Introduction
As a common pregnancy-related complication,
pregnancy-induced hypertension is one of the main causes of
perinatal morbidity and mortality (1). Reports show that the prevalence of
pregnancy-induced hypertension is 4.6-13.1% worldwide, and the
neonatal mortality is 9.2% (2).
Studies have shown that pregnancy-induced hypertension not only
causes serious complications during the perinatal period, but also
increases the incidence of cardiovascular and cerebrovascular
diseases, seriously affecting the health and safety of pregnant
women (3,4). Moreover, due to the particularity of
drug action mechanism when pregnant women and fetuses take drugs
(5), it is particularly important to
choose a safe and effective drug treatment.
Magnesium sulfate could relieve spasmolysis,
diuresis and pain (6). It could also
relax the vascular smooth muscle, dilate the spasmolysis peripheral
blood vessels, reduce blood pressure, which is suitable for the
treatment of patients with moderate and severe pregnancy-induced
hypertension when receiving intravenous injection (7,8).
Nifedipine is a type of dihydropyridine calcium antagonist, it is
applicable to various types of hypertension (9-11)
and has a good effect on intractable and severe hypertension. Wen
and Li (12) found that the combined
use of the above two drugs had a good effect in treating
pregnancy-induced hypertension. A previous study confirmed that
inflammation response may be involved in the occurrence and
development of hypertension (13).
Suppressors of cytokine signaling-3 (SOCS-3) is a cytokine signal
inhibitor. It inhibits various inflammatory factors by mediating
JAK/STAT cytokine signal transduction pathway, playing a role in
inhibiting inflammatory reactions (14). By staining, Wang et al
(15) found expression of SOCS-3 in
the vascular endothelium of pregnant women with preeclampsia, and
the expression of SOCS-3 was lower than that of healthy pregnant
women. Interleukins play important roles in many processes,
including information transmission, activation and regulation of
immune cells, activation of mediating cells T and B, proliferation
and differentiation, and inflammatory response (16). Interleukin-10 (IL-10) is a key
biomarker for the diagnosis and treatment of adverse preeclampsia,
a common complication of pregnancy-induced hypertension (17). Krishnan et al (18) proved that hypertension was closely
related to the abnormal expression of interleukin-18 (IL-18).
At present, the therapeutic effect of magnesium
sulfate combined with nifedipine on pregnancy-induced hypertension
has been confirmed in the clinic, but there are few studies on the
related changes of patients' inflammatory factors. We speculated
that the changes in inflammatory factors in patients can better
assess the clinical efficacy and adverse reactions. In order to
confirm our conjecture, this investigation explored the changes of
serum inflammatory factors during the treatment of
pregnancy-induced hypertension syndrome with magnesium sulfate
combined with nifedipine. It is expected that the progress of
pregnancy-induced hypertension syndrome will be predicted by the
level of inflammatory factors.
Patients and methods
General data
This study is a prospective non-randomized
controlled study. A total of 188 cases were collected as research
objects. These patients had pregnancy-induced hypertension, and
were admitted to the hospital from June 2016 to February 2018.
Among them, 94 cases were treated with magnesium sulfate and were
the control group, and further 94 cases of patients were treated
with magnesium sulfate with nifedipine and were the study group.
The study was approved by the Ethics Committee of The First
People's Hospital of Yunnan Province (The Affiliated Hospital of
Kunming University of Science and Technology). Signed informed
consents were obtained from the patients and/or guardians.
Inclusion and exclusion criteria
Inclusion criteria: patients who met the diagnostic
criteria and were diagnosed with pregnancy-induced hypertension;
patients received treatment in the hospital; patients aged 18-40
years; patients who cooperated with research; patients or their
immediate family member signed the informed consent; patients with
complete medical records.
Exclusion criteria: patients with heart, liver,
spleen, lung, kidney and other important organs injury; patients
combined with mental illness and speech dysfunction; patients with
diabetes; patients with lymph node, organ and tissue metastasis or
drug allergy.
Therapies
Both groups received routine antihypertensive
therapy, and continuous ambulatory blood pressure and vital signs
of mothers and infants were monitored. Pregnant women were required
to take a left lying position to improve uterine placental blood
supply. Control group: patients in the control group received 25%
magnesium sulfate injection (60 ml) (Tianjin KingYork
Pharmaceutical Co., Ltd., SFDA approval no. H12020994), and 5%
glucose solution (500 ml) was added for intravenous infusion. Study
group: after being given the same intravenous infusion of magnesium
sulfate as the control group, patients received nifedipine
controlled-release tablet (Shanghai Xiandai Pharmaceutical Co.,
Ltd., SFDA approval no. H12020994), 3 mg/t.i.d. orally. The
treatment in both groups continued for more than half a month. Both
groups were treated for seven continuous days.
Curative effect assessment
Evaluation standards were formulated based on
reference (19). Marked effect:
edema, proteinuria and other clinical symptoms disappeared, blood
pressure was <140/90 mmHg. Effective: edema, proteinuria and
other major clinical symptoms were significantly reduced, with
blood pressure of 140-150/90-100 mmHg. Ineffective: clinical
symptoms and blood pressure such as edema and proteinuria were not
significantly improved compared with before treatment, or even
aggravated. Total effectiveness = marked effect + effective. For
patients with large thrombus and patients who failed to respond to
conservative treatment, lower extremity venous thrombectomy and
lower extremity venous ligation were taken into consideration.
Determination of inflammatory factors
in serum
Blood samples were collected before treatment (on
the day of admission) and after treatment (on the day of delivery).
The samples were sent to the clinical laboratory of the hospital
for determination in the morning of the same day. Inflammatory
indexes included SOCS-3 (USCNK, SEB684Hu-1), IL-10 (FineTest,
ESH0009), and IL-18 (FineTest, ECH0039). Blood samples were added
with 0.2 ml of 2% coagulant, centrifuged at 1,505 x g, 4˚C for 30
min, and stored at -20˚C. Enzyme-linked immunosorbent assay (ELISA)
was used for detection, and the procedure was strictly in
accordance with the kit instructions.
Observational indexes
Clinical efficacy were observed and compared between
the two groups. Delivery and incidence of adverse reactions were
compared between the two groups. The levels of serum SOCS-3, IL-10
and IL-18, and the predictive value of SOCS-3, IL-10, and IL-18 for
therapeutic effects in the two groups were compared before and
after treatment.
Statistical analysis
SPSS 24.0 statistical software (Shanghai Yuchuang
Network Technology Co., Ltd.) was used for statistical calculation
of all experimental results. Graphpad 8 (Softhead Inc.) was used
for drawing all images and for checking the results twice.
Enumeration data were all expressed in the form of (rate), and
χ2 test was used for comparison between groups.
Measurement data were all expressed in the form of (mean ± SD), and
t-test was used for comparison between groups. The predictive value
was analyzed by ROC curve. P<0.050 was considered statistically
significant.
Results
Comparison of general data
There were no significant differences in age, BMI,
gestational age, fertility circumstance, mean arterial pressure,
place of residence, ethnicity, smoking history, the degree of
edema, proteinuria, drinking history and exercise habits in the
study group and the control group (P>0.050), indicating that the
two groups of patients were comparable (Table I).
 | Table IComparison of clinical data [mean ±
SD, n (%)]. |
Table I
Comparison of clinical data [mean ±
SD, n (%)].
| Characteristics | Research group
(n=94) | Control group
(n=94) | χ2 or
t | P-value |
|---|
| Age (years) | | | 1.751 | 0.0815 |
| | 29.38±1.64 | 28.91±2.02 | | |
| BMI
(kg/m2) | | | 0.594 | 0.553 |
| | 25.42±2.39 | 25.61±1.97 | | |
| Gestational age
(weeks) | | | 0.432 | 0.665 |
| | 31.13±3.16 | 31.32±2.85 | | |
| Mean arterial
pressure (mmHg) | | | 1.319 | 0.189 |
| | 154.62±8.62 | 152.85±9.74 | | |
| Fertility
circumstance | | | 0.447 | 0.503 |
|
Unipara | 72 (76.60) | 68 (72.34) | | |
|
Multipara | 22 (23.40) | 26 (27.66) | | |
| Place of
residence | | | 0.223 | 0.636 |
|
City | 83 (88.30) | 85 (90.43) | | |
|
Countryside | 11 (11.70) | 9 (9.57) | | |
| Ethnicity | | | 0.495 | 0.483 |
|
Han | 75 (79.78) | 71 (75.53) | | |
|
Minority | 19 (20.21) | 23 (24.47) | | |
| Smoking history | | | 0.046 | 0.829 |
|
Yes | 13 (13.83) | 12 (12.77) | | |
|
No | 81 (86.17) | 81 (87.23) | | |
| Drinking
history | | | 0.367 | 0.544 |
|
With | 16 (17.02) | 13 (13.83) | | |
|
Without | 78 (82.98) | 81 (86.17) | | |
| Exercise habit | | | 0.101 | 0.74 |
|
With | 27 (28.72) | 29 (30.85) | | |
|
Without | 67 (71.28) | 65 (69.15) | | |
| Proteinuria (g/24
h) | | | 1.190 | 0.236 |
| | 1.84±0.54 | 1.94±0.61 | | |
| Degree of
edema | | | 0.426 | 0.808 |
|
+ (extended
to the thigh) | 24 (25.53) | 28 (29.79) | | |
|
++ (extends
to the vulva and abdomen) | 54 (57.45) | 51 (54.26) | | |
|
+++
(systemic edema or ascites) | 16 (17.02) | 15 (15.96) | | |
Efficacy evaluation and adverse
occurrence comparison
The total effective rate of clinical efficacy was
95.74% in the study group, which was significantly higher than that
in the control group (82.97%), P<0.05 (Table II).
 | Table IIComparison of clinical efficacy
between the two groups [n (%)]. |
Table II
Comparison of clinical efficacy
between the two groups [n (%)].
| Curative
effect | Research group
(n=94) | Control group
(n=94) | χ2 | P-value |
|---|
| Significant
effect | 74 (78.72) | 55 (58.51) | | |
| Effective | 16 (17.02) | 23 (24.47) | | |
| No effect | 4 (4.26) | 16 (17.02) | | |
| Overall
response | 90 (95.74) | 78 (82.97) | 8.057 | 0.004 |
The eutocia rate of the study group was higher than
that of the control group, and the cesarean section rate was lower
than that of the control group, P<0.05 (Table III).
 | Table IIIComparison of labor outcomes between
the two groups [n (%)]. |
Table III
Comparison of labor outcomes between
the two groups [n (%)].
| Groups | Eutocia | Cesarean
section | Premature
birth |
|---|
| Research group
(n=94) | 75 (79.78) | 13 (13.82) | 6 (6.38) |
| Control group
(n=94) | 61 (64.89) | 24 (25.53) | 11 (11.70) |
| χ2 | 5.21 | 4.072 | 1.617 |
| P-value | 0.02 | 0.043 | 0.203 |
There were a total of 9 adverse reactions in the
study group, including 3 cases of postpartum hemorrhage, 1 case of
eclampsia, 1 case of pulmonary edema, and 4 cases of cardiovascular
disease. In the control group, there were 4 cases of eclampsia, 5
cases of postpartum hemorrhage, 2 cases of placental abruption, 3
cases of pulmonary edema, and 5 cases of cardiovascular diseases, a
total of 19 cases of adverse reactions occurred in the control
group. The incidence of adverse reactions was significantly lower
in the study group than that in the control group, P<0.05
(Table IV).
 | Table IVComparison of adverse reactions of
patients in the two groups [n (%)]. |
Table IV
Comparison of adverse reactions of
patients in the two groups [n (%)].
| | Research group
(n=94) | Control group
(n=94) | χ2 | P-value |
|---|
| Eclampsia | 1 (1.06) | 4 (4.25) | | |
| Postpartum
hemorrhage | 3 (3.19) | 5 (5.32) | | |
| Placental
abruption | 0 (0.00) | 2 (2.12) | | |
| Pulmonary
edema | 1 (1.06) | 3 (3.19) | | |
| Cardiovascular
disease | 4 (4.25) | 5 (5.32) | | |
| Total adverse
reactions | 9 (9.57) | 19 (20.21) | 4.196 | 0.040 |
Comparison of SOCS-3, IL-10 and IL-18
levels between the two groups before and after treatment
Before treatment, no differences were found in
SOCS-3, IL-10 and IL-18 between the two groups (P>0.050). After
treatment, the levels of SOCS-3 and IL-10 were significantly
increased in the two groups, and they were higher in the study
group than those in the control group (P<0.050). The IL-18
levels of the two groups were significantly decreased, and they
were significantly lower in the study group than those in the
control group (P<0.050 (Fig.
1).
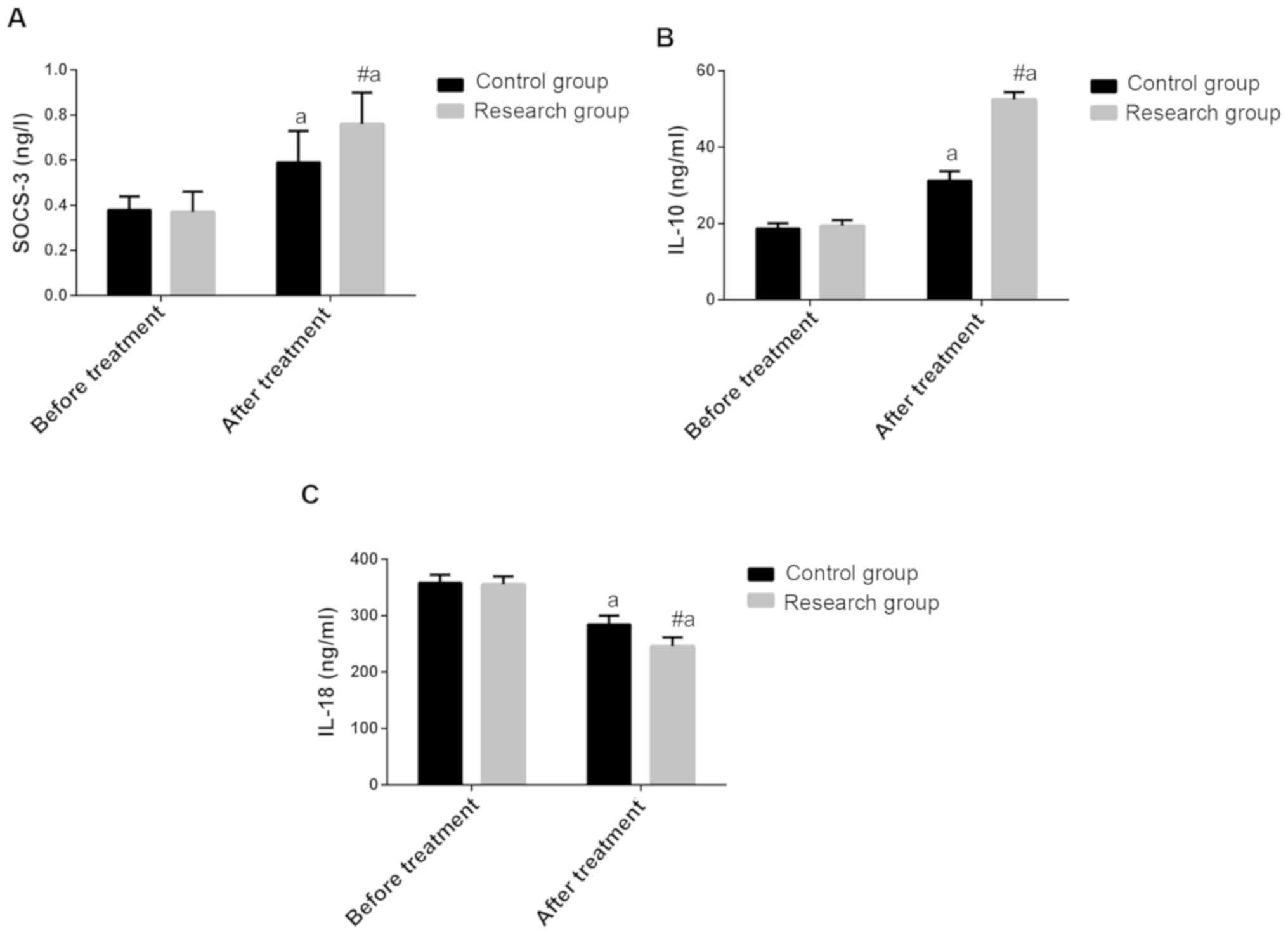 | Figure 1Curative effect of magnesium sulfate
combined with nifedipine on pregnancy-induced hypertension and the
effect of serum inflammatory factors. (A) Comparison of SOCS-3
levels before and after treatment between the two groups. Before
treatment, there were no differences in SOCS-3 between the two
groups (P>0.050). After treatment, SOCS-3 was significantly
increased in both groups, and this in the study group was higher
than that in the control group (P<0.050). (B) Comparison of
IL-10 levels before and after treatment in both groups. Before
treatment, there were no differences in IL-10 between the two
groups (P>0.050). After treatment, IL-10 increased significantly
in both groups, and this in the study group was higher than that in
the control group (P<0.050). (C) Comparison of IL-18 levels
before and after treatment in both groups. Before treatment, there
were no differences in IL-18 between the two groups (P>0.050).
After treatment, IL-18 decreased significantly in both groups, and
this in the study group was lower than that in the control group
(P<0.050). #P<0.05, compared with the control
group; aP<0.05, compared with before treatment.
SOCS-3, cytokine signaling-3; IL-10, interleukin-10; IL-18,
interleukin-18. |
Predictive value of SOCS-3, IL-10 and
IL-18 in patients with poor efficacy
All the patients were divided into good efficacy
group (n=168) and poor efficacy group (n=20) in accordance with
efficacy. According to the expression of SOCS-3, IL-10 and IL-18 in
the two groups, ROC curve was drawn to analyze its predictive value
for efficacy. The area under the curve (AUC) of SOCS-3 was 0.717
(95% CI, 0.577-0.857). When the cut-off value was <0.553 ng/l,
the optimal specificity was 76.19%, the sensitivity was 70.00%, and
the Youden index was 46.19. The AUC of IL-10 was 0.727 (95% CI,
0.6356-0.817). When the cut-off value was <48.06 ng/ml, the
optimal specificity was 52.98%, the sensitivity was 94.74%, and the
Youden index was 47.71. The AUC of IL-18 curve was 0.725 (95% CI,
0.630-0.821). When the cut-off value was <269.4 ng/ml, the
optimal specificity was 61.90%, the sensitivity was 85.00%, and the
Youden index was 46.90 (Fig. 2).
Discussion
Pregnancy-induced hypertension is an idiopathic
disease and prone to occur in the third trimester of pregnancy
(20,21). Severe complications such as eclampsia
appear in intensive cases (22,23),
which seriously affects the health and safety of pregnant women and
infants. Early intervention can effectively prevent the occurrence
of severe symptoms. The level of inflammatory factors in serum were
studied to carry out early screening for pregnancy-induced
hypertension and early drug intervention. In previous research,
indexes such as IL-1, IL-2, IL-6, and PCT have been studied
thoroughly, and were found with abnormal manifestations in many
diseases (24-28),
with low specificity.
Our study found that the total effective rate of
clinical efficacy in the study group was 95.74%, which was
significantly higher than that in the control group (82.97%), and
the eutocia rate in the study group was higher than that in the
control group, with better pregnancy results. It suggested that
magnesium sulfate combined with nifedipine could effectively
improve the clinical efficacy of in pregnancy-induced hypertension.
Then the incidence of adverse reactions between the two groups were
compared, and it was found that the incidence of adverse reactions
in the study group was significantly lower than that in the control
group, and there was only one case of adverse reactions (eclampsia)
in the study group. This suggested that combination therapy reduces
the incidence of adverse reactions, and some studies have shown
that magnesium sulfate and nifedipine in the treatment of eclampsia
does not increase the risk of serious magnesium-related effects,
confirming the safety of combination therapy (29). In this study, we first analyzed the
changes of SOCS-3, IL-10 and IL-18 levels in observation group and
control group before and after treatment. It was found that SOCS-3,
IL-10 levels in both groups increased significantly after
treatment, and the level of IL-18 in both groups decreased
significantly. The levels in the study group were significantly
lower than those in the control group. This is consistent with the
study of Chen et al (30)
that IL-6 level in pregnant women with preeclampsia treated with
magnesium sulfate combined with nifedipine decreased. SOCS-3 is an
important molecule that regulates the function of trophoblasts
during placental development (31).
Clinical studies have shown that SOCS-3 is the target of
miR-203(32), and the decreased
expression of SOCS-3 has been confirmed in the maternal vascular
endothelium of preeclampsia. Wang et al (33) found that the expression of miR-203
was increased in the maternal vascular endothelium of women with
preeclampsia, and the overexpression of miR-203 could down-regulate
the expression of SOCS-3 in the endothelial cells, accompanied by
inhibition of TNF-α-induced increase in adhesion of neutrophils to
endothelial cells. This suggests that the down-regulation of SOCS-3
and increased inflammatory response in the maternal vascular system
may be the result of increased expression of miR-203 in
preeclampsia. Subsequently, ROC curve analysis of the curative
effect was performed and found that the AUC of the predictive value
of SOCS-3, IL-10 and IL-18 in pregnancy-induced hypertension was
0.717, 0.727 and 0.725, respectively. The best specificity was
76.19, 52.98 and 61.90%, respectively, when the cut-off value was
<0.553 ng/l, 48.06 ng/ml and 269.46 ng/ml, and the sensitivity
was 70.00, 94.74 and 85.00%, respectively. This suggests that
SOCO-3, IL-10 and IL-18 have certain value in predicting the
efficacy of pregnancy-induced hypertension, and may be used as
biomarkers to predict the clinical efficacy. We speculate that the
combined detection of the above indicators in the future clinical
practice can effectively determine the clinical efficacy of
patients.
Although this study observed changes in inflammatory
factors before and after treatment in the two groups of patients,
we did not specifically analyze the treatment time and efficacy,
leaving certain limitations. Thus, further studies are required on
the relationship between SOCS-3, IL-10 and IL-18 and
pregnancy-induced hypertension, to further corroborate the results
of this study.
In conclusion, magnesium sulfate combined with
nifedipine can significantly improve the prevalence of
pregnancy-induced hypertension. The levels of SOCS-3, IL-10 and
IL-18 in patients are related to the curative effect of
pregnancy-induced hypertension, suggesting that they have important
value in the treatment and monitoring of gestational
hypertension.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
FZ, FA and XD conceived and designed the study, and
drafted the manuscript. FZ, FA, JW and XD collected, analyzed and
interpreted the experimental data. FZ revised the manuscript for
important intellectual content. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The First People's Hospital of Yunnan Province (The Affiliated
Hospital of Kunming University of Science and Technology). Signed
informed consents were obtained from the patients and/or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
George JN and Amaresh A: Neonatal
mortality and morbidity in pregnancy induced hypertension: A
prospective observational study. J Evol Med Dent Sci. 3:5238–5246.
2014.
|
|
2
|
Zhuang C, Gao J, Liu J, Wang X, He J, Sun
J, Liu X and Liao S: Risk factors and potential protective factors
of pregnancy-induced hypertension in China: A cross-sectional
study. J Clin Hypertens (Greenwich). 21:618–623. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kintiraki E, Papakatsika S, Kotronis G,
Goulis DG and Kotsis V: Pregnancy-induced hypertension. Hormones
(Athens). 14:211–223. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Andrews L and Patel N: Correlation of
serum lactate dehydrogenase and pregnancy induced hypertension with
its adverse outcomes. Int J Res Med Sci. 4:1347–1350. 2016.
|
|
5
|
Easterling TR: Pharmacological management
of hypertension in pregnancy. Semin Perinatol. 38:487–495.
2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chen C and Tao R: The impact of magnesium
sulfate on pain control after laparoscopic cholecystectomy: A
meta-analysis of randomized controlled studies. Surg Laparosc
Endosc Percutan Tech. 28:349–353. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lamarca B, Brewer J and Wallace K:
IL-6-induced pathophysiology during pre-eclampsia: Potential
therapeutic role for magnesium sulfate? Int J Interferon Cytokine
Mediat Res. 2011:59–64. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Alexander JM, McIntire DD, Leveno KJ and
Cunningham FG: Selective magnesium sulfate prophylaxis for the
prevention of eclampsia in women with gestational hypertension.
Obstet Gynecol. 108:826–832. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sharma KJ, Greene N and Kilpatrick SJ:
Oral labetalol compared to oral nifedipine for postpartum
hypertension: A randomized controlled trial. Hypertens Pregnancy.
36:44–47. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Webster LM, Myers JE, Nelson-Piercy C,
Harding K, Cruickshank JK, Watt-Coote I, Khalil A, Wiesender C,
Seed PT and Chappell LC: Labetalol versus nifedipine as
antihypertensive treatment for chronic hypertension in pregnancy: A
randomized controlled trial. Hypertension. 70:915–922.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Martínez-Jiménez C, Cruz-Angeles J, Videa
M and Martínez LM: Co-amorphous simvastatin-nifedipine with
enhanced solubility for possible use in combination therapy of
hypertension and hypercholesterolemia. Molecules.
23(2161)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wen J and Li X: Effect of magnesium
sulfate combined with phentolamine and nifedipine for gestational
hypertension and serum levels of LIF and apelin. J Coll Physicians
Surg Pak. 29:231–234. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sun Q, Xin F, Wen X, Lu C, Chen R and Ruan
G: Protective effects of different kinds of filtered water on
hypertensive mouse by suppressing oxidative stress and
inflammation. Oxid Med Cell Longev. 2018(2917387)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Niwa Y, Kanda H, Shikauchi Y, Saiura A,
Matsubara K, Kitagawa T, Yamamoto J, Kubo T and Yoshikawa H:
Methylation silencing of SOCS-3 promotes cell growth and migration
by enhancing JAK/STAT and FAK signalings in human hepatocellular
carcinoma. Oncogene. 24:6406–6417. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang Y, Lewis DF, Gu Y, Zhao S and Groome
LJ: Elevated maternal soluble Gp130 and IL-6 levels and reduced
Gp130 and SOCS-3 expressions in women complicated with
preeclampsia. Hypertension. 57:336–342. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yang GX, Sun Y, Tsuneyama K, Zhang W,
Leung PS, He XS, Ansari AA, Bowlus C, Ridgway WM and Gershwin ME:
Endogenous interleukin-22 protects against inflammatory bowel
disease but not autoimmune cholangitis in dominant negative form of
transforming growth factor beta receptor type II mice. Clin Exp
Immunol. 185:154–164. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cubro H, Kashyap S, Nath MC, Ackerman AW
and Garovic VD: The role of interleukin-10 in the pathophysiology
of preeclampsia. Curr Hypertens Rep. 20(36)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Krishnan SM, Sobey CG, Latz E, Mansell A
and Drummond GR: IL-1β and IL-18: Inflammatory markers or mediators
of hypertension? Br J Pharmacol. 171:5589–5602. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu LS: Writing Group of 2010 Chinese
Guidelines for the Management of Hypertension. 2010 Chinese
guidelines for the management of hypertension. Zhonghua Xin Xue
Guan Bing Za Zhi. 39:579–615. 2011.PubMed/NCBI(In Chinese).
|
|
20
|
Świątkowska-Stodulska R, Kmieć P,
Stefańska K and Sworczak K: Renin-Angiotensin-aldosterone system in
the pathogenesis of pregnancy-induced hypertension. Exp Clin
Endocrinol Diabetes. 126:362–366. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cai G, Zhang B, Weng W, Yang L, Shi G, Xue
S and Fu X: Associations of pregnancy-associated plasma protein-A
level with essential hypertension and hypertensive disorders in
pregnancy in Chinese population: A meta-analysis of 20 research
studies involving 3332 individuals. BMJ Open.
5(e008210)2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xu Q, Fan D, Li F and Zhang Z: Influence
of serum HMW adiponectin level in patients with pregnancy-induced
hypertension syndrome on the occurrence of eclampsia in secondary
pregnancy. Exp Ther Med. 14:4972–4976. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhou Y, Chen ZH, Zhao XL, Qiao YH and Guo
RX: Effect of serum high molecular weight adiponectin level on the
occurrence of eclampsia during subsequent pregnancy in patients
with primary pregnancy induced hypertension. Eur Rev Med Pharmacol
Sci. 21:213–218. 2017.PubMed/NCBI
|
|
24
|
Alves S, Churlaud G, Audrain M,
Michaelsen-Preusse K, Fol R, Souchet B, Braudeau J, Korte M,
Klatzmann D and Cartier N: Interleukin-2 improves amyloid
pathology, synaptic failure and memory in Alzheimer's disease mice.
Brain. 140:826–842. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Parulekar AD, Kao CC, Diamant Z and
Hanania NA: Targeting the interleukin-4 and interleukin-13 pathways
in severe asthma: Current knowledge and future needs. Curr Opin
Pulm Med. 24:50–55. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Dinarello CA: Interleukin-1 mediated
autoinflammation from heart disease to cancer. In: Textbook of
Autoinflammation. Hashkes PJ, Laxer RM and Simon A (eds). Springer,
Cham, pp711-725, 2019.
|
|
27
|
Ridker PM, Libby P, MacFadyen JG, Thuren
T, Ballantyne C, Fonseca F, Koenig W, Shimokawa H, Everett BM and
Glynn RJ: Modulation of the interleukin-6 signalling pathway and
incidence rates of atherosclerotic events and all-cause mortality:
Analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes
Study (CANTOS). Eur Heart J. 39:3499–3507. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Vijayan AL, Vanimaya Ravindran S, Saikant
R, Lakshmi S, Kartik R and Manoj G: Procalcitonin: A promising
diagnostic marker for sepsis and antibiotic therapy. J Intensive
Care. 5(51)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Magee LA, Miremadi S, Li J, Cheng C, Ensom
MH, Carleton B, Côté AM and von Dadelszen P: Therapy with both
magnesium sulfate and nifedipine does not increase the risk of
serious magnesium-related maternal side effects in women with
preeclampsia. Am J Obstet Gynecol. 193:153–163. 2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chen Q, Zhao M, Guo F, Yin YX, Xiao JP,
Stone PR and Chamley LW: The reduction of circulating levels of
IL-6 in pregnant women with preeclampsia by magnesium sulphate and
nifedipine: In vitro evidence for potential mechanisms. Placenta.
36:661–666. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhao S, Gu Y, Dong Q, Fan R and Wang Y:
Altered interleukin-6 receptor, IL-6R and gp130, production and
expression and decreased SOCS-3 expression in placentas from women
with pre-eclampsia. Placenta. 29:1024–1028. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wei T, Orfanidis K, Xu N, Janson P, Ståhle
M, Pivarcsi A and Sonkoly E: The expression of microRNA-203 during
human skin morphogenesis. Exp Dermatol. 19:854–856. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang Y, Dong Q, Gu Y and Groome LJ:
Up-regulation of miR-203 expression induces endothelial
inflammatory response: Potential role in preeclampsia. Am J Reprod
Immunol. 76:482–490. 2016.PubMed/NCBI View Article : Google Scholar
|
















