Introduction
Anterior communicating artery (AComA) aneurysm is
the most common type of anterior circulation aneurysm (1-3). It
is symptomatic when rupture occurs, resulting in subarachnoid
hemorrhage (SAH), which mostly accumulates in the suprasellar
cistern, cerebral longitudinal fissure or cisterna lamia terminal
is; at times, it may be associated with hematoma, particularly
under the frontal lobe or in lateral ventricles (4).
In the field of neurosurgery, the minimally invasive
keyhole approach has been gradually developed in the past 30 years.
In the surgical treatment of anterior circulation aneurysms, with
the deepening of the understanding of local microanatomy,
continuous improvement of microsurgical techniques and the
improvement of microsurgical instruments, the popularity of the
keyhole approach for clipping aneurysms is increasing. Certain
better alternatives to the pterional approach, including the
supraorbital eyebrow approach (5),
mini-pterional approach (6),
mini-supraorbital approach (7) and
lateral supraorbital approach (8)
are also gradually used in anterior circulation aneurysm surgery.
Skeptics of the supraorbital keyhole approach (SOEK) argue that it
is difficult to approach ruptured aneurysms due to brain swelling
(9-11).
Furthermore, if an unexpected rupture occurs during the operation
prior to proximal control of the feeding artery, it may not be
possible for the surgeon to properly manage the bleeding. For this
reason, SOEK surgery is mainly performed in patients with
unruptured intracranial aneurysms and studies reporting on aneurysm
rupture during SOEK are rare. Therefore, the feasibility of
applying this type of keyhole surgery for ruptured intracranial
aneurysm has not been determined.
The present study reported on the routine use of
SOEK in the treatment of ruptured AComA aneurysms.
Materials and methods
Study design, setting and
participants
The present retrospective study was approved by the
Institutional Review Board and ethics committee of the Third
Affiliated Hospital of Sun Yat-Sen University (Guangzhou, China).
Written informed consent was obtained from all patients or their
custodians. Between September 2010 and October 2018, 543 patients
with intracranial aneurysms were admitted to the Department of
Neurosurgery of the Third Affiliated Hospital of Sun Yat-Sen
University (Guangzhou, China). According to the patients' condition
and choice, microsurgical clipping or interventional embolization
were selected for treatment. Furthermore, if patients exhibited
reduced consciousness then permission was obtained from their
guardians. Among them, 85 patients with ruptured AComA aneurysm
underwent microsurgical clipping via the SOEK approach. All 85
patients were diagnosed as having ruptured AComA aneurysm with SAH
within 48 h after onset by CT angiography (CTA; 320-slice spiral
CT, Aquilion ONE; Toshiba Medical Systems).
Variables and data sources
The clinical data collected included age, sex, Hunt
and Hess scores (12), World
Federation of Neurological surgeons (WNSF) scores (13), Fisher scores (14), aneurysm size, aneurysm location,
aneurysm direction, operation time and complications.
Intra-operative indocyanine green angiography (ICGA) was used to
evaluate the success rate of clipping. The operation time was
calculated from induction of anesthesia to completion of skin
closure. Ischemic complications were confirmed by including new
neurological deficits or changes in consciousness. Sudden
neurological impairment during the peri-operative period,
indicative of new infarction, was confirmed by CT scan/MRI.
Surgical techniques Selection of
surgical approach
Initially, the SOEK approach was selected regardless
of the size, characteristics and complexity of the AComA aneurysm.
After several years' experience of using the SOEK approach at our
department, most ruptured AComA aneurysms were clipped via the SOEK
approach except for cases associated with severe brain swelling and
with a large amount of intracerebral hemorrhage (ICH). In those
cases associated with severe brain swelling and with an ICH of
>100 cc, where decompression and evacuation of hemorrhage were
required, the pterional approach was selected.
Operative timing and methods
All subjects underwent surgery within 48 h of the
first SAH to reduce the risk of rebleeding. Of the 85 subjects, 21
underwent extraventricular drainage (EVD) prior to surgery. In
general, the right surgical approach was used. In order to secure
the parent artery in advance, the A1 dominant blood supply side was
selected in the surgical approach when AComA complex variation was
present.
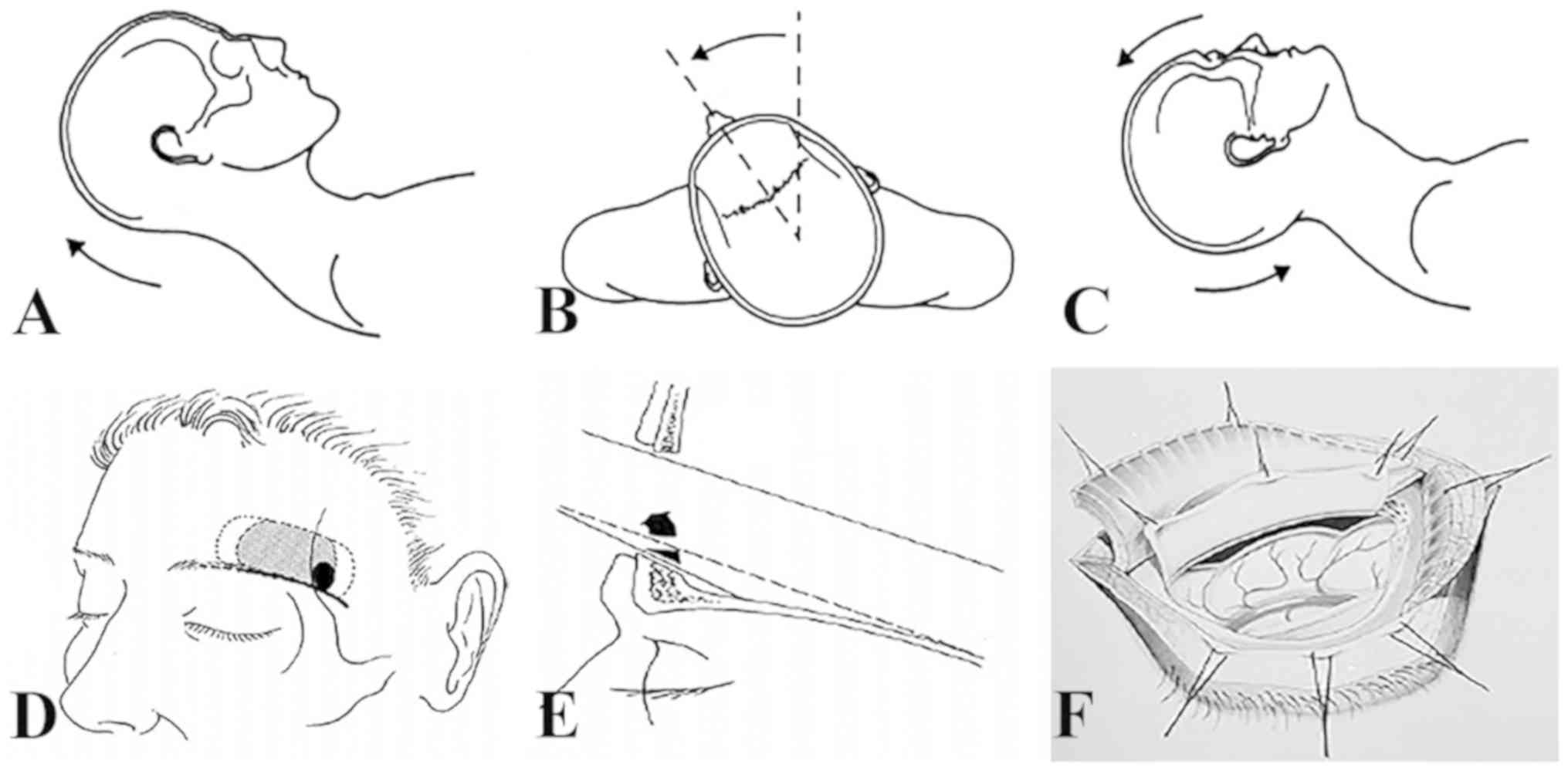
Posture and anesthesia
All operations were performed under general
anesthesia with tracheal intubation. The patient was placed in the
supine position; the head was elevated to 15 degrees, then rotated
~30 degrees to the contralateral side, and finally tilted back to
~20 degrees. This caused the frontal lobe to retract downward due
to gravity, thereby reducing brain retraction (Fig. 1).
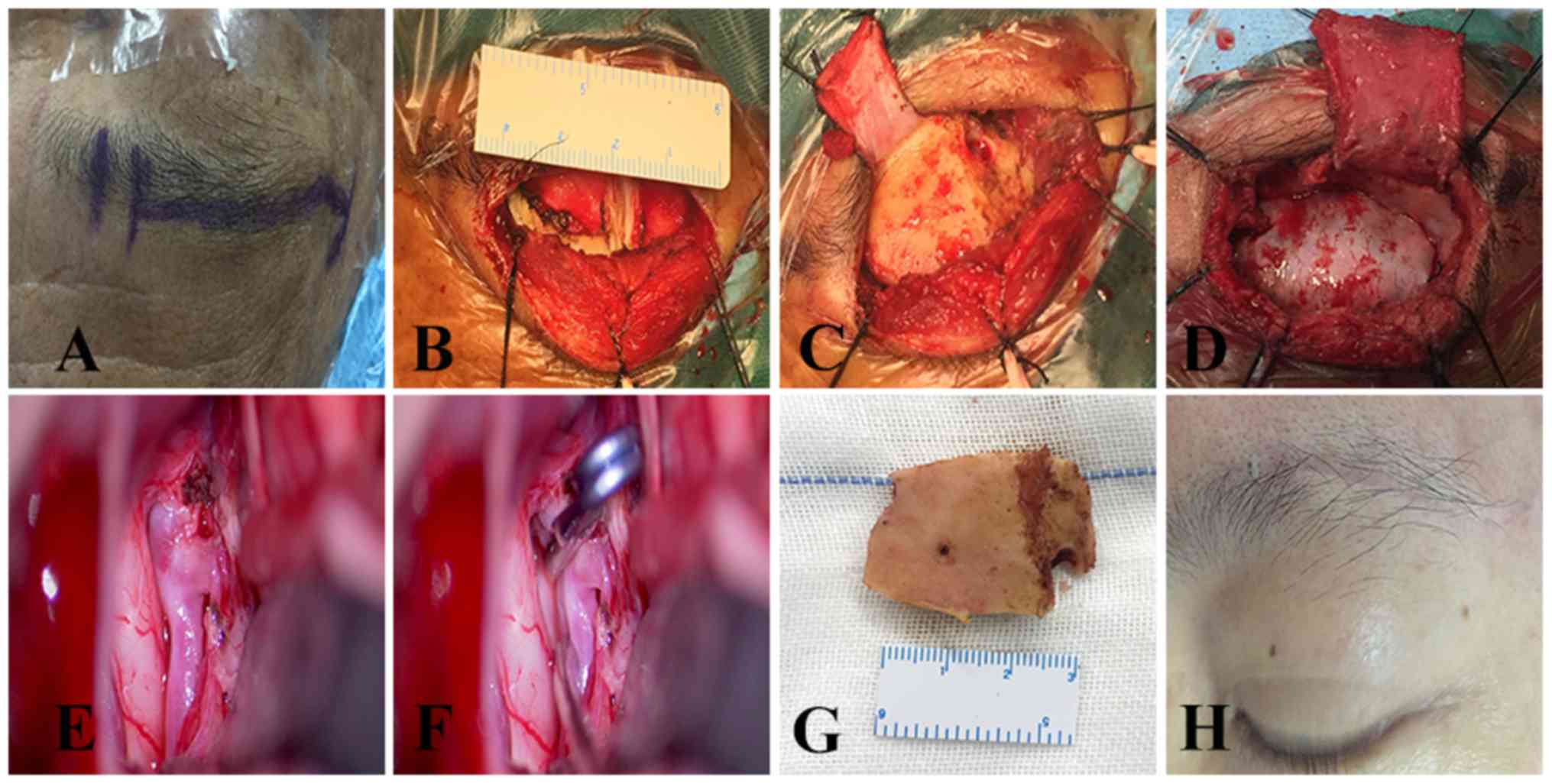
Skin incision
Skin and subcutaneous fascia were incised along the
lateral supraorbital foramen (to avoid injury to supraorbital nerve
and blood vessel) along the superior orbital margin of the eyebrow,
4-5 cm in length. The upper forehead skin flap was pulled using a
rubber band and the temporal muscle was pulled outward to expose
the zygomatic process of the frontal bone. Similarly, the inferior,
frontal and orbicularis oculi muscles were pushed down to the
orbital margin and fixed with silk thread. The pericranium remained
intact above the orbital margin. The fascial opening was opened
downward to avoid the injury of the frontal branch of the facial
nerve. The frontal is and orbital muscles were cut in a parallel
fashion. The frontal is and orbicularis muscles were peeled from
the bony insertion and gently pushed.
Craniotomy
A Burr hole was made behind the zygomatic process of
the frontal bone, and the frontal bone was opened along the
supraorbital margin from the outside to the inside. Next, a 3x2.5
cm semilunar bone flap was fabricated (Fig. 2). The superior orbital margin and
anterior skull base processes were drilled. If the frontal sinus
was ruptured, the mucosa was removed, thoroughly cleaned with
iodine solution and sealed with bone wax. Subsequently, the dura
was opened in semilunar shape with its base facing towards the
skull base. From this stage of the operation onwards, the
microscope (Leica M530 OH6; Leica Microsystems GmbH) was used.
Intradural dissection
Upon opening of the lateral fissure, carotid
cistern, optic chiasmatic cistern, basal cistern and endplate
cistern, the cerebrospinal fluid (CSF) was fully released. At
times, the subarachnoid space was filled with blood clots rather
than CSF. In this case, it was difficult to release sufficient CSF
to relax the brain tissue. At this time, CSF was drained in advance
by inserting an EVD tube in the anterior horn of the contralateral
ventricle. After opening the dura mater, slow release further
provided CSF drainage to fully relax the brain tissue if required.
It was possible to fully observe the end plate behind the carotid
cistern and optic chiasma by placing an automatic retractor at the
bottom of the frontal lobe and gradually exposing the frontal lobe
to the optic nerve. At times, gyrus rectus resection may also help
to fully expose the AComA complex. Dissection began from the
internal carotid artery-A1 on the operative side; the distal end of
the aneurysm-bearing artery A1 was dissected and the contralateral
A1 was dissected. Subsequently, the adjacent A1, A2, AComA and the
recurrent artery were gradually approached and separated from the
aneurysmal neck. If necessary, temporary clipping of the
ipsilateral and/or contralateral A1was performed. The decision on
whether or not to dissect the aneurysm may be made under
consideration of the projection, size and adhesion of the aneurysm.
Aneurysm clips of appropriate shape and size were selected and
accurate clipping of the aneurysm was implemented. While clipping
the aneurysm pointing downwards and forward through the SOEK
approach, the exposure of the aneurysmal neck is obscured from the
operative field, making the top of aneurysm dome more likely to
rupture. In the case of adhesion between the dome of the aneurysm
and the skull base caused by bleeding, inappropriate pulling of the
frontal lobe may easily lead to rupture of the aneurysm during the
operation. To avoid intra-operative rupture, the following points
may be considered: i) Surgical approach from the side of the
dominant blood supply, which is more conducive to controlling the
aneurysm-bearing artery; ii) when exposure of the aneurysm neck is
difficult, CSF should be released to obtain adequate space for
clipping the AComA aneurysm. In the case of intra-operative
rupture, the A1 segment may be temporarily occluded and the
aneurysm neck may be rapidly separated followed by clipping of the
aneurysm with an appropriate clip.
Closure
Prior to the closure of the dura, papaverine saline
was used to fill the subdural space. Following careful hemostasis,
the dura was sutured with silk thread in a waterproof manner. The
frontal sinus, if open, was exteriorized, cleaned with iodine
solution and sealed with bone wax. Bone flaps were fixed with a
titanium plate and screws. The muscle was sutured in layers without
a drainage catheter and the subcutaneous suture was made using
prolene 4-0. All patients underwent surgery via the SOEK approach.
None of these surgeries was assisted by endoscopy. All of the 85
patients underwent CTA examination the day after the operation.
Post-operative management
Conventional prophylactic intravenous drip of
nimodipine was administered to prevent cerebral vasospasm. If
necessary, adjuvants including dexamethasone and osmotic diuretics
were used to control brain swelling. Routine lumbar puncture was
performed to replace blood-infused cerebrospinal fluid. EVD was
performed in patients with hydrocephalus. All patients were
monitored at the post-operative intensive care unit.
Follow-up
All patients were followed up by neurosurgeons.
Outpatient follow-up was the major method. Patients who were not
able to visit the outpatient clinic were contacted by telephone to
assess their neurological function. Clinical outcomes at 3, 6 and
12 months post-operatively were assessed using a modified Rankin
scale (mRS). A favorable outcome was defined as a mRS score of ≤2.
Furthermore, the risk factors of adverse prognosis (mRS >3)
after 1 year were analyzed and the conventional CTA/digital
subtraction angiography (DSA) imaging evaluation was performed at 3
months after the operation.
Statistical analysis
Continuous variables are expressed as the mean ±
standard deviation and categorical variables are expressed as n
(%). Differences were analyzed using Student's t-test for
continuous variables and Fisher's exact test or χ2 test
for categorical parameters. In the univariate logistic regression
analysis [analyzed using SPSS 21.0 software (IBM Corp.)],
statistical variables were determined to identify risk factors for
poor clinical grade, including the odds ratio (ORs) and 95% CI.
P<0.05 was considered to indicate a statistically significant
difference. SPSS software (version 21.0; IBM Corp.) was used for
statistical analysis.
Results
General information
In the present study, 85 patients with ruptured
AComA aneurysms were treated using the SOEK approach. The cohort
comprised were 50 males and 35 females. The age of the patients
ranged from 28 to 78 years (mean age, 52.69±9.94 years; Table I).
 | Table ICharacteristics of patients with
AComA aneurysm (n=85). |
Table I
Characteristics of patients with
AComA aneurysm (n=85).
|
Characteristics | Value |
|---|
| Sex
(male/female) | 50/35 |
| Age (years) | 52.69±9.94
(28-78) |
| AComA complex
variation |
|
Right
dominant blood supply | 16 |
|
Left
dominant blood supply | 36 |
|
No marked
variation | 33 |
| Associated ICH
grade |
|
I (no
ICH) | 63 |
|
II (<50
cc) | 14 |
|
III (50-100
cc) | 8 |
| Pre-operative Hunt
and Hess grade |
|
1 | 17 (20.0) |
|
2 | 32 (37.6) |
|
3 | 25 (29.4) |
|
4 | 9 (10.6) |
|
5 | 2 (2.4) |
| World Federation of
Neurosurgical Societies grade |
|
I | 28 (32.9) |
|
II | 20 (23.5) |
|
III | 12 (14.1) |
|
IV | 23 (27.1) |
|
V | 2 (2.4) |
| Pre-operative
Fisher grade on CT |
|
1 | 0 (0.0) |
|
2 | 42 (49.4) |
|
3 | 13 (15.3) |
|
4 | 30 (35.3) |
| Aneurysm size
(mm) |
|
Small
(<7) | 71 (83.5) |
|
Medium
(7-12) | 13 (15.3) |
|
Large
(>12-<25) | 1 (1.2) |
| Aneurysmal
directionality |
|
Superior | 28 |
|
Inferior | 14 |
|
Anterior | 16 |
|
Posterior | 3 |
|
Complex | 24 |
Pre-operative evaluation
According to Fisher's grade of head CT prior to the
operation, the cohort comprised 0 cases of grade I, 42 cases of
grade II, 13 cases of grade III and 30 cases of grade IV.
Furthermore, 17 patients had a Hunt and Hess grade of 1, 32
patients were grade 2, 25 patients were grade 3, 9 patients were
grade 4 and 2 patients were grade 5. A total of 28 patients had a
WFNS score I, 20 were WFNS score II, 12 were WFNS score III, 23
were WFNS score IV and 2 were WFNS score V. Table I summarizes the demographic
characteristics of the 85 patients treated via the SOEK
approach.
Aneurysmal number and size
All 85 patients were diagnosed with AComA aneurysm
by CTA and 320-slice CT (Aquillion One; Toshiba). A total of 85
aneurysms were detected in the 85 patients with an average diameter
of 5.07±2.36 mm (range, 2-11 mm). Among them, the diameter of the
small aneurysm (<7 mm), medium aneurysm (7-12 mm) and large
aneurysm (>12 mm) were 83.5, 15.3 and 1.2%, respectively
(Table I).
Aneurysmal directionality
According to the Yasargil typing method (15), the aneurysm pointed superiorly in 28
patients, inferiorly in 14 patients, anteriorly in 16 patients,
posteriorly in 3 patients and toward multiple directions in 24
patients (Table I).
Association between aneurysmal
directionality and pre-operative ICH
Pre-operative ICH was more prevalent in aneurysms
pointing anteriorly than in the other aneurysms (P<0.014; data
not shown).
Association between Fisher grade and
blood loss
Severe blood loss was more frequent in patients with
poor Fisher grade (Grade III/IV; P<0.005).
AComA complex variation
Right-side dominant blood supply with congenital
absence or hypoplasia of the left A1 was present in 16 patients,
whereas the left-side dominant blood supply with congenital absence
or hypoplasia of right A1 was present in 36 patients. Furthermore,
33 patients had no marked variation.
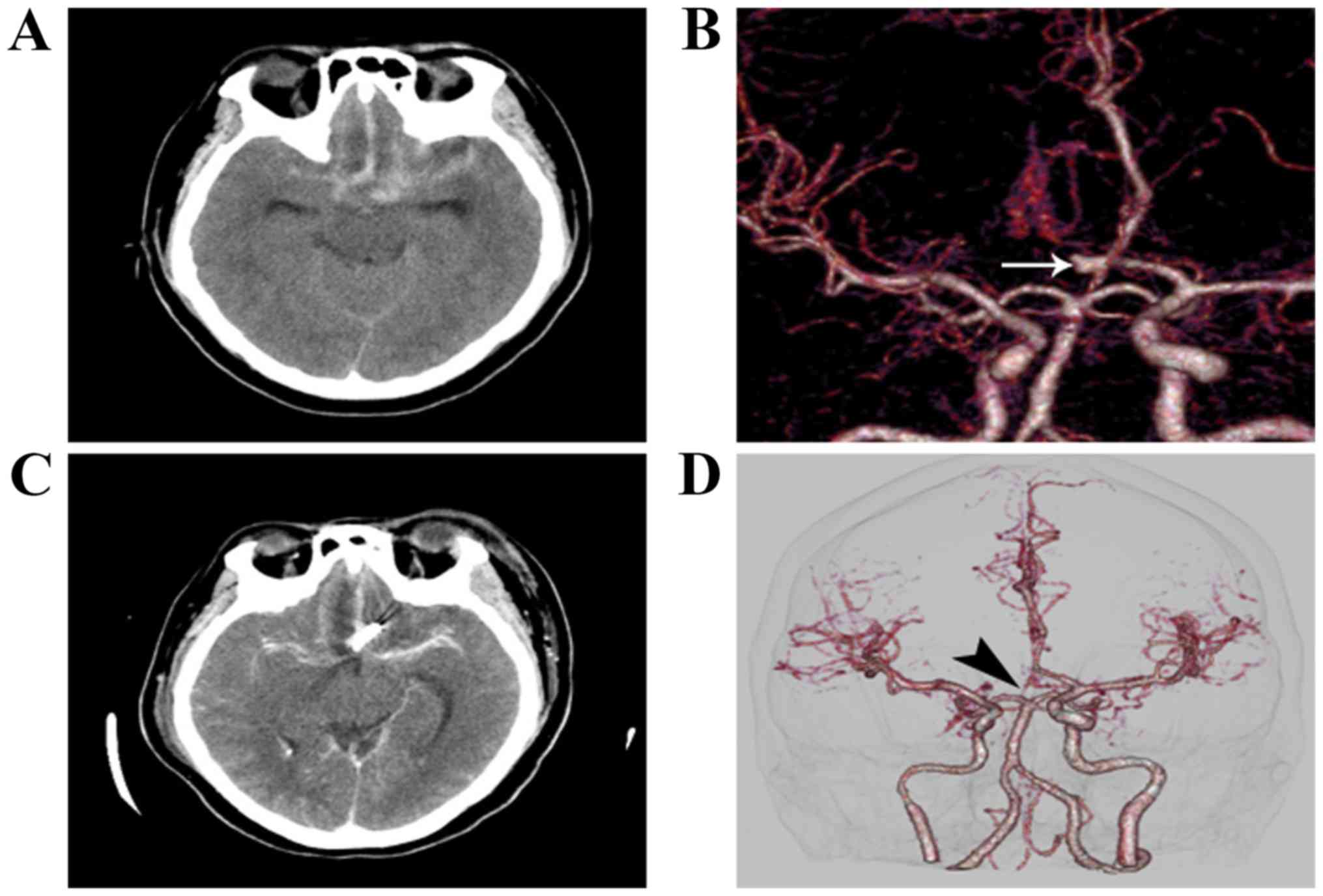
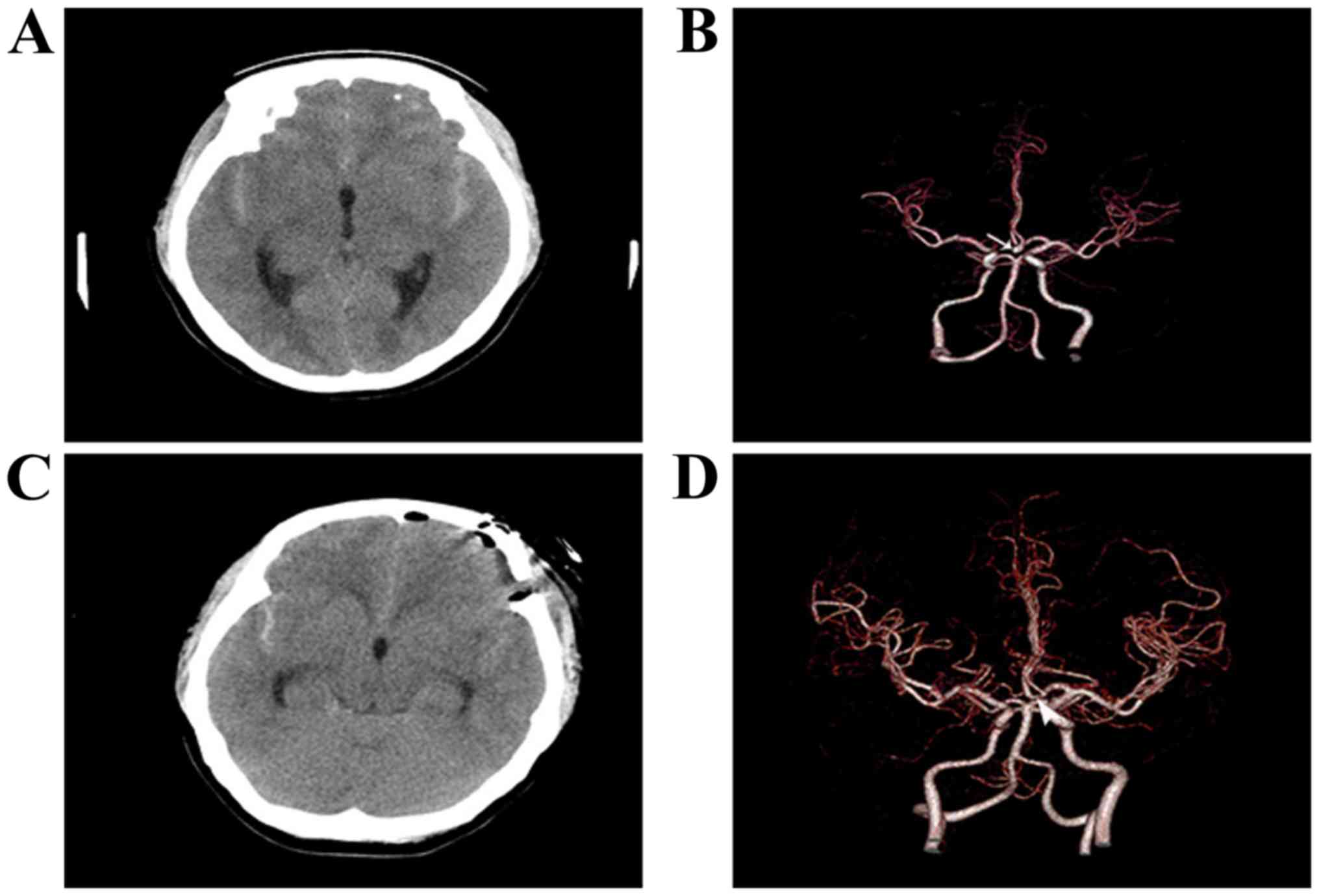
Surgical results
None of the patients undergoing surgery via the SOEK
approach required conversion to a pterional microsurgical approach
(Figs. 3 and 4). No aneurysm recurred after the
operation. The average operation time (from induction of anesthesia
to completion of skin closure) was 268.2±82.8 min (range, 60-360
min). Pre-operative hydrocephalus was identified in 23 cases
(27.1%) and intracerebral hematoma in 31 cases (36.5%).
Pre-operative EVD was observed in 21 (24.7%) cases. The incidence
of frontal sinus opening occurred in 2 cases (2.4%). However,
despite the opening of the frontal sinus, no cerebrospinal fluid
leakage occurred after proper treatment. Temporary clipping was
performed in 25 patients (29.4%). A total of 5 patients underwent
partial excision of the gyrus rectus. Intra-operative rupture of
the aneurysm during the operation occurred in 3 cases. The
aneurysm-bearing artery was temporarily blocked and the aneurysm
was then separated, followed by adequate clipping of the aneurysm
neck. Delayed vasospasm occurred in 42 cases (49.4%). A
post-operative ventriculoperitoneal (VP) shunt was placed in 9
cases (10.6%). No frontalis muscle palsy occurred in any of the
patients.
Risk factors for early post-operative
poor prognosis
A total of 85 patients were included in the
univariate logistic regression analysis to investigate the risk
factors associated with adverse clinical outcomes (mRS 3-6;
Table II). Risk factors that were
significant predicators of poor prognosis (mRS 3-6) included the
Hunt & Hess score, Fisher grade, WFNS score, ICH, pre-operative
EVD, pre-operative hydrocephalus and VP shunt (Table III). These factors were determined
using the χ2 test or Fisher's exact test (P=0.020,
P=0.003, P=0.004, P=0.001, P=0.005, P=0.002 and P=0.006). Other
risk factors, including age (P=0.387), sex (P=0.330), aneurysm size
(P=0.330), Anesthesia-to-skin time (P=0.065), vasospasm (P=0.889),
gyrus rectus resection (P=0.436) and temporary clipping (P=0.777)
during operation, had no significant influence on the prognosis
(Table II).
 | Table IICharacteristics of patients with
poor-grade outcomes (mRS score, 3-6). |
Table II
Characteristics of patients with
poor-grade outcomes (mRS score, 3-6).
|
Characteristics | Total (n=85) | mRS score |
P-valuea |
|---|
| Good grade
(n=69) | Poor grade
(n=16) |
|---|
| Age (years) | | | | 0.387 |
| Mean ± SD | 53±11.38 | 52.51±9.13 | 53.5±13.2 | |
| Median (range) | 50 (29-78) | 52 (29-78) | 51.5 (28-76) | |
| Sex | | | | 0.330 |
|
Male | 50 (58.8) | 38 (55.1) | 12 (75.0) | |
|
Female | 35 (41.2) | 31 (44.9) | 4 (25.0) | |
| Hunt and Hess
grade | | | | 0.020 |
|
1-3 | 74 (87.1) | 66 (95.7) | 8(50) | |
|
4/5 | 11 (12.9) | 3 (4.3) | 8(50) | |
| Fisher grade | | | | 0.003 |
|
1/2 | 42 (49.4) | 40 (58.0) | 2 (12.5) | |
|
3/4 | 43 (50.6) | 29 (42.0) | 14 (87.5) | |
| WFNS scale | | | | 0.004 |
|
I-III | 60 (70.6) | 54 (78.3) | 6 (37.5) | |
|
IV/V | 25 (29.4) | 15 (21.7) | 10 (62.5) | |
| Aneurysm size
(mm) | | | | 0.169 |
|
Small
(<7) | 71 (83.5) | 60 (87.0) | 11 (68.8) | |
|
Medium
(7-12) | 13 (15.3) | 8 (11.6) | 5 (31.3) | |
|
Large
(>12-<25) | 1 (1.2) | 1 (1.4) | 0 (0.0) | |
| Anesthesia-to-skin
time (h) | | | | 0.065 |
|
Mean ±
SD | 4.5±1.38 | 4.35±1.17 | 5.13±1.97 | |
|
Median
(range) | 4.2 (1-8.75) | 4.1 (1-7.0) | 5.07 (2-8.75) | |
| Pre-operative
EVD | | | | 0.005 |
|
No | 64 (75.3) | 57 (82.6) | 7 (43.8) | |
|
Yes | 21 (24.7) | 12 (17.4) | 9 (56.3) | |
| Vasospasm | | | | 0.089 |
|
No | 39 (45.9) | 37 (53.6) | 2 (12.5) | |
|
Yes | 46 (54.1) | 32 (46.4) | 14 (87.5) | |
| Hydrocephalus | | | | 0.002 |
|
No | 62 (72.9) | 57 (82.6) | 5 (31.3) | |
|
Yes | 23 (27.1) | 12 (17.4) | 11 (68.8) | |
| ICH | | | | 0.001 |
|
No | 63 (74.1) | 57 (82.6) | 6 (37.5) | |
|
Yes | 22 (25.9) | 12 (17.4) | 10 (62.5) | |
| Gyrus rectus
resection | | | | 0.436 |
|
No | 80 (94.1) | 66 (95.7) | 14 (87.5) | |
|
Yes | 5 (5.9) | 3 (4.3) | 2 (12.5) | |
| Temporary
clipping | | | | 0.777 |
|
No | 60 (70.6) | 51 (73.9) | 9 (56.3) | |
|
Yes | 25 (29.4) | 18 (26.1) | 7 (43.8) | |
| VP shunt | | | | 0.006 |
|
No | 76 (89.4) | 64 (92.8) | 12 (75.0) | |
|
Yes | 9 (10.6) | 5 (7.2) | 4 (25.0) | |
| Frontal sinus
opening | | | | 0.204 |
|
No | 83 (97.6) | 68 (98.6) | 15 (93.8) | |
|
Yes | 2 (2.4) | 1 (1.4) | 1 (6.3) | |
 | Table IIIUnivariate analysis of risk factors
associated with poor-grade outcomes (modified Rankin scale score,
3-6). |
Table III
Univariate analysis of risk factors
associated with poor-grade outcomes (modified Rankin scale score,
3-6).
| Item | OR (95% CI) | P-value |
|---|
| Hunt and Hess grade
(4/5 vs. 1-3) | 22.0
(4.80-100.20) | 0.001 |
| Fisher grade (3/4
vs. 1/2) | 10.88
(2.29-51.74) | 0.003 |
| WFNS scale (4/5 vs.
1-3) | 6.0
(1.88-19.19) | 0.003 |
| ICH (yes vs.
no) | 7.9
(2.41-25.97) | 0.001 |
| Pre-operative EVD
(yes vs. no) | 6.1
(1.90-19.62) | 0.002 |
| Hydrocephalus (yes
vs. no) | 10.45
(3.06-35.63) | 0.001 |
| VP shunt (yes vs.
no) | 4.26
(0.90-18.22) | 0.050 |
Risk factors for poor long-term
prognosis after surgery
Regarding poor-grade outcomes (mRS 3-6) at 12 months
after surgery, univariate analysis suggested that the Hunt &
Hessgrade (OR=22.0, 95% CI: 4.8-100.2, P=0.001), Fisher grade
(OR=10.88, 95% CI: 2.292-51.74, P=0.003), WFNS score (OR=6.0, 95%
CI: 1.88-19.19, P=0.003), ICH (OR=7.9, 95% CI: 2.41-25.97,
P=0.001), pre-operative EVD (OR=6.1, 95% CI: 1.90-19.62, P=0.002),
pre-operative hydrocephalus (OR=10.45, 95% CI: 3.06-35.63, P=0.001)
and post-operative VP shunt (OR=4.26, 95% CI: 0.90-18.22, P=0.050;
Table III) were identified as
influencing factors with statistical significance, but there was no
statistical significance for operation time, vasospasm, gyrus
rectus resection and temporary clipping (results not shown).
Occlusion rate of aneurysms and
mortality
A complete occlusion rate of 98.8% (84/85) was
achieved, as determined by intra-operative ICGA and follow-up
imaging (CTA or DSA performed on the day after surgery for all
cases). Aneurysm wrapping was performed in one case. Two patients
with pre-operative Hunt & Hess grade V died. At one year after
treatment, 69 (81.2%) patients attained a favorable outcome (mRS
≤2; Table IV). Furthermore, 95.23%
of the 42 patients with a Fisher grade of 2 had a favorable outcome
(Table II).
 | Table IVmRS scores of patients at 12 months
post-operatively. |
Table IV
mRS scores of patients at 12 months
post-operatively.
| mRS score | Patients, n
(%) |
|---|
| 0-No symptoms | 11 (12.9) |
| 1-No significant
disability | 24 (28.2) |
| 2-Slight
disability | 34 (40.0) |
| 3-Moderate
disability | 3 (3.5) |
| 4-Moderately severe
disability | 3 (3.5) |
| 5-Severe
disability | 8 (2.4) |
| 6-Death (combined
systemic disease) | 2 (2.4) |
| Total/Loss to
follow-up | 83/2 |
Surgery-associated complications
A total of 9 patients (10.5%) had surgery-associated
complications. Procedural complications included 7
intracranial/wound infections (8.23%) and 17 ischemic events. A
total of 7 patients recovered completely within 6 months (11.8%;
Table V). Early or late re-bleeding
was not observed in any of the patients.
 | Table VAnalysis of outcomes. |
Table V
Analysis of outcomes.
| Outcome | Value |
|---|
| Pre-operative
hydrocephalus | 23 (27.0) |
| Pre-operative
EVD | 21 (24.7) |
| Operating time
(mean minutes) | 268.2 (60-360) |
| Intra-operative
blood loss (mean ml) | 165.26
(50-600) |
| Frontal sinus
opening | 2 (2.4) |
| Temporary
clipping | 25 (29.4) |
| Gyrus rectus
resection | 5 (5.9) |
| Post-operative
EVD | 9(10.6) |
| Post-operative VP
shunt | 9 (10.6) |
| Intraoperative
aneurysm rerupture | 3 (3.5) |
| Vasospasm
(delayed) | 44 (51.7) |
| Procedural
complications | |
|
Intracerebral
hematoma | 7 (8.2) |
|
Ischemic
events | 17 (20.0) |
|
Intracranial
infection | 4 (4.7) |
|
Intracranial/wound
infection | 3 (3.5) |
|
Duration of
hospitalization (days) | 37.9±24.5 |
|
Mean
follow-up (months) | 28.4 (2-113) |
|
Favorable
outcome at follow-up | 81.2 |
Post-operative follow-up
A total of 83 patients (n=85-2 cases of mortality)
were followed up for 6-88 months, with a mean follow-up time of
37.9±24.5 months (Table V). There
were no cases of wound infection, recurrent bleeding or
neurological deterioration (data not shown).
Discussion
In recent years, endovascular therapy has increased
in popularity among cerebrovascular surgeons. Therefore, in the
surgical treatment of aneurysms, microclipping technology has
gradually been replaced by endovascular therapy (16-20).
During a 10-year follow-up, both the residual and
recurrence rates in the International Subarachnoid Aneurysm Trial
(ISAT) (21) (34%) and Barrow
Ruptured Aneurysm Trial (BRAT) (22)
(52%) increased when compared with microsurgical clipping. In these
two trials, ~17% of patients required retreatment. As reported by
Campi et al (21), the
frequency of retreatment at the late stage of embolization
increased nearly seven times as compared with the data of ISAT
patients. Even with the use of novel equipment and technology,
similar results have been obtained. For instance, Shapiro et
al (23) reviewed 39 studies on
the use of stent-assisted coils including 1,517 patients and
indicated that the first complete occlusion rate of aneurysms was
only 45%. A recent study suggested that complete occlusion was
achieved in only 56.1% of patients with wide-necked bifurcation
aneurysms (24).
In addition to the recurrence rate, complications
are also an important issue. In the ISAT, the rebleeding rate
following interventional embolization of aneurysms was 2.5 times
higher than that of microsurgical clipping (21). Moore et al (25) recently reported that the re-rupture
rate of ruptured aneurysms after embolization was 7.7% and the
residual or recurrence rate was 82%. However, in the present study,
no rebleeding was encountered.
The status of microsurgical clamping in the
treatment of aneurysm has been gradually improved, particularly in
the treatment of recurrent aneurysm after embolism. Daou et
al (26) reported on 111 cases
of recurrent aneurysm after embolism was surgically treated. AComA
aneurysm accounted for 49.5%. After clipping again, 97.3% of the
aneurysms were completely occluded and 90% of the patients had good
results at the final follow-up. While there is an absence of clear
and complete treatment guidelines, microsurgical clipping appears
to be a better choice for the treatment of recurrent aneurysms
after embolization compared with coil embolization.
The major purpose of the keyhole approach is to
minimize the trauma of craniotomy and obtain the best intracranial
exposure without affecting the safety and effectiveness of surgical
treatment. According to the experience of numerous neurosurgeons,
the supraorbital keyhole approach has the following advantages: i)
Small skin incision on the eyebrow ensures a good cosmetic effect;
ii) reduction of brain tissue exposure; iii) short operation time
and reduced use of general anesthetics; iv) rapid recovery after
the operation; v) preservation of superficial temporal artery and
frontal branch of the facial nerve; vi) lower incision
complications; vii) shortened hospitalization time and reduced
hospitalization costs (27,28).
In the present study, the average operation time was
268.2±1.38 min (range, 60-360 min) and the average number of days
of hospitalization was 28±19.05 days (range, 2-113 days).
The posterior wall of frontal sinus may be injured
during craniotomy via the SOEK approach. This defect may be the
cause of CSF leakage and wound infection after the operation. To
avoid frontal sinus wall injury during craniotomy, the lateral
range of the frontal sinus should be assessed on the pre-operative
CT. However, 2 patients had frontal sinus opening due to the
prominent size of their sinus. If the frontal sinus is breached,
the mucosa is removed, thoroughly cleaned with iodine solution and
sealed with bone wax. Therefore, a wide frontal sinus is not an
absolute contraindication for the keyhole approach.
Due to the ‘keyhole effect’, the SOEK approach
provides sufficient exposure of the lateral fissure and is
sufficient for arachnoid dissection and CSF drainage (29,30). At
times, in the case of a ruptured aneurysm, it is difficult to drain
sufficient CSF, as the subarachnoid space is filled with blood clot
instead of CSF.
In 2005, Paladino et al (31) compared keyhole craniotomy with
pterional craniotomy. The keyhole group consisted of 482 patients
with 565 aneurysms, while 146 patients with 167 aneurysms underwent
pterional craniotomy. The mortality rate in the keyhole group was
0.83% (4 cases) and that in the standard craniotomy group was 2.05%
(3 cases). In the present study, a good therapeutic effect was
achieved in 69 of 85 patients (81.2%) and 2 deaths occurred. The
first case was a 68-year-old female with WFNS grade V and Fisher
grade IV; aneurysm had a complex direction (multiple directions,
the prominent direction was superior). On the pre-operative CT
scans, this patient had an aneurysm of medium size (8 mm), which
was associated with grade III bleeding (50-100 ml). The patient
also suffered severe bleeding during the operation and then
developed a delayed ischemic defect due to perforator injury. The
patient also contracted an intracranial infection. The second
patient was a 67-year-old male with WFNS grade 4 and Fisher grade
4. The aneurysm featured anterior projection. On the pre-operative
CT, a small aneurysm (6.4 mm) was identified, which was associated
with grade II bleeding (<50 ml). The operation time was 7.2 h
and a delayed ischemic defect occurred. The two patients had
pre-operative hydrocephalus and EVD was performed prior to
surgery.
Yu et al (32)
also performed a randomized controlled trial on keyhole craniotomy
and pterional craniotomy for ruptured AComA aneurysm. Each group
contained 70 cases. The operation time of the keyhole group was
141.9 min, which was significantly lower than that of the pterional
group (184.5 min). The incidence of surgical complications in the
keyhole group was 10.5%, which was lower than that in the pterional
group (32.9%). The long-term efficacy was comparable. In addition,
the SOEK approach with only a single burr hole and small craniotomy
may shorten the overall operation time and reduce
craniotomy-associated complications, including CSF leakage,
post-operative epidural hematoma and infection.
In 2014, Gupta et al (33) reported on the long-term prognosis of
patients surviving ruptured intracranial aneurysm. Of the 494
patients with microsurgical clipping, 74 (15%) had a poor prognosis
at 1 year (mRS 3-6). In the BRAT, at 6 months of follow-up, 62 of
the 170 patients (36.5%) had poor outcomes (mRS 3-6) (22). In the present study, 16 patients
(18.8%) had poor outcome (mRS 3-6). The present results are similar
to those of other studies (Table
VI).
 | Table VIClinical outcomes of aneurysm surgery
by supraorbital craniotomy. |
Table VI
Clinical outcomes of aneurysm surgery
by supraorbital craniotomy.
| First author
(year) | Patients (n) | Unruptured
aneurysm, n (%) | Intra-operative
rupture (%) | Length of stay
(days) | Good outcome
(%) | Peri-operative
complications | (Refs.) |
|---|
| Paladino
(1998) | 37 | NA | 3 (8.1) | NA | 100 | Infection
(n=1) | (40) |
| van Lindert
(1998) | 139 | NA | 3 (2.1) | NA | NA | None | (5) |
| Chen (2009) | 88 | 0 (0) | 23 (26.1) | NA | 89 | Infection
(n=10) | (39) |
| Tang (2013) | 76 | 6 (7.9) | 8 (10.5) | NA | 95 | CSF leak (n=3) | (41) |
| Seizure (n=4) |
| Hyposmia (n=6) |
| Chalouhi
(2014) | 47 | 0 (0) | 5 (10.6) | NA | 77 | Post-op hematoma
(n=1) | (42) |
| Infection
(n=1) |
| Infarction
(n=4) |
| Park (2018) | 188 | 0 (0) | 2 (1.0) | NA | 91.5 | Infection
(n=3) | (38) |
| Anosmia (n=11) |
| Infarction
(n=1) |
| Yu (2018) | 70 | 0 (0) | 20 (28.6) | 15.5 | 83.6 | Infarction
(n=11) | (32) |
| Infection
(n=7) |
Accidental rupture of aneurysms is a major risk in
all aneurysm surgeries. Van Lindert et al (5) reported an accidental rupture of an
aneurysm during supraorbital keyhole surgery in 4 of 139 patients
(3%). In their experience report on a keyhole technique to clip
aneurysms, Fischer et al (34) reported an intra-operative rupture
rate of 7.7%, which was associated with aneurysm size and amount of
SAH.
The ISAT indicated that, under the condition that
the majority of enrolled patients (88%) were WFNS grade 1 or 2, the
rate of survivors with favorable outcomes (mRS score ≤2) after 5
years was similar between surgical clipping (82%) and coil
embolization (83%) (35). In the
BRAT, 75% of patients treated by coil embolization and 72% of
patients treated with clipping for anterior circulation aneurysms
had an mRS score of ≤2 at 1 year. After 6 years, this difference
was still not significant (22,36). In
the present study, 81.2% of patients with ruptured aneurysm
achieved good results; only 56.4% of patients in the present study
were WFNS grade 1 and 2. Furthermore, 47.1% of patients in this
group had a Fisher grade 3 or 4. As factors associated with adverse
outcomes, the Hunt & Hess score (OR=22.0, 95%CI: 4.8-100.2,
P=0.001), Fisher grade (OR=10.88, 95%CI: 2.292-51.74, P=0.003),
WFNS score (OR 6.0, 95%CI: 1.88-19.19, P=0.003), ICH (OR=7.9,
95%CI: 2.41-25.97, P=0.001), pre-operative EVD (OR=6.1, 95%CI:
1.90-19.62, P=0.002), hydrocephalus (OR=10.45, 95% CI: 3.06-35.63,
P=0.001) and VP shunt (OR=4.26, 95% CI: 0.9-18.22, P=0.050) were
identified. Ogilvy and Carter (37)
also reported similar results.
In the present study, surgical complications
occurred in 10.5% of cases. Park et al (38) reported similar results. Their study
described the experience of treating patients with ruptured
anterior circulation aneurysm with SOEK. Chen et al
(39) used the SOEK approach to
treat ruptured anterior circulation aneurysm in a prospective,
single-center study; the results were similar to those by Park
et al (38), with a high rate
of clipping. A favorable outcome was achieved in 88.6% of cases, no
mortalities occurred and 10 (11.4%) patients developed intracranial
or wound infection.
However, there is no doubt that the SOEK method is a
more challenging and technically demanding procedure with a steep
learning curve, which limits its wide applicability to a certain
extent. The SOEK method also has certain indications. In general,
it is suitable for patients with a Hunt & Hess grade of I-III,
including conscious patients and patients without any severe
intracranial pressure. Although it is also suitable for patients
with Hunt & Hess grade IV, its applicability depends on the
state of brain cisterns, sulci and ventricles on CT imaging.
However, it is generally not suitable for patients with a Hunt
& Hess grade with severe complications, including intracranial
hypertension, brain hernia and requirement for enlargement of the
bone window for decompression. In the SOEK approach, sufficient
space may be obtained for clipping aneurysms by completely removing
the skull base and releasing cerebrospinal fluid from the cistern.
In addition, if necessary, pre-operative external ventricular
drainage and/or resection of part of the gyrus rectus may be
performed intra-operatively.
The major limitations of the present study include
the small sample size, retrospective design, lack of randomization
and outcome assessment performed by clinical surgeons. Finally, the
present study reports on the clinical experience of specific
surgical techniques and schemes at a single center, which limits
the generalizability of the results. Furthermore, it may not be
possible to extrapolate the present results to unruptured or
posterior circulation aneurysms.
In conclusion, advancements in endovascular
interventional techniques have reduced surgical clipping of
aneurysms. Although the technology of endovascular interventional
therapy is developing continuously, it does not provide an exact
method for the treatment of residual aneurysms and retreatment
remains necessary in a certain ratio of cases. With the advancement
of minimally invasive microsurgery, small incision and bone window
provide ideal exposure to clip AComA aneurysms. The present study
indicated that the SOEK approach is an effective and ideal approach
for clipping of AComA aneurysm; it has numerous advantages,
including less trauma, a better therapeutic effect, fewer
complications and lower cost when compared with coil embolization.
However, due to the small operative space and difficult maneuvering
of surgical instruments, a number of obstacles may be encountered
during the operation. Therefore, it may be suggested that the
individual treatment for patients with AComA should be selected
based on pre-operative analysis.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the Science
and Technology Program of Guangzhou, China (201604020080) and
Guangdong Basic and Applied Basic Research
Foundation(2018B0303110014)
Availability of data and materials
The datasets used and/or analyzed during the present
study are available on reasonable request from the corresponding
author.
Authors' contributions
RB and YG conceived and designed the current study.
TCH, CC, HW and XJN acquired the data. RB and CFL analyzed and
interpreted the data, and drafted the manuscript. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Ethics approval was obtained from the Institutional
Review Board and ethics committee of the Third Affiliated Hospital
of Sun Yat-Sen University (Guangzhou, China) and all participants
provided written informed consent prior to enrolment in the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kassell NF, Torner JC, Haley EC Jr, Jane
JA, Adams HP and Kongable GL: The international cooperative study
on the timing of aneurysm surgery. Part 1: Overall management
results. J Neurosurg. 73:18–36. 1990.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yasargil MG: Anterior cerebral and
anterior communicating artery aneurysms. In Yasargil MG, (ed):
Microneurosurgery, New York, Georg Thieme Verlag. Vol 2:pp180–185.
1984.
|
|
3
|
Lai LT, Gragnaniello C and Morgan MK:
Outcomes for a case series of unruptured anterior communicating
artery aneurysm surgery. J Clin Neurosci. 20:1688–1692.
2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Abla AA, Wilson DA, Williamson RW, Nakaji
P, McDougall CG, Zabramski JM, Albuque FC and Spetzler RF: The
relationship between ruptured aneurysm location, subarachnoid
hemorrhage clot thickness, and incidence of radiographic or
symptomatic vasospasm in patients enrolled in a prospective
randomized controlled trial. J Neurosurg. 120:391–397.
2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
van Lindert E, Perneczky A, Fries G and
Pierangeli E: The supraorbital keyhole approach to supratentorial
aneurysms: Concept and technique. Surg Neurol. 49:481–490.
1998.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Figueiredo EG, Deshmukh P, Nakaji P,
Crusius MU, Crawford N, Spetzler RF and Preul MC: The minipterional
craniotomy: Technical description and anatomic assessment.
Neurosurgery. 61 (5 Suppl 2)(S256-S265)2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Figueiredo EG, Deshmukh V, Nakaji P,
Deshmukh P, Crusius MU, Crawford N, Spetzler RF and Preul MC: An
anatomical evaluation of the mini-supraorbital approach and
comparison with standard craniotomies. Neurosurgery. 59 (4 Suppl
2)(ONS212-ONS220)2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hernesniemi J, Ishii K, Niemelä M, Smrcka
M, Kivipelto L, Fujiki M and Shen H: Lateral supraorbital approach
as an alternative to the classical pterional approach. Acta
Neurochir Suppl. 94:17–21. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nakajima H, Kamiyama H, Nakamura T,
Takizawa K and Ohata K: Direct surgical treatment of giant
intracranial aneurysms on the anterior communicating artery or
anterior cerebral artery. Neurol Med Chir (Tokyo). 53:153–156.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Choi JH, Kang MJ and Huh JT: Influence of
clinical and anatomic features on treatment decisions for anterior
communicating artery aneurysms. J Korean Neurosurg Soc. 50:81–88.
2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Froelich S, Cebula H, Debry C and Boyer P:
Anterior communicating artery aneurysm clipped via an endonasal
approach: Technical note. Neurosurgery. 68 (2 Suppl
Operative):310–316. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hunt WE and Hess RM: Surgical risk as
related to time of intervention in the repair of intracranial
aneurysms. J Neurosurg. 28:14–20. 1968.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Report of World Federation of Neurological
Surgeons Committee on a Universal Subarachnoid Hemorrhage Grading
Scale. J Neurosurg: 68, 1988. Available from: https://thejns.org/view/journals/j-neurosurg/68/6/article-jns.1988.68.6.0985.xml.xml.
|
|
14
|
Fisher CM, Kistler JP and Davis JM:
Relation of cerebral vasospasm to subarachnoid hemorrhage
visualized by computerized tomographic scanning. Neurosurgery.
6:1–9. 1980.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yasargil MG: Microneurosurgery. Thieme
Stratton Inc. 2:169–184. 1984.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lin N, Cahill KS, Frerichs KU, Friedlander
RM and Claus EB: Treatment of ruptured and unruptured cerebral
aneurysms in the USA: A paradigm shift. J Neurointerv Surg.
4:182–189. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Huang MC, Baaj AA, Downes K, Youssef AS,
Sauvageau E, van Loveren HR and Agazzi S: Paradoxical trends in the
management of unruptured cerebral aneurysms in the United States:
Analysis of nationwide database over a 10-year period. Stroke.
42:1730–1735. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kozba-Gosztyla M, Czapiga B and
Jarmundowicz W: Aneurismal subarachnoid hemorrhage: Who remains for
surgical treatment in the post-ISAT era? Arch Med Sci. 11:536–543.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Koh KM, Ng Z, Low SY, Chua HZ, Chou N, Low
SW and Yeo TT: Management of ruptured intracranial aneurysms in the
post-ISAT era: Outcome of surgical clipping versus endovascular
coiling in a Singapore tertiary institution. Singapore Med J.
54:332–338. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Niemela M, Koivisto T, Kivipelto L, Ishii
K, Rinne J, Ronkainen A, Kivisaari R, Shen H, Karatas A, Lehecka M,
et al: Microsurgical clipping of cerebral aneurysms after the ISAT
Study. Acta Neurochir Suppl. 94:3–6. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Campi A, Ramzi N, Molyneux AJ, Summers PE,
Kerr RS, Sneade M, Yarnold JA, Rischmiller J and Byrne JV:
Retreatment of ruptured cerebral aneurysms in patients randomized
by coiling or clipping in the International Subarachnoid Aneurysm
Trial (ISAT). Stroke. 38:1538–1544. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Spetzler RF, McDougall CG, Zabramski JM,
Albuquerque FC, Hills NK, Russin JJ, Partovi S, Nakaji P and
Wallace RC: The Barrow ruptured aneurysm trial: 6-year results. J
Neurosurg. 123:609–617. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shapiro M, Becske T, Sahlein D, Babb J and
Nelson PK: Stent-supported aneurysm coiling: A literature survey of
treatment and follow-up. AJNR Am J Neuroradiol. 33:159–163.
2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pierot L, Costalat V, Moret J, Szikora I,
Klisch J, Herbreteau D, Holtmannspötter M, Weber W, Januel AC,
Liebig T, et al: Safety and efficacy of aneurysm treatment with
WEB: Results of the WEBCAST study. J Neurosurg. 124:1250–1256.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Moore N, Lang M and Bain M: 603.
Rehemorrhage and recurrence rates of small ACOM aneurysms post
embolization. Oral Presentations 2016 AANS Annual Scientific
Meeting Chicago, IL. J Neurosurg. 124(A1152)2016.
|
|
26
|
Daou B, Chalouhi N, Starke RM, Barros G,
Ya'qoub L, Do J, Tjoumakaris S, Rosenwasser RH and Jabbour P:
Clipping of previously coiled cerebral aneurysms: Efficacy, safety,
and predictors in a cohort of 111 patients. J Neurosurg.
125:1337–1343. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Reisch R, Perneczky A and Filippi R:
Surgical technique of the supraorbital key-hole craniotomy. Surg
Neurol. 59:223–227. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yasargil MG, Reichman MV and Kubik S:
Preservation of the frontotemporal branch of the facial nerve using
the interfascial temporalis flap for pterional craniotomy.
Technical article. J Neurosurg. 67:463–466. 1987.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cheng CM, Noguchi A, Dogan A, Anderson GJ,
Hsu FP, McMenomey SO and Delashaw JB Jr: Quantitative verification
of the keyhole concept: A comparison of area of exposure in the
parasellar region via supraorbital keyhole, frontotemporal
pterional, and supraorbital approaches. J Neurosurg. 118:264–269.
2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yagmurlu K, Safavi-Abbasi S, Belykh E,
Kalani MYS, Nakaji P, Rhoton AL Jr, Spetzler RF and Preul MC:
Quantitative anatomical analysis and clinical experience with
mini-pterional and mini-orbitozygomatic approaches for intracranial
aneurysm surgery. J Neurosurg. 127:646–659. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Paladino J, Mrak G, Miklić P, Jednacak H
and Mihaljević D: The keyhole concept in aneurysm surgery-a
comparative study: Keyhole versus standard craniotomy. Minim
Invasive Neurosurg. 48:251–258. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yu LB, Huang Z, Ren ZG, Shao JS, Zhang Y,
Wang R and Zhang D: Supraorbital keyhole versus pterional
craniotomies for ruptured anterior communicating artery aneurysms:
A propensity score-matched analysis. Neurosurgical Rev. Nov 10.
2018.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
33
|
Gupta SK, Chhabra R, Mohindra S, Sharma A,
Mathuriya SN, Pathak A, Tewari MK, Mukherji KK, Singla N, Salunke
P, et al: Long-term outcome in surviving patients after clipping of
intracranial aneurysms. World Neurosurg. 81:316–321.
2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fischer G, Stadie A, Reisch R, Hopf NJ,
Fries G, Böcher-Schwarz H, van Lindert E, Ungersböck K, Knosp E,
Oertel J and Perneczky A: The keyhole concept in aneurysm surgery:
Results of the past 20 years. Neurosurgery. 68 (1 Suppl
Operative):45–51. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Molyneux AJ, Kerr RS, Birks J, Ramzi N,
Yarnold J, Sneade M and Rischmiller J: ISAT Collaborators: Risk of
recurrent subarachnoid haemorrhage, death, or dependence and
standardized mortality ratios after clipping or coiling of an
intracranial aneurysm in the International Subarachnoid Aneurysm
Trial (ISAT): Long-term follow-up. Lancet Neurol. 8:427–433.
2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Spetzler RF, McDougall CG, Albuquerque FC,
Zabramski JM, Hills NK, Partovi S, Nakaji P and Wallace RC: The
barrow ruptured aneurysm trial: 3-year results. J Neurosurg.
119:146–157. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ogilvy CS and Carter BS: A proposed
comprehensive grading system to predict outcome for surgical
management of intracranial aneurysms. Neurosurgery. 42:959–970.
1998.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Park JS, Kim H, Baik MW and Park IS: Risk
factor analysis for poor outcomes in supraorbital keyhole aneurysm
clipping for ruptured anterior circulation aneurysms. World
Neurosurgery. 111(e386-e394)2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen L, Tian X, Zhang J, Huang Y, Chen E
and Lan Q: Is eyebrow approach suitable for ruptured anterior
circulation aneurysms on early stage: A prospective study at a
single institute. Acta Neurochir (Wien). 151:781–784.
2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Paladino J, Pirker N, Štimac D and
Stern-Padovan R: Eyebrow keyhole approach in vascular neurosurgery.
Minim Invasive Neurosurg. 41:200–203. 1998.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tang C, Sun J, Xue H, Yu Y and Xu F:
Supraorbital keyhole approach for anterior circulation aneurysms.
Turk Neurosurg. 23:434–438. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chalouhi N, Thakkar V, Tjoumakaris S,
Fernando Gonzalez L, Hasan D, Rosenwasser R, Singhal S and Jabbour
PM: Microsurgical clipping of large and giant cerebral aneurysms: A
single-center contemporary experience. J Clin Neurosci.
21:1424–1427. 2014.PubMed/NCBI View Article : Google Scholar
|