Introduction
Alzheimer's disease (AD) is the currently most
common neurodegenerative disease. According to the official
statistics in 2015, 110,561 individuals succumbed to the disease,
rendering AD the 6th most common cause of mortality in the United
States and the 5th cause of mortality for Americans aged ≥65 years.
The number of patients with AD in the United States are expected to
increase to 13.8 million by the middle of the century (1,2).
Patients with AD have a number of neurofibrillary tangles and
amyloid plaques in their brains. Plaques, astrocyte proliferation,
neuronal dystrophy, neuronal loss and vascular variations are signs
of the disease. AD results in the loss of mental, behavioral and
cognitive functions and learning ability, which causes great
distress to patients and nursing staff (3). Patients with AD are mainly treated with
acetylcholinesterase inhibitors, which enhance cholinergic
neurotransmission in the brain by reducing the degradation rate of
acetylcholine in the synaptic cleft (4). Donepezil and rivastigmine are commonly
used acetylcholinesterase inhibitors that can improve the treatment
of patients with AD (5-8).
There are a number of studies available on the separate application
of these two drugs. For example, it has been reported that
rivastigmine significantly improves cognitive function and daily
living ability in patients with AD compared with those administered
the placebo (9). In addition,
previous research has indicated that compared with the placebo,
donepezil significantly improves the overall function of patients
with AD, and is safer and well-tolerated (10). At present, however, to the best of
our knowledge, there is no evidence available on the effects of the
combination of rivastigmine and donepezil in the treatment of
AD.
The kallikrein-kinin system (KKS) is considered as
an important pathophysiological mediator of cerebrovascular
dysfunction, neuroinflammation and amyloid β-protein (Aβ) pathology
in AD (11). Bradykinin released by
KKS is a pro-inflammatory mediator with a series of physiological
actions in the periphery (12). In a
previous study, the chronic administration of bradykinin B1 and B2
receptor antagonists was shown to improve amyloidosis-related
cerebral hypoperfusion and vascular reactivity, thereby relieving
the symptoms of patients with AD (13). Another study demonstrated that
bradykinin increased the intracellular calcium concentration
[(Ca2+)i] in astrocytes, while donepezil reduced this
increase. Donepezil inhibited bradykinin-induced inflammatory
responses through the nicotinic acetylcholine receptor (nAChR) and
PI3K/Akt pathways in astrocytes, so as to treat AD (14). Therefore, rivastigmine hydrogen
tartrate and donepezil hydrochloride may suppress the symptoms of
AD by inhibiting the bradykinin level.
In the present study, changes in the cognitive
functions and mental behavior of patients with AD were observed
through rivastigmine hydrogen tartrate and donepezil hydrochloride,
so as to compare their efficacy on AD and to compare the effects
between the two drugs. In addition, the effects of the drugs on
serum bradykinin levels in the patients were examined.
Patients and methods
Inclusion and exclusion criteria
The present study was a non-randomized controlled
trial. A total of 126 patients with AD admitted to Luoyang Central
Hospital from January, 2018 to December, 2018 were enrolled.
Patients treated with the single-agent donepezil were included in
the monotherapy group (n=56), and patients receiving donepezil plus
rivastigmine were in the combination group (n=70). Patients were
divided into different groups according to the treatment they
selected.
The inclusion criteria were as follows: Patients who
met the diagnostic criteria for AD (15); patients<80 years of age, but
>50 years of age; patients who had a primary school education or
higher, and were able to complete the treatments and follow-up
tests; patients who did not receive antipsychotic drugs or
cholinesterase inhibitors 4 weeks prior to enrollment; patients
with a Minimum Mental State Examination (MMSE) score (16) between 12-27 points. The exclusion
criteria were as follows: Patients with allergy or
contraindications to donepezil and rivastigmine; patients with
mental illnesses other than AD; patients with poor treatment
compliance; patients with heart, liver and kidney insufficiency;
patients who had suffered severe head injuries. In the present
study, patients and their families were informed and signed an
informed consent form. The study was approved by the Medical Ethics
Committee of Luoyang Central Hospital.
Grouping and treatment methods
According to the severity of the patients' symptoms,
patients in the monotherapy group were administered 1-2 tablets (5
mg/1 tablet) of donepezil hydrochloride tablets (purchased from
Chongqing Zein Pharmaceutical Co., Ltd.), once/day. On the basis of
the monotherapy group, patients in the combination group were
administered rivastigmine hydrogen tartrate capsules (purchased
from Novartis) 1.5 mg/time and twice/day. The dosage could be
changed to 6 mg/time and once/day according to the patients'
tolerance. Patients in both groups were treated for 6 months.
Scoring standards
Before and after treatment, the MMSE (14) was used to score the patients' memory,
attention, language competence and other cognitive functions in the
monotherapy and combination groups. A total of 27-30 points
indicated normal cognitive functions, whereas <27 points
indicated cognitive impairment. The lower the score was, the more
severe the cognitive impairment was.
Before and after treatment, the Blessed-Roth
Dementia Scale (BRDS) (17) was used
to evaluate the patients' ability of social/daily living, cognition
of common sense and character changes in the monotherapy and
combination groups. A score of ≤7 points indicated no dementia,
whereas a score of >7 points indicated dementia. The higher the
score was, the more severe the dementia was.
Before and after treatment, the Alzheimer's Disease
Assessment Scale-Cognitive Subscale (ADAS-Cog) (18) was used to score the patients' memory,
language competence, ability to use, inferential capability,
orientation and other abilities in the monotherapy and combination
groups. The scoring system was between 0-70 points. The higher the
score was, the more severe the injury was.
Before and after treatment, the AD quality of life
scale (QOL-AD) (19) was used to
evaluate the quality of life of patients in the 2 groups, including
physical condition, energy, mood, memory and other 13 items, with a
total score of 52. The higher the score, the greater the quality of
life.
Detection of serum bradykinin level
before and after treatment
Before and after treatment, 5 ml of fasting venous
blood was extracted in the morning from patients in the 2 groups,
placed in centrifuge tubes and centrifuged (1,500 x g, 4˚C, 10 min)
for multiple times to obtain the supernatant, namely serum. The
serum was stored in a refrigerator at -80˚C for later use. Before
and after treatment, the serum bradykinin level was determined by
enzyme-linked immunosorbent assay (ELISA). An ELISA kit was
purchased from Abcam. Standard wells, sample wells to be tested and
blank control wells were set up on an ELISA plate. The standard
well was supplemented with 50 µl of standard substances with
various concentrations, while the sample well to be tested was
added with 40 µl of sample diluent and then 10 µl of the samples to
be tested. The plate was sealed with a microplate sealer and
incubated at 37˚C for 1 h. After the sealer was removed and the
liquid in the wells was discarded, the plate was washed with
washing liquid over 5 min 3 times and dried with absorbent paper
towels. Subsequently, the standard well and the sample well to be
tested were added with 50 µl of enzyme-labeled reagents. The plate
was sealed with a microplate sealer and incubated at 37˚C for 1 h.
After the sealer was removed and the liquid in the wells was
discarded, the plate was washed with washing liquid over 5 min 3
times and dried with absorbent paper towels. Subsequently, each
well was supplemented with 50 µl of substrates
H2O2 and TMB in turn, and then developed at
37˚C for 10-15 min in the dark after the substrates were mixed
evenly. Finally, each well was supplemented with 50 µl of stop
solution to cease the reaction. The optical density values of each
well were detected at 450 nm using a multifunctional microplate
reader (CLARIOstar, BMG LABTECH).
Statistical analysis
SPSS 18.0 (IBM Corp.) was used for statistical
analysis. In the present study, count data, such as place of
residence and the number of adverse reactions were expressed in
terms of number/percentage [n (%)]. The comparisons between
technical data were performed using the Chi-squared test or
Fisher's exact test. ADAS-Cog MMSE scores were expressed as the
means ± standard deviation (SD). One-way ANOVA followed by Tukey's
HSD test was used for comparison of measurement data, expressed by
F values. P<0.05 was considered to indicate a statistically
significant difference.
Results
Comparison of general information
No significant differences were observed between the
combination and monotherapy groups as regards age, exercise habits,
place of residence, nationality, educational level, body weight,
marital status and food preference (P>0.05). Further details are
presented in Table I.
 | Table IComparison of general information
[n(%)]. |
Table I
Comparison of general information
[n(%)].
| Groups | Monotherapy group
(n=56) | Combination group
(n=70) | χ2 | P-value |
|---|
| Age | | | 0.202 | 0.653 |
|
<65 years
old | 23 (41.07) | 26 (37.14) | | |
|
≥65 years
old | 33 (58.93) | 44 (62.86) | | |
| Exercise | | | 0.015 | 0.902 |
|
Yes | 21 (37.50) | 27 (38.57) | | |
|
No | 35 (62.50) | 43 (61.43) | | |
| Place of
residence | | | 0.161 | 0.689 |
|
City | 30 (53.57) | 40 (57.14) | | |
|
Countryside | 26 (46.43) | 30 (42.86) | | |
| Nationality | | | 0.358 | 0.550 |
|
Han | 50 (89.29) | 60 (85.71) | | |
|
National
minorities | 6 (10.71) | 10 (15.29) | | |
| Educational
level | | | 0.129 | 0.720 |
|
<Senior
high school | 40 (71.43) | 52 (74.29) | | |
|
≥Senior high
school | 16 (28.57) | 18 (25.71) | | |
| Body weight | | | 0.082 | 0.775 |
|
<55
KG | 21 (37.50) | 28 (40.00) | | |
|
≥55 KG | 35 (62.50) | 42 (60.00) | | |
| Marital status | | | 1.994 | 0.369 |
|
Married | 47 (83.93) | 52 (74.29) | | |
|
Unmarried | 6 (10.71) | 10 (14.29) | | |
|
Widowed | 3 (5.36) | 8 (11.42) | | |
| Food
preference | | | 0.113 | 0.737 |
|
Bland | 36 (64.29) | 47 (67.14) | | |
|
Spicy | 20 (35.71) | 23 (32.86) | | |
Comparison of MMSE score before and
after treatment
Patients in both groups cooperated to complete the
treatment. Following treatment, the MMSE score in the monotherapy
group increased from 16.12±1.74 to 22.23±1.99 (P<0.05), and the
score in the combination group increased from 15.85±2.19 to
26.23±2.17 (P<0.05). Following treatment, the MMSE score in the
combination group was significantly higher than that in the
monotherapy group (P<0.05). Further details are presented in
Table II.
 | Table IIComparison of MMSE score before and
after treatment (means ± SD). |
Table II
Comparison of MMSE score before and
after treatment (means ± SD).
| Groups | Monotherapy group
(n=56) | Combination group
(n=70) |
|---|
| Before
treatment | 16.12±1.74 | 15.85±2.19 |
| After
treatment |
22.23±1.99a |
26.23±2.17a,b |
| F value | 16.894 | 27.783 |
| P-value | <0.001 | <0.001 |
Comparison of the BRDS score before
and after treatment
Following treatment, the BRDS score in the
monotherapy group decreased from 14.78±1.67 to 9.23±1.28
(P<0.05), and the score in the combination group decreased from
14.33±1.81 to 7.18±0.95 (P<0.05). Following treatment, the BRDS
score in the combination group was significantly lower than that in
the monotherapy group (P<0.05). Further details are presented in
Table III.
 | Table IIIComparison of BRDS score before and
after treatment (means ± SD). |
Table III
Comparison of BRDS score before and
after treatment (means ± SD).
| Groups | Monotherapy group
(n=56) | Combination group
(n=70) |
|---|
| Before
treatment | 14.78±1.67 | 14.33±1.81 |
| After
treatment |
9.23±1.28a |
7.18±0.95a,b |
| F value | 18.768 | 28.398 |
| P-value | <0.001 | <0.001 |
Comparison of the ADAS-Cog score
before and after treatment
Following treatment, the ADAS-Cog score in the
monotherapy group decreased from 29.67±3.03 to 22.24±3.98
(P<0.05), and the score in the combination group decreased from
30.15±2.89 to 18.24±3.67 (P<0.05). Following treatment, the
ADAS-Cog score in the combination group was significantly lower
than that in the monotherapy group (P<0.05). Further details are
presented in Table IV.
 | Table IVComparison of ADAS-Cog score before
and after treatment (means ± SD). |
Table IV
Comparison of ADAS-Cog score before
and after treatment (means ± SD).
| Groups | Monotherapy group
(n=56) | Combination group
(n=70) |
|---|
| Before
treatment | 29.67±3.03 | 30.15±2.89 |
| After
treatment |
22.24±3.98a |
18.24±3.67a,b |
| F value | 10.752 | 19.278 |
| P-value | <0.001 | <0.001 |
Comparison of the QOL-AD score before
and after treatment
Following treatment, the QOL-AD score in the
monotherapy group decreased from 26.24±5.89 to 35.24±5.78
(P<0.05), and the score in the combination group decreased from
25.28±6.45 to 43.24±5.35 (P<0.05). Following treatment, the
QOL-AD score in the combination group was significantly higher than
that in the monotherapy group (P<0.05). Further details are
presented in Table V.
 | Table VComparison of QOL-AD score before and
after treatment (means ± SD). |
Table V
Comparison of QOL-AD score before and
after treatment (means ± SD).
| Groups | Monotherapy group
(n=56) | Combination group
(n=70) |
|---|
| Before
treatment | 26.24±5.89 | 25.28±6.45 |
| After
treatment |
35.24±5.78a |
43.24±5.35a,b |
| F value | 8.852 | 16.759 |
| P-value | <0.001 | <0.001 |
Comparison of adverse reactions
No allergic reactions occurred during the treatment
period in the 2 groups, and adverse reactions were treated with
symptomatic treatment. No significant differences were observed in
the main adverse reactions in appetite loss, headache, vomiting,
muscle spasm, insomnia, sleepiness and diarrhea between the 2
groups, and there were no significant differences in the total
number of affected patients (with at least 1 adverse event) between
the two groups (P>0.05). Further details are presented in
Table VI.
 | Table VIComparison of adverse reactions
[cases (%)]. |
Table VI
Comparison of adverse reactions
[cases (%)].
| Groups | Monotherapy group,
n=56 [n (%)] | Combination group,
n=70 [n (%)] | χ2 | P-value |
|---|
| Decreased food
appetite | 3 (5.36) | 5 (7.14) | 0.167 | 0.683 |
| Headache | 2 (3.57) | 4 (5.71) | 0.315 | 0.575 |
| Vomiting | 4 (7.14) | 7 (10.00) | 0.319 | 0.572 |
| Muscle spasms | 2 (3.57) | 5 (7.14) | 0.756 | 0.385 |
| Insomnia | 3 (5.36) | 6 (8.57) | 0.485 | 0.486 |
| Drowsiness and
fatigue | 5 (8.93) | 4 (5.13) | 0.120 | 0.729 |
| Diarrhea | 2 (3.57) | 6 (8.57) | 1.308 | 0.253 |
| Total number of
affected patients | 21 (37.50) | 37 (52.86) | 2.954 | 0.086 |
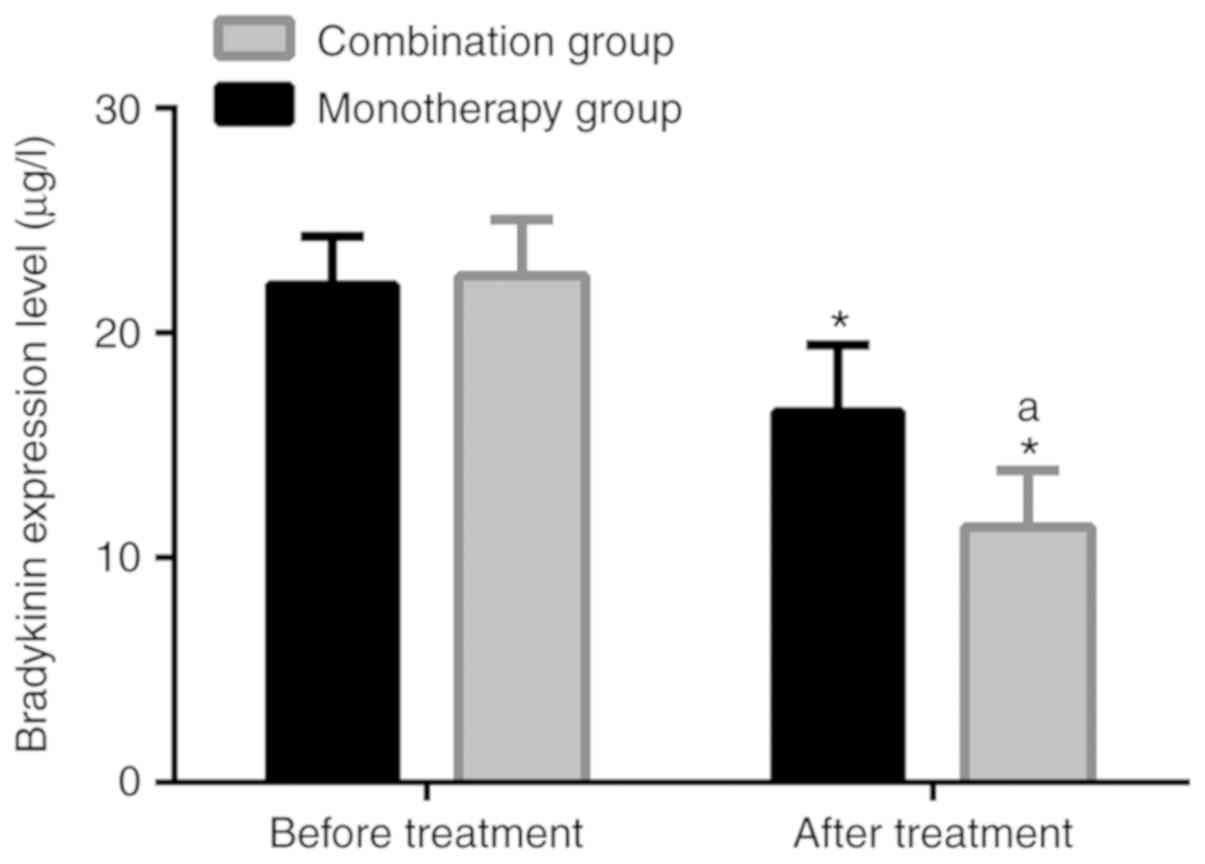
Comparison of bradykinin level
Following treatment, the bradykinin level in the
monotherapy group was 16.48±2.99 µg/l, significantly lower than
22.13±2.18 µg/l before treatment (P<0.05). Following treatment,
the level in the combination group was 11.37±2.51 µg/l,
significantly lower than 22.55±2.49 µg/l before treatment
(P<0.05). Following treatment, the bradykinin level in the
combination group was significantly lower than that in the
monotherapy group (P<0.05). Further details are presented in
Table VII and Fig. 1.
 | Table VIIComparison of bradykinin level (µg/l,
means ± SD). |
Table VII
Comparison of bradykinin level (µg/l,
means ± SD).
| Groups | Monotherapy group
(n=56) | Combination group
(n=70) |
|---|
| Before
treatment | 22.13±2.18 | 22.55±2.49 |
| After
treatment |
16.48±2.99a |
11.37±2.51a,b |
| F value | 11.273 | 25.573 |
| P-value | <0.001 | <0.001 |
Discussion
There are currently 47 million patients with AD
worldwide. The morbidity rate of the disease increases with the
aging of the population, which brings great mental and economic
burdens to families. It is estimated that 74.7 million individuals
will suffer from dementia by the year 2030, and the nursing
expenses for these patients will increase to about 2 trillion US
dollars (20,21). At present, there is no cure available
for AD, although the symptoms can be relieved by drug interventions
(4). Therefore, it is of great
significance for patients with AD and their families to identify
therapeutic regimens with which to attenuate the deterioration of
AD symptoms.
Patients with AD exhibit significant choline
deficiency (22). Therefore,
cholinesterase inhibitors are mainly used to reduce the metabolic
rate of acetylcholine and suppress its hydrolysis, thus increasing
acetylcholine in the body and improving the choline deficiency, and
relieving AD symptoms (23). As a
selective and reversible cholinesterase inhibitor widely used in
clinical practice, donepezil hydrochloride inhibits the hydrolysis
of acetylcholine and increases its concentration in the human body,
thereby improving information transfer in the brain (24,25). As
a dual cholinesterase inhibitor, rivastigmine hydrogen tartrate
inhibits cholinesterase activity and reduces the loss of
presynaptic cholinergic function, thus reducing the degradation of
acetylcholine in synaptic cleft and increasing cholinergic signal
transduction, and then improving cognition and memory (26,27).
There are number of previous studies available confirming the
efficacy of rivastigmine hydrogen tartrate and donepezil
hydrochloride in the treatment of AD, however, their combined use
in the treatment of the disease has been rarely studied (28-30).
The results of the present study demonstrated that the MMSE, BRDS,
ADAS-Cog, and QOL-AD scores of the 2 groups were significantly
improved following treatment, and the improvement of the
combination group was more significant. No significant differences
were found between the 2 groups in the occurrence of adverse
reactions. These findings suggest that compared with donepezil
hydrochloride alone, rivastigmine hydrogen tartrate combined with
donepezil hydrochloride can relieve the symptoms and improve the
quality of life of patients with AD more effectively, and the
adverse reactions are not increased.
Recently, it has been indicated that bradykinin
released by KKS plays an important role in the central nervous
system (31). Brain inflammation
aggravates the pathology of AD, and bradykinin B1 receptors play a
regulatory role in brain inflammation and in amyloid deposition of
AD in mice. Therefore, bradykinin B1 receptors may involve
microglia/macrophages, thus affecting the progression of AD
(32). Bradykinin B2 receptor
antagonists provide significant protection for synaptic loss and
cognitive impairment induced by Aβ1-40 in mice, by reducing the
activation of microglia and pro-inflammatory protein levels, as
well as inhibiting MAPK signaling pathway and the activation of
transcription factors (33).
According to the study by Ashby et al, the bradykinin level
was significantly higher in patients with AD, and it may affect
cerebral blood flow and vascular permeability (34). This indicates that the reduction of
the bradykinin level can relieve the symptoms of patients with AD.
In the present study, following treatment, the bradykinin level in
the 2 groups significantly decreased and the AD symptoms were
relieved, which was consistent with the results of the
above-mentioned studies. The decrease in the combination group was
stronger compared with that in the monotherapy group, suggesting
that the two drugs may relieve AD symptoms by reducing bradykinin
level.
In conclusion, compared to treatment with donepezil
hydrochloride alone, rivastigmine hydrogen tartrate combined with
donepezil hydrochloride can relieve the symptoms and improve the
quality of life of patients with AD more effectively, which may be
related to the reduction of the bradykinin level. However, in the
present study, the efficacy was not compared between the 2 drugs
and other drugs. Additionally, the mechanisms of action between
rivastigmine hydrogen tartrate, donepezil hydrochloride and
bradykinin were not explored. Therefore, the conclusions presented
herein are limited. In the future, large-scale randomized
controlled trials or prospective cohort studies need to be
conducted, and individual differences and potential interference
factors will be controlled, in order to further confirm the
reliability of the conclusions of the present study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ conceived the study and wrote the manuscript. RY
analyzed and interpreted the patient general data. HW performed
ELISA. RZ was responsible for observation indicators analysis. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Luoyang Central Hospital. Patients who participated in this
research, signed the informed consent and had complete clinical
data. Signed written informed consents were obtained from the
patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Anand R, Gill KD and Mahdi AA:
Therapeutics of Alzheimer's disease: Past, present and future.
Neuropharmacology. 76:27–50. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Alzheimer's Association: 2018 Alzheimer's
disease facts and figures. Alzheimer Dement 14: 367-429, 2018.
|
|
3
|
Lombardo S and Maskos U: Role of the
nicotinic acetylcholine receptor in Alzheimer's disease pathology
and treatment. Neuropharmacology. 96:255–262. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Birks JS, Chong LY and Grimley Evans J:
Rivastigmine for Alzheimer's disease. Cochrane Database Syst Rev.
9(CD001191)2015.(Epub ahead of print). PubMed/NCBI View Article : Google Scholar
|
|
5
|
Islam MM, Gurung AB, Bhattacharjee A,
Aguan K and Mitra S: Human serum albumin reduces the potency of
acetylcholinesterase inhibitor based drugs for Alzheimer's disease.
Chem Biol Interact. 249:1–9. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tsoi KK, Chan JY, Leung NW, Hirai HW, Wong
SY and Kwok TC: Combination therapy showed limited superiority over
monotherapy for Alzheimer disease: A meta-analysis of 14 randomized
trials. J Am Med Dir Assoc. 17:863.e1–e8. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
D'onofrio G, Sancarlo D, Addante F,
Ciccone F, Cascavilla L, Paris F, Elia AC, Nuzzaci C, Picoco M,
Greco A, et al: A pilot randomized controlled trial evaluating an
integrated treatment of rivastigmine transdermal patch and
cognitive stimulation in patients with Alzheimer's disease. Int J
Geriatr Psychiatry. 30:965–975. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Han HJ, Kwon JC, Kim JE, Kim SG, Park JM,
Park KW, Park KC, Park KH, Moon SY, Seo SW, et al: Effect of
rivastigmine or memantine add-on therapy is affected by
butyrylcholinesterase genotype in patients with probable
Alzheimer's disease. Eur Neurol. 73:23–28. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Birks JS and Grimley Evans J: Rivastigmine
for Alzheimer's disease. Cochrane Database Syst Rev.
2015(CD001191)2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Maher-Edwards G, Dixon R, Hunter J, Gold
M, Hopton G, Jacobs G, Hunter J and Williams P: SB-742457 and
donepezil in Alzheimer disease: A randomized, placebo-controlled
study. Int J Geriatr Psychiatry. 26:536–544. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Nokkari A, Abou-El-Hassan H, Mechref Y,
Mondello S, Kindy MS, Jaffa AA and Kobeissy F: Implication of the
Kallikrein-Kinin system in neurological disorders: Quest for
potential biomarkers and mechanisms. Prog Neurobiol. 165-167:26–50.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhao WQ and Alkon DL: Alzheimer's disease
diagnosis based on mitogen-activated protein kinase
phosphorylation. U.S. Pat Appl. 9:188–595. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ni R, Kindler DR, Waag R, Rouault M,
Ravikumar P, Nitsch R, Rudin M, Camici GG, Liberale L, Kulic L and
Klohs J: fMRI reveals mitigation of cerebrovascular dysfunction by
bradykinin receptors 1 and 2 inhibitor noscapine in a mouse model
of cerebral amyloidosis. Front Aging Neurosci.
11(27)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Makitani K, Nakagawa S, Izumi Y, Akaike A
and Kume T: Inhibitory effect of donepezil on bradykinin-induced
increase in the intracellular calcium concentration in cultured
cortical astrocytes. J Pharmacol Sci. 134:37–44. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
McKhann GM, Knopman DS, Chertkow H, Hyman
BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux
R, et al: The diagnosis of dementia due to Alzheimer's disease:
Recommendations from the National Institute on Aging-Alzheimer's
Association workgroups on diagnostic guidelines for Alzheimer's
disease. Alzheimers Dement. 7:263–269. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Arevalo-Rodriguez I, Smailagic N, Roqué I
Figuls M, Ciapponi A, Sanchez-Perez E, Giannakou A, Pedraza OL,
Bonfill Cosp X and Cullum S: Mini-mental state examination (MMSE)
for the detection of Alzheimer's disease and other dementias in
people with mild cognitive impairment (MCI). Cochrane Database Syst
Rev. 2015(CD010783)2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Blessed G, Tomlinson BE and Roth M:
Blessed-Roth dementia scale (DS). Psychopharmacol Bull. 24:705–708.
1988.PubMed/NCBI
|
|
18
|
Podhorna J, Krahnke T, Shear M and
Harrison JE: Alzheimer's Disease Neuroimaging Initiative:
Alzheimer's disease assessment scale-cognitive subscale variants in
mild cognitive impairment and mild Alzheimer's disease: Change over
time and the effect of enrichment strategies. Alzheimers Res Ther.
8(8)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Logsdon RG, Gibbons LE, McCurry SM and
Teri L: Quality of life in Alzheimer's disease: Patient and
caregiver reports. J Ment health Aging. 5:21–32. 1999.
|
|
20
|
Winblad B, Amouyel P, Andrieu S, Ballard
C, Brayne C, Brodaty H, Cedazo-Minguez A, Dubois B, Edvardsson D,
Feldman H, et al: Defeating Alzheimer's disease and other
dementias: A priority for European science and society. Lancet
Neurol. 15:455–532. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cummings J, Aisen PS, DuBois B, Frölich L,
Jack CR Jr, Jones RW, Morris JC, Raskin J, Dowsett SA and Scheltens
P: Drug development in Alzheimer's disease: The path to 2025.
Alzheimers Res Ther. 8(39)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shao ZQ: Comparison of the efficacy of
four cholinesterase inhibitors in combination with memantine for
the treatment of Alzheimer's disease. Int J Clin Exp Med.
8:2944–2948. 2015.PubMed/NCBI
|
|
23
|
Godyń J, Jończyk J, Panek D and Malawska
B: Therapeutic strategies for Alzheimer's disease in clinical
trials. Pharmacol Rep. 68:127–138. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chase TN, Farlow MR and Clarence-Smith K:
Donepezil plus Solifenacin (CPC-201) treatment for Alzheimer's
disease. Neurotherapeutics. 14:405–416. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kearney MC, Caffarel-Salvador E, Fallows
SJ, McCarthy HO and Donnelly RF: Microneedle-mediated delivery of
donepezil: Potential for improved treatment options in Alzheimer's
disease. Eur J Pharm Biopharm. 103:43–50. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mohamed LA, Keller JN and Kaddoumi A: Role
of P-glycoprotein in mediating rivastigmine effect on amyloid-β
brain load and related pathology in Alzheimer's disease mouse
model. Biochim Biophys Acta. 1862:778–787. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Oh YS, Kim JS and Lee PH: Effect of
rivastigmine on behavioral and psychiatric symptoms of Parkinson's
disease dementia. J Mov Disord. 8:98–102. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cummings J, Lai TJ, Hemrungrojn S,
Mohandas E, Yun Kim S, Nair G and Dash A: Role of donepezil in the
management of neuropsychiatric symptoms in Alzheimer's disease and
dementia with Lewy bodies. CNS Neurosci Ther. 22:159–166.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nieto RA, Deardorff WJ and Grossberg GT:
Efficacy of rivastigmine tartrate, transdermal system, in
Alzheimer's disease. Expert Opin Pharmacother. 17:861–870.
2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Montero-Odasso M, Muir-Hunter SW,
Oteng-Amoako A, Gopaul K, Islam A, Borrie M, Wells J and Speechley
M: Donepezil improves gait performance in older adults with mild
Alzheimer's disease: A phase II clinical trial. J Alzheimers Dis.
43:193–199. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bitencourt RM, Guerra de Souza AC, Bicca
MA, Pamplona FA, de Mello N, Passos GF, Medeiros R, Takahashi RN,
Calixto JB and Prediger RD: Blockade of hippocampal bradykinin B1
receptors improves spatial learning and memory deficits in
middle-aged rats. Behav Brain Res. 316:74–81. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Asraf K, Torika N, Danon A and
Fleisher-Berkovich S: Involvement of the bradykinin B1
receptor in microglial activation: In vitro and in vivo studies.
Front Endocrinol (Lausanne). 8(82)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bicca MA, Costa R, Loch-Neckel G,
Figueiredo CP, Medeiros R and Calixto JB: B2 receptor
blockage prevents Aβ-induced cognitive impairment by
neuroinflammation inhibition. Behav Brain Res. 278:482–491.
2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ashby EL, Love S and Kehoe PG: Assessment
of activation of the plasma kallikrein-kinin system in frontal and
temporal cortex in Alzheimer's disease and vascular dementia.
Neurobiol Aging. 33:1345–1355. 2012.PubMed/NCBI View Article : Google Scholar
|