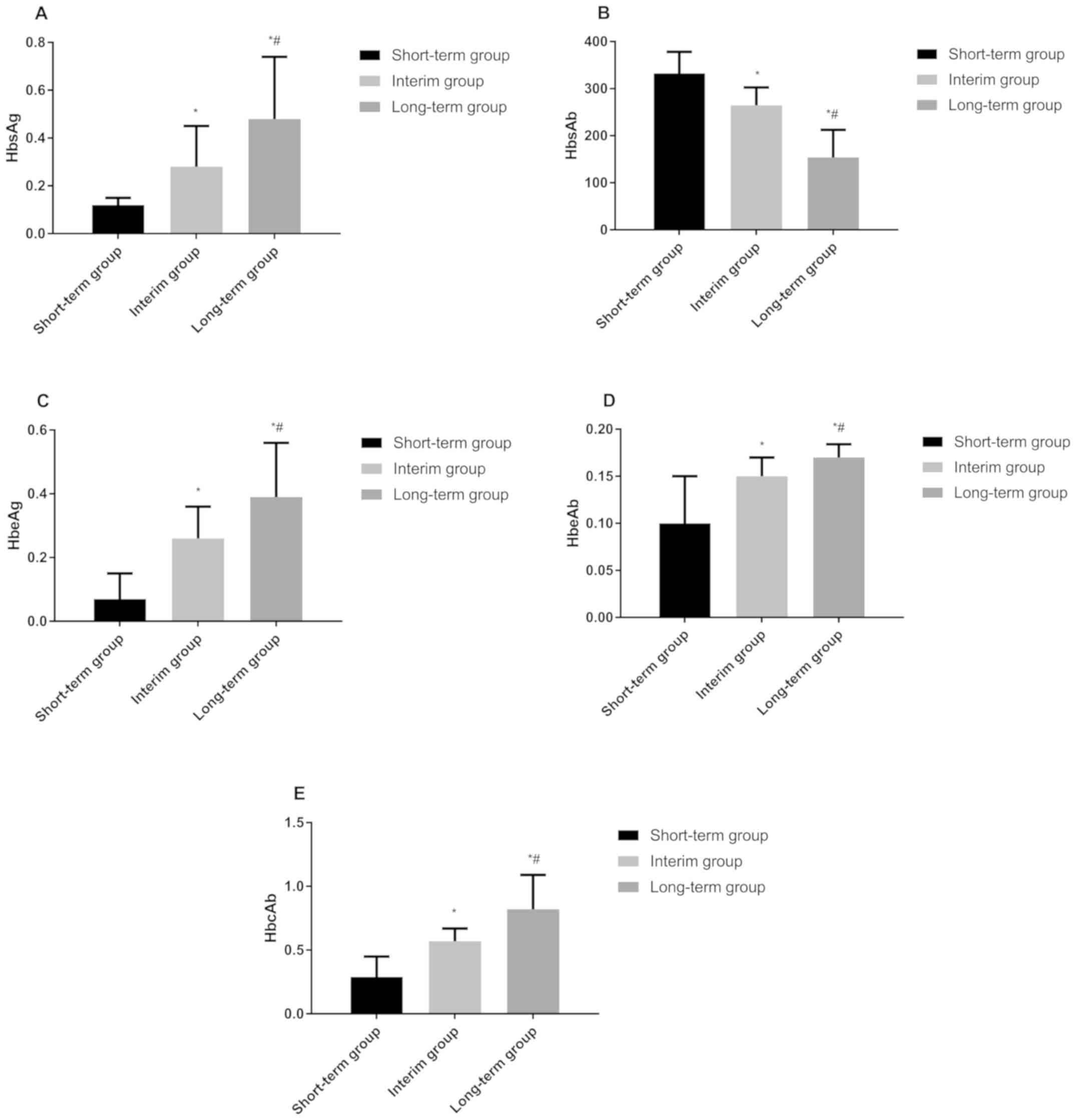 | Figure 1Quantitative results of five HBV
markers. (A) Quantitative results of HBsAg in patients of the three
groups. The HBsAg in long-term group was significantly higher than
that in medium-term and short-term group, and that in medium-term
group was significantly higher than that in short-term group.
*P<0.050, compared with the quantitative detection
results of HBsAg in short-term group; #P<0.050,
compared with the quantitative detection results of HBsAg in
medium-term group. (B) Quantitative results of HBsAb in patients of
the three groups. The HBsAb in long-term group was significantly
higher than that in medium-term and short-term group, and that in
medium-term group was significantly higher than that in short-term
group. *P<0.050, compared with the quantitative
detection results of HBsAb in short-term group;
#P<0.050, compared with the quantitative detection
results of HBsAb in medium-term group. (C) Quantitative results of
HBeAg in patients of the three groups. The HBeAg in long-term group
was significantly higher than that in medium-term and short-term
group, and that in medium-term group was significantly higher than
that in short-term group. *P<0.050, compared with the
quantitative detection results of HBeAg in short-term group;
#P<0.050, compared with the quantitative detection
results of HBeAg in medium-term group. (D) Quantitative results of
HBeAb in patients of the three groups. The HBeAb in long-term group
was significantly higher than that in medium-term and short-term
group, and that in medium-term group was significantly higher than
that in short-term group. *P<0.050, compared with the
quantitative detection results of HBeAb in short-term group;
#P<0.050, compared with the quantitative detection
results of HBeAb in medium-term group. (E) Quantitative results of
HBcAb in patients of the three groups. The HBcAb in long-term group
was significantly higher than that in medium-term and short-term
group, and that in medium-term group was significantly higher than
that in short-term group. *P<0.050, compared with the
quantitative detection results of HBcAb in short-term group;
#P<0.050, compared with the quantitative detection
results of HBcAb in medium-term group. HBV, hepatitis B virus;
HBsAg, hepatitis B surface antigen; HBsAb, hepatitis B surface
antibody; HBeAg, hepatitis Be antigen; HBeAb, hepatitis Be
antibody; HBcAb, hepatitis B core antibody. |
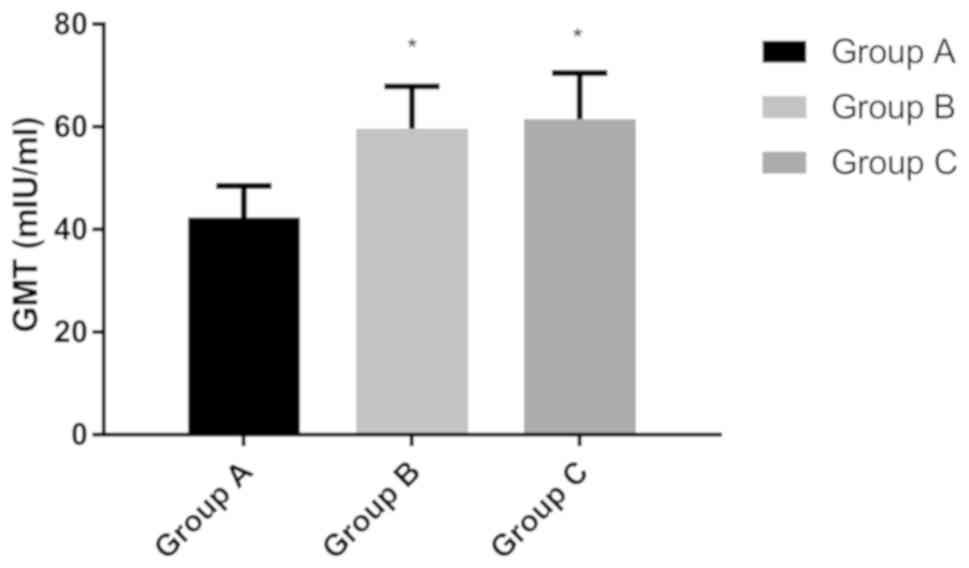
GMT experiment
The results of qualitative and quantitative analysis
of hepatitis B five markers showed that, among the three groups,
the results of the long-term group were the worst, so the patients
in this group were selected for further analysis. According to the
previous time of the hepatitis B antibody vaccination, the patients
in the long-term group were subdivided into three groups: Group A
(previous vaccination time: 10-13 years, n=420); group B (previous
vaccination time: 13-15 years, n=377); group C (last vaccination
time >15 years, n=406). Results of GMT showed that GMT in group
A was 42.37±6.14 mIU/ml, that in group B was 59.63±8.24 mIU/ml, and
that in group C was 61.51±8.97 mIU/ml. There was no significant
difference in GMT between groups B and C (P<0.050), but GMT in
group A was significantly lower than that in the other two groups
(P<0.050) (Fig. 2).
Discussion
Hepatitis B, one of the most common infectious
diseases in clinical practice, is highly infectious, and because of
its slow onset and insignificant specific signs, most patients not
only suffer from the disease, but also greatly increase the
probability of disease transmission (14,15). HBV
can cause a series of immune responses after invading the body, the
most severe of which is the change of hepatocytes (16). Moreover, it can also kill hepatitis B
cells and cause hepatocyte inflammation, fibrosis and necrosis.
Serious dysfunction of hepatic metabolic circulation directly leads
to yellow urine, jaundice, fatigue, abnormal digestive tract, and
even death directly in severe cases (17-19).
At present, the incidence of hepatitis B is mainly divided into
acute and chronic. About 90% of patients with acute hepatitis B can
recover by themselves, but need isolation treatment (20,21).
However, there is no significant treatment for chronic hepatitis B,
which can only be limited by long time antibiotics use (3-5 years
or more) (22,23). At present, chronic hepatitis B
accounts for the vast majority in clinic (24). Therefore, in order to reduce the harm
of hepatitis B to human body, the most effective method is to
vaccinate hepatitis B antibody in time. Since there is no reference
study at present to point out how long the best period of hepatitis
B vaccination is, this study, through experimental analysis and
taking hepatitis B five markers as research direction, analyzed the
re-vaccination time of hepatitis B vaccine in order to provide
reference and guidance for clinical diagnosis and treatment of
hepatitis B.
Examination of five HBV markers is the most
effective detection method for hepatitis B in clinical practice.
HBsAg-positive represents the existence of complete HBV particles
in the patient's body; HBsAb-positive represents the existence of
immune and protective antibodies against HBV in the patient's body;
HBeAg is an antigen within HBV, and its positive expression
indicates the activation of HBV in patients; HBeAb-positive means
that HBV is already in a stable or rehabilitative stage; and as a
marker of hepatitis B infection, the positive expression of HBcAb
indicates that the patient has or has had hepatitis B (25). In the qualitative results of this
study, there was no significant difference between the medium-term
and the long-term groups of HBsAb-positive patients, which were
significantly more than the short-term group, suggesting that there
were significantly more patients with hepatitis B virus in the
medium-term and long-term groups than in the short-term group. The
likelihood of HBV infection in patients after more than 5 years
after vaccination with hepatitis B began to increase significantly.
There were no significant differences between the short-term group
and the medium-term group of HBsAb-positive patients, which were
significantly more than the long-term group, suggesting that there
was a relatively stable hepatitis B resistance in the body within
10 years after vaccination. After 10 years, hepatitis B antibodies
gradually disappeared, and the risk of contracting hepatitis B
began to increase significantly. The comparison of the positive
cases of HBeAg in the three groups further confirmed our
hypothesis. None of the patients in the short-term group were
HBeAg-positive, while there were only 0.40% HBeAg-positive cases in
the medium-term group and 2.41% HBeAg-positive cases in the
long-term group. This also suggests that after 5 years of hepatitis
B vaccination, hepatitis B patients will begin to appear, and the
infection rate will increase significantly after 10 years of
vaccination. The comparison of HBeAb and HBcAb in the three groups
showed that the long-term group had the most HBeAb and HBcAb
positive patients, which further proves that patients are at their
worst after 10 years of hepatitis B vaccination. In summary, the
qualitative analysis of five hepatitis B showed that patients who
had been vaccinated with hepatitis B vaccine for more than 10 years
began to experience a significant decrease in the ability of the
hepatitis B antibody vaccine to work in the body, and a significant
increase in the risk of hepatitis B infection. The results of the
quantitative analysis are also consistent with the results of the
above examination, and can verify our experimental results. GMT, as
an excellent indicator for evaluating the concentration of virus in
the blood, is currently widely used in clinical practice to detect
viral infections in patients (26-28).
The greater the test results, the stronger the ability to represent
viruses or toxins. The study by Folaranmi et al (29) found the recommendation of the
vaccination time of the meningococcal vaccine of type B by GMT, and
Benoit et al (30) confirmed
that GMT was closely related to the viral infection of seasonal
influenza. In order to determine the exact time of antibody decline
after vaccination with hepatitis B vaccine, we performed a GMT test
on patients in the long-term group. The results showed that
patients with vaccination over 13 years have significantly higher
GMT than patients with less than 13 years. It is suggested that the
resistance of hepatitis B antibody vaccine begins to decrease
significantly after 13 years, and patients should be revaccinated
13 years after hepatitis B vaccination.
Examination of the five HBV markers is very
sensitive to the situation of hepatitis B infection, but it is
easily affected by external environmental factors in the process of
detection. Yang et al (31)
pointed out that hemolysis, blood vessel pollution, incomplete
washing, fibrinogen and other factors might cause differences in
the results of five HBV markers examination. In this study,
inspectors were all senior examiners at the director level in the
hospital, and factors that might affect the outcome were avoided as
much as possible to further enhance the accuracy of the
experimental results. The results of this study show that the
protective ability of hepatitis B antibody vaccine begins to
decrease 10 years after vaccination, and its mechanism needs to be
further studied.
The determination of re-vaccination time of
hepatitis B antibody vaccine by five HBV markers was analyzed in
this experiment. However, due to the limited experimental
conditions, there were still some shortcomings. The subjects were
of relatively similar origin, not excluding the possibility that
there might be differences in the results of examination among
different ethnic groups. Other human or environmental factors were
not excluded which might have an effect on the experimental
results.
In conclusion, the protective effect of hepatitis B
antibody vaccine is satisfactory within 10 years after vaccination,
and re-vaccination is recommended after more than 13 years of
vaccination when the virus begins to increase significantly, in
order to prevent the occurrence of hepatitis B.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QJ and FY wrote the manuscript and performed ELISA.
CM and QZ collected and analyzed the patients’ general data. XC and
ZG were responsible for the analysis of the observation indicators.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Women and Children's Health Care Hospital of Linyi (Linyi, China).
Signed informed consents were obtained from the patients and/or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sarin SK, Kumar M, Lau GK, Abbas Z, Chan
HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, et al:
Asian-Pacific clinical practice guidelines on the management of
hepatitis B: A 2015 update. Hepatol Int. 10:1–98. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Terrault NA, Bzowej NH, Chang KM, Hwang
JP, Jonas MM and Murad MH: American Association for the Study of
Liver Diseases. AASLD guidelines for treatment of chronic hepatitis
B. Hepatology. 63:261–283. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lok AS, McMahon BJ, Brown RS Jr, Wong JB,
Ahmed AT, Farah W, Almasri J, Alahdab F, Benkhadra K, Mouchli MA,
et al: Antiviral therapy for chronic hepatitis B viral infection in
adults: A systematic review and meta-analysis. Hepatology.
63:284–306. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang C, Ji D, Chen J, Shao Q, Li B, Liu J,
Wu V, Wong A, Wang Y, Zhang X, et al: Hepatitis due to reactivation
of hepatitis B virus in endemic areas among patients with hepatitis
C treated with direct-acting antiviral agents. Clin Gastroenterol
Hepatol. 15:132–136. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Brown RS Jr, McMahon BJ, Lok AS, Wong JB,
Ahmed AT, Mouchli MA, Wang Z, Prokop LJ, Murad MH and Mohammed K:
Antiviral therapy in chronic hepatitis B viral infection during
pregnancy: A systematic review and meta-analysis. Hepatology.
63:319–333. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ye B, Liu X, Li X, Kong H, Tian L and Chen
Y: T-cell exhaustion in chronic hepatitis B infection: Current
knowledge and clinical significance. Cell Death Dis.
6(e1694)2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bruce MG, Bruden D, Hurlburt D, Zanis C,
Thompson G, Rea L, Toomey M, Townshend-Bulson L, Rudolph K, Bulkow
L, et al: Antibody levels and protection after hepatitis B vaccine:
Results of a 30-year follow-up study and response to a booster
dose. J Infect Dis. 214:16–22. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hernán MA, Jick SS, Olek MJ and Jick H:
Recombinant hepatitis B vaccine and the risk of multiple sclerosis:
A prospective study. Neurology. 63:838–842. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Van Damme P: Long-term protection after
hepatitis B vaccine. J Infect Dis. 214:1–3. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Qiu Y, Guo L, Zhang S, Xu B, Gao Y, Hu Y,
Hou J, Bai B, Shen H and Mao P: DNA-based vaccination against
hepatitis B virus using dissolving microneedle arrays adjuvanted by
cationic liposomes and CpG ODN. Drug Deliv. 23:2391–2398.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nelson NP, Easterbrook PJ and McMahon BJ:
Epidemiology of hepatitis B virus infection and impact of
vaccination on disease. Clin Liver Dis. 20:607–628. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Guirguis-Blake JM, Senger CA, Webber EM,
Mularski RA and Whitlock EP: Screening for chronic obstructive
pulmonary disease: Evidence report and systematic review for the US
preventive services task force. JAMA. 315:1378–1393.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Eichelberger MC, Couzens L, Gao Y, Levine
M, Katz J, Wagner R, Thompson CI, Höschler K, Laurie K, Bai T, et
al: ELLA study participants: Comparability of neuraminidase
inhibition antibody titers measured by enzyme-linked lectin assay
(ELLA) for the analysis of influenza vaccine immunogenicity.
Vaccine. 34:458–465. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lampertico P, Agarwal K, Berg T, Buti M,
Janssen HLA, Papatheodoridis G, Zoulim F and Tacke F: European
Association for the Study of the Liver. Electronic address:
easloffice@easloffice.eu; European Association for the Study of the
Liver. EASL 2017 Clinical Practice Guidelines on the management of
hepatitis B virus infection. J Hepatol. 67:370–398. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kim WR, Berg T, Asselah T, Flisiak R, Fung
S, Gordon SC, Janssen HL, Lampertico P, Lau D, Bornstein JD, et al:
Evaluation of APRI and FIB-4 scoring systems for non-invasive
assessment of hepatic fibrosis in chronic hepatitis B patients. J
Hepatol. 64:773–780. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Buti M, Gane E, Seto WK, Chan HL, Chuang
WL, Stepanova T, Hui AJ, Lim YS, Mehta R, Janssen HL, et al:
GS-US-320-0108 Investigators: Tenofovir alafenamide versus
tenofovir disoproxil fumarate for the treatment of patients with
HBeAg-negative chronic hepatitis B virus infection: A randomised,
double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol
Hepatol. 1:196–206. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Roberts H, Kruszon-Moran D, Ly KN, Hughes
E, Iqbal K, Jiles RB and Holmberg SD: Prevalence of chronic
hepatitis B virus (HBV) infection in U.S. households: National
Health and Nutrition Examination Survey (NHANES), 1988-2012.
Hepatology. 63:388–397. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Paul S, Saxena A, Terrin N, Viveiros K,
Balk EM and Wong JB: Hepatitis B virus reactivation and prophylaxis
during solid tumor chemotherapy: A systematic review and
meta-analysis. Ann Intern Med. 164:30–40. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Decorsière A, Mueller H, van Breugel PC,
Abdul F, Gerossier L, Beran RK, Livingston CM, Niu C, Fletcher SP,
Hantz O, et al: Hepatitis B virus X protein identifies the Smc5/6
complex as a host restriction factor. Nature. 531:386–389.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lok AS, Pan CQ, Han SH, Trinh HN, Fessel
WJ, Rodell T, Massetto B, Lin L, Gaggar A, Subramanian GM, et al:
Randomized phase II study of GS-4774 as a therapeutic vaccine in
virally suppressed patients with chronic hepatitis B. J Hepatol.
65:509–516. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Harris AM, Iqbal K, Schillie S, Britton J,
Kainer MA, Tressler S and Vellozzi C: Increases in acute hepatitis
B virus infections - Kentucky, Tennessee, and West Virginia,
2006-2013. MMWR Morb Mortal Wkly Rep. 65:47–50. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Park JJ, Wong DK, Wahed AS, Lee WM, Feld
JJ, Terrault N, Khalili M, Sterling RK, Kowdley KV, Bzowej N, et
al: Hepatitis B Research Network: Hepatitis B Virus-specific and
global T-cell dysfunction in chronic hepatitis B. Gastroenterology.
150:684–695.e5. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Takayama H, Sato T, Ikeda F and Fujiki S:
Reactivation of hepatitis B virus during interferon-free therapy
with daclatasvir and asunaprevir in patient with hepatitis B
virus/hepatitis C virus co-infection. Hepatol Res. 46:489–491.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hayashi K, Ishigami M, Ishizu Y, Kuzuya T,
Honda T, Nishimura D, Goto H and Hirooka Y: A case of acute
hepatitis B in a chronic hepatitis C patient after daclatasvir and
asunaprevir combination therapy: Hepatitis B virus reactivation or
acute self-limited hepatitis? Clin J Gastroenterol. 9:252–256.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xiang KH, Michailidis E, Ding H, Peng YQ,
Su MZ, Li Y, Liu XE, Dao Thi VL, Wu XF, Schneider WM, et al:
Effects of amino acid substitutions in hepatitis B virus surface
protein on virion secretion, antigenicity, HBsAg and viral DNA. J
Hepatol. 66:288–296. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fries LF, Smith GE and Glenn GM: A
recombinant viruslike particle influenza A (H7N9) vaccine. N Engl J
Med. 369:2564–2566. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Patel M, Glass RI, Jiang B, Santosham M,
Lopman B and Parashar U: A systematic review of anti-rotavirus
serum IgA antibody titer as a potential correlate of rotavirus
vaccine efficacy. J Infect Dis. 208:284–294. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
van Assen S, Holvast A, Telgt DSC, Benne
CA, de Haan A, Westra J, Kallenberg CG and Bijl M: Patients with
humoral primary immunodeficiency do not develop protective
anti-influenza antibody titers after vaccination with trivalent
subunit influenza vaccine. Clin Immunol. 136:228–235.
2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Folaranmi T, Rubin L, Martin SW, Patel M
and MacNeil JR: Centers for Disease Control (CDC). Use of serogroup
B meningococcal vaccines in persons aged ≥10 years at increased
risk for serogroup B meningococcal disease: Recommendations of the
Advisory Committee on Immunization Practices, 2015. MMWR Morb
Mortal Wkly Rep. 64:608–612. 2015.PubMed/NCBI
|
|
30
|
Benoit A, Beran J, Devaster JM, Esen M,
Launay O, Leroux-Roels G, McElhaney JE, Oostvogels L, van Esse GA
and Gaglani M: Hemagglutination inhibition antibody titers as a
correlate of protection against seasonal A/H3N2 influenza disease.
Open Forum Infect Dis 2: ofv067. doi: 10.1093/ofid/ofv067.
eCollection 2015 Apr.
|
|
31
|
Yang S, Tian G, Cui Y, Ding C, Deng M, Yu
C, Xu K, Ren J, Yao J, Li Y, et al: Factors influencing immunologic
response to hepatitis B vaccine in adults. Sci Rep.
6(27251)2016.PubMed/NCBI View Article : Google Scholar
|