Introduction
Acute kidney injury (AKI) is a life-threatening
disease syndrome characterized by rapid loss of renal excretion
function (1). This serious
complication often occurs in critically ill patients with
devastating consequences (2).
Dialysis is an important choice for the treatment of acute renal
failure in clinical treatment, but maintenance of dialysis leads to
a higher daily medicine burden and peritoneal dialysis patients are
at great risk of infection (3). At
present, many drugs used for preventing and treating AKI have shown
benefits in clinical models, but there is no specific drug for a
curative effect in treatment of humans (4). The drug treatment of acute kidney
injury needs further investigation.
Syndecan-1 (SDC-1) is a ubiquitous and
multifunctional extracellular matrix proteoglycan, which can
mediate the binding and activity of basic fibroblast growth factor
(bFGF) and plays an important role in cell adhesion and maintenance
of epithelial integrity (5). Lu
et al (6) confirmed that
ischemic AKI can be effectively prevented by inhibiting shedding of
SDC-. Junior et al (7) also
found that SDC-1 is a new biomarker for renal injury and
endothelial dysfunction in HIV patients. The abscisic enzymes of
matrix metalloproteinase (MMP) 7(8),
MP9(9) and other SDC-1 proteases
participate in the proteolysis of SDC-1 extracellular domain.
GM6001 is a broad-spectrum MMP inhibitor (10), which acts on MMP-1, MMP-2, MMP-3 and
MMP-8. It was considered that the application of GM6001 can reduce
the proteolysis of SDC-1 by inhibiting the expression of MMPs, thus
achieving the purpose of inhibiting SDC-1 to treat or prevent the
development of AKI and effectively protect renal tissues of
patients.
In this study, the expression of SDC-1 in the kidney
tissues of rats was detected after the application of GM6001 to
explore the protective effect of GM6001 on SDC-1 and kidney by
establishing the rat model of acute kidney injury.
Materials and methods
Animals
Fifty clean grade 2 week-old SD rats, weighing
~180-250 g, were purchased from Kay Biological Technology
(Shanghai) Co., Ltd. The rats were raised at the temperature of
24.00±2.00˚C, humidity 50.00±5.00%, natural light, free access to
food and drink. The experiment was approved by the animal ethics
committee of Weifang People's Hospital (Weifang, China).
Methods Experimental methods
The rats after adaptive feeding were randomly
assigned as CG (n=15), TCG (n=10), MG (n=15) and TG (n=10).
Modeling method: Rats were anesthetized with phenobarbital.
Bilateral renal arteries and renal veins were bluntly separated
through the abdominal median incision. Bilateral renal arteries
were clamped with non-invasive vascular clamps. The vascular clamps
were loosened after 1 h. Renal artery blood flow was restored and
abdomen was closed layer by layer. Then the other groups of rats
were treated: In TG, after pretreatment of intraperitoneal
injection of GM6001 one day before modeling, the kidney injury
model of rats was established according to the above method on the
day of modeling. In MG, the same amount of saline was injected
intraperitoneally one day before modeling and the same treatment
was done on the day of modeling. In CG, the same amount of saline
was injected intraperitoneally one day before modeling but the
model was not made on the day of modeling. In TCG, rats were
pretreated with intraperitoneal injection of GM6001 one day before
modeling and the model was not made on the day of modeling. The
contents of blood urea nitrogen (BUN), serum creatinine (SCR), uric
acid (UA) and blood β2-microglobulin (β2-MG) in the above four
groups were detected, respectively, before modeling and 24 h after
successful modeling. All rats were sacrificed and the kidney
tissues of the rats were stripped off. One piece of tissue was
taken from each part and placed in 10% neutral formaldehyde at 4˚C
overnight for fixation to detect the contents of SDC-1 in kidney
tissue.
Samples detection QRT-PCR: Detection
of the contents of renal serum SDC-1
The collected serum was accelerated to coagulate,
left to stand for 30 min and centrifuged at 4˚C for 5 min at 400 x
g. The serum was taken and stored in a 1.5 ml centrifuge tube in a
refrigerator at-80˚C for testing. Total RNA was extracted. The
concentration and purity of the extracted RNA were determined by
using an ultraviolet spectrophotometer. The A260/A280 ratio should
be between 1.8 and 2.1. The RNA concentration was calculated for
further experiments. Then TransScript Green miRNA Two-Step qRT-PCR
SuperMix was used for reverse transcription into cDNA. After the
reaction, the cDNA sample was used as the template of qRT-PCR
(Thermo Fisher Scientific - CN) reaction and put into the
refrigerator for testing. The reaction conditions were cDNA 1 µl,
forward primer 0.4 µl, Universal miRNA qPCR Primer 0.4 µl, 2X
TransStart Tip Green qPCR SuperMix 10 µl, Passive Reference Dye
(50X) (Optional) 0.4 µl and total volume 20 µl. ddH2O
was used to complete to 20 µl. The conditions were as follows:
pre-denaturation at 95˚C for 5 min, then denaturation at 90˚C for
15 sec, annealing and extension at 60˚C for 30 sec and the cycle
was repeated 40 times. The relative expression of SDC-1 was
expressed by 2-∆Ct. All the tests were repeated 3 times
and the results were averaged (Table
I).
 | Table IPrimer sequences. |
Table I
Primer sequences.
| | Upstream sequence
(5'-3') | Downstream sequence
(5'-3') |
|---|
| SDC-1 |
CAGCAGCAACACCGAGAC |
GATTGGCAGTTCCATCCTC |
| GAPDH |
TGGCAAAGTGGAGATTGTT |
CTTCTGGGTGGCAGTGAT |
Western blotting: Detection of the
contents of SDC-1 in kidney tissue
After the collected rat kidney tissues were fully
ground, the total protein was extracted by RIPA lysis. The protein
concentration was detected by BCA (NC-BIO, LCB004). The protein
concentration was adjusted to 4 µg/µl. 12% SDS-PAGE electrophoresis
separation was carried out. The membrane was transferred to PVDF
membrane after ionization and soaked with PBST for 5 min for
washing. 5% skim milk powder was used for sealing for 2 h and first
antibody (1:1,000 Abcam, ab34164) was added for sealing overnight
at 4˚C. The first antibody was removed by washing the film.
Horseradish peroxidase-labeled sheep anti-mouse second antibody
(1:5,000 LD, LD-BJ-101891) was added, incubated at 37˚C for 1 h and
rinsed 3 times with PBS for 5 min each time, and then developed in
the darkroom. The excess liquid on the film was dried with a filter
paper. The ECL was illuminated and developed. The protein bands
were scanned and the gray values were analyzed in the Quantity One.
Relative expression level of its protein = the gray value of the
target protein band / the gray value of the β-actin protein
band.
ELISA: Detection of renal injury
indexes in rat blood
Renal function indexes include BUN, SCR, UA and
β2-MG. The detection was performed by using automatic biochemistry
analyzer. Detection kit was BUN (Shanghai Kanglang Biological
Technology Co., Ltd., KLJC0193), SCR (Shanghai Yuanye Biological
Technology Co., Ltd., S83553), UA (Chundu Biological Technology
Co., Ltd., CD-1161-LIN) and blood β2-MG (SenBeiJia, SBJ-R0215).
Observation indexes
Levels of BUN, SCR, UA and β2-MG of rats in four
groups before and after injection. SDC-1 levels of rats in four
groups after treatment.
Statistical methods
All the experimental results were statistically
calculated by using SPSS 24.0 statistics (Shanghai Yuchuang Network
Technology Co., Ltd.). Graphpad 8 (Shenzhen Tianruiqi Software
Technology Co., Ltd.) was used to draw graphs and check the
statistical calculation. The results of the experiments were all
expressed in the form of (mean number ± standard deviation). Single
factor analysis of variance was used for comparison among groups.
t-test was used for comparison between two groups of data
conforming to normal distribution. Nonparametric test was used for
data of non-normal distribution. Paired t-test was used for
comparison before and after modeling. The difference was
statistically significant at P<0.050.
Results
Modeling results
Among the 50 rats, 25 were modeled and 1 died in MG.
The success rate of modeling was 96%.
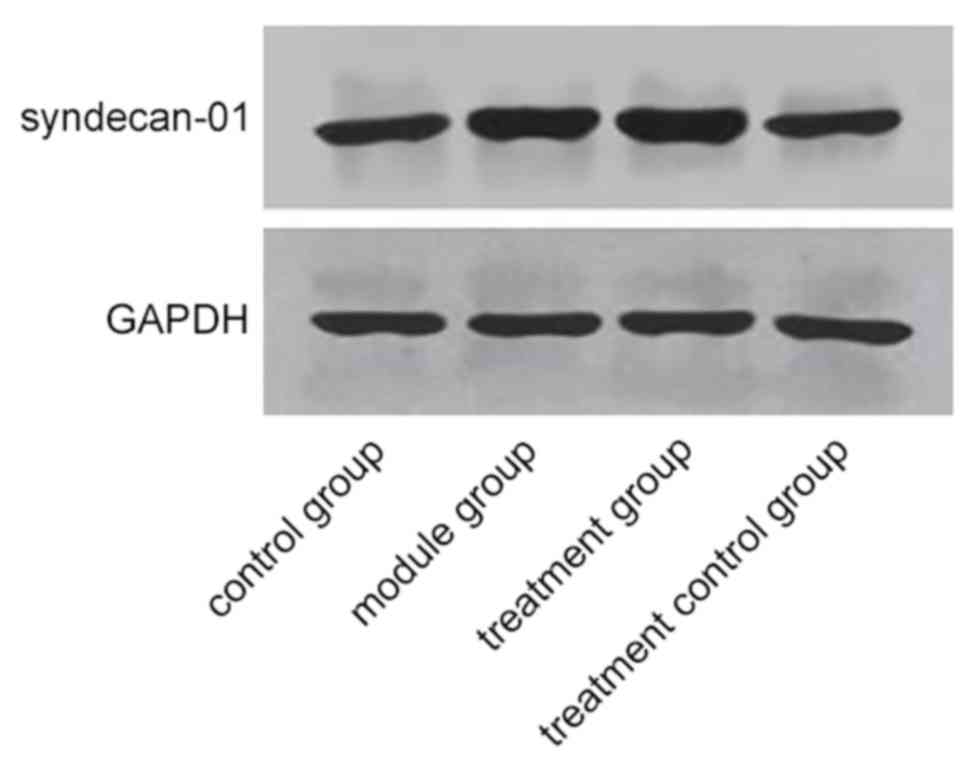
Effect of GM6001 on SDC-1 expression
in TG and TCG
The expression of SDC-1 protein in TG was 5.68±0.74
and it was the highest among the three groups (P<0.001).
Expression of SDC-1 protein in CG was 1.49±0.34, which was not
significantly different from the expression of SDC-1 protein in TCG
of 1.45±0.48 (P>0.050) and was significantly lower than that in
the other two groups (P>0.001) (Fig.
1).
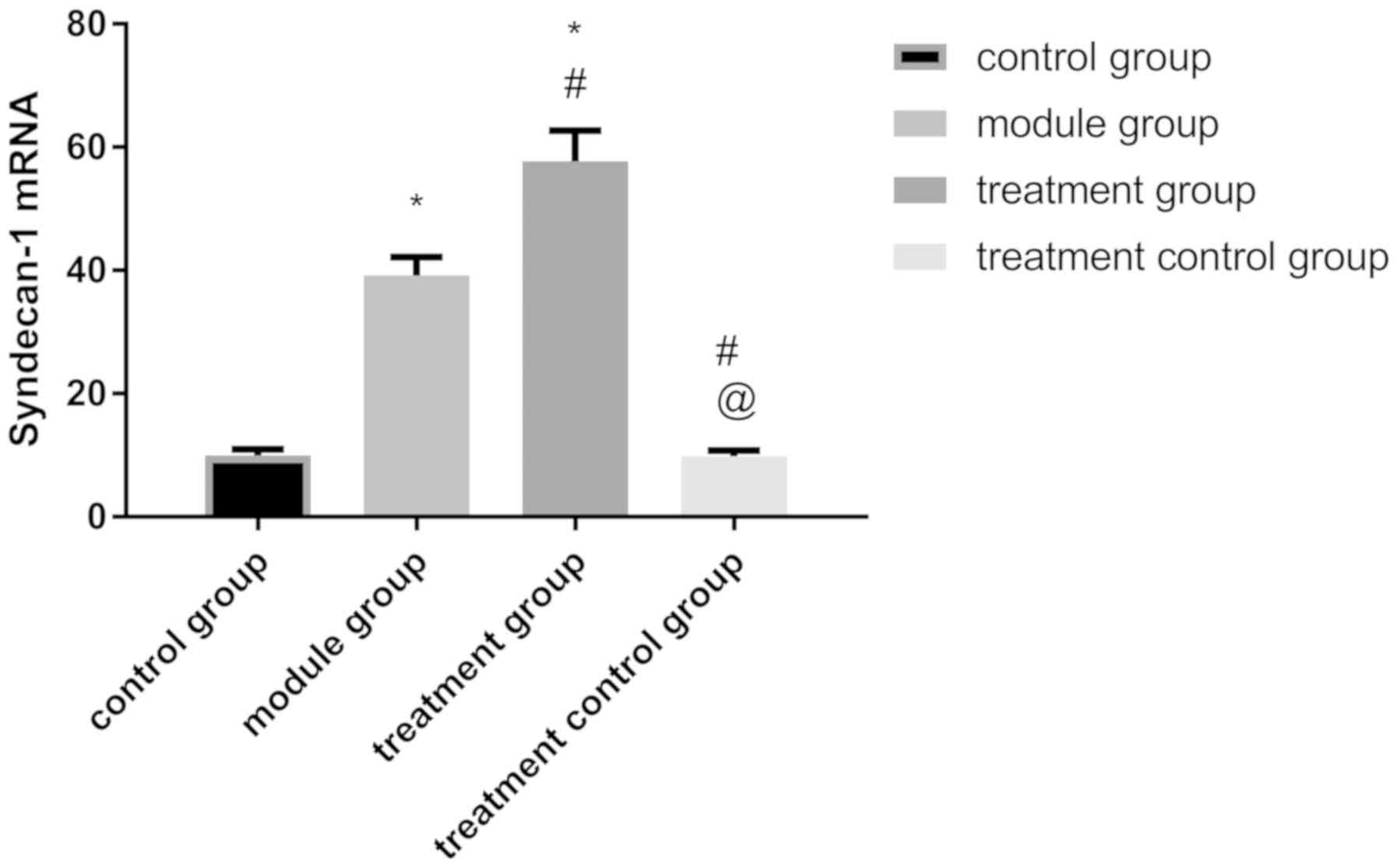
The levels of SDC-1 mRNA in TG were 54.39±5.34 and
it was also the highest among the three groups (P<0.001). The
levels of SDC-1 mRNA in CG was 9.74±1.39, which was not
significantly different from the levels of SDC-1 mRNA in TCG of
9.61±1.87 (P>0.050) and was lower than that in the other two
groups (P>0.001) (Fig. 2).
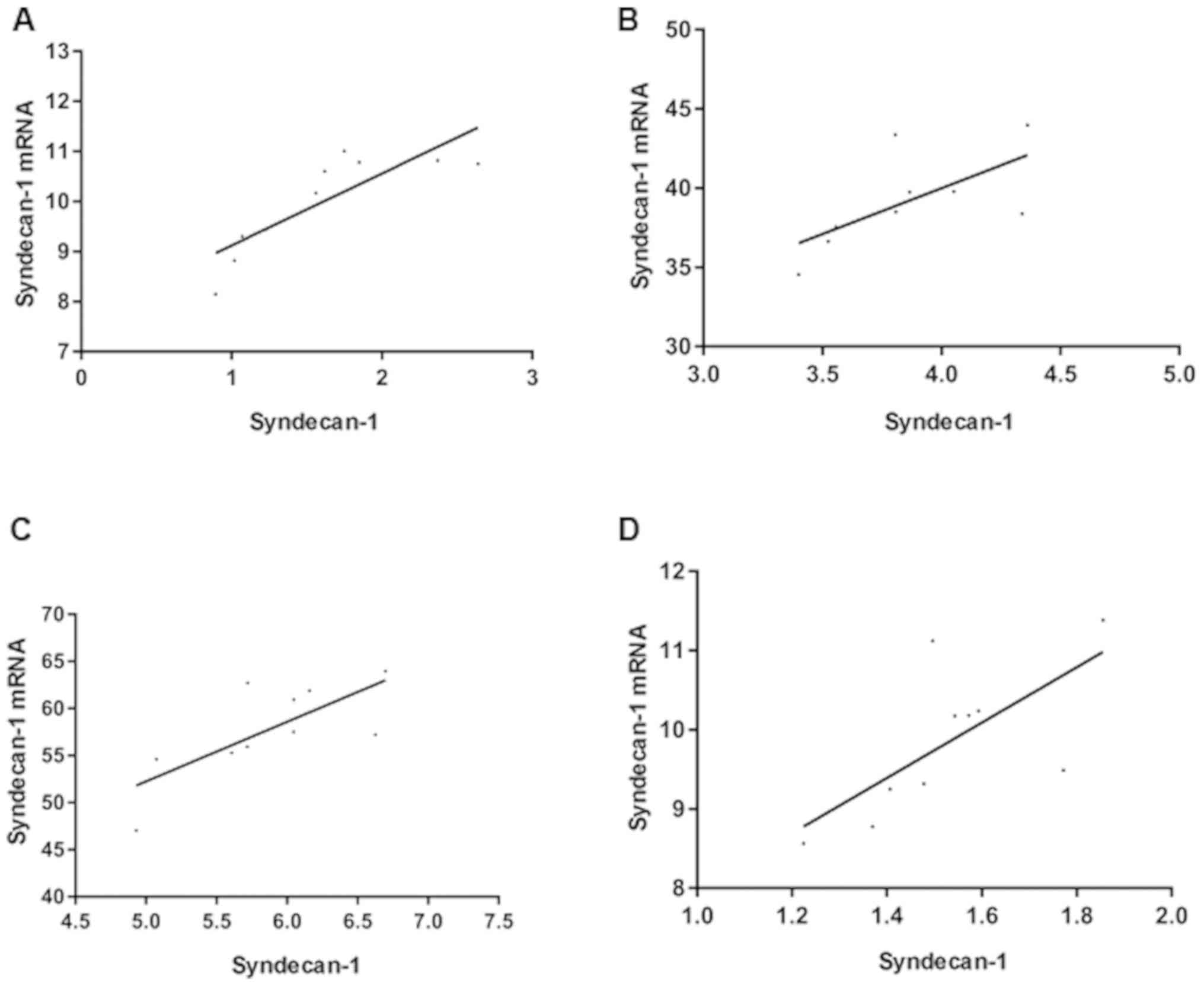
Correlation between SDC-1 protein
expression in tissues and SDC-1 mRNA levels in serum
Through Pearson correlation analysis of the
relationship between SDC-1 protein expression in rat tissues and
SDC-1 mRNA levels in serum of each group, it was found that SDC-1
protein expression in rat tissues was positively correlated with
SDC-1 mRNA levels in serum (Fig.
3).
Changes of renal function indexes of
rats in each group before and after treatment
There was no significant difference in BUN, SCR, UA
and β2-MG contents among the groups before modeling (P>0.05).
Compared with before modeling, there was no significant difference
in the contents of BUN, SCR, UA and β2-MG between CG and TCG after
modeling (P>0.05). Compared with before modeling, the contents
of BUN, SCR, UA and β2-MG in MG and TG increased (P<0.05). After
modeling, there was no significant difference between CG and TCG
(P>0.050), while the contents of BUN, SCR, UA and β2-MG in TG
were significantly lower than those in MG and higher than those in
CG and the TCG (P<0.05) (Table
II).
 | Table IIChanges of renal function indexes
before and after treatment. |
Table II
Changes of renal function indexes
before and after treatment.
| | CG | MG | TG | TCG | F | P-value |
|---|
| Before modeling |
|
BUN
(mmol/l) | 5.32±1.39 | 5.34±1.42 | 5.38±1.37 | 5.33±1.41 | 0.004 | 0.999 |
|
SCR
(µmmol/l) | 85.52±6.34 | 83.46±5.97 | 83.48±6.51 | 83.33±5.94 | 0.002 | 0.999 |
|
UA
(µmol/l) | 128.45±14.37 | 127.25±15.540 | 127.32±12.53 | 128.39±13.59 | 0.024 | 0.995 |
|
β2-MG
(mg/l) | 0.089±0.028 | 0.090±0.027 | 0.089±0.029 | 0.087±0.029 | 0.019 | 0.996 |
| After modeling |
|
BUN
(mmol/l) | 5.32±1.41 |
12.53±2.52a,d |
9.24±1.94a,b,d |
5.38±1.32b,c | 33.600 | <0.001 |
|
SCR
(µmmol/l) | 83.63±6.39 |
139.43±13.45a,d |
114.50±16.35a,b,d |
83.47±5.45b,c | 53.96 | <0.001 |
|
UA
(µmol/l) | 129.73±15.74 |
198.35±31.53a,d |
169.36±29.45a,b,d |
127.46±13.94b,c | 19.470 | <0.001 |
|
β2-MG
(mg/l) | 0.089±0.027 |
0.213±0.059a,d |
0.175±0.045a,b,d |
0.088±0.028b,c | 22.130 | <0.001 |
Discussion
AKI is an extremely painful and costly disease that
affects more than 13 million people each year, 85% of whom live in
developing countries (10). However,
the prevalence of acute kidney injury is also increasing in
developed countries. The incidence rate is estimated to be as high
as 15% in hospital patients and it is more common in severe
patients, with the prevalence rate estimated to be as high as 60%
(11). How to treat acute kidney
injury is currently a major medical problem in the world.
SDC-1 is a member of the transmembrane sulfate acid
heparin proteoglycan family. Through its sulfate acid heparin
chain, SDC-1 covalently binds to a variety of extracellular ligands
(12,13). It mediates cell adhesion to several
extracellular matrix molecules and promotes cell proliferation
(14). Relevant reports have also
proven that down-regulation of SDC-1 induces glomerular endothelial
cell dysfunction by regulating the internalization of VEGFR-2,
suggesting that SDC-1 may be a new target for the treatment of AKI
(15). BUN and Cr are important
indicators of the severity of renal function injury (16-18).
The increase of β2-MG in urine can predict residual renal function
(19), which is currently the most
commonly used renal function detection indicator in clinical and
animal research and can reflect renal state to some extent. In this
study, AKI rats were treated with different treatment methods and
the expression of SDC-1 and renal function levels in rat kidney
tissue were measured to explore the protective effect of GM6001 on
the kidneys.
The results of this experiment showed that compared
with CG, the expression of SDC-1 protein and mRNA in MG were
increased and the levels of renal function were decreased. The
difference was statistically significant, indicating that SDC-1
participates in the development and progression of AKI. According
to the study of de Melo Bezerra Cavalcante et al (20), the expression levels of SDC-1 in AKI
caused by pediatric cardiac surgery were increased, which is
approximately consistent with the results of this study. In studies
of Mosaad et al on adolescent systemic lupus erythematosus
patients (21), it was found that
the serum levels of SDC-1 in patients were higher than that in
healthy control group (CG), which can further support the results
of this study. Our research also showed that the expression of
SDC-1 protein in rat tissues was positively correlated with the
levels of SDC-1 mRNA in serum, which further confirms the
relationship between the two. However, we found that the SDC-1
content in TG was higher than that in MG and the renal function
level in TG is better than that in MG, suggesting that the
application of GM6001 before AKI can inhibit SDC-1 hydrolysis and
has a protective effect on renal function level. However, there was
no significant difference in renal function between TCG and CG,
which also indicated that GM6001 has no negative effect on renal
function of rats and can be used for treatment of AKI. The reason
is that soluble SDC-1 can participate in the repair process of
damaged tissues and promote the regeneration and repair of renal
tubules when AKI occurs in the body (22). After the development of AKI, renal
expression levels of MMP-2 and MMP-9 significantly increased
(23), while matrix
metalloproteinases such as MMP-7(24) belong to hydrolase and participate in
the down-regulation of SDC-1, thus reducing the levels of SDC-1 in
the body and weakening the repair function of renal tissue. GM6001
has been proved to be able to inhibit MMP extensively (25). GM6001 changes the three-dimensional
structure of the enzyme by binding with MMPs polypeptide to inhibit
MMPs activity (26), reduce SDC-1
hydrolysis and to improve the renal protection role of SDC-1.
This study found that GM6001 can reduce SDC-1
hydrolysis by inhibiting MMP expression by establishing the AKI rat
model. However, due to the short experimental period, the long-term
effect of GM6001 on AKI were not observed. In this experiment, PCR
was used for analysis. As the best quantitative detection method
currently recognized in clinical practice, PCR is highly
representative. In order to make the experimental data reliable, we
used tissue testing, which can only be carried out when the rats
were sacrificed. Therefore, no detailed multi-time point data was
possible.
In conclusion, the increasing concentration of SDC-1
indicates that renal dysfunction is gradually aggravated. GM6001
can effectively improve renal function in rats after the treatment
of AKI and is expected to become an effective drug for treating
AKI.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KZ wrote the manuscript. KZ and RL conceived and
designed the study. RL and GX were responsible for the collection
and analysis of the experimental data. GX and HH interpreted the
data and drafted the manuscript. LQ performed PCR, Western blot
analysis and ELISA. KZ and LQ revised the manuscript critically for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Weifang People's Hospital (Weifang, China).
Patient consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no competing
interests.
References
|
1
|
Bellomo R, Kellum JA and Ronco C: Acute
kidney injury. Lancet. 380:756–766. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Vijayan A, Faubel S, Askenazi DJ, Cerda J,
Fissell WH, Heung M, Humphreys BD, Koyner JL, Liu KD, Mour G, et
al: American Society of Nephrology Acute Kidney Injury Advisory
Group: Clinical use of the urine biomarker [TIMP-2]x[IGFBP7] for
acute kidney injury risk assessment. Am J Kidney Dis. 68:19–28.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Szeto CC, Li PK, Johnson DW, Bernardini J,
Dong J, Figueiredo AE, Ito Y, Kazancioglu R, Moraes T, Van Esch S,
et al: ISPD catheter-related infection recommendations: 2017
update. Perit Dial Int. 37:141–154. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Doi K and Rabb H: Impact of acute kidney
injury on distant organ function: Recent findings and potential
therapeutic targets. Kidney Int. 89:555–564. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mennerich D, Vogel A, Klaman I, Dahl E,
Lichtner RB, Rosenthal A, Pohlenz HD, Thierauch KH and Sommer A:
Shift of syndecan-1 expression from epithelial to stromal cells
during progression of solid tumours. Eur J Cancer. 40:1373–1382.
2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lu Z, Song N, Shen B, Xu X, Fang Y, Shi Y,
Ning Y, Hu J, Dai Y, Ding X, et al: Syndecan-1 shedding inhibition
to protect against ischemic acute kidney injury through HGF target
signaling pathway. Transplantation. 102:e331–e344. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Junior GS, Sobral D, Cavalcante MG,
Meneses G, Martins A and Daher E: Novel biomarkers of kidney injury
and endothelial dysfunction among HIV patients. Int J Infect Dis.
73(252)2018.
|
|
8
|
Gill SE, Nadler ST, Li Q, Frevert CW, Park
PW, Chen P and Parks WC: Shedding of syndecan-1/CXCL1 complexes by
matrix metalloproteinase 7 functions as an epithelial checkpoint of
neutrophil activation. Am J Respir Cell Mol Biol. 55:243–251.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang X, Zuo D, Chen Y, Li W, Liu R, He Y,
Ren L, Zhou L, Deng T, Wang X, et al: Shed Syndecan-1 is involved
in chemotherapy resistance via the EGFR pathway in colorectal
cancer. Br J Cancer. 111:1965–1976. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li F, Majd H, Weir MD, Arola DD and Xu HH:
Inhibition of matrix metalloproteinase activity in human dentin via
novel antibacterial monomer. Dent Mater. 31:284–292.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Makris K and Spanou L: Acute kidney
injury: Definition, pathophysiology and clinical phenotypes. Clin
Biochem Rev. 37:85–98. 2016.PubMed/NCBI
|
|
12
|
Brauer R, Ge L, Schlesinger SY, Birkland
TP, Huang Y, Parimon T, Lee V, McKinney BL, McGuire JK, Parks WC,
et al: Syndecan-1 attenuates lung injury during influenza infection
by potentiating c-met signaling to suppress epithelial apoptosis.
Am J Respir Crit Care Med. 194:333–344. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gandley RE, Althouse A, Jeyabalan A,
Bregand-White JM, McGonigal S, Myerski AC, Gallaher M, Powers RW
and Hubel CA: Low soluble syndecan-1 precedes preeclampsia. PLoS
One. 11(e0157608)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Götte M, Kersting C, Ruggiero M, Tio J,
Tulusan AH, Kiesel L and Wülfing P: Predictive value of syndecan-1
expression for the response to neoadjuvant chemotherapy of primary
breast cancer. Anticancer Res. 26B:621–627. 2006.PubMed/NCBI
|
|
15
|
Jing Z, Wei-Jie Y, Yi-Feng ZG and Jing H:
Downregulation of Syndecan-1 induce glomerular endothelial cell
dysfunction through modulating internalization of VEGFR-2. Cell
Signal. 28:826–837. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Schmidt M, Mansfield KE, Bhaskaran K,
Nitsch D, Sørensen HT, Smeeth L and Tomlinson LA: Serum creatinine
elevation after renin-angiotensin system blockade and long term
cardiorenal risks: cohort study. BMJ356. (j791)2017.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Zhang Z, Zhao J, Dong W, Remer E, Li J,
Demirjian S, Zabell J and Campbell SC: Acute kidney injury after
partial nephrectomy: Role of parenchymal mass reduction and
ischemia and impact on subsequent functional recovery. Eur Urol.
69:745–752. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tsuji T, Ohishi K, Takeda A, Goto D, Sato
T, Ohashi N, Fujigaki Y, Kato A and Yasuda H: The impact of serum
uric acid reduction on renal function and blood pressure in chronic
kidney disease patients with hyperuricemia. Clin Exp Nephrol.
22:1300–1308. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wong J, Sridharan S, Berdeprado J, Vilar
E, Viljoen A, Wellsted D and Farrington K: Predicting residual
kidney function in hemodialysis patients using serum β-trace
protein and β2-microglobulin. Kidney Int. 89:1090–1098.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
de Melo Bezerra Cavalcante CT, Castelo
Branco KM, Pinto Júnior VC, Meneses GC, de Oliveira Neves FM, de
Souza NM, Penaforte KL, Martins AM and Libório AB: Syndecan-1
improves severe acute kidney injury prediction after pediatric
cardiac surgery. J Thorac Cardiovasc Surg. 152:178–186.e2.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mosaad NA, Lotfy HM, Farag YM, Mahfouz RH
and Shahin RM: Study of serum syndecan-1 levels in a group of
Egyptian juvenile systemic lupus erythematosus patients. Immunol
Lett. 181:16–19. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kato M, Wang H, Kainulainen V, Fitzgerald
ML, Ledbetter S, Ornitz DM and Bernfield M: Physiological
degradation converts the soluble syndecan-1 ectodomain from an
inhibitor to a potent activator of FGF-2. Nat Med. 4:691–697.
1998.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Xiong C, Zang X, Zhou X, Liu L, Masucci
MV, Tang J, Li X, Liu N, Bayliss G, Zhao TC, et al: Pharmacological
inhibition of Src kinase protects against acute kidney injury in a
murine model of renal ischemia/reperfusion. Oncotarget.
8:31238–31253. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zeng Y, Yao X, Chen L, Yan Z, Liu J, Zhang
Y, Feng T, Wu J and Liu X: Sphingosine-1-phosphate induced
epithelial-mesenchymal transition of hepatocellular carcinoma via
an MMP-7/ syndecan-1/TGF-β autocrine loop. Oncotarget.
7:63324–63337. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dolmatov IY, Shulga AP, Ginanova TT,
Eliseikina MG and Lamash NE: Metalloproteinase inhibitor GM6001
delays regeneration in holothurians. Tissue Cell. 59:1–9.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liu X and Han Q: Efficacy of GM6001 as an
adjuvant to ceftriaxone in a neonatal rat model of Streptococcus
pneumoniae meningitis. Acta Neurobiol Exp (Wars). 74:489–496.
2014.PubMed/NCBI
|