Introduction
The incidence of joint replacement is very high.
Over one million of hip and knee replacement operations are
performed in the United States each year (1). Due to the aging population and the
demand for treatment quality and quality of life, the number of
joint replacement operations will further increase (2) in the future. Therefore, risks of
anesthesia and death during perioperative period will obviously
increase (3,4).
Anesthesia is an essential step in the operation.
Appropriate anesthesia method is of decisive significance to
maintain vital signs of patients and help them to survive the
perioperative period (5). Anesthesia
procedure is an independent factor that affects the mortality of
elderly orthopedic patients. Peripheral nerve block can reduce the
incidence and mortality of perioperative complications (6,7). Lumbar
plexus combined with sciatic nerve block anesthesia has advantages
of small hemodynamic impact and good postoperative analgesic
effects (8). However, if tissue
damage during surgery is extensive and often affects adjacent
nerves, patients may experience immunosuppression for up to one
week, significantly increasing risks of infection and other
pathological complications (9).
Dexmedetomidine is an α2 adrenergic receptor agonist with high
specificity. Besides sedation and abirritation, dexmedetomidine
also has anti-sympathetic and anti-anxiety effects (10). In recent years, many studies have
reported that dexmedetomidine-assisted anesthesia has good effects
on protection of immune function of patients after various surgical
treatments. Wang et al (11)
reported that the supplement of dexmedetomidine anesthesia in
gastrectomy could reduce surgical stimulation and inflammatory
response, maintain the balance of Th1/Th2, and protect the immune
function of patients. Jang et al (12) further analyzed the analgesic effect
of dexmedetomidine on immune regulation under pain condition
through an animal model. They established a mouse model of
formalin-induced pain, and discovered that dexmedetomidine could
effectively improve the pain response of formalin-induced pain in
mice in acute pain stage 1 and hyperalgesia stage 2, and inhibit
the activation of natural killer cells under pain conditions.
Moreover, dexmedetomidine has no toxic effect on splenocytes and
can protect immune function more effectively. But there are few
reports on protection of immune function of dexmedetomidine in
joint replacement, which still needs to be verified.
This study analyzed the application of
dexmedetomidine-assisted intravertebral anesthesia in hip
replacement and its influence on T-lymphocyte subsets, providing
reference for clinical anesthesia treatment.
Patients and methods
Subjects of study
A total of 186 patients undergoing elective hip
replacement surgery from January 2013 to February 2017 in The Sixth
Affiliated Hospital of the Sixth Clinical Medical School of
Xinjiang Medical University (Urumqi, China) were selected, aged
55-70 years. Patients were divided into two groups according to
different anesthesia methods. Patients undergoing intravertebral
anesthesia were as group A (86 cases), and patients undergoing
intravertebral anesthesia combined with dexmedetomidine were
included in group B (100 cases). Inclusion criteria: The patients
were unable to relieve hip pain with conservative treatment, and
were treated with hip replacement for the first time. The patients
were unilateral hip disease and ASA grade I-III. Exclusion
criteria: There were serious organic diseases such as heart, lung
and liver, uncontrolled hypertension and diabetes, abnormal
metabolism of water and electrolytes, osteoporosis, abnormal mental
state and inability to communicate, patients with active infection
including hip joint. This study was in line with the Helsinki
Declaration.
The study was approved by the Ethics Committee of
the Sixth Affiliated Hospital of the Sixth Clinical Medical School
of Xinjiang Medical University. Patients who participated in this
research, signed an informed consent and had complete clinical
data.
Anesthesia methods
The patients fasted for 12 h before surgery and did
not take any drugs. All patients received lumbar nerve block
anesthesia. Arterial pressure, central venous pressure,
electrocardiogram, blood pressure, heart rate, pulse and oxygen
protection were monitored. After determining the lumbar nerve
location under ultrasound guidance, 25 ml of 0.375% ropivacaine was
injected around it for anesthesia. Intravenous sufentanil 0.35
µg/kg + midazolam 0.05 mg/kg + vecuronium bromide 0.15 mg/kg +
propofol 1.5 mg/kg were used for anesthesia induction, remifentanil
0.3 µg/kg/min + propofol 1.5 mg/kg/h + vecuronium bromide 2 mg were
used for anesthesia maintenance, and bispectral index (BIS) 40-60
were maintained. Patients in group B were injected 0.6 µg/kg
dexmedetomidine intravenously 15 min before anesthesia
induction.
Observation indicators
Ramsay sedation scores 5 min before anesthesia (T0),
immediately after skin incision (T1) and after surgery (T2) were
compared between the groups. General operation conditions of both
groups were recorded, including operation time, anesthesia time,
bleeding volume and recovery time of infusion volume. Changes of
T-lymphocyte subsets before surgery and 24 h after surgery were
measured by flow cytometry. Changes of interleukin-6 (IL-6) and
tumor necrosis factor-α (TNF-α) in the serum of peripheral blood in
the two groups before and 24 h after operation were detected by
enzyme-linked immunosorbent assay (ELISA), and hemodynamic changes
of T0, T1 and T2 were recorded. visual analogue scale (VAS) scores
and mini-mental state examination (MMSE) scores were used to
evaluate changes of pain and cognitive function of patients in both
groups before surgery and 24 h after surgery, and the incidence of
postoperative complications of patients in both groups was
recorded. IL-6 and TNF-α test kits were all purchased from Wuhan
Elabscience Biotechnology Co., Ltd., and the number of kits were
E-EL-H0102c and E-EL-H0109c, respectively. CytoFLE S flow cytometry
was purchased from Beckman Coulter.
Ramsay sedation score
Two to four is an ideal sedation, less than 2 is
ineffective sedation, and more than 4 is excessive sedation
(Table I).
 | Table IRamsay sedation score. |
Table I
Ramsay sedation score.
| 1 point | It is easy to create
anxiety and irritability. |
| 2 points | Patients are sober,
able to cooperate with medical work, and relatively calm |
| 3 points | Sleepiness, only
response to commands. |
| 4 points | Shallow sleep, can be
sensitive to mild shaking or loud sound stimulation. |
| 5 points | Deep sleep, response
to noxious stimulation. |
Statistical analysis
SPSS 19.0 (Asia Analytics Formerly SPSS China) was
used. The measurement data were expressed as [n(%)], and comparison
of rates between the groups adopted χ2 test. The
counting data were expressed as mean ± SD. Comparison between both
groups was conducted by independent-samples t-test, comparison at
different time-points in the group was conducted by repeated
measures analysis of variance, and back testing was conducted by
LSD test. P<0.05 indicates a statistically significant
difference.
Results
General information
There were 86 patients in group A, including 34 male
and 52 female patients, with an age of 61.4±7.3 years; there were
100 patients in group B, including 47 male and 53 female patients,
with an age of 62.9±7.8 years. There was no significant difference
in sex ratio and age between the groups (P>0.05). Analysis of
results of other data in both groups, such as weight, ASA grading,
and history of hypertension, also showed no significant difference
(P>0.05) (Table II).
 | Table IIAnalysis of general data. |
Table II
Analysis of general data.
| Patient
characteristics | Group A (n=86) | Group B (n=100) | χ2/t
value | P-value |
|---|
| Sex [n(%)] | | | 1.048 | 0.306 |
|
Male | 34 (39.53) | 47 (47.00) | | |
|
Female | 52 (60.47) | 53 (53.00) | | |
| Age (years) | 61.4±7.3 | 62.9±7.8 | 1.347 | 0.180 |
| Weight (kg) | 62.42±9.17 | 60.58±8.24 | 1.441 | 0.151 |
| ASA classification
[n(%)] | | | 0.241 | 0.624 |
|
I | 63 (73.26) | 70 (70.00) | | |
|
II-III | 23 (26.74) | 30 (30.00) | | |
| History of
hypertension [n(%)] | | | 1.048 | 0.306 |
|
Yes | 52 (60.47) | 53 (53.00) | | |
|
No | 34 (39.53) | 47 (47.00) | | |
| History of diabetes
[n(%)] | | | 1.565 | 0.211 |
|
Yes | 17 (19.77) | 13 (13.00) | | |
|
No | 69 (80.23) | 87 (87.00) | | |
| Other surgical
history [n(%)] | | | 0.290 | 0.590 |
|
Yes | 20 (23.26) | 20 (20.00) | | |
|
No | 66 (76.74) | 80 (80.00) | | |
| Operative site
[n(%)] | | | 0.116 | 0.733 |
|
Left | 34 (39.53) | 42 (42.00) | | |
|
Right | 52 (60.47) | 58 (58.00) | | |
| Years of
education | 11.3±4.5 | 12.4±5.3 | | |
Comparison of Ramsay sedation score of
patients between the groups
Ramsay sedation score represented that there was no
statistical difference in Ramsay sedation score of patients between
the groups at each time-point (P>0.05); Ramsay sedation score
was higher than that at T1 and T2 (P<0.05), and there was no
statistical difference between T1 and T2 (P>0.05) (Table III).
 | Table IIIComparison of Ramsay sedation score
of patients between the two groups. |
Table III
Comparison of Ramsay sedation score
of patients between the two groups.
| Times | Group A (n=86) | Group B
(n=100) | t-value | P-value |
|---|
| T0 | 2.14±0.11 | 2.16±0.12 | 1.178 | 0.241 |
| T1 |
3.12±0.75a |
3.24±0.63a | 1.186 | 0.237 |
| T2 |
3.26±0.62a |
3.30±0.59a | 0.450 | 0.653 |
Analysis of general conditions of
surgery for patients in both groups
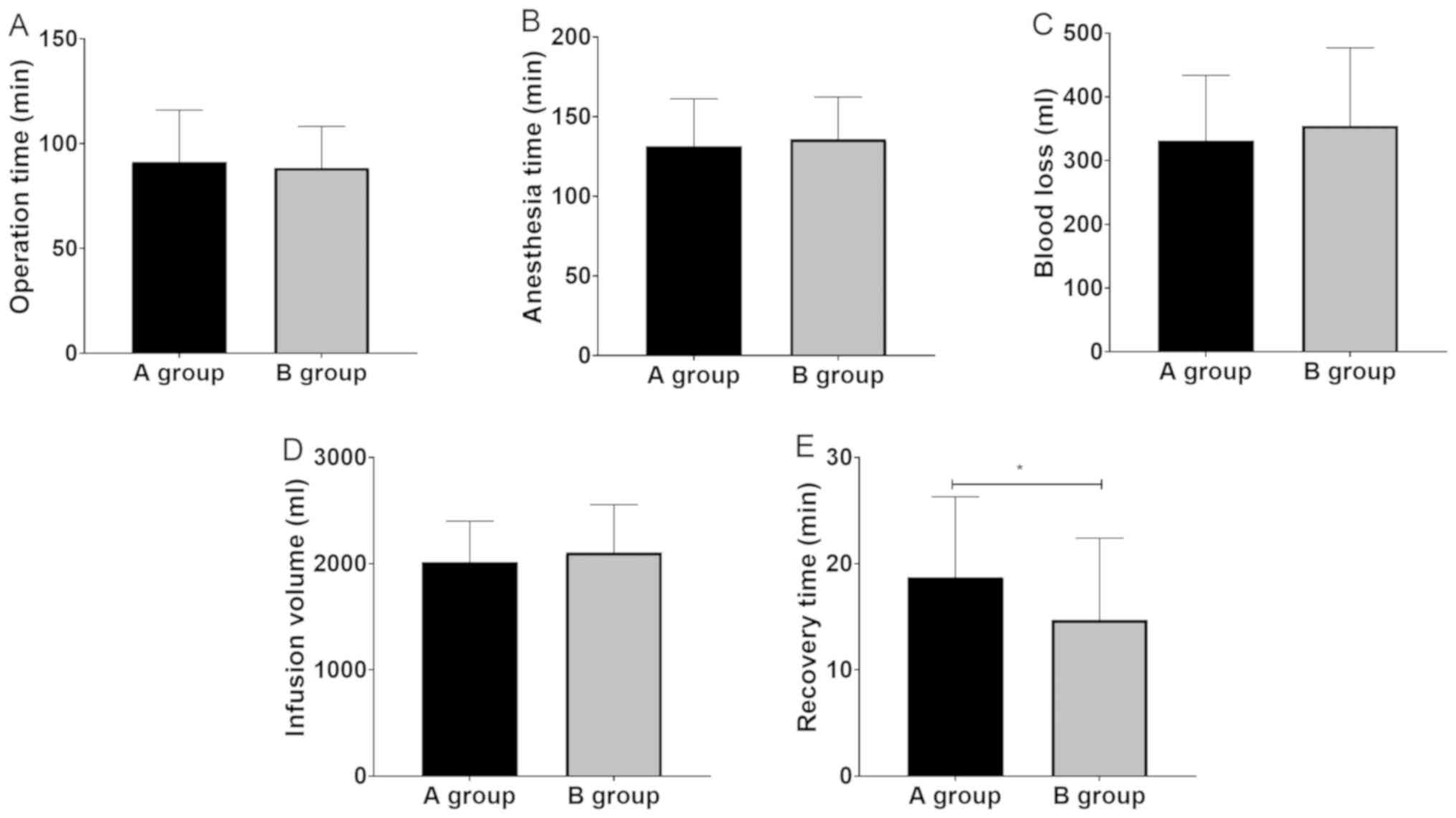
Operation time, anesthesia time, hemorrhage volume
and infusion volume of both groups had no statistical difference
(P>0.05), but the recovery time of group B was shorter than that
of group A (P<0.05) (Fig. 1).
Hemodynamic changes of patients in
both groups during surgery
Hemodynamic monitoring results showed that there was
no statistical difference in systolic blood pressure (SBP),
diastolic blood pressure (DBP) and heart rate between the groups at
T0 (P>0.05). There was no statistical difference in SBP between
the groups at T1 (P>0.05), but DBP and heart rate (HR) in group
B were lower than those in group A (P<0.05), while SBP, DBP and
heart rate in group B were lower than those in group A at T2
(P<0.05). Intra-group comparison results showed that SBP and DBP
of patients in both groups at T1 were higher than those at T0
(P<0.05), the heart rate of patients in group A was also higher
than that at T0 (P<0.05), while patients in group B had no
significant change (P>0.05). By T2, SBP and DBP of patients in
both groups were both lower than those at T1 (P<0.05), and the
heart rate had no significant changes compared with that at T1
(P<0.05) (Table IV).
 | Table IVHemodynamic changes of patients in
the two groups during operation. |
Table IV
Hemodynamic changes of patients in
the two groups during operation.
| Hemodynamic
changes | Group A (n=86) | Group B
(n=100) | t-value | P-value |
|---|
| SBP (mmHg) |
|
T0 | 138.47±10.81 | 137.82±10.79 | 0.409 | 0.683 |
|
T1 |
151.73±12.38a |
148.86±12.71a | 1.554 | 0.122 |
|
T2 |
147.28±13.75a,b |
125.73±14.61a,b | 10.305 | <0.001 |
| DBP (mmHg) |
|
T0 | 85.42±6.71 | 85.38±7.33 | 0.048 | 0.962 |
|
T1 |
104.83±7.48a |
95.67±9.42a | 7.261 | <0.001 |
|
T2 |
95.32±10.85a,b |
83.42±10.26a,b | 7.680 | <0.001 |
| HR (times/min) |
|
T0 | 85.46±5.15 | 85.49±5.07 | 0.040 | 0.968 |
|
T1 |
98.85±11.52a |
86.83±5.26a | 9.364 | <0.001 |
|
T2 |
97.43±10.35a |
85.76±8.73a | 8.342 | <0.001 |
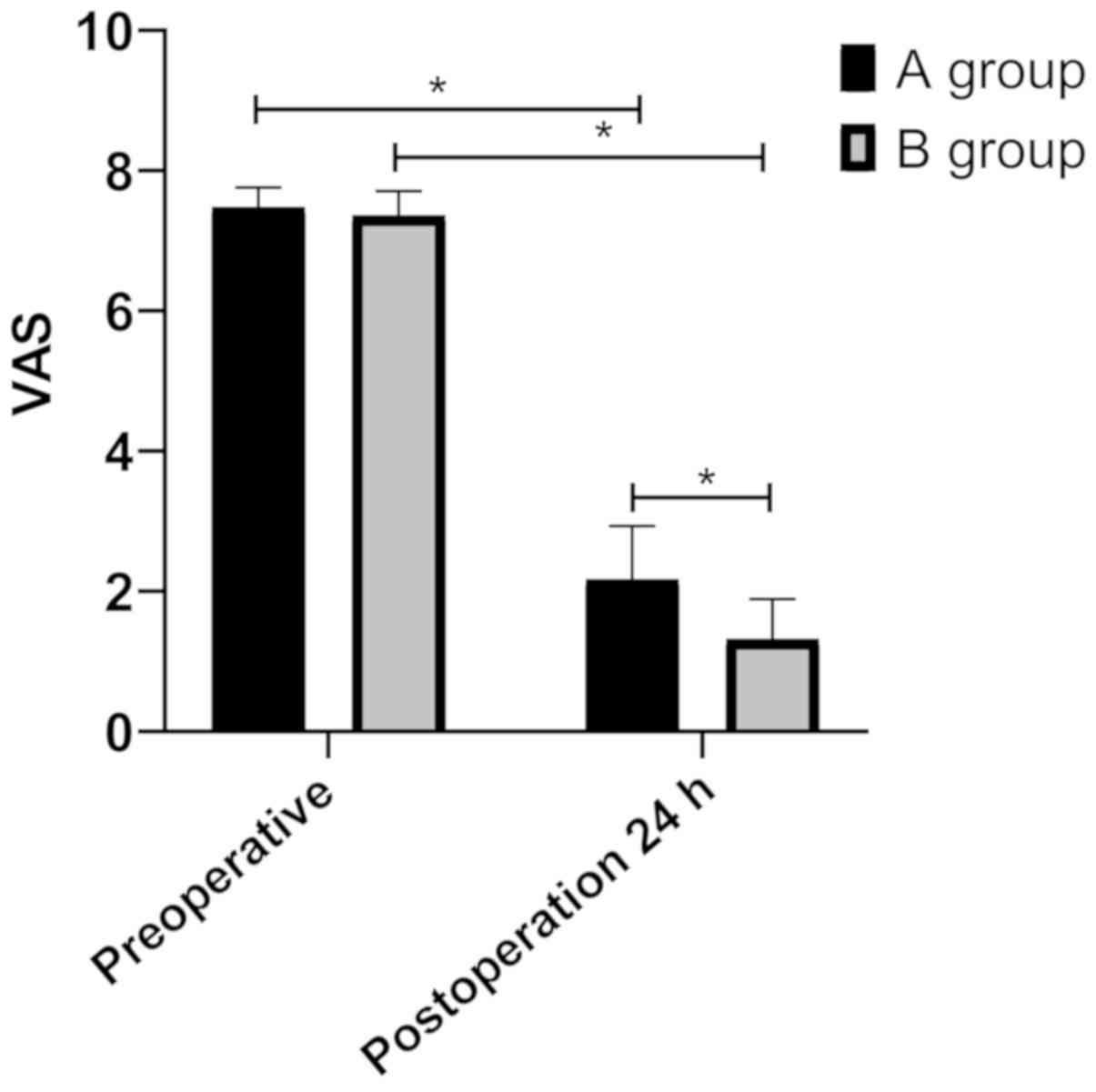
Differences in postoperative VAS
scores of patients between the groups
VAS score results indicated that there was no
statistically significant difference in VAS score of patients
between the groups before surgery (P>0.05). VAS scores of
patients in both groups were significantly reduced 24 h after
surgery (P<0.05), but those in group B were lower than those in
group A (P<0.05) (Fig. 2).
Analysis of changes of inflammatory
response related factors after surgery of patients in both
groups
The results of ELISA showed that there was no
significant difference between the groups in IL-6 and TNF-α before
surgery (P>0.05). Twenty-four hours after surgery, IL-6 and
TNF-α of patients in both groups were higher than those before
surgery (P<0.05), but those in group B were lower than those in
group A (P<0.05) (Table V).
 | Table VAnalysis on changes of inflammatory
response related factors after operation of patients in the two
groups (pg/ml). |
Table V
Analysis on changes of inflammatory
response related factors after operation of patients in the two
groups (pg/ml).
| Inflammatory
factors | Group A (n=86) | Group B
(n=100) | t-value | P-value |
|---|
| IL-6 |
|
Before
operation | 25.22±4.29 | 25.38±4.32 | 0.253 | 0.801 |
|
24 h after
operation |
40.13±5.25a |
32.27±4.88a | 10.574 | <0.001 |
| TNF-α |
|
Before
operation | 1.58±0.23 | 1.51±0.28 | 1.844 | 0.067 |
|
24 h after
operation |
3.79±1.03a |
2.22±0.83a | 11.507 | <0.001 |
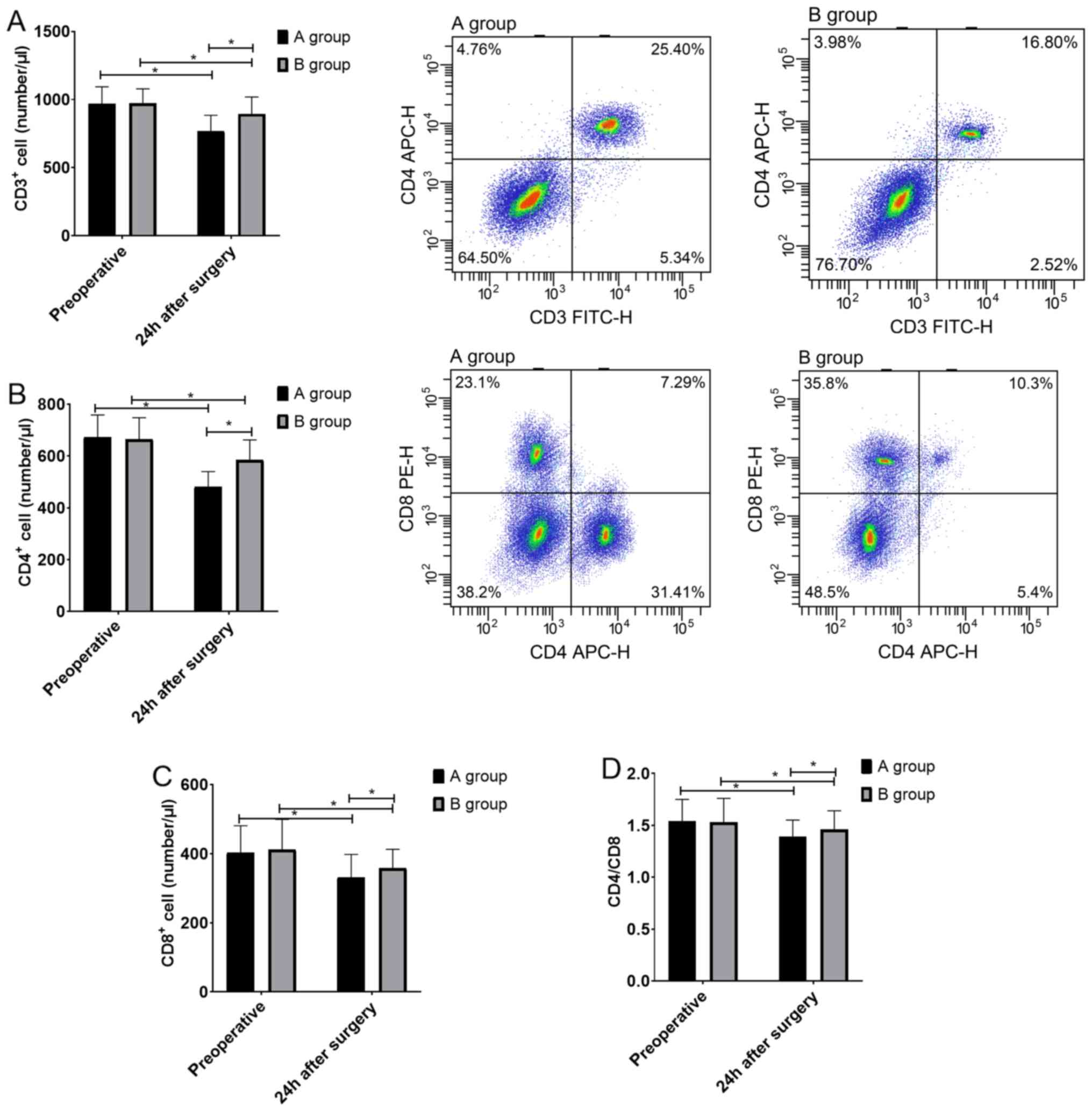
Analysis on changes of T-lymphocyte
subsets of patients in both groups
The results of flow cytometry revealed that 24 h
after surgery, CD3+ cells, CD4+ cells,
CD8+ cells and CD4/CD8 ratio of patients in both groups
were lower than those before surgery (P<0.05), but those in
group B were higher than those in group A (P<0.05) (Fig. 3).
Analysis of complications 24 h after
surgery
There was no statistical difference in the incidence
of vertigo and increase of blood pressure 24 h after surgery of
patients between the groups (P>0.05), but the incidence of
gastrointestinal reaction and postoperative cognitive dysfunction
(POCD) in group B was lower than that in group A (P<0.05)
(Table VI).
 | Table VIAnalysis of complications 24 h after
operation. |
Table VI
Analysis of complications 24 h after
operation.
| Complication | Group A (n=86) | Group B
(n=100) | Z value | P-value |
|---|
| Gastrointestinal
reaction | 8 (9.3) | 1 (1.00) | 2.288 | 0.022 |
| Vertigo | 6 (6.98) | 2 (2.00) | 1.306 | 0.192 |
| Increase of blood
pressure | 6 (6.98) | 4 (4.00) | 0.571 | 0.568 |
| POCD | 14 (16.28) | 6 (6.00) | 2.019 | 0.044 |
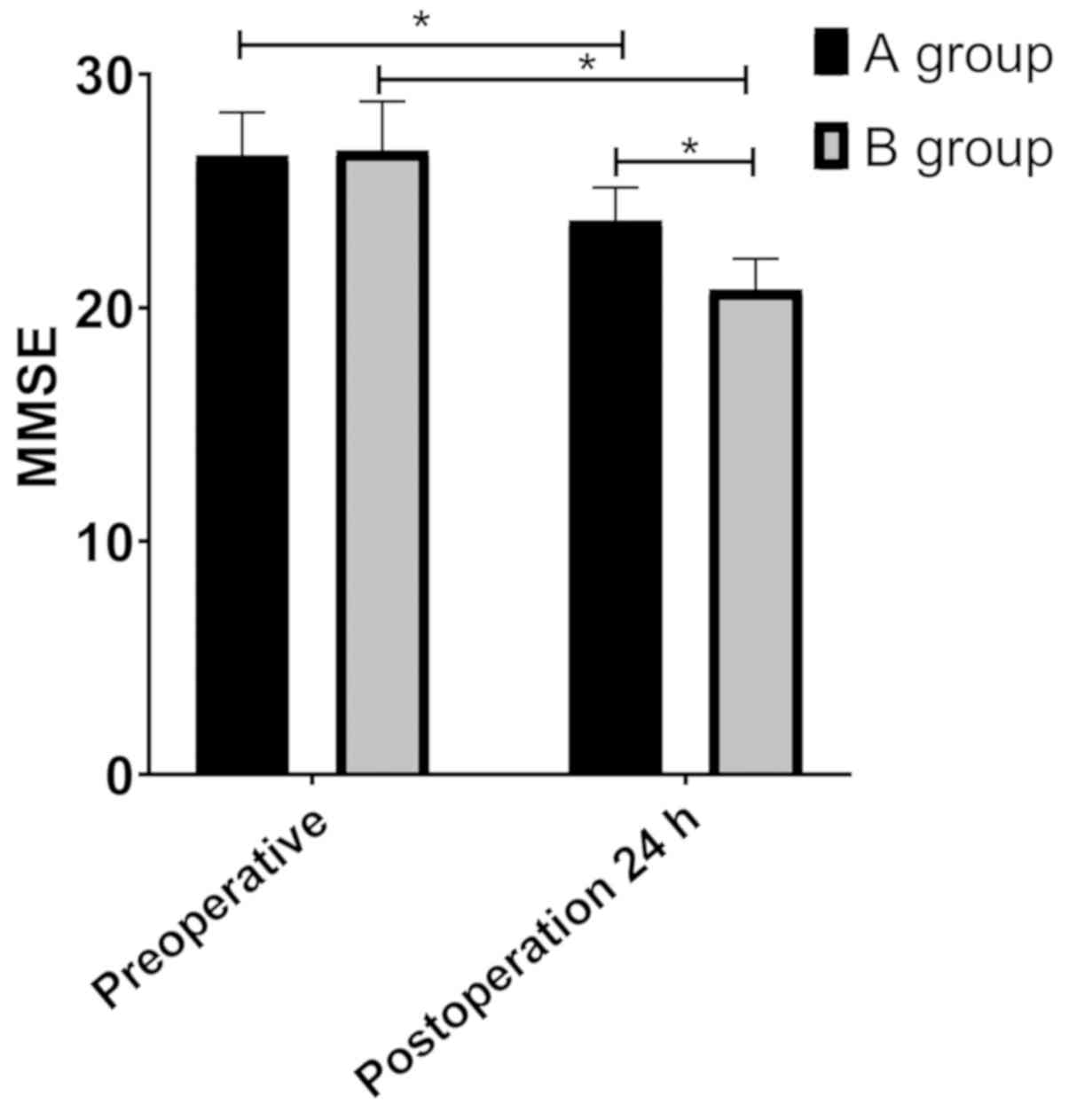
Changes of MMSE scores of patients in
the groups
MMSE scores displayed that there was no significant
difference in MMSE scores of patients between the groups before
surgery (P>0.05). MMSE scores in group B were significantly
higher than those in group A 24 h after surgery (P<0.05), but
MMSE scores in both groups were lower than those before surgery
(P<0.05) (Fig. 4).
Discussion
Anesthesia of patients in both groups in this study
was good. Ramsay sedation score was between 2 and 4 points. From
the results of this study, administration of 0.6 µg/kg
dexmedetomidine before anesthesia induction can effectively shorten
postoperative recovery time of patients and stabilize their
hemodynamic changes during surgery, which was consistent with the
results reported in previous studies. Zhou et al (13) reported that administration of
dexmedetomidine could stabilize hemodynamics, reduce postoperative
recovery time, and also shorten extubation time. Nayagam et
al (14) also reported
advantages of dexmedetomidine in stabilizing hemodynamics during
surgery. Patients with hip joint diseases are often accompanied by
unbearable pain (15,16). Even if this situation can be
effectively improved after surgery, due to invasive operation and
other reasons, patients may still have severe pain after
anesthesia, which affects their recovery (17). Dexmedetomidine is a selective α
receptor agonist with rapid onset, long duration of motor and
sensory block, and can effectively prolong the analgesic time
(18). This study indicated that VAS
pain score of patients in group B was significantly lower than that
of patients in group A 24 h after surgery, which also reflected
advantages of dexmedetomidine in analgesia. There are similar
reports in some studies (19,20)
stating that dexmedetomidine can effectively improve postoperative
pain of patients.
Increased inflammatory response caused by surgical
stress is an important cause of postoperative pain in patients
(21), and anti-inflammatory therapy
is also an important way to relieve their postoperative pain
(22). Based on the results of this
study, levels of inflammatory factors IL-6 and TNF-α of patients in
group B 24 h after surgery were significantly lower than those in
group A, which suggested that dexmedetomidine 0.6 µg/kg before
anesthesia induction could also reduce the production of their
inflammatory mediators. Dexmedetomidine also played an effective
anti-inflammatory role in other surgical treatments. A
meta-analysis showed that perioperative addition of dexmedetomidine
could significantly reduce levels of serum IL-6, IL-8 and TNF-α as
well as inflammatory response of patients (23). In a study on artificial tooth
implantation, a conclusion that dexmedetomidine provided better
postoperative analgesia through anti-inflammatory and antioxidant
pathways was further put forward (24).
Postoperative pain is an important reason for the
decline of patients' immune function, which is an important factor
affecting their prognosis after surgery (25). This study showed that CD3+
cells, CD4+ cells, CD8+ cells and CD4/CD8
ratio of patients in group B were higher than those in group A 24 h
after surgery, suggesting that dexmedetomidine had protective
effects on their immune function after surgery. There may be two
reasons for this. First, dexmedetomidine can relieve postoperative
pain of patients, thus improving the inhibition of pain on their
immune function. Second, dexmedetomidine can directly reduce the
inhibition of surgery on patients' immune function. Many reports
have showed improvement of dexmedetomidine on patients'
postoperative immune function. Wang et al (11) reported that dexmedetomidine could
reduce the pressure of radical operation for stomach cancer,
maintain Th1/Th2 balance, reduce inflammatory reaction and play an
immune protective role. Yang et al (26) also reported that intravenous
dexmedetomidine could significantly reduce the inhibition of
cellular immune function in patients undergoing radical mastectomy,
which was of great significance to maintain and improve the immune
function of the body, as well as their postoperative rehabilitation
and prognosis.
We analyzed the safety of dexmedetomidine
application in hip replacement. The results showed that
gastrointestinal reaction and POCD incidence rate of patients in
group B were lower than those in group A, which was also reported
in some other studies. As a supplement to peripheral nerve block,
dexmedetomidine sedation during operation can reduce the incidence
of postoperative psychosis in patients with hip replacement and is
conducive to reducing the incidence of early POCD (27,28).
This may be related to the anti-inflammatory effects of
dexmedetomidine. Li et al (29) reported that the use of
dexmedetomidine to reduce the incidence rate of early POCD during
anesthesia of laparoscopic cholecystectomy might be realized
through the mechanism of reducing the level of inflammatory
response. Similar conclusions were also reported in the study by
Chen et al (30). A
meta-analysis also revealed that postoperative psychosis and POCD
were indeed related to the concentration of inflammatory markers in
peripheral blood and cerebrospinal fluid, such as CRP and IL-6,
which played a certain role in postoperative psychosis and POCD
(31). Therefore, the safety of
dexmedetomidine in hip replacement could be assured. However, the
effect of dextromethopyrimidine might be different between the
sexes. Jang et al (32)
analyzed and found that dexmedetomidine accelerated the extinction
of fear memory and reduced anxiety in rats, but it was more
effective on female rats than male rats. Li et al (33) reported that the effect of
dexmedetomidine on acute postoperative pain in male patients is
better than that in female patients. Thus, further analysis is
required on this.
In conclusion, dexmedetomidine administration during
anesthesia for patients with hip replacement can effectively
shorten the recovery time, stabilize the intraoperative
hemodynamics, protect the immune function, and reduce postoperative
pain and POCD occurrence rate, which may be related to
anti-inflammatory effects of dexmedetomidine.
Acknowledgements
Not applicable.
Funding
This study is funded by the project ‘A new mechanism
of dexmedetomidine regulating IL-17A expression in subacute phase
inflammatory response to protect organs from injury’ (item no.
2017D01C264).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YX wrote the manuscript, interpreted and analyzed
the patient data. XD performed flow cytometry and ELISA, and was
responsible for observation indicators analysis. Both authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Sixth Affiliated Hospital of the Sixth Clinical Medical School
of Xinjiang Medical University (Urumqi, China). Patients who
participated in this research, signed an informed consent and had
complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kremers HM, Larson DR, Crowson CS, Kremers
WK, Washington RE, Steiner CA, Jiranek WA and Berry DJ: Prevalence
of total hip and knee replacement in the United States. J Bone
Joint Surg Am. 97:1386–1397. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kurtz SM, Lau E, Ong K, Zhao K, Kelly M
and Bozic KJ: Future young patient demand for primary and revision
joint replacement: National projections from 2010 to 2030. Clin
Orthop Relat Res. 467:2606–2612. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liu XW, Zi Y, Xiang LB and Wang Y: Total
hip arthroplasty: Areview of advances, advantages and limitations.
Int J Clin Exp Med. 8:27–36. 2015.PubMed/NCBI
|
|
4
|
Bilsel K, Erdil M, Gulabi D, Elmadag M,
Cengiz O and Sen C: Factors affecting mortality after hip fracture
surgery: A retrospective analysis of 578 patients. Eur J Orthop
Surg Traumatol. 23:895–900. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kadry B, Feaster WW, Macario A and
Ehrenfeld JM: Anesthesia information management systems: Past,
present, and future of anesthesia records. Mt Sinai J Med.
79:154–165. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Flack S and Anderson C: Ultrasound guided
lower extremity blocks. Paediatr Anaesth. 22:72–80. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Karaca S, Ayhan E, Kesmezacar H and Uysal
O: Hip fracture mortality: Is it affected by anesthesia techniques?
Anesthesiol Res Pract. 2012(708754)2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shi ZY, Jiang CN and Shao G: Application
of lower limb nerve block combined with slow induction of light
general anesthesia and tracheal induction in elderly hip surgery.
Medicine (Baltimore). 97(e12581)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gu CY, Zhang J, Qian YN and Tang QF:
Effects of epidural anesthesia and postoperative epidural analgesia
on immune function in esophageal carcinoma patients undergoing
thoracic surgery. Mol Clin Oncol. 3:190–196. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Suthar O, Sethi P and Sharma UD:
Comparison of dexmedetomidine and clonidine as an adjuvant to
intrathecal bupivacaine in lower limb surgery: A randomised,
double-blind, placebo controlled trial. Anaesth Pain Intensive
Care. 18:149–154. 2015.
|
|
11
|
Wang Y, Xu X, Liu H and Ji F: Effects of
dexmedetomidine on patients undergoing radical gastrectomy. J Surg
Res. 194:147–153. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jang Y, Yeom MY, Kang ES, Kang JW and Song
HK: The antinociceptive effect of dexmedetomidine modulates spleen
cell immunity in mice. Int J Med Sci. 11:226–233. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhou J, Wang J and Meng F: The use of
dexmedetomidine in aged patients with total hip replacement surgery
under general anesthesia: Changes in hemodynamics, cerebral state
index and wakening quality. Afr J Pharm Pharmacol. 6:1833–1836.
2012.
|
|
14
|
Nayagam HA, Singh NR and Singh HS: A
prospective randomised double blind study of intrathecal fentanyl
and dexmedetomidine added to low dose bupivacaine for spinal
anesthesia for lower abdominal surgeries. Indian J Anaesth.
58:430–435. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Park SH, Kim AR and Lee HS: Effects of
short-term corrective exercise on pain, hip joint range of motion
and trunk muscle strength of a patient with anterior pelvic tilt: A
case study. Off J Korean Acad Kinesiol. 18:85–93. 2016.
|
|
16
|
Iidaka T, Muraki S, Oka H, Kodama R,
Tanaka S, Kawaguchi H, Nakamura K, Akune T and Yoshimura N:
Radiographic measurements of the hip joint and their associations
with hip pain in Japanese men and women: The Research on
Osteoarthritis/osteoporosis Against Disability (ROAD) study.
Osteoarthritis Cartilage. 25:2072–2079. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wylde V, Sayers A, Lenguerrand E,
Gooberman-Hill R, Pyke M, Beswick AD, Dieppe P and Blom AW:
Preoperative widespread pain sensitization and chronic pain after
hip and knee replacement: a cohort analysis. Pain. 156:47–54.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hua X, Hu Y, Chen D, Xiao Y and Luo L:
Efficacy and safety of ultrasound-guided fascia iliaca compartment
block using dexmedetomidine combined with ropivacaine in aged
patients undergoing hip replacement. Int J Clin Exp Med.
10:16484–16491. 2017.
|
|
19
|
Wu ZL, Zhou ZF, Xu LX and She SZ: Effect
of dexmedetomidine on patient-controlled intravenous analgesia with
fentanyl in elderly patients after total hip replacement. Nan Fang
Yi Ke Da Xue Xue Bao. 31:701–704. 2011.(In Chinese). PubMed/NCBI
|
|
20
|
Peng K, Liu HY, Wu SR, Cheng H and Ji FH:
Effects of combining dexmedetomidine and opioids for postoperative
intravenous patient-controlled analgesia. Clin J Pain.
31:1097–1104. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Reddi D: Preventing chronic postoperative
pain. Anaesthesia. 71 (Suppl 1):64–71. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang Z, Xu H, Zhang Y, Li W, Yang Y, Han
T, Wei Z, Xu X and Gao J: Nonsteroidal anti-inflammatory drugs for
postoperative pain control after lumbar spine surgery: A
meta-analysis of randomized controlled trials. J Clin Anesth.
43:84–89. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li B, Li Y, Tian S, Wang H, Wu H, Zhang A
and Gao C: Anti-inflammatory effects of perioperative
dexmedetomidine administered as an adjunct to general anesthesia: A
meta-analysis. Sci Rep. 5(12342)2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li S, Yang Y, Yu C, Yao Y, Wu Y, Qian L
and Cheung CW: Dexmedetomidine analgesia effects in patients
undergoing dental implant surgery and its impact on postoperative
inflammatory and oxidative stress. Oxid Med Cell Longev.
2015(186736)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Beilin B, Shavit Y, Trabekin E, Mordashev
B, Mayburd E, Zeidel A and Bessler H: The effects of postoperative
pain management on immune response to surgery. Anesth Analg.
97:822–827. 2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yang XH, Bai Q, Lv MM, Fu HG, Dong TL and
Zhou Z: Effect of dexmedetomidine on immune function of patients
undergoing radical mastectomy: A double blind and placebo control
study. Eur Rev Med Pharmacol Sci. 21:1112–1116. 2017.PubMed/NCBI
|
|
27
|
Mei B, Meng G, Xu G, Cheng X, Chen S,
Zhang Y, Zhang M, Liu X and Gu E: Intraoperative sedation with
dexmedetomidine is superior to propofol for elderly patients
undergoing hip arthroplasty. Clin J Pain. 34:811–817.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu Y, Ma L, Gao M, Guo W and Ma Y:
Dexmedetomidine reduces postoperative delirium after joint
replacement in elderly patients with mild cognitive impairment.
Aging Clin Exp Res. 28:729–736. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li Y, He R, Chen S and Qu Y: Effect of
dexmedetomidine on early postoperative cognitive dysfunction and
peri-operative inflammation in elderly patients undergoing
laparoscopic cholecystectomy. Exp Ther Med. 10:1635–1642.
2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chen W, Liu B, Zhang F, Xue P, Cui R and
Lei W: The effects of dexmedetomidine on post-operative cognitive
dysfunction and inflammatory factors in senile patients. Int J Clin
Exp Med. 8:4601–4605. 2015.PubMed/NCBI
|
|
31
|
Liu X, Yu Y and Zhu S: Inflammatory
markers in postoperative delirium (POD) and cognitive dysfunction
(POCD): A meta-analysis of observational studies. PLoS One.
13(e0195659)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jang M, Jung T, Kim SH and Noh J: Sex
differential effect of dexmedetomidine on fear memory extinction
and anxiety behavior in adolescent rats. Neurosci Res. 149:29–37.
2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li YY, Ge DJ, Li JY and Qi B: Sex
differences in the morphine-sparing effects of intraoperative
dexmedetomidine in patient-controlled analgesia following general
anesthesia: A consort-prospective, randomized, controlled clinical
trial. Medicine (Baltimore). 95(e3619)2016.PubMed/NCBI View Article : Google Scholar
|