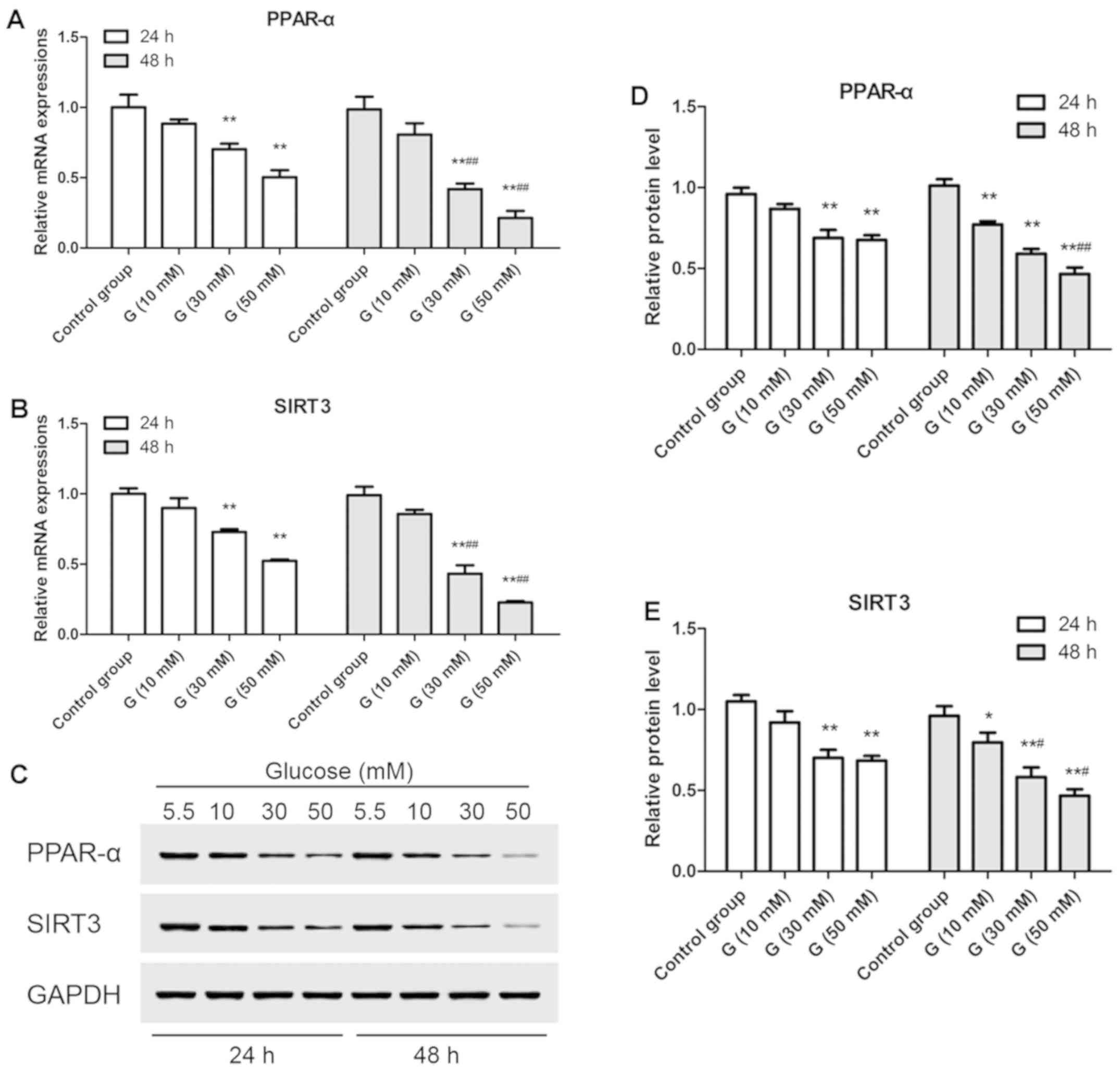
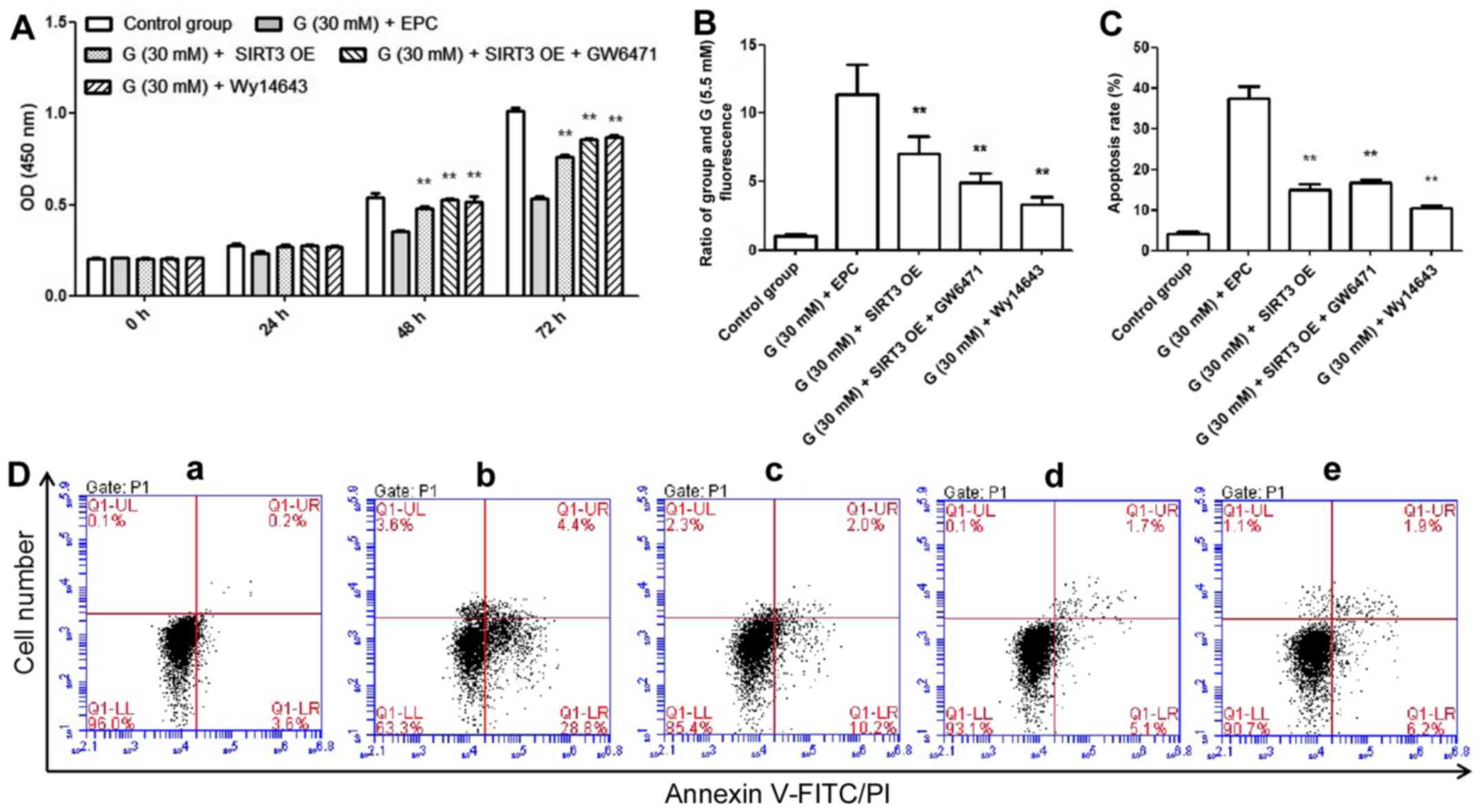
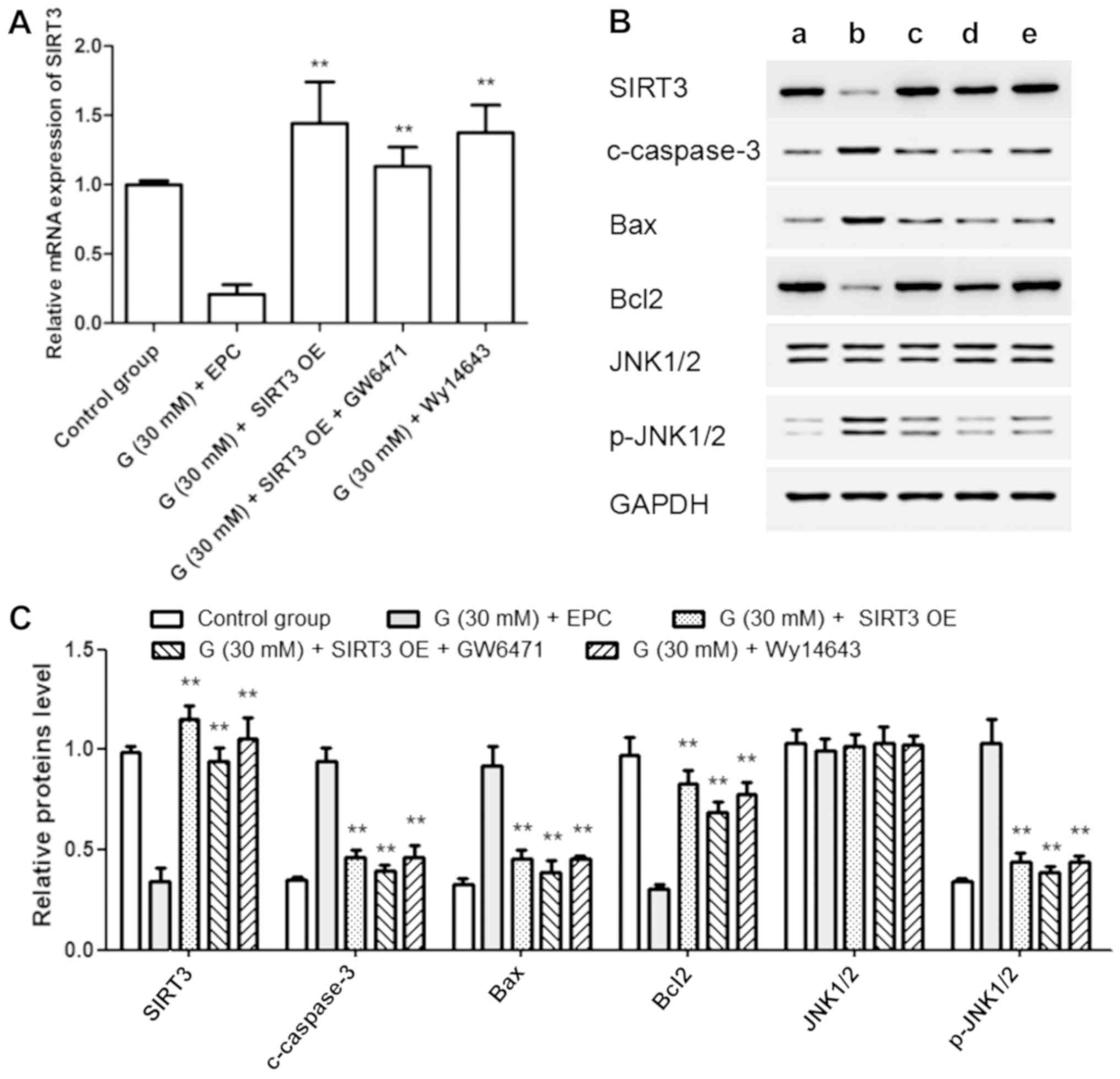
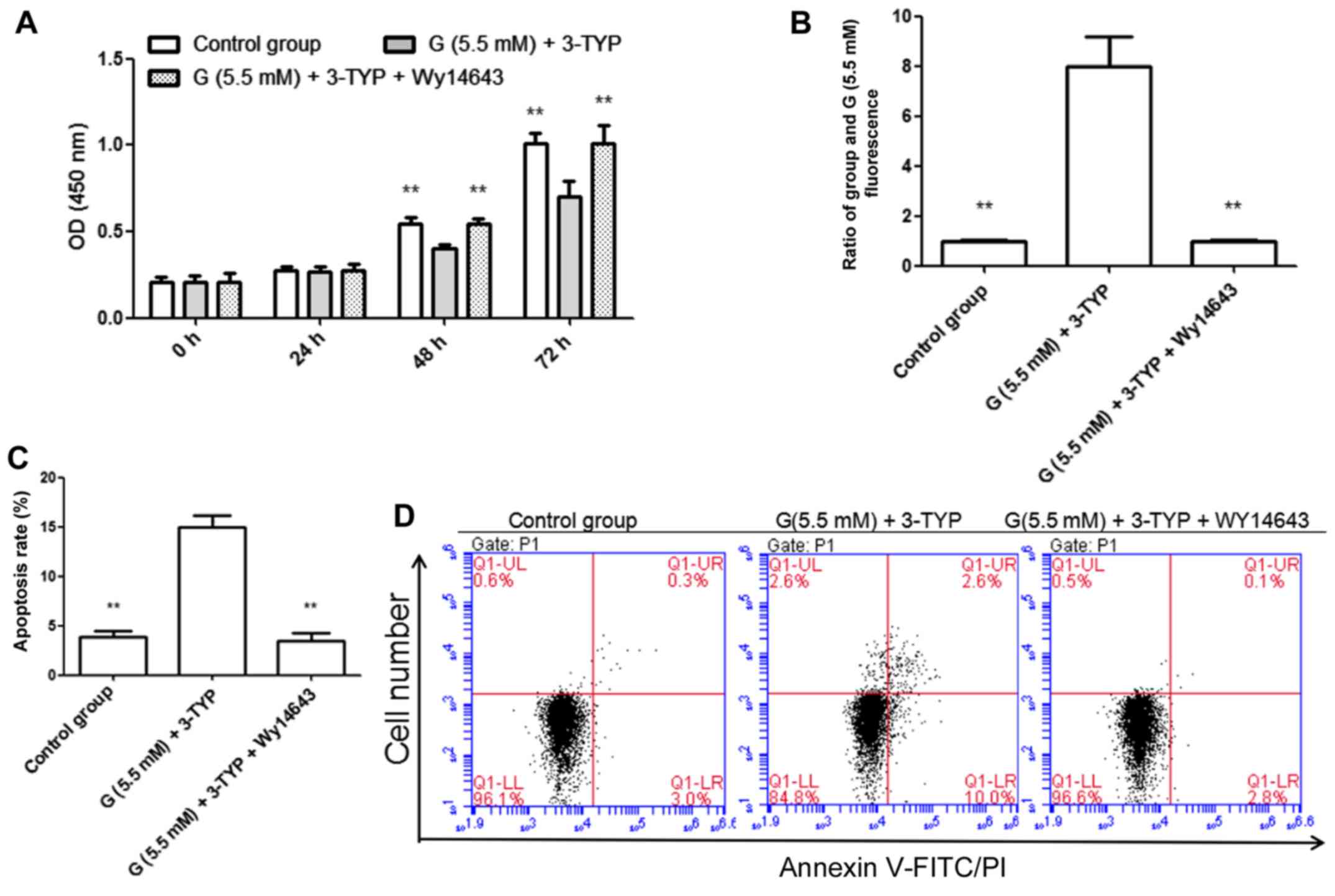
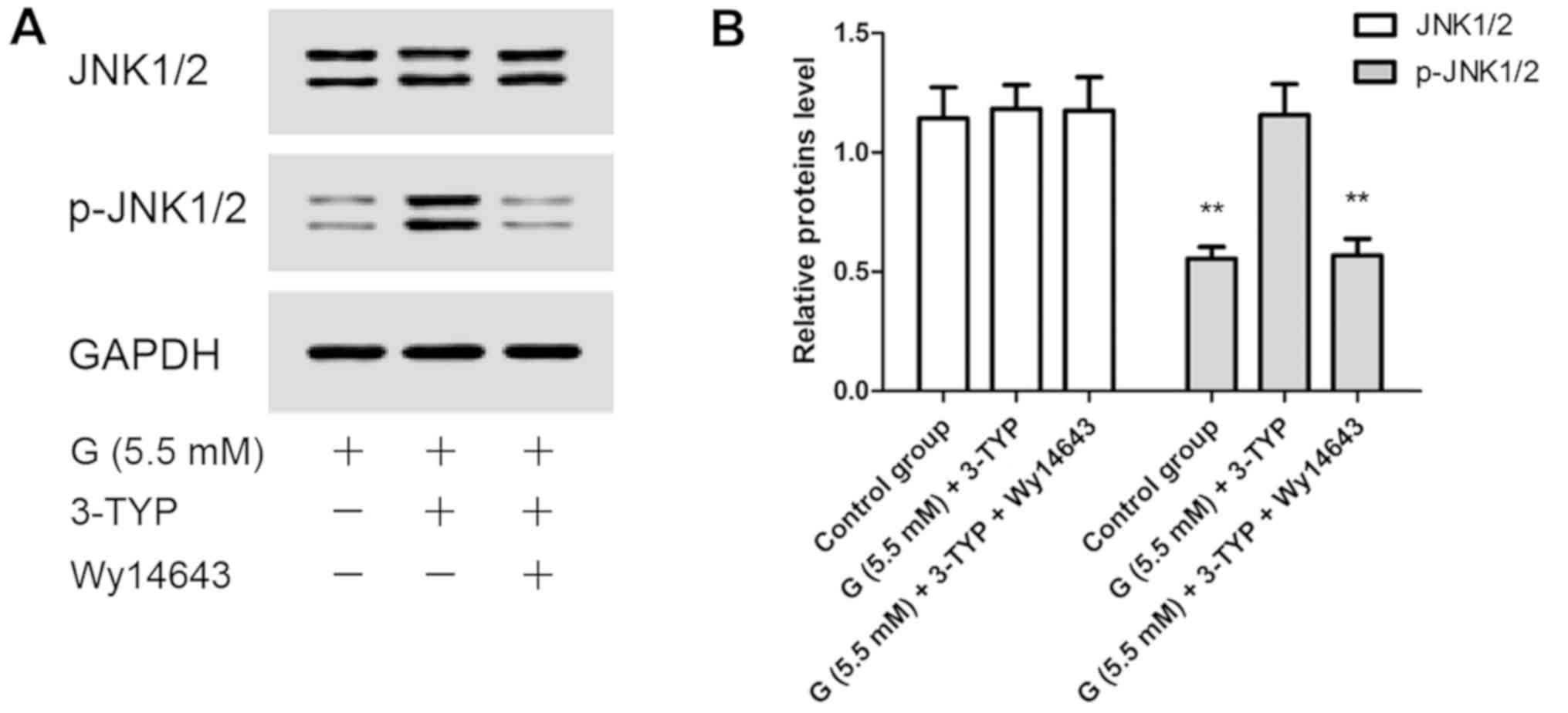
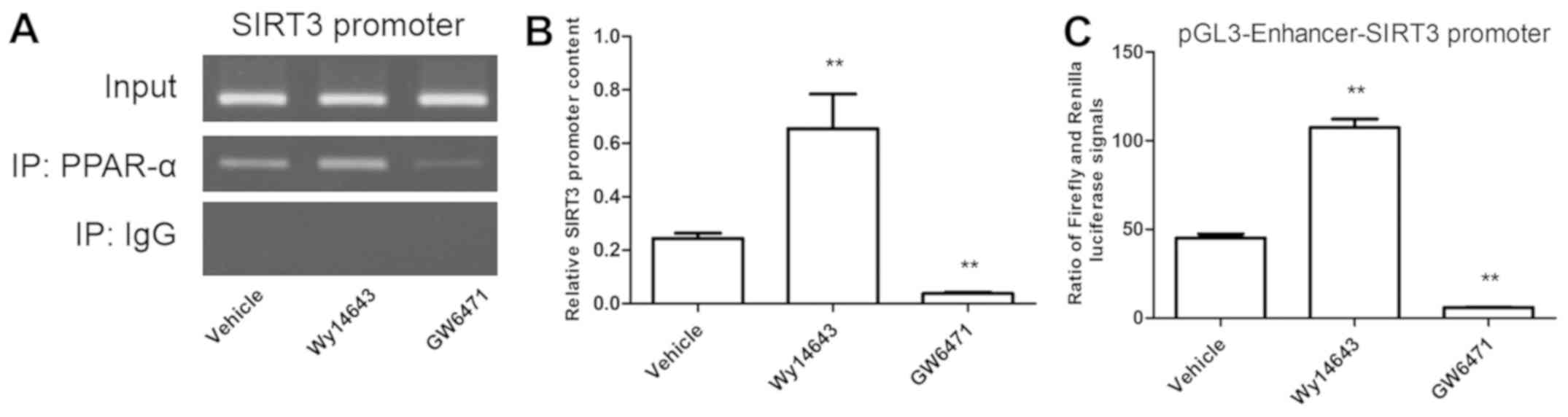
|
1
|
Li D, Han N and Cui Z: Application of
latent class model in the classification of the patients with
diabetes vulnerability. Chin J Health Stat. 1:11–13. 2018.
|
|
2
|
Pappachan JM, Varughese GI, Sriraman R and
Arunagirinathan G: Diabetic cardiomyopathy: Pathophysiology,
diagnostic evaluation and management. World J Diabetes. 4:177–189.
2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rubler S, Dlugash J, Yuceoglu YZ, Kumral
T, Branwood AW and Grishman A: New type of cardiomyopathy
associated with diabetic glomerulosclerosis. Am J Cardiol.
30:595–602. 1972.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rutter MK, Parise H, Benjamin EJ, Levy D,
Larson MG, Meigs JB, Nesto RW, Wilson PW and Vasan RS: Impact of
glucose intolerance and insulin resistance on cardiac structure and
function: Sex-related differences in the Framingham Heart Study.
Circulation. 107:448–454. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Aneja A, Tang WH, Bansilal S, Garcia MJ
and Farkouh ME: Diabetic cardiomyopathy: Insights into
pathogenesis, diagnostic challenges, and therapeutic options. Am J
Med. 121:748–757. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rossen JD: Abnormal microvascular function
in diabetes: Relationship to diabetic cardiomyopathy. Coron Artery
Dis. 7:133–138. 1996.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hattori Y, Kawasaki H, Abe K and Kanno M:
Superoxide dismutase recovers altered endothelium-dependent
relaxation in diabetic rat aorta. Am J Physiol. 261:H1086–H1094.
1991.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bucala R, Tracey KJ and Cerami A: Advanced
glycosylation products quench nitric oxide and mediate defective
endothelium-dependent vasodilatation in experimental diabetes. J
Clin Invest. 87:432–438. 1991.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tarquini R, Lazzeri C, Pala L, Rotella CM
and Gensini GF: The diabetic cardiomyopathy. Acta Diabetol.
48:173–181. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Huynh K, Bernardo BC, McMullen JR and
Ritchie RH: Diabetic cardiomyopathy: Mechanisms and new treatment
strategies targeting antioxidant signaling pathways. Pharmacol
Ther. 142:375–415. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cai L, Li W, Wang G, Guo L, Jiang Y and
Kang YJ: Hyperglycemia-induced apoptosis in mouse myocardium:
Mitochondrial cytochrome C-mediated caspase-3 activation pathway.
Diabetes. 51:1938–1948. 2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dreyer C, Krey G, Keller H, Givel F,
Helftenbein G and Wahli W: Control of the peroxisomal
beta-oxidation pathway by a novel family of nuclear hormone
receptors. Cell. 68:879–887. 1992.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Finck BN, Lehman JJ, Leone TC, Welch MJ,
Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, et
al: The cardiac phenotype induced by PPARalpha overexpression
mimics that caused by diabetes mellitus. J Clin Invest.
109:121–130. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Finley LWS, Haas W, Desquiret-Dumas V,
Wallace DC, Procaccio V, Gygi SP and Haigis MC: Succinate
dehydrogenase is a direct target of sirtuin 3 deacetylase activity.
PLoS One. 6(e23295)2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Qiu X, Brown K, Hirschey MD, Verdin E and
Chen D: Calorie restriction reduces oxidative stress by
SIRT3-mediated SOD2 activation. Cell Metab. 12:662–667.
2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tao R, Coleman MC, Pennington JD, Ozden O,
Park SH, Jiang H, Kim HS, Flynn CR, Hill S, Hayes McDonald W, et
al: Sirt3-mediated deacetylation of evolutionarily conserved lysine
122 regulates MnSOD activity in response to stress. Mol Cell.
40:893–904. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lombard DB, Alt FW, Cheng HL, Bunkenborg
J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D,
Murphy A, et al: Mammalian Sir2 homolog SIRT3 regulates global
mitochondrial lysine acetylation. Mol Cell Biol. 27:8807–8814.
2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kendrick AA, Choudhury M, Rahman SM,
McCurdy CE, Friederich M, Van Hove JL, Watson PA, Birdsey N, Bao J,
Gius D, et al: Fatty liver is associated with reduced SIRT3
activity and mitochondrial protein hyperacetylation. Biochem J.
433:505–514. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Caldefie-Chézet F, Walrand S, Moinard C,
Tridon A, Chassagne J and Vasson MP: Is the neutrophil reactive
oxygen species production measured by luminol and lucigenin
chemiluminescence intra or extracellular? Comparison with DCFH-DA
flow cytometry and cytochrome c reduction. Clin Chim Acta.
319:9–17. 2002.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gilmour DS and Lis JT: RNA polymerase II
interacts with the promoter region of the noninduced hsp70 gene in
Drosophila melanogaster cells. Mol Cell Biol. 6:3984–3989.
1986.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Us-Camas R and De-la-Peña C: Chromatin
immunoprecipitation (ChiP) protocol for the analysis of gene
regulation by histone modifications in Agave angustifolia
haw. Methods Mol Biol. 1815:371–383. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cai L, Wang Y, Zhou G, Chen T, Song Y, Li
X and Kang YJ: Attenuation by metallothionein of early cardiac cell
death via suppression of mitochondrial oxidative stress results in
a prevention of diabetic cardiomyopathy. J Am Coll Cardiol.
48:1688–1697. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zheng J, Shi L, Liang F, Xu W, Li T, Gao
L, Sun Z, Yu J and Zhang J: Sirt3 ameliorates oxidative stress and
mitochondrial dysfunction after intracerebral hemorrhage in
diabetic rats. Front Neurosci. 12(414)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang G, Fu XL, Wang JJ, Guan R, Sun Y and
Tony To SS: Inhibition of glycolytic metabolism in glioblastoma
cells by Pt3glc combinated with PI3K inhibitor via SIRT3-mediated
mitochondrial and PI3K/Akt-MAPK pathway. J Cell Physiol.
234:5888–5903. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bause AS and Haigis MC: SIRT3 regulation
of mitochondrial oxidative stress. Exp Gerontol. 48:634–639.
2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lee TI, Kao YH, Chen YC, Huang JH, Hsiao
FC and Chen YJ: Peroxisome proliferator-activated receptors
modulate cardiac dysfunction in diabetic cardiomyopathy. Diabetes
Res Clin Pract. 100:330–339. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Desvergne B and Wahli W: Peroxisome
proliferator-activated receptors: Nuclear control of metabolism.
Endocr Rev. 20:649–688. 1999.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Barger PM and Kelly DP: PPAR signaling in
the control of cardiac energy metabolism. Trends Cardiovasc Med.
10:238–245. 2000.PubMed/NCBI View Article : Google Scholar
|