Introduction
The ovarian function and estrogen level continuously
decline in women relative to age, ultimately leading to
osteoporosis (OP) (1). OP is one of
the common complications of patients with type 2 diabetes mellitus
(T2DM), causing great morbidity and seriously affecting the quality
of life of patients (2-4).
Currently, the main therapeutic methods for elderly patients with
T2DM and OP include calcium and vitamin D supplements, physical
exercise, specified diet and high quality nursing, yet the
occurrence and progression of OP in elderly patients with T2DM
cannot be effectively controlled (5,6).
Therefore, it is of great clinical significance to explore new
therapeutic methods and drugs for patients with T2DM and OP.
Microribonucleic acids (microRNAs or miRs) are a
type of single-stranded, non-coding and small-molecule RNAs
approximately 18-25 nucleotides in length, which are involved in
the occurrence and progression of various diseases (7,8). It has
been reported that some miRs can participate in bone turnover, bone
metabolism and bone development, thereby regulating the occurrence
and progression of OP (9-11).
However, whether there is a certain association between miRs and
T2DM + OP has not yet been reported.
In previous experiments, several miRNAs related to
OP have been screened, and it was found that miR-205 had a certain
association with T2DM + OP in elderly patients (9-11).
In the present study, therefore, the expression of miR-205 in bone
tissues and serum of elderly female patients with T2DM + OP was
explored, and the effect of miR-205 on osteogenesis/adipogenesis of
bone marrow mesenchymal stem cells (BMSCs) and its specific
mechanism in elderly female mice with T2DM + OP were investigated,
so as to provide new insight for the clinical treatment of elderly
female patients with T2DM + OP.
Materials and methods
Patients and data
A total of 24 female patients with T2DM + OP treated
at The Third Affiliated Hospital of Qiqihar Medical University
(Qiqihar, Heilongjiang, China) from October 2016 to June 2017 were
selected. The patients were aged from 60 to 80 years with an
average age of 72.42±13.41 years. All patients met the clinical
diagnostic criteria for T2DM and the WHO diagnostic criteria for
OP. WHO diagnostic criteria for T2DM included: Plasma glucose ≥11.1
mmol/l, fasting plasma glucose (FPG) ≥7.0 mmol/l, or oral glucose
tolerance test 2-h postprandial blood glucose (OGTT2hPG) ≥11.1
mmol/l at any time. WHO diagnostic criteria for OP included: A bone
mineral density (BMD) measured by dual-energy X-ray absorptiometry
to be 2.5 standard deviations below that in the local region and of
the same gender and ethnic group (T value ≤-2.5). According to
studies (12-17),
when studying orthopedic-related diseases, the selected controls
are usually patients admitted to the hospital for trauma. In this
study, trauma that required emergency surgery due to multiple
trauma to the bone was defined as severe trauma. At the same time,
24 patients with normal blood glucose and BMD who were treated at
this hospital due to severe trauma were selected as the control
group. During surgery of the elderly female patients with T2DM + OP
and patients in the control group, the bone tissues were collected
from the femur or tibia and immediately stored in liquid nitrogen.
In addition, the fasting venous blood was collected in both groups
in the morning, and the serum was separated and stored in a
refrigerator at -80˚C.
Exclusion criteria included patients with T1DM,
special type of diabetes or autoimmune diseases, patients who
presented with severe hematological diseases, those who received
estrogen, calcitonin, vitamin K and other drugs that may affect
bone metabolism for a long period prior to the study, those
complicated with severe kidney disease, tumors or gout, patients
who presented with other congenital OP, those who had poor mental
or consciousness state or failed to cooperate in the study.
Establishment of an elderly female
mouse model of T2DM + OP
All mice were maintained at a temperature between 20
and 26˚C, with a humidity of 40 to 60%, and a 12-h light/12-h dark
cycle, and free access to food and water. Animal health and
behavior were monitored each morning and evening.
A total of 54 C57BL/6J female mice were obtained
from Beijing Vital River Laboratory Animal Technology Co., Ltd. for
this study, and 24 C57BL/6J female mice, 8 weeks of age, were used
as the controls. Bone tissue and serum samples were collected from
the mice. Thirty C57BL/6J female mice, age 16 months were used to
construct the models. One week after acclimatization, a mouse model
of osteoporosis was constructed. All mice were anesthetized with 50
mg/kg of 1% sodium pentobarbital. After anesthesia, under sterile
conditions, ophthalmic scissors, ophthalmic tweezers, hemostatic
forceps and other instruments were used to remove the bilateral
ovaries of the mice. After suture, the mice were rested on a clean
table, until they could move freely. Bilateral ovaries of the mice
were removed. After 4 weeks of breeding, if the mice exhibited
phenotypes such as reduced bone density, increased bone separation,
and decreased number of trabeculae, the osteoporosis mouse model
was deemed as successfully constructed. At the same time, the mice
were fed with high-fat and high-glucose diets for 6 weeks, and 2%
streptozotocin (STZ) solution (pH 4.4) was intraperitoneally (i.p.)
injected (35 mg/kg/time) after fasting for 12 h, so as to establish
the T2DM model. A FPG level of >11.1 mmol/l within 2 weeks after
STZ injection indicated T2DM. Finally, 24 mice met the criteria,
and the elderly female mouse model of T2DM + OP was achieved after
8 weeks. The bone tissues and serum samples were collected in both
groups for subsequent experiment. Once it was clear that the 6 mice
did not meet the requirement of subsequent experiment, the
experiments on the 6 mice were stopped immediately, and euthanasia
was performed. In ‘Guide for the Care and Use of Laboratory
Animals’ of the National Institutes of Health, endpoints for
experiments are defined. It is highlighted that euthanasia is
performed to reduce animal pain and suffering before death. Dying
is the state before the death of the animal, which can replace
death as the experimental endpoint. The criteria for the dying end
point include the following: i) weight loss; ii) body temperature
reduction; iii) obvious inactivity or remain motionless; iv) arched
back posture; v) hair stains; vi) tumor ulceration and bleeding;
vii) dyspnea. In this experiment, we found that 6 mice did not meet
the requirement of subsequent experiment, and because of turbid
hair and arched back, they were euthanized. The other 6 mice were
sacrificed by injection of 200 mg/kg sodium pentobarbital.
To summarize, in the present study, 54 female
C57BL/6J mice were utilized, and 24 8-week-old control mice were
also sacrificed by cervical dislocation after anesthesia. Thirty
16-month-old mice were used to construct the model of type 2
diabetes with osteoporosis; among them, 6 mice did not meet the
standard and were euthanized, and 24 mice were sacrificed by
cervical dislocation after anesthesia.
Total RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from bone tissues using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and
the miRNA was extracted from serum using a serum miRNA extraction
kit (Foregene). The RNA purity and concentration were detected
using a Nanodrop spectrophotometer (Thermo Fisher Scientific,
Inc.). Then RNA was synthesized into cDNA using the RevertAid™
First Strand cDNA Synthesis kit (Fermentas) and amplified using the
SYBR Green Master Mix (Roche) on the ABI7500 PCR instrument
(Applied Biosystems; Thermo Fisher Scientific, Inc.) to detect the
expression of the target gene. All primer sequences used in this
experiment are shown in Table I.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Forward
(5'-3') | Reverse
(5'-3') |
|---|
| Murine miR-205 |
CACGTCCCAGGCTCCA |
CGGGCATCGAAACTGCCAATT |
| Human miR-205 |
CTTGTCCTTCATTCCACCGGA |
TGCCGCCTGAACTTCACTCC |
| Murine
U6 |
GCGCGTCGTGAAGCGTTC |
GTGCAGGGTCCGAGGT |
| Human U6 |
CGCTTCGGCAGCACATATA |
TTCACGAATTTGCGTGTCAT |
| Murine
ALP |
AGAACCCCAAAGGCTTCTTC |
CTTGGCTTTTCCTTCATGGT |
| Murine
OCN |
AGGGCAGCGAGGTAGTGAA |
TCCTGAAAGCCGATGTGGT |
| Murine
PPARγ |
TCTGAGTCTGTATGGAGTGACAT |
CCAAGTCGTTCACATCTAGTTCA |
| Murine
FABP4 |
TGCCTTTGTGGGAACCTG |
CCTGTCGTCTGCGGTGAT |
| Murine
Runx2 |
TCTACTATGGCACTTCGTCAGG |
GCTTCCATCAGCGTCAACAC |
| Murine
β-actin |
TAAAGACCTCTATGCCAACACAGT |
CACGATGGAGGGGCCGGACTCATC |
Separation and culture of mouse
BMSCs
After the successful establishment of the elderly
female mouse model of T2DM + OP, mouse bone marrow mesenchymal stem
cells (BMSCs) were extracted according to a previous study
(18). First, the mice were
anesthetized by an i.p. injection of sodium barbiturate
(Sigma-Aldrich; Merck KGaA), and then the mice were sacrificed by
cervical dislocation. The bilateral femur and tibia were isolated
under sterile conditions; muscle tissue was removed, and the femur
and tibia were exposed. Then BMSCs were cultured in high-glucose
DMEM (Corning) containing 10% fetal bovine serum (FBS) (Cyagen),
100 U/ml penicillin (Beyotime) and 100 µg/ml streptomycin
(Beyotime). The medullary cavity was rinsed repeatedly, the cells
were extracted out of the marrow cavity, centrifuged with a
high-speed centrifuge (Thermo Fisher Scientific, Inc.) for 5 min at
200 x g, and the cell pellet was placed in cell bottles. Then the
cell pellet was placed in an incubator (Thermo Fisher Scientific,
Inc.) with 5% CO2 at 37˚C. BMSCs were purified by
differential adherence method; culture medium was replaced every
three days, and non-adherent cells were removed; the splitting
ratio was 1:3. The above steps were repeated and passed to the
third generation for subsequent experiments. The study was approved
by the Animal Ethics Care Committee of Qiqihar Medical University
(QMU-AECC-2019-51).
Cell transfection
The third-generation BMSCs in the logarithmic growth
phase were inoculated into a 24-well plate
(2x104/cm2), and transiently transfected
using the transfection reagent X-treme (Vazyme). After transfection
with the miR-205 mimic (final concentration: 50 nM) and miR-205
inhibitor (final concentration: 100 nM) for 24 h, subsequent
experiments were performed. The sequences were: miR-205 mimic,
5'-UCCUUCAUUCCACCGGAGUCUG-3' and NC-mimic,
5'-UUCUCCGAACGUGUCACGUTT-3'; miR-205 inhibitor,
5'-CAGACUCCGGUGGAAUGAAGGA-3' and NC-inhibitor,
5'-CAGUACUUUUGUGUAGUACAA-3'.
CCK-8 assay
BMSCs in the third-generation logarithmic growth
phase were selected and seeded in 96-well plates at a density of
1x104 cells/cm2. The cells were transfected
with miR-205. After 24, 48 and 72 h, 10 µl of CCK-8 solution and
100 µl of serum-free medium were added to each well. The cells were
cultured for 2 h in an incubator, and the absorbance value (OD
value) of each well was measured at 450 nm using a microplate
reader (Tecan).
Osteogenic and adipogenic induction of
BMSCs
The third-generation BMSCs in the logarithmic growth
phase were inoculated into a 24-well plate
(2x104/cm2) and transfected with miR-205.
Then osteogenic induction medium containing 10% FBS, 10 mmol/l
sodium 3-phosphoglycerate, 10 mol/l dexamethasone and 50 µg/ml
ascorbic acid was added for osteogenic induction for 21 days, and
the original medium was replaced with fresh medium once every 3
days. After that, alizarin red S (ARS) staining was performed.
First, the induction medium was discarded, and the cells were
washed with PBS repeatedly, fixed with 4% paraformaldehyde for 30
min, stained with ARS dye (Cyagen) for 30 min and washed again with
PBS, followed by observation under an inverted microscope
(magnification, x10).
In addition, the third-generation BMSCs in the
logarithmic growth phase were inoculated into a 24-well plate and
transfected with miR-205. Then adipogenic induction medium
containing 1 µM of dexamethasone, 200 µM of indometacin, 0.5 mM of
IBMX and 10 µM of insulin for adipogenic induction for 24 days, and
the original medium was replaced with the fresh medium once every 3
days. After that, oil red O (ORO) staining was performed. First,
the induction medium was discarded, and the cells were washed with
PBS 3 times, fixed with 4% paraformaldehyde at room temperature for
30 min, stained with ORO dye (Cyagen) for 30 min and washed again
with PBS, followed by observation under the inverted microscope
(magnification, x10).
Target prediction
The target genes of miR-205 were predicted and
analyzed using the online websites TargetScan (http://www.targetscan.org/vert_72/) and miRanda
(http://www.microrna.org/src/jmiranda/).
Western blotting
The total protein was lysed using protein lysis
buffer (Beyotime) and centrifuged at 2,500 x g and 4˚C for 5 min.
The supernatant was transferred into another EP tube and
cryopreserved in a refrigerator at -80˚C. The protein concentration
was measured using the Bradford protein quantification kit, and
loading buffer was added to the samples and boiled. After 12.5%
SDS-PAGE, the protein samples were transferred onto a PVDF
membrane, sealed with skim milk for 2 h, and incubated with the
primary antibodies, Runx2 (dilution 1:1,000, cat. no. AF2593;
Beyotime) or GAPDH (dilution 1:1,000, cat. no. AF1186; Beyotime) on
a shaker at 4˚C overnight. On the next day, the protein samples
were incubated with the anti-rabbit secondary antibody (dilution,
1:500; cat. no. A0279; Beyotime) at room temperature. After 1 h,
the fluorescence signals were collected using the luminescence
detector and analyzed by Image-Pro Plus 6 software (Media
Cybernetics).
Luciferase reporter assay
The miR-205 precursor sequences and the
3'-untranslated region (3'UTR) of the mouse Runx2 gene were
cloned into the psi-CHECK2 vector through PCR, enzyme digestion,
ligation and transformation, and 0.5 µg of 3'UTR and 1 µg of miR
were co-transfected into BMSCs. After 48 h, the cells were
collected and lysed. According to the instructions of the
dual-luciferase reporter gene kit (Promega), the firefly luciferase
activity and Renilla luciferase activity were detected for
luciferase reporter assay.
Statistical methods
All data are expressed as mean ± standard error of
measurement, and a t-test was performed for the comparison of
sample means. GraphPad 7.0 software (GraphPad Software, Inc.) was
used for the statistical processing of all data, and the t-test for
statistical analysis. *P<0.05, **P<0.01
and ***P<0.001 were indicative of statistically
significant differences as shown in the figures and defined in the
figure legends.
Results
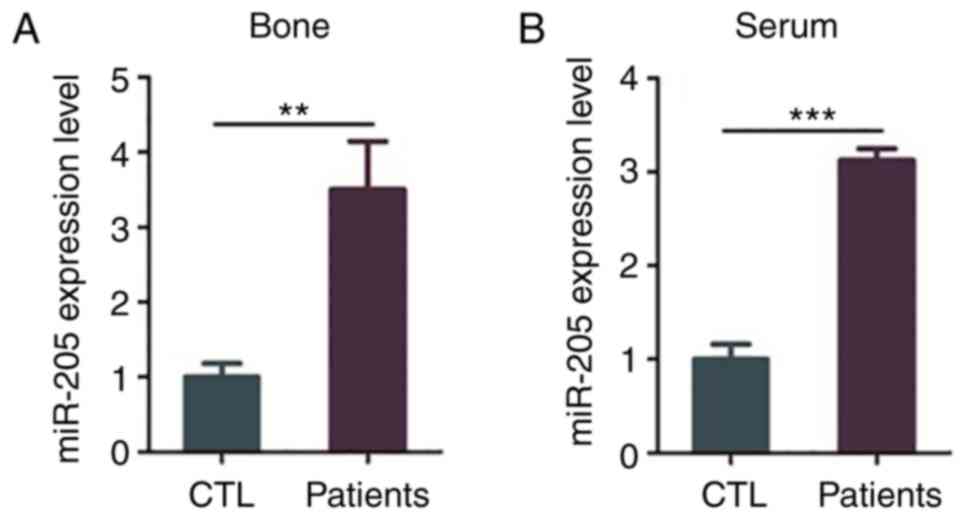
Expression of miR-205 is increased in
elderly female patients with T2DM + OP
Compared with the control group, the expression
level of miR-205 was significantly increased in the bone tissues
and serum of elderly female patients with T2DM + OP (P=0.0098 and
P=0.001) (Fig. 1A and B).
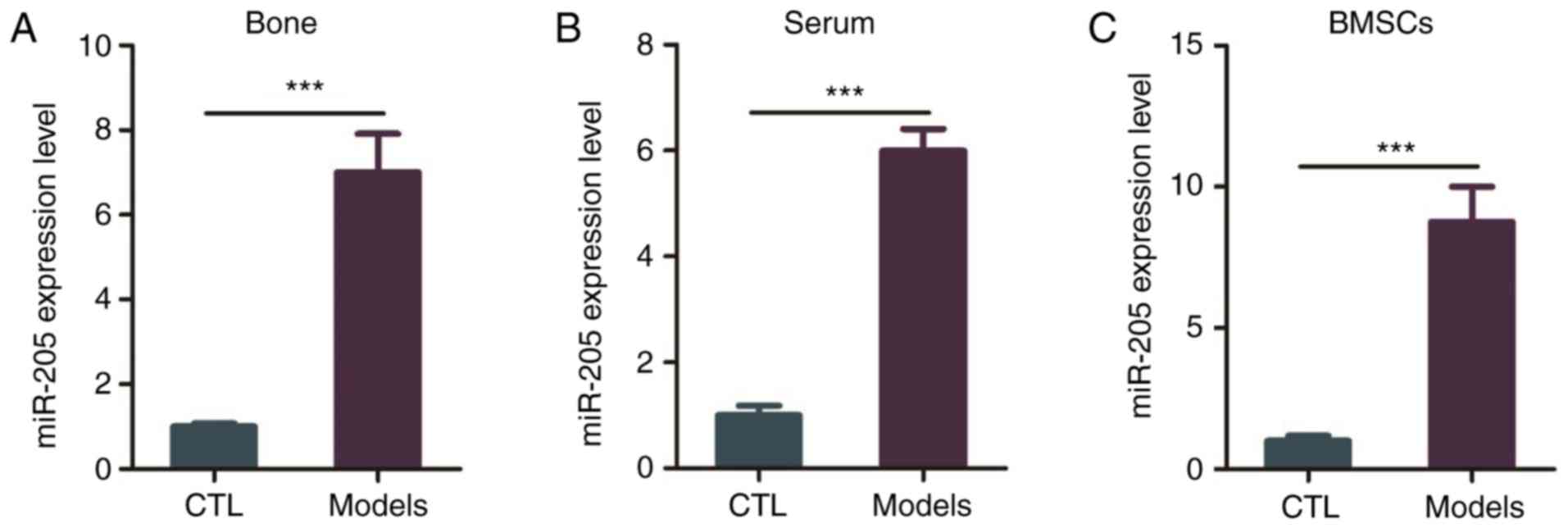
Expression of miR-205 is increased in
the elderly female mouse model of T2DM + OP
The expression level of miR-205 was higher in the
bone tissues, serum and BMSCs of the elderly female mice with T2DM
+ OP than this level in the control group (Fig. 2A-C).
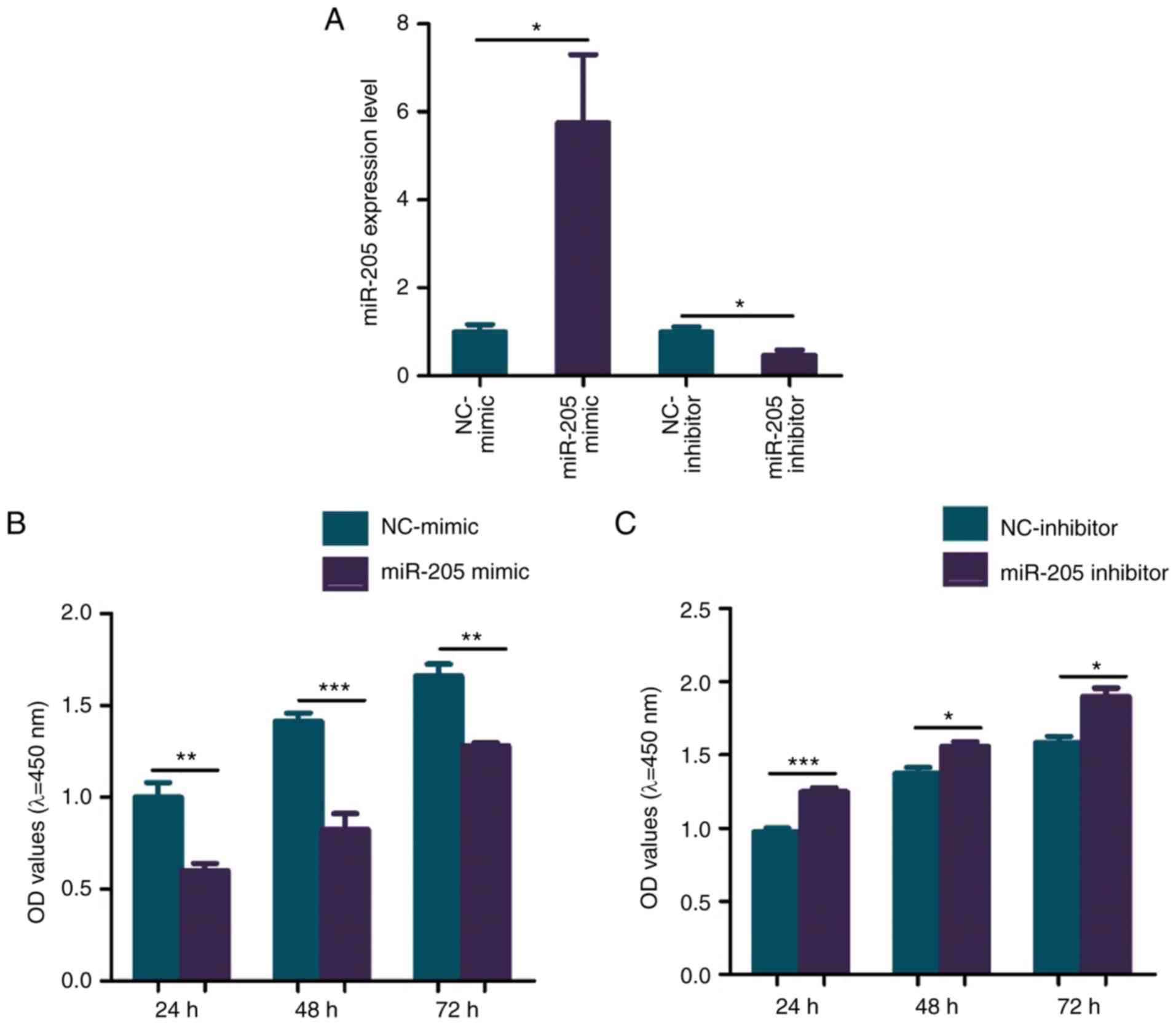
Effect of miR-205 on BMSC viability in
the elderly female mice with T2DM + OP
To investigate the effect of miR-205 overexpression
on the biological function of BMSCs in elderly female type 2
diabetic mice with OP, the cells were transfected with the miR-205
mimic and the transfection was deemed successful (Fig. 3A). CCK-8 assay was performed to
explore the effect of miR-205 on the cell viability of BMSCs
extracted from the elderly female mice with T2DM + OP. The results
showed that overexpression of miR-205 significantly inhibited the
viability of BMSCs in the elderly female mice with T2DM + OP when
compared to the negative control (NC) group (Fig. 3B). To investigate the effect of
miR-205 knockdown on the biological function of BMSCs in elderly
female type 2 diabetic mice with OP, the cells were transfected
with the miR-205 inhibitor and the transfection was deemed
successful (Fig. 3A). Knockdown of
miR-205 significantly increased the viability of BMSCs in elderly
female mice with T2DM + OP when compared to the NC group (Fig. 3C).
Effects of overexpression of miR-205
on osteogenic/adipogenic differentiation of BMSCs in elderly female
mice with T2DM + OP
The results of ARS staining showed that
overexpression of miR-205 obviously inhibited osteogenic
differentiation of BMSCs in the elderly female mice with T2DM + OP
(Fig. 4A). In addition, the results
of RT-PCR revealed that overexpression of miR-205 obviously reduced
the expression of the osteogenesis-related genes, alkaline
phosphatase, biomineralization associated (ALP) and
osteocalcin (OCN) in BMSCs of elderly female mice with T2DM
+ OP (Fig. 4B).
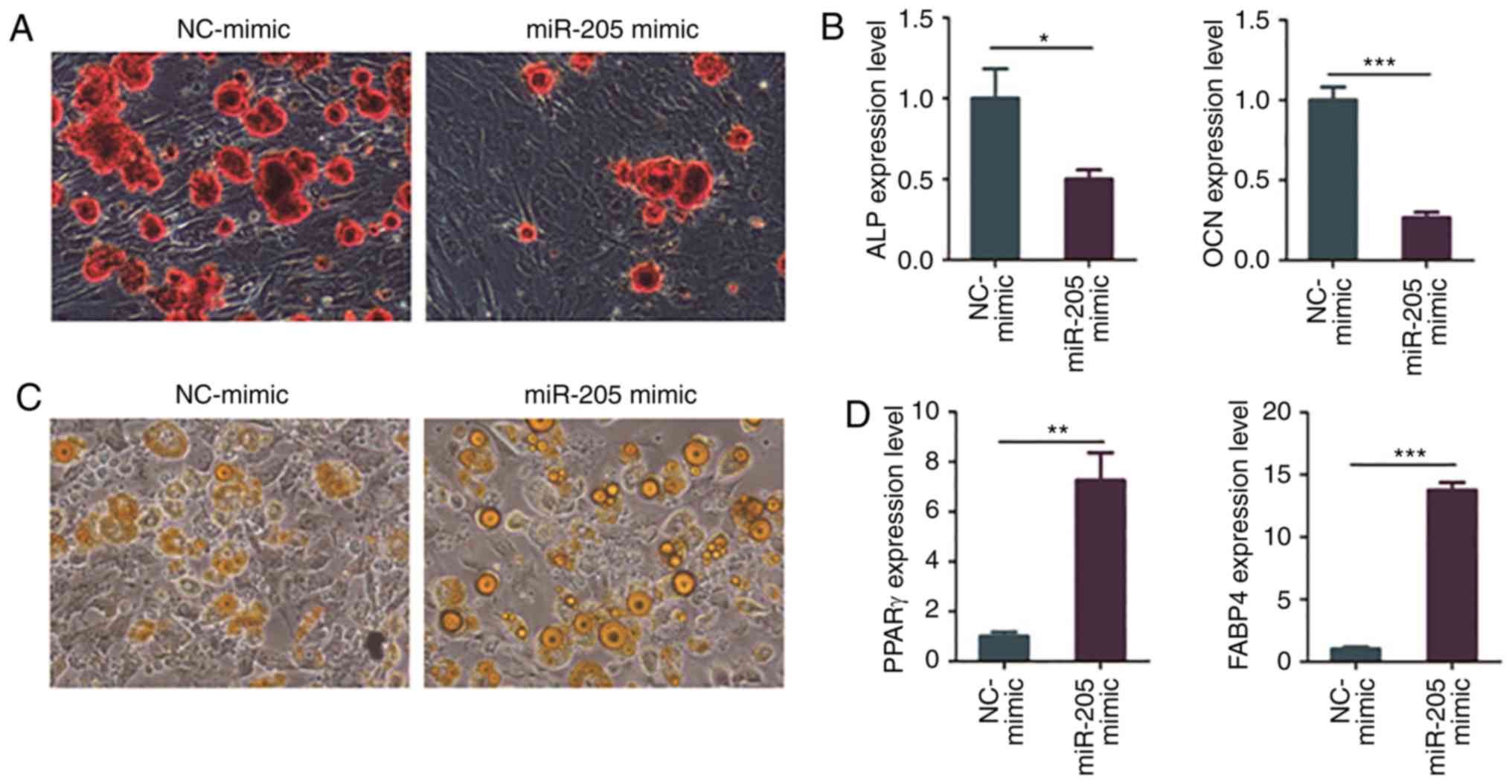 | Figure 4Overexpression of miR-205 inhibits
osteogenic differentiation and promotes adipogenic differentiation
of BMSCs in elderly female mice with T2DM + OP. (A) Osteogenic
differentiation of BMSCs as detected via ARS staining. (B)
Expression levels of osteogenesis genes, ALP and OCN,
as detected via RT-PCR. (C) Adipogenic differentiation as detected
via ORO staining. (D) Expression levels of adipogenesis genes,
PPARγ and FABP4, as detected via RT-PCR.
*P<0.05, **P<0.01 and
***P<0.001 compared to the NC-mimic group. BMSCs,
bone marrow mesenchymal stem cells; T2DM, type 2 diabetes mellitus;
OP, osteoporosis; ARS, alizarin red S; ORO, oil red O; ALP,
alkaline phosphatase, biomineralization associated; OCN,
osteocalcin; PPARγ, peroxisome proliferator-activated receptor-γ;
FABP4, fatty acid binding protein 4. |
Moreover, BMSCs of elderly female mice with T2DM +
OP were transfected with miR-205 mimic and negative control
(NC)-mimic, and ORO staining and RT-PCR were carried out after
adipogenic induction for 24 days. The results of ORO staining
showed that overexpression of miR-205 obviously promoted adipogenic
induction of BMSCs in elderly female mice with T2DM + OP (Fig. 4C). In addition, the results of RT-PCR
revealed that overexpression of miR-205 obviously increased the
expression of adipogenesis-related genes PPARγ and
FABP4 in BMSCs of elderly female mice with T2DM + OP
(Fig. 4D).
Effects of knockdown of miR-205 on
osteogenic/adipogenic differentiation of BMSCs in elderly female
mice with T2DM + OP
BMSCs of elderly female mice with T2DM + OP were
transfected with miR-205 inhibitor and NC-inhibitor, and ARS
staining and RT-PCR were performed after osteogenic induction for
21 days. The results of ARS staining showed that knockdown of
miR-205 markedly promoted osteogenic differentiation of BMSCs in
elderly female mice with T2DM + OP (Fig.
5A). In addition, the results of RT-PCR revealed that knockdown
of miR-205 significantly upregulated the expression of
osteogenesis-related genes ALP and OCN (Fig. 5B).
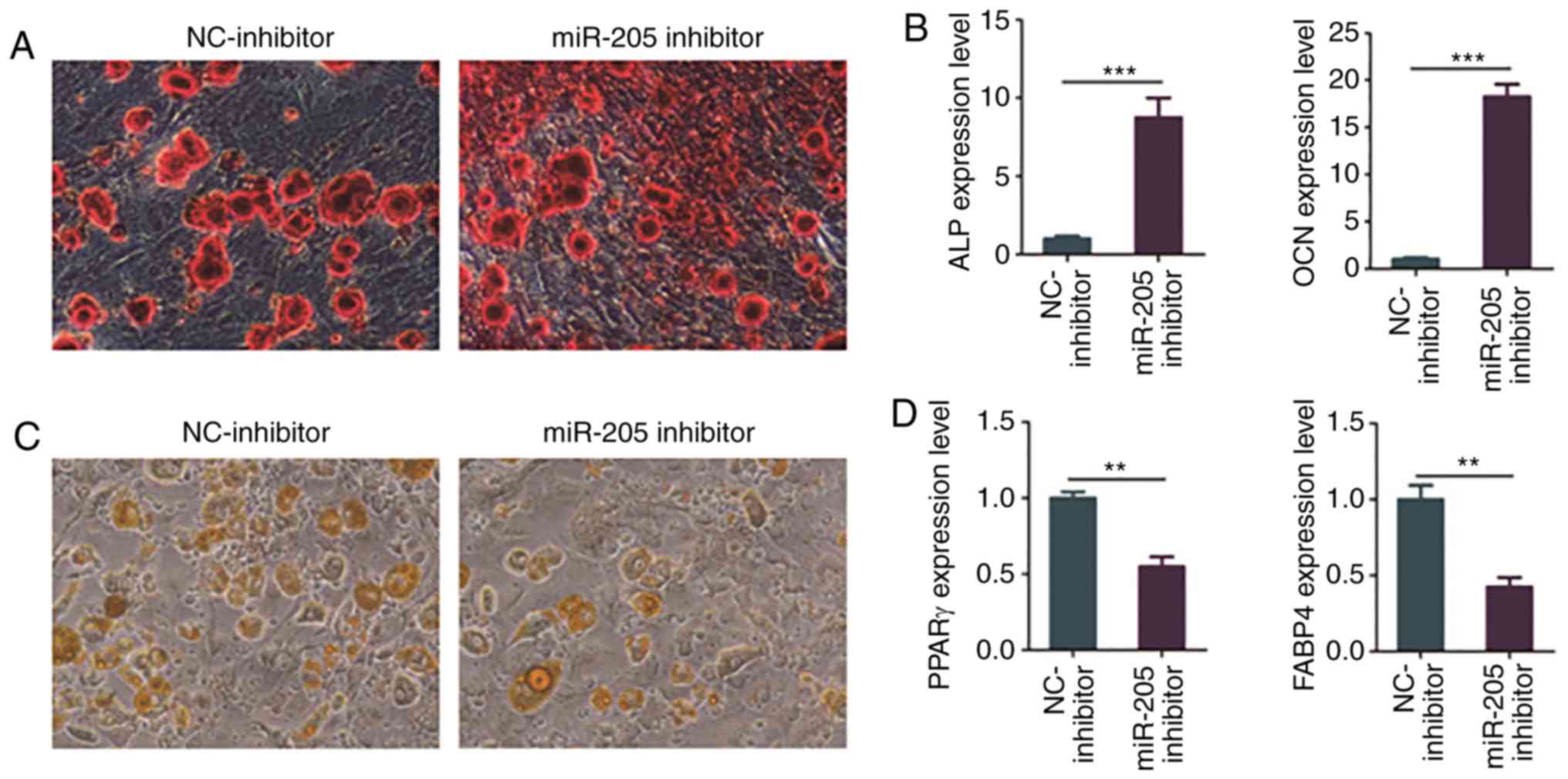 | Figure 5Knockdown of miR-205 promotes
osteogenic differentiation and inhibits adipogenic differentiation
of BMSCs in elderly female mice with T2DM + OP. (A) Osteogenic
differentiation of BMSCs detected via ARS staining. (B) Expression
levels of osteogenesis genes, ALP and OCN, as
detected via RT-PCR. (C) Adipogenic differentiation as detected via
ORO staining. (D) Expression levels of adipogenesis genes,
PPARγ and FABP4, as detected via RT-PCR.
**P<0.01 and ***P<0.001 compared to the
NC-inhibitor group. BMSCs, bone marrow mesenchymal stem cells;
T2DM, type 2 diabetes mellitus; OP, osteoporosis; ARS, alizarin red
S; ORO, oil red O; ALP, alkaline phosphatase,
biomineralization associated; OCN, osteocalcin; PPARγ,
peroxisome proliferator-activated receptor-γ; FABP4, fatty
acid binding protein 4. |
Moreover, BMSCs of elderly female mice with T2DM +
OP were transfected with miR-205 inhibitor and NC-inhibitor, and
ORO staining and RT-PCR were conducted after adipogenic induction
for 24 days. The results of ORO staining showed that the knockdown
of miR-205 markedly suppressed adipogenic induction of BMSCs in
elderly female mice with T2DM + OP (Fig.
5C). In addition, the results of RT-PCR revealed that the
knockdown of miR-205 significantly downregulated the expressions of
adipogenesis-related genes PPARγ and FABP4 (Fig. 5D).
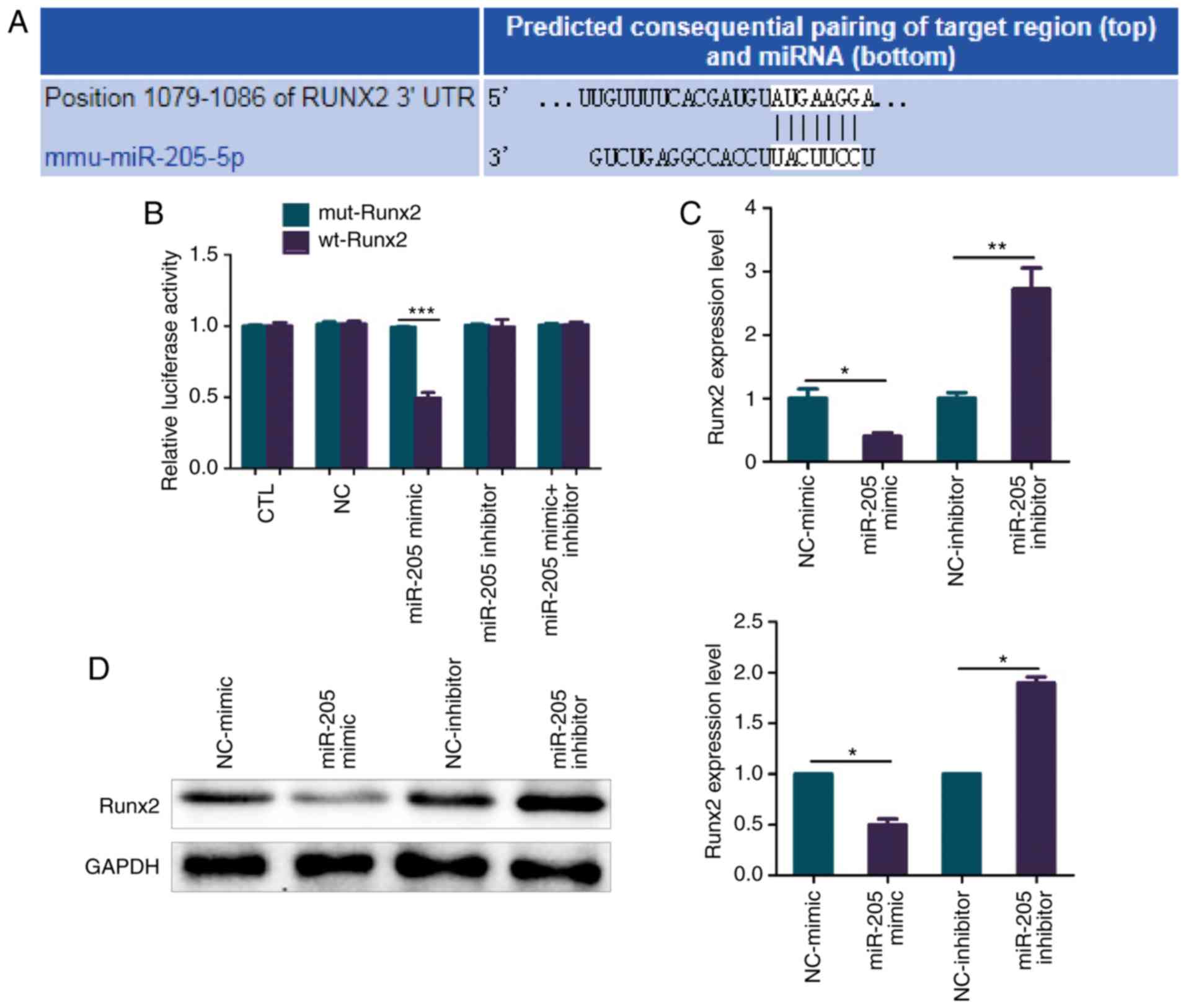
miR-205 inhibits Runx2 expression in a
targeted manner
The target genes of miR-205 were predicted using
online websites TargetScan and miRanda, and the binding sites for
miR-205 and Runx2 are shown in Fig.
6A. Luciferase reporter assay confirmed that there was a
regulatory relationship between miR-205 and Runx2 (Fig. 6B). BMSCs of elderly female mice with
T2DM + OP were transfected with miR-205 mimic, NC-mimic, miR-205
inhibitor and NC-inhibitor, after which the mRNA and protein levels
of Runx2 were determined. It was found that overexpression of
miR-205 significantly suppressed the mRNA expression of
Runx2, while knockdown of miR-205 significantly increased
the mRNA expression of Runx2 (Fig. 6C). As shown in Fig. 6D, the results of western blotting
were consistent with those of RT-PCR. Overexpression of miR-205
significantly suppressed the protein expression of Runx2, while
knockdown of miR-205 significantly increased the protein expression
of Runx2 (Fig. 6D).
Discussion
Osteoblasts are the most important cells in the bone
microenvironment, accounting for approximately 95% of the total
cell population in bone tissues, and they can determine the
proportion of components in bone tissues, whose main source is bone
marrow mesenchymal stem cells (BMSCs) (19,20).
Under certain conditions, BMSCs can differentiate into osteoblasts,
adipocytes and chondrocytes, and there is a certain dynamic balance
between osteogenic and adipogenic differentiation of BMSCs under
normal conditions, which plays an important role during bone
development (21-23).
Studies have found that miRNAs, as ubiquitous
molecules that regulate gene expression, play important regulatory
roles in bone development and bone formation (24). For example, Yang et al found
that miR-21 was able to facilitate bone formation and bone
regeneration through PTEN/PI3K/Akt/HIF-1α (25). Therefore, whether there is an miRNA
able to regulate osteogenic/adipogenic differentiation of BMSCs in
elderly female patients with T2DM + OP was explored in the present
study.
In previous studies, the expression levels of miRNAs
(miR-205, miR-188, miR-23a, miR-26a and miR-214) related to bone
development and bone formation were detected in elderly female
patients with T2DM + OP (9-11),
and the results showed that the expression of miR-205 was
significantly upregulated in bone tissues and serum of elderly
female patients with T2DM + OP, thus miR-205 was selected as the
object of further study. It is reported that miR-205 can regulate
the biological functions of a variety of cancer cells and
participate in bone formation. However, the correlation between
miR-205 and osteogenic/adipogenic differentiation of BMSCs in
elderly female patients with T2DM + OP has been sparsely studied.
Therefore, the expression of miR-205 in bone tissues, serum and
BMSCs of elderly female mice with T2DM + OP and its effect on
osteogenic/adipogenic differentiation of BMSCs was further
investigated. It was found that the expression of miR-205 was
obviously increased in elderly female patients with T2DM + OP and
elderly female mice with T2DM + OP, which provides an important
target and marker for the early prediction and diagnosis of T2DM +
OP in the elderly. Hu et al (26) found that the miR-205 expression
obviously declined during osteogenic differentiation of normal
BMSCs, consistent with our conjecture. In the present study,
overexpression of miR-205 inhibited osteogenic differentiation and
promoted adipogenic differentiation of BMSCs in elderly female mice
with T2DM + OP, while knockdown of miR-205 promoted osteogenic
differentiation and inhibited adipogenic differentiation of BMSCs
in elderly female mice with T2DM + OP. Hu et al (26) found that miR-205 can weaken
osteogenic differentiation and enhance adipogenic differentiation
of normal BMSCs. Therefore, we conclude that miR-205 inhibits
osteogenic differentiation and promotes adipogenic differentiation
of BMSCs in normal mice and elderly female mice with T2DM + OP. In
addition, the results of luciferase reporter assay, RT-PCR and
western blotting confirmed that miR-205 could directly inhibit the
expression of its target gene Runx2. Runx2 is an important
regulator of osteogenic differentiation and is able to regulate the
transcription of various osteogenesis-related genes (27,28).
Inhibiting Runx2 expression may affect osteogenic differentiation
of BMSCs, thereby impacting the formation of osteoblasts and
ultimately affecting bone development and bone formation (29). We will reorganize the phenotype of
BMSCs in vitro in future research, and perform subcutaneous
implantation experiments of BMSCs from nude mice with different
expression levels of miR-205. We suspect that the subcutaneous
implantation of BMSCs with overexpressing miR-205 can inhibit the
formation of subcutaneous bone in nude mice, and the subcutaneous
implantation of BMSCs with silenced miR-205 can promote the
formation of subcutaneous bone in nude mice. Therefore, it was
found in the present study that miR-205 is involved in the
osteogenic/adipogenic differentiation of BMSCs in elderly female
mice with T2DM + OP by targeted inhibition of the expression of its
target gene Runx2.
In conclusion, the expression level of miR-205 is
obviously increased in female patients with T2DM + OP and elderly
female mouse model of T2DM + OP. In addition, miR-205 can regulate
the osteogenic differentiation of BMSCs, and miR-205/Runx2 may
serve as a new method and target for the treatment of female
patients with T2DM + OP, which provides theoretical support and
experimental basis for the research on bone loss in female patients
with T2DM + OP.
Acknowledgements
Not applicable.
Funding
This study was supported by the Project of Qiqihar
Science and Technology (no. SFZD-2017029).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
GZ, HL, WZ, ML, LT and WJ conceived and designed
this study. GZ, HL, WZ, ML, WJ and XL conducted the data
collection, analysis and summary. GZ, HL, WZ, LT, WJ and XL were
responsible for data analysis and interpretation. GZ wrote the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Animal Ethics Care Committee of Qiqihar Medical University
(QMU-AECC-2019-51).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Picke AK, Campbell G, Napoli N, Hofbauer
LC and Rauner M: Update on the impact of type 2 diabetes mellitus
on bone metabolism and material properties. Endocr Connect.
8:R55–R70. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Liu M, Lu Y, Cheng X, Ma L, Miao X, Li N,
Sun B, Yan S, Li J and Li C: Relationship between abnormal glucose
metabolism and osteoporosis in Han Chinese men over the age of 50
years. Clin Interv Aging. 14:445–451. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen FP, Kuo SF, Lin YC, Fan CM and Chen
JF: Status of bone strength and factors associated with vertebral
fracture in postmenopausal women with type 2 diabetes. Menopause.
26:182–188. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pagnotti GM, Styner M, Uzer G, Patel VS,
Wright LE, Ness KK, Guise TA, Rubin J and Rubin CT: Combating
osteoporosis and obesity with exercise: Leveraging cell
mechanosensitivity. Nat Rev Endocrinol. 15:339–355. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Schepper JD, Irwin R, Kang J, Dagenais K,
Lemon T, Shinouskis A, Parameswaran N and McCabe LR: Probiotics in
Gut-bone signaling. Adv Exp Med Biol. 1033:225–247. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rizzo S, Farlay D, Akhter M, Boskey A,
Recker R, Lappe J and Boivin G: Variables reflecting the
mineralization of bone tissue from fracturing versus nonfracturing
postmenopausal nonosteoporotic Women. JBMR Plus. 2:323–327.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hadjiargyrou M and Komatsu DE: The
therapeutic potential of microRNAs as orthobiologics for skeletal
fractures. J Bone Miner Res. 34:797–809. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pan BL, Tong ZW, Li SD, Wu L, Liao JL,
Yang YX, Li HH, Dai YJ, Li JE and Pan L: Decreased microRNA-182-5p
helps alendronate promote osteoblast proliferation and
differentiation in osteoporosis via the Rap1/MAPK pathway. Biosci
Rep. 38(pii: BSR20180696)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cui Q, Xing J, Yu M, Wang Y, Xu J, Gu Y,
Nan X, Ma W, Liu H and Zhao H: Mmu-miR-185 depletion promotes
osteogenic differentiation and suppresses bone loss in osteoporosis
through the Bgn-mediated BMP/Smad pathway. Cell Death Dis.
10(172)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li H, Fan J, Fan L, Li T, Yang Y, Xu H,
Deng L, Li J, Li T, Weng X, et al: MiRNA-10b reciprocally
stimulates osteogenesis and inhibits adipogenesis partly through
the TGF-β/SMAD2 signaling pathway. Aging Dis. 9:1058–1073.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li X, Ning L, Zhao X and Wan S:
MicroRNA-543 promotes ovariectomy-induced osteoporosis through
inhibition of AKT/p38 MAPK signaling pathway by targeting YAF2. J
Cell Biochem: Dec 2, 2018 doi: 10.1002/jcb.28143 (Epub ahead of
print).
|
|
12
|
Kim BJ, Lee JY, Park SJ, Lee SH, Kim SJ,
Yoo HJ, Rivera De Pena SI, McGee-Lawrence M, Isales CM, Koh JM and
Hamrick MW: Elevated ceramides 18:0 and 24:1 with aging are
associated with hip fracture risk through increased bone
resorption. Aging (Albany NY). 11:9388–9404. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sun Y, Xiong Y, Yan C, Chen L, Chen D, Mi
B and Liu G: Downregulation of microRNA-16-5p accelerates fracture
healing by promoting proliferation and inhibiting apoptosis of
osteoblasts in patients with traumatic brain injury. Am J Transl
Res. 11:4746–4760. 2019.PubMed/NCBI
|
|
14
|
Lozano Calderón SA, Garbutt C, Kim J,
Lietz CE, Chen YL, Bernstein K, Chebib I, Nielsen GP, Deshpande V,
Rubio R, et al: Clinical and molecular analysis of pathologic
fracture-associated osteosarcoma: MicroRNA profile Is different and
correlates with prognosis. Clin Orthop Relat Res. 477:2114–2126.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang Y, Chen H and Zhang H: Tanshinone IIA
exerts beneficial effects on fracture healing in vitro and in vivo.
Chem Biol Interact. 310(108748)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kaneko T, Okamura K, Yonemoto Y, Okura C,
Suto T, Tachibana M, Sakane H, Inoue M and Chikuda H: Effects of
denosumab on bone mineral density and bone turnover markers in
rheumatoid arthritis patients switching from bisphosphonates. J Exp
Orthop. 6(41)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cehic M, Lerner RG, Achten J, Griffin XL,
Prieto-Alhambra D and Costa ML: Prescribing and adherence to bone
protection medications following hip fracture in the United
Kingdom: Results from the World Hip Trauma Evaluation (WHiTE)
cohort study. Bone Joint J. 101-B:1402–1407. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yang F, Yang L, Li Y, Yan G, Feng C, Liu
T, Gong R, Yuan Y, Wang N, Idiiatullina E, et al: Melatonin
protects bone marrow mesenchymal stem cells against iron
overload-induced aberrant differentiation and senescence. J Pineal
Res. 63(e12422)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lopes D, Martins-Cruz C, Oliveira MB and
Mano JF: Bone physiology as inspiration for tissue regenerative
therapies. Biomaterials. 185:240–275. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Muruganandan S, Govindarajan R and Sinal
CJ: Bone marrow adipose tissue and skeletal health. Curr Osteoporos
Rep. 16:434–442. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fakhry M, Hamade E, Badran B, Buchet R and
Magne D: Molecular mechanisms of mesenchymal stem cell
differentiation towards osteoblasts. World J Stem Cells. 5:136–148.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sun MH, Wang WJ, Li Q, Yuan T and Weng WJ:
Autologous oxygen release nano bionic scaffold composite miR-106a
induced BMSCs enhances osteoblast conversion and promotes bone
repair through regulating BMP-2. Eur Rev Med Pharmacol Sci.
22:7148–7155. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Luo Y, Zhang Y, Miao G, Zhang Y, Liu Y and
Huang Y: Runx1 regulates osteogenic differentiation of BMSCs by
inhibiting adipogenesis through Wnt/β-catenin pathway. Arch Oral
Biol. 97:176–184. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Qing Y, Huang M, Cao Y, Du T and Song K:
Effects of miRNA-342-3p in modulating Hedgehog signaling pathway of
human umbilical cord mesenchymal stem cells by down-regulating
Sufu. Oral Dis. 25:1147–1157. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yang C, Liu X, Zhao K, Zhu Y, Hu B, Zhou
Y, Wang M, Wu Y, Zhang C, Xu J, et al: miRNA-21 promotes
osteogenesis via the PTEN/PI3K/Akt/HIF-1α pathway and enhances bone
regeneration in critical size defects. Stem Cell Res Ther.
10(65)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hu N, Feng C, Jiang Y, Miao Q and Liu H:
Regulative effect of Mir-205 on osteogenic differentiation of bone
mesenchymal stem cells (BMSCs): Possible role of SATB2/Runx2 and
ERK/MAPK pathway. Int J Mol Sci. 16:10491–10506. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shi J, Folwaczny M, Wichelhaus A and
Baumert U: Differences in RUNX2 and P2RX7 gene expression between
mono- and coculture of human periodontal ligament cells and human
osteoblasts under compressive force application. Orthod Craniofac
Res. 22:168–176. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yang L, Zeng Z, Kang N, Yang JC, Wei X and
Hai Y: Circ-VANGL1 promotes the progression of osteoporosis by
absorbing miRNA-217 to regulate RUNX2 expression. Eur Rev Med
Pharmacol Sci. 23:949–957. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Polo-Corrales L, Latorre-Esteves M and
Ramirez-Vick JE: Scaffold design for bone regeneration. J Nanosci
Nanotechnol. 14:15–56. 2014.PubMed/NCBI View Article : Google Scholar
|