Introduction
Non-alcoholic fatty liver disease (NAFLD) has become
one of the most common disorders due to its growing prevalence and
latent progression in severe liver disease (1). During the past decades, consumption of
fructose has risen markedly owing to the use of sucrose and
high-fructose corn syrup in beverages and processed foods (2). Furthermore, increased fructose
consumption is considered to play an important role in the
development of metabolic diseases (3). In humans and other animals, chronically
high consumption of fructose can cause various symptoms of the
metabolic syndrome, such as impaired glucose tolerance, insulin
resistance, hypertension, hypertriglyceridemia, dyslipidemia and
fatty liver, which are associated with NAFLD (4-6).
Many studies have reported the underlying mechanisms
of hepatic steatosis, which include hepatic fatty acid uptake,
de novo lipogenesis, β-oxidation and export of hepatic lipid
accumulation in fructose induced NAFLD (4,6,7). The increase in hepatic de novo
lipogenesis is an important provider of lipids in fructose-induced
fatty livers (8,9). Fructose-derived precursors act as
nutritional regulators of the transcription factors, including
carbohydrate response element binding protein (ChREBP) and Sterol
regulatory element-binding protein (SREBP) 1c (10) that regulate the expression of de
novo lipogenesis genes. These two transcription factors
activate the upstream and downstream targets: liver X receptor,
acetyl-CoA carboxylase (ACC)1, fatty acid synthase and stearoyl-CoA
desaturase (SCD)1 to upregulate hepatic lipogenic genes, which are
associated with fructose-induced fatty acid synthesis (11-17).
Also, impaired lipid disposal pathways including fatty acid
oxidation (FAO), and export of lipids in very low-density
lipoproteins (VLDL) contributed to the development of hepatic
steatosis in the NAFLD (18). It has
previously been revealed that several of the enzymes involved in
hepatic FAO are influenced by PPARs, particularly PPARα (19). Moreover, high expression levels of
PPARα and its target genes PGC1α and carnitine
palmitoyl-transferase-1 (CPT1α) are responsible for mitochondrial
and peroxisomal FAO to reduce hepatic lipid accumulation (20). Through the modulation of PPAR
activity, the activity of SIRT1 controls hepatic lipid metabolism
(21,22). In addition, inflammation serves an
important role in NAFLD. Previous studies have reported that NAFLD
promotes liver inflammation to induce the downregulation of PPAR-α,
which in turn increases the activation of the pro-inflammatory
NF-κB as a priming signal leading to inflammasome activation
(23,24). As the increasing influence of NAFLD,
more and more therapies focus on the comorbdities associated with
NAFLD, particularly obesity, hyperglycemia, dyslipidemia and
hypertension, or rely on diet and lifestyle changes (25). However, there are still no approved
drugs for the treatment of NAFLD. Many natural products and herbal
medicines have great antioxidant, anti-inflammatory,
anti-apoptotic, and anti-adipogenic effects that allow them to be
possible therapeutic agents in NAFLD treatment (26,27).
Apple pomace is a by-product of apple processing
used for beverages and desserts; however, due to the lack of
awareness of apple pomace recycling, large amounts of resources are
wasted (28,29). Apple pomace is a rich source of
various nutrients, including phytochemicals, vitamins and dietary
minerals, and is particularly high in non-digestible carbohydrates
and dietary fibers, indicating that it may elevate hepatic
multi-unsaturated fatty acid content, increase circulating bile
acids and attenuate hepatic steatosis (30,31). In
addition, apple pomace consumption has been demonstrated to improve
lipid profiles (31) and endurance
in the exercise performance of mice (32), as well as to ameliorate glucose
metabolism in an oral glucose tolerance test in healthy volunteers
(33).
Rosemary, which is an aromatic evergreen shrub grown
in several parts of the world, is generally used as a spice and
flavoring agent in food processing (34). It has also been reported that
rosemary may regulate glucose and lipid metabolism in diabetic
animals (35-37).
Furthermore, rosemary extract along with moderate exercise training
may ameliorate streptozotocin-induced oxidative damage, which help
prevent the formation of diabetes-induced oxidative stress by
upregulating superoxide dismutase (SOD), glutathione peroxidase
(GSH-Px) and catalase levels in the erythrocytes of rats (35).
As apple pomace and rosemary (AR) individually
attenuate metabolic disorders, it was hypothesized that a mixture
of these compounds may have an anti-steatosis function in liver.
Our recent study demonstrated that treatment with AR for 5 weeks
attenuated chronic liquid fructose consumption-induced insulin
resistance via modulation of sarcolemmal CD36 and glucose
transporter 4 (GLUT4) in rats (38).
Therefore, the present study examined whether AR may ameliorate
hepatic steatosis in fructose-fed rats.
The aim of the present study was to investigate the
effect of AR on fructose overconsumption-induced fatty liver and
identify the underlying molecular mechanisms.
Materials and methods
Animals, diet and experimental
protocol
This study was approved by the Animal Ethics
Committee of Chongqing Medical University (Chongqing, China). Male
Sprague-Dawley (SD) rats (weight, 210-230 g; age, 12 weeks) and a
standard diet were supplied by the Laboratory Animal Center of
Chongqing Medical University. Rats were allowed free access to
water and standard chow, and housed under specific pathogen-free
conditions in an air-conditioned room (temperature, 21±1˚C;
relative humidity, 55±5%) with a 12 h light/dark cycle. The animal
model used in the present study was constructed as previously
described (39-43).
In total, 32 SD rats were initially divided into two groups, water
control (n=6) and fructose (n=26). Rats in water control group had
free access to water, and rats in fructose group had free access to
10% fructose solution (w/v, prepared every day). In the pilot
experiment, it was observed that the animals treated with 20%
fructose drank a reduced amount of fructose liquid compared with
the water control group (data not shown), and it was considered
that treatment with 20% fructose may cause dehydration in rats.
Therefore, to investigate NAFLD induced by sugar-sweetened
non-alcoholic beverages (~10% sugar), 10% fructose was used in the
study.
AR was made up of apple pomace (provided by
Professor Johji Yamahara) and rosemary extract (Sami Labs Limited)
at a ratio of 10:1 as previously described (38). Our previous study (38) reported that AR treatment at the doses
of 100 and 500 mg/kg had a positive effect on the high level of
plasma insulin and Homeostatic model assessment-index; however, AR
exerted an improved anti-hepatic steatosis effect at 100 mg/kg
compared with 500 mg/kg. Therefore, in the present study, rats in
AR-treated groups were treated with 50 and 100 mg/kg of AR.
After 13 weeks, the fructose group was divided into
three groups for the last 5 weeks: i) Fructose control (AR 0 mg/kg;
n=8); ii) fructose AR 50 mg/kg (suspended in 5% gum arabic; gavage
once daily; n=9); and iii) fructose AR 100 mg/kg (suspended in 5%
gum arabic, gavage once daily; n=9). The rats in the water- and
fructose-control groups received vehicle (5% gum arabic; cat. no.
MC0124B1013J; Shanghai Bioengineering Co., Ltd.) alone.
Chow and fructose remaining in the feeder were
weighed daily and the amount consumed per day was calculated. At
the end of week 4 of treatment with vehicle or AR, the rats were
fasted overnight, and blood samples (500 µl per rat) were collected
to determine the plasma triglyceride using a and total cholesterol
levels using a kit from Nanjing Jiancheng Bioengineering institute
(cat. no. F001, F002). At the end of the experiment, the rats were
anesthetized with inhaled isoflurane at an induction concentration
of 3-4% and a maintenance concentration of 2-2.5%. Blood (5 ml per
rat) was collected through the posterior orbital vein with a blood
collection tube, and the rats were weighed and sacrificed by
decapitation. The liver was collected and weighed, and segments of
the liver were stored in liquid nitrogen and 4% formalin for
subsequent testing.
Determination of plasma and liver
triglyceride and cholesterol
The plasma and liver levels of total cholesterol
(cat. no. F002 Nanjing Jiancheng Bioengineering Institute) and
triglyceride (cat. no. F001 Nanjing Jiancheng Bioengineering
Institute) were measured enzymatically using commercial kits
according to the manufacturer's instructions. The liver
triglyceride and total cholesterol content was determined as
previously described (39,40). First, 100 mg of liver tissue was
homogenated and dissolved in 2 ml 100% isopropanol. Then, following
3,000 x g centrifugation at 4˚C for 20 min, the supernatants were
used to detect triglyceride and total cholesterol.
Histological examination
To assess lipid droplet accumulation, a portion of
the liver fixed with 4% formalin for 24 h was collected, and 6-µm
frozen sections were cut and incubated with Oil Red O for 30 min at
room temperature to examine the liver histology under a light
microscope (BX-53; Olympus Corporation) at x40 magnification. In
total, 40 fields in three individual sections were randomly
selected, and the Oil Red O-stained and total tissue areas were
measured using ImageJ 1.43 software (National Institutes of
Health). The Oil Red O-stained to total tissue area ratio was
calculated (%).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from the liver tissues using
RNAiso Plus (cat. no. 9109; Takara Biotechnology Co., Ltd.)
according to the manufacturer's instructions. Subsequently, 1 µg of
total RNA from each sample was used to generate cDNA by linear
amplification using PrimeScript™ RT reagent Kit with gDNA Eraser
(cat. no. RR047A; Takara Biotechnology Co., Ltd.). RNA from each
sample was eliminated theDNAgenome at room temperature for 30 min
and then reverse transcribed into cDNA at 37˚C for 15 min. The PCR
reaction was performed in a total volume of 10 µl containing 1 µl
cDNA template, 5 µl 2x TB Green Premix Ex Taq II (cat. no. RR420A;
Takara Biotechnology Co., Ltd.), 0.4 µl forward primer, 0.4 µl
reverse primer and 3.2 µl dH2O. The PCR program was as
follows: Initial denaturation at 95˚C for 30 sec, followed by 40
cycles of 95˚C for 5 sec and 60˚C for 34 sec. The sequences of the
primers used for RT-qPCR are listed in Table I. Relative gene expression data were
analyzed using the 2-ΔΔCq method
(44). Gene expression for each
sample was analyzed in duplicate and normalized against the
internal control gene β-actin.
 | Table IPrimers sequences of reverse
transcription-quantitative PCR assays. |
Table I
Primers sequences of reverse
transcription-quantitative PCR assays.
| Gene | Sequences
(5'-3') | GenBank code | Product length,
bp | Tm |
|---|
| β-actin | F:
ACGGTCAGGTCATCACTATCG | NM031144 | 155 | 60˚C |
| R:
GGCATAGAGGTCTTTACGGATG |
| ACC-1 | F:
AACATCCCGCACCTTCTTCTAC | NM022193 | 138 | 60˚C |
| R:
CTTCCACAAACCAGCGTCTC |
| ACO | F:
CCCAAGACCCAAGAGTTCATTC | NM017340 | 113 | 60˚C |
| R:
TCACGGATAGGGACAACAAAGG |
| ChREBP | F:
GAAGACCCAAAGACCAAGATGC | FN432819 | 169 | 60˚C |
| R:
TCTGACAACAAAGCAGGAGGTG |
| CPT-1α | F:
CTGCTGTATCGTCGCACATTAG | NM031559 | 120 | 60˚C |
| R:
GTTGGATGGTGTCTGTCTCTTCC |
| FAS | F:
ACCTCATCACTAGAAGCCACCAG | NM017332 | 116 | 60˚C |
| R:
GTGGTACTTGGCCTTGGGTTTA |
| LPK | F:
GACCCGAAGTTCCAGACAAGG | NM012624 | 110 | 60˚C |
| R:
ATGAGCCCGTCGTCAATGTAG |
| PPARα | F:
GTCATCACAGACACCCTCTCCC | HM117640 | 124 | 60˚C |
| R:
TGTCCCCACATATTCGACACTC |
| SCD-1 | F:
CAGTTCCTACACGACCACCACTA | NM139192 | 111 | 60˚C |
| R:
GGACGGATGTCTTCTTCCAGAT |
| SREBP-1c | F:
CTGTCGTCTACCATAAGCTGCAC | NM001276707 | 121 | 60˚C |
| R:
ATAGCATCTCCTGCACACTCAGC |
| LXR | F:
AGAAACTGAAGCGTCAAGAAGAGG | NM031627 | 131 | 60˚C |
| R:
GGCAGCCACCAACTTCTCAA |
| DGAT1 | F:
GGCAGCCACCAACTTCTCAA | NM053437 | 136 | 60˚C |
| R:
CAGCATCACCACGCACCAAT |
| DGAT2 | F:
CCTGGCAAGAACGCAGTCAC | NM001012345 | 137 | 60˚C |
| R:
CCTGGCAAGAACGCAGTCAC |
| MGAT2 | F:
GCGACAAAGGAAGAACGACG | NM053604 | 105 | 60˚C |
| R:
GCGACAAAGGAAGAACGACG |
| HSL | F:
TTCGGGGAACACTACAAACGC | NM012859 | 179 | 60˚C |
| R:
AGCACCTCGATCTCCGTGATATTC |
| ATGL | F:
CTGATGACCACCCTTTCCAAC | NM001108509 | 169 | 60˚C |
| R:
AGATGCTACCTGTCTGCTCCTTC |
| AMPK | F:
CTCAACCGTTCTATTGCCACTCT | NM019142 | 179 | 60˚C |
| R:
AGGAAAGAGGTAACTGGGCAAAT |
| PGC-1α | F:
TGACCACAAACGATGACCCTC | NM031347 | 207 | 60˚C |
| R:
GACTGCGGTTGTGTATGGGAC |
| G6PC3 | F:
GAGTGGCTCAACCTCGTCTTC | NM176077 | 112 | 60˚C |
| R:
AAGGGAACTGGTGAATCTGGAC |
| IRS | F:
CTTCTGTTACACCTCAAGGGGC | NM012969 | 120 | 60˚C |
| R:
GGTTATGGTTGGGACTTAGGTTCA |
| PEPCK | F:
CGAGAGATCAACTGGGAAGAGC | NM001276721 | 136 | 60˚C |
| R:
TGTCAGCGAACGATAGCCG |
| MTTP | F:
TTCATTCAGCACCTCCGCACTTC | NM001107727 | 123 | 60˚C |
| R:
AGTCCAGGATGGCTTCCAGTGAG |
| FGF21 | F:
TCTCCTGCTGCCTGTCTTCCTG | NM130752 | 129 | 60˚C |
| R:
TCGGTGTCCTGGTCGTCATCTG |
| SIRT1 | F:
AGGGAACCTCTGCCTCATCTAC | NM001372090 | 99 | 60˚C |
| R:
GGCATACTCGCCACCTAACCT |
Western blot analysis
Total protein from livers was prepared individually
using the T-PER™ Tissue Protein Extraction Reagent (cat. no. 78510;
Thermo Fisher Scientific, Inc.). Protein concentration was
determined using the bicinchoninic acid protein concentration assay
kit (cat. no. P0010S; Biyuntian Biotechnology Research Institute.
Protein samples (30 µg/lane) were subjected to 10% SDS-PAGE and
then electrotransferred to PVDF membranes (GE Healthcare Life
Sciences). Membranes were incubated in blocking buffer (5% non-fat
milk) for 2 h at room temperature and probed with the following
antibodies: anti-SIRT1 (cat. no. 19A7AB4; 1:4,000; Abcam);
anti-CPT1α (cat. no. ab83862; 1:800; Abcam), anti-PGC1α (cat. no.
ab54481; 1:1,000; Abcam); anti-PPARα (cat. no. ab24509; Abcam);
anti-adenosine 5'-monophosphate (AMP)-activated protein kinase
(AMPK) (cat. no. ab3759; 1:500; Abcam); anti-phospho-AMPK (cat. no.
2535; 1:800; Cell Signaling Technology, Inc.); NF-κB p65 (cat. no.
ab16502; 1:1,000; Abcam) and tumor necrosis factor (TNF)-α (cat.
no. ab66579; 1:500; Abcam) at 4˚C overnight. Subsequently, the
membranes were incubated with horseradish peroxidase-conjugated
goat anti-mouse (cat. no. BA1050; 1:5,000; Boster Biological
Technology) and goat anti-rabbit IgG (cat. no. BA1054; 1:5,000;
Boster Biological Technology) secondary antibodies at room
temperature for 1.5 h. A polyclonal rabbit GAPDH antibody (cat. no.
sc-AP0063; 1:10,000; Cell Signaling Technology, Inc.) and rabbit
β-actin antibody (cat. no. 4970, 1:5,000; Cell Signaling
Technology, Inc.) were used as the loading controls to normalize
the signals obtained for proteins. Signal detection was performed
using the ECL western blot detection kit (cat. no. M29050; Meng
Bio) and the density was evaluated using ImageJ 1.43.
Determination of malondialdehyde (MDA)
and GSH-Px content, and total SOD (T-SOD) activity in the
liver
First, 30 mg liver was rinsed with ice-cold 0.9%
physiological saline, and the liver and 0.86% normal saline were
mixed at the ratio of 1:9 or 1:90 (weight/volume) and homogenized
to obtain 10 and 1% liver homogenates. After centrifugation at
1,200 x g for 15 min at 4˚C, the supernatant was used to detect
T-SOD (cat. no. A001-3; Nanjing Jiancheng Bioengineering Institute)
and succinate dehydrogenase (SDH) activity (cat. no. A022; Nanjing
Jiancheng Bioengineering Institute), MDA (cat. no. A003-3; Nanjing
Jiancheng Bioengineering Institute) and GSH-PX content (cat. no.
A005; Nanjing Jiancheng Bioengineering Institute) according to the
manufacturer's instructions.
Statistical analysis
Data are presented as the mean ± SEM of at least
three independent experiments. Data obtained from experiments with
>2 groups of animals were analyzed by one-way ANOVA followed by
Bonferroni's post hoc test for n>3 groups, while Student
Newman-Keuls test was used for n<3 groups. All the statistical
analyses were performed using GraphPad Prism software (version
6.02; GraphPad Software, Inc.) P<0.05 was considered to indicate
a statistically significant difference.
Results
General parameters of rats in each
group
Total chow intake of each rat over 5 weeks in the
fructose groups decreased compared with that the water control
group (Table II). However, no
significant differences were observed in fructose and chow intake
between the fructose control and the AR treatment groups (Table II). In addition, no differences were
observed in the body weight, liver weight and ratio of liver to
body weight among all groups at the end of the experiment (Table II). However, rats in the fructose
groups had a higher plasma concentration of triglyceride compared
with the water control group rats (Table II), but there was no significant
difference in plasma total cholesterol concentration (Table II). It was also demonstrated that AR
treatment did not suppress the increase in plasma triglyceride
induced by fructose overconsumption (Table II).
 | Table IIGeneral parameters of rats in each
group. |
Table II
General parameters of rats in each
group.
| Parameter | Water control | Fructose
control | Fructose AR (50
mg/kg) | Fructose AR (100
mg/kg) |
|---|
| Chow intake,
g/rat/5 weeks |
821.0±17.0a | 457.3±17.3 | 476.0±17.7 | 485.0±17.3 |
| Fructose intake,
g/rat/5 weeks | 0 | 384.0±12.0 | 394.3±11.3 | 377.8±11.7 |
| Energy intake,
kcal | 3447±30 | 3461±32 | 3583±32 | 3547±33 |
| Body weight, g | 362.2±17.0 | 361.0±14.9 | 360.7±16.3 | 365.0±16.2 |
| Liver weight,
g | 9.3±0.4 | 9.5±0.4 | 10.2±0.5 | 9.8±0.4 |
| Liver/body weight,
mg/g | 25.7±0.6 | 26.8±0.7 | 26.9±1.3 | 27.0±1.1 |
| Plasma total
cholesterol, mmol/l | 2.1±0.4 | 2.4±0.2 | 2.4±0.2 | 2.3±0.2 |
| Plasma
triglyceride, mmol/l |
0.5±0.04a | 0.8±0.03 | 0.8±0.05 | 0.8±0.03 |
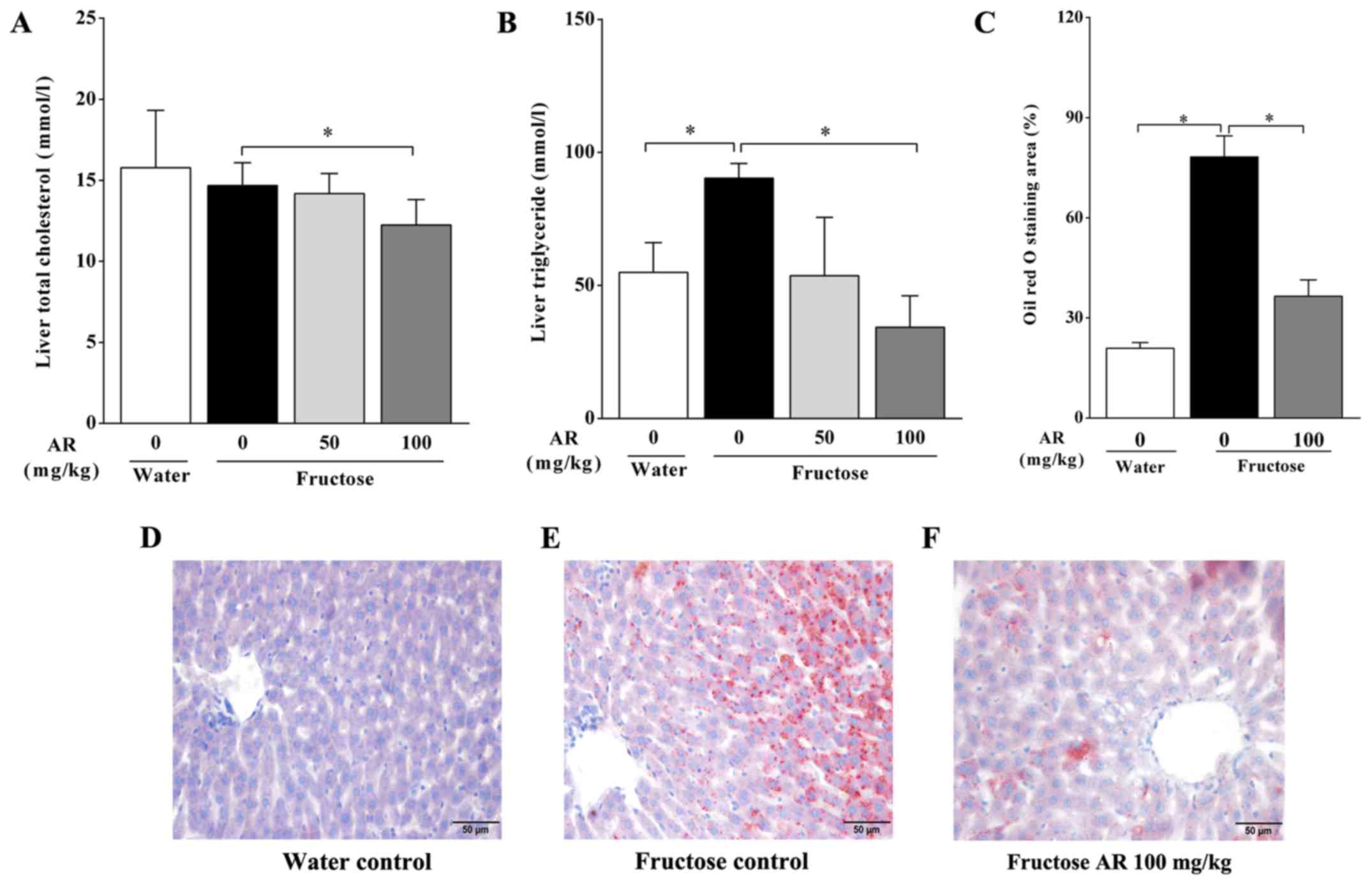
AR affects lipid accumulation in the
livers of fructose-fed rats
Compared with the water control group, the rats in
the fructose control group exhibited a higher hepatic triglyceride
level (Fig. 1B), whereas no
significant difference was present in the total cholesterol
concentration in the liver (Fig.
1A). Furthermore, 100 mg/kg, but not 50 mg/kg of AR
significantly diminished hepatic triglyceride accumulation induced
by fructose overconsumption (Fig.
1B). In accordance with these results, an increased Oil Red O
staining area was observed during histological examination of liver
sections from fructose-fed rats compared with that in the water
control group, indicating excess lipid droplet accumulation induced
by fructose feeding (Fig. 1C-E). In
rats treated with 100 mg/kg of AR, the Oil Red O staining area in
the liver was lower compared with that in untreated fructose-fed
rats (Fig. 1C, E and F).
Therefore, the histological and biochemical examination results in
livers suggested that 100 mg/kg of AR attenuated hepatic lipid
accumulation in fructose-fed rats.
Expression of genes involved in fatty
acid synthesis and metabolism
In the present study, fructose intake significantly
stimulated hepatic upregulation of genes associated with fatty acid
synthesis, such as SREBP1c, ACC1 and SCD1 (P<0.05), but had
little effect on genes involved in triglyceride synthesis, such as
MGAT2, DGAT1 and DGAT2, in the livers of rats (Fig. 2A). However, 100 mg/kg of AR treatment
did not affect the expression levels of these genes. Therefore,
these results suggested that AR-mediated amelioration of hepatic
lipid accumulation may bypass the pathway of de novo
lipogenesis.
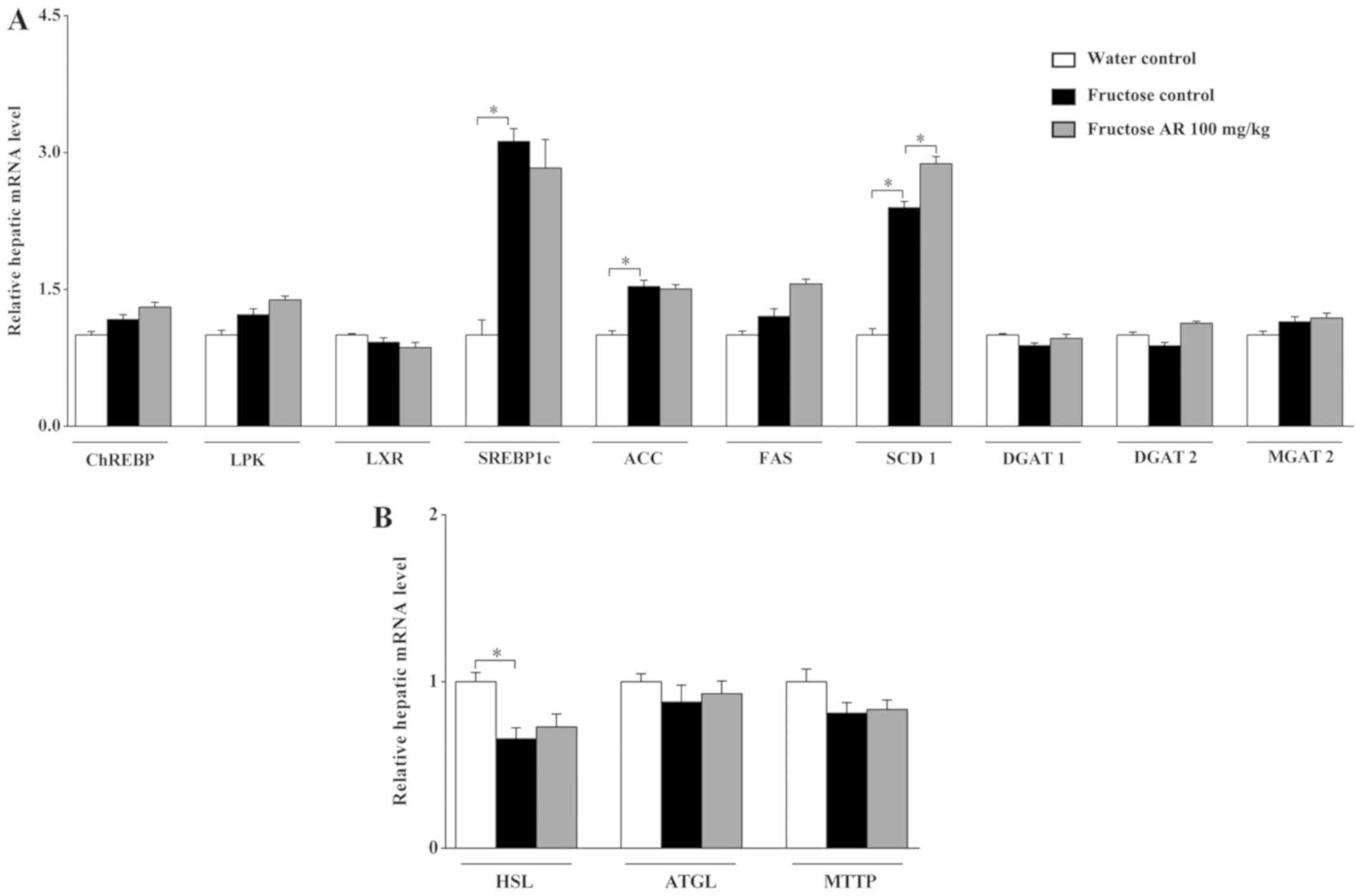 | Figure 2Expression of genes involved in fatty
acid synthesis and metabolism, as well as lipolysis and
mobilization. (A and B) Expression levels of genes responsible for
(A) lipogenesis and (B) lipid mobilization in the livers of rats
from each group were determined by reverse
transcription-quantitative PCR and normalized to β-actin. Data are
presented as the mean ± SEM, n=6-9 per group. *P<0.05
vs. fructose control. CHREBP, carbohydrate response element binding
protein; LPK, liver pyruvate kinase; LXR, liver X receptor;
SREBP-1c, sterol regulatory element-binding protein-1c; ACC,
acetyl-CoA carboxylase; FAS, fatty acid synthase; SCD-1,
stearoyl-CoA desaturase-1; DGAT-1, diacylglycerol
acyltransferases-1; DGAT-2, diacylglycerol acyltransferases-2;
MGAT-2, monoacylglycerol acyltransferase-2; HSL, hormone-sensitive
lipase; ATGL, adipose triglyceride lipase; MTTP, microsomal
triglyceride transfer protein; AR, apple pomace and rosemary. |
Expression of genes involved in fatty
acid lipolysis and mobilization
Microsomal triglyceride transfer protein (MTTP) is
the major lipid transfer protein that transfers triacylglycerols,
phospholipids and cholesteryl esters in vitro between
vesicles to assemble lipoproteins (45,8).
Adipose triglyceride lipase (ATGL) and hormone-sensitive lipase
(HSL) can coordinate to liberate fatty acids, and the fatty acids
are then transported into the mitochondria to undergo β-oxidation
(46). The results of the present
study indicated no significant differences in the gene expression
levels of ATGL and MTTP among all groups (Fig. 2B). Fructose feeding decreased the
expression of HSL, but no significant changes were observed between
the fructose control and fructose AR 100 mg/kg group (Fig. 2B). Collectively, these results
suggested that the anti-steatotic effect of AR may not be caused by
modulation of genes associated with fatty acid lipolysis and
mobilization.
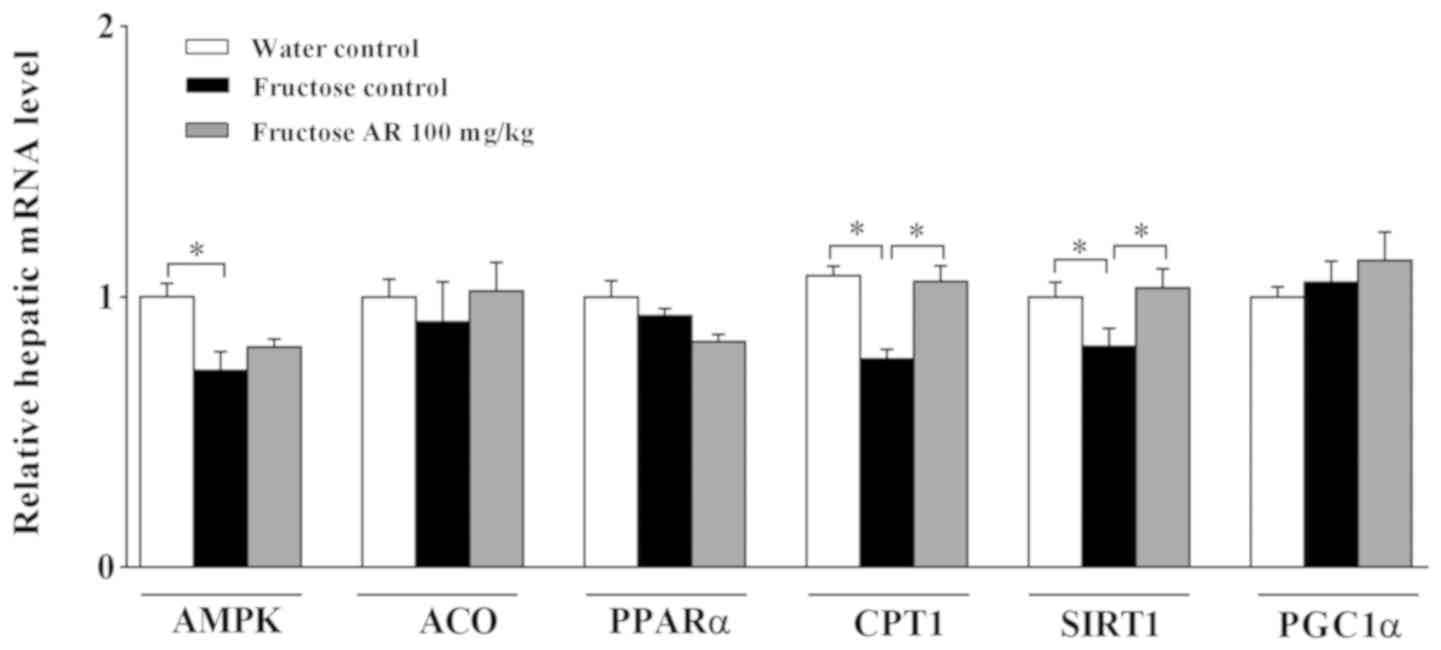
Expression of genes and proteins
responsible for FAO
The RT-qPCR results demonstrated that the mRNA
expression levels of CPT1α and SIRT1 were decreased in the livers
of fructose-fed rats compared with those in the water control
group, but significantly reversed by AR (Fig. 3). However, AMPK mRNA expression was
significantly decreased in the fructose control group compared with
the water control group, but the decreation was little restored by
AR (Fig. 3). The results of western
blotting also identified that AR treatment increased the expression
of pAMPK/AMPK, CPT1α, PPARα, SIRT1 and PGC1α at the protein level
in the livers of fructose-fed rats (P<0.05; Fig. 4), suggesting that the restoration of
PPARα and PGC-1α at translation, but not transcription level,
improved liver lipid accumulation (Fig.
4C and F).
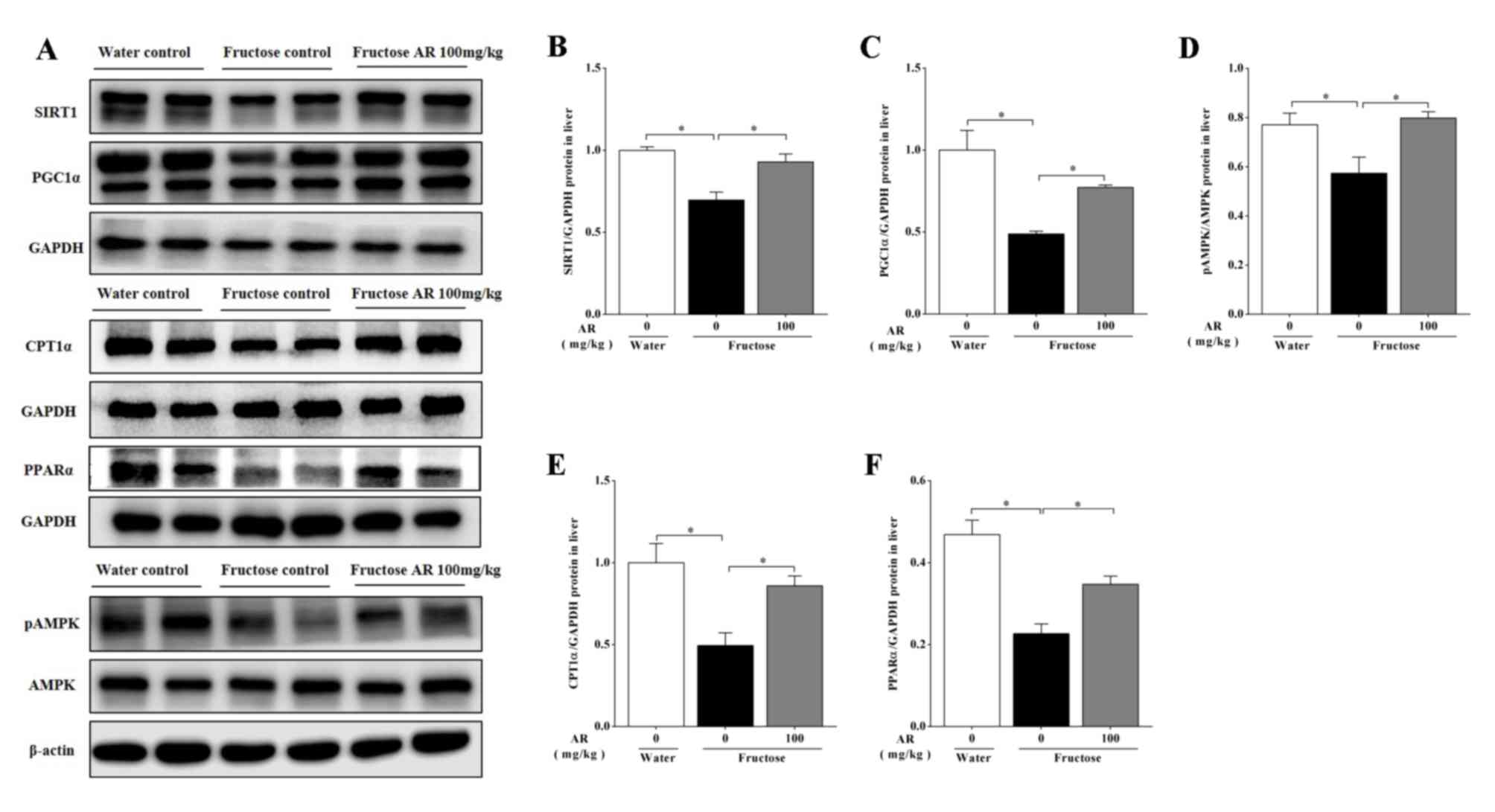 | Figure 4Expression of proteins involved in
fatty acid oxidation. (A) A representative image of western blot
analysis of SIRT1, PGC-1α, CPT-1α, PPAR-α, pAMPK and AMPK in the
liver. (B) SIRT1 protein expression. (C) PGC-1α protein expression.
(D) Ratio of pAMPK/AMPK protein expression levels. (E) CPT-1α
protein expression. (F) PPAR-α protein expression. Protein
expression was analyzed by western blotting and normalized to GAPDH
and β-actin. Data are presented as the mean ± SEM, n=6-9 per group.
Water, water control; Fructose, contains fructose control and
fructose AR (100 mg/kg). *P<0.05 vs. fructose
control. SIRT1, sirtuin 1; PGC-1α, peroxisome
proliferator-activated receptor-γ coactivator-1α; PPARα, peroxisome
proliferator-activated receptor-α; CPT1, carnitine
palmitoyl-transferase-1; pAMPK, phospho-adenosine 5'-monophosphate
(AMP)-activated protein kinase; AMPK, adenosine AMP-activated
protein kinase; AR, apple pomace and rosemary. |
Lipid peroxidation was measured as the content of
MDA in whole homogenates of the rat livers among the three groups.
In addition, the activities of enzymes responsible for
mitochondrial function, including SOD, GSH and SDH, were detected.
Compared with the water control group, GSH, which was used to
assess oxidative stress in the liver, decreased significantly after
fructose treatment alone, whereas treatment with AR had no effect
on GSH compared with the fructose control group (Fig. 5A). It was also demonstrated that
fructose administration increased SDH activity in the liver, and AR
treatment had a minimal effect on this (Fig. 5B). Furthermore, no differences in the
MDA and SOD content were observed under any condition (Fig. 5C and D).
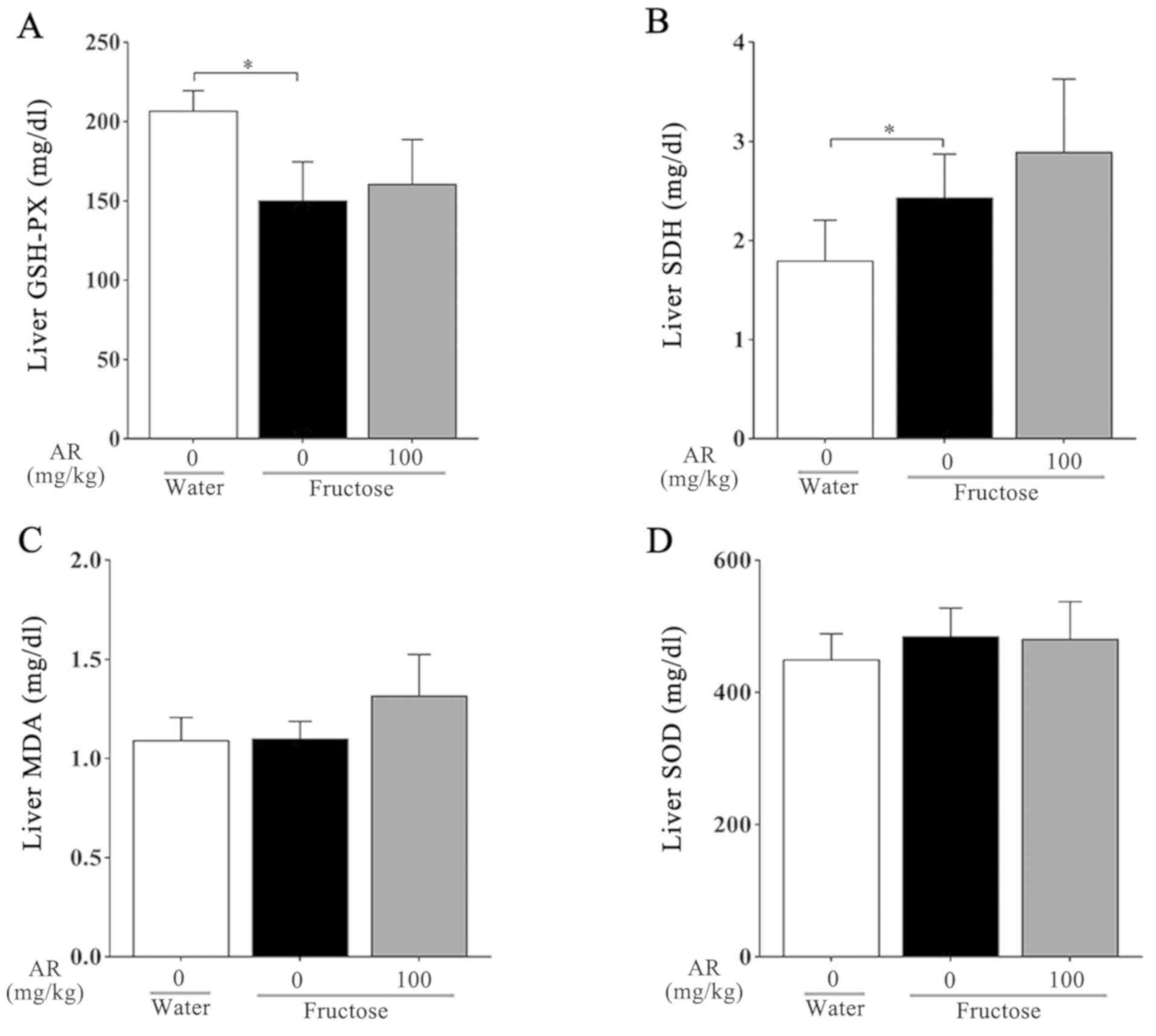 | Figure 5Detection of mitochondrial enzyme
functional activity. (A-D) Contents of (A) GSH-Px, (B) SDH, (C) MDA
and (D) SOD in the livers of rats from each group. Data are
presented as the mean ± SEM, n=6-9 per group. Water, water control;
fructose, contains fructose control and fructose AR (100 mg/kg).
*P<0.05 vs. fructose control group. GSH-Px,
glutathione peroxidase; SDH, succinate dehydrogenase; MDA,
malondialdehyde; SOD, superoxide dismutase; AR, apple pomace and
rosemary. |
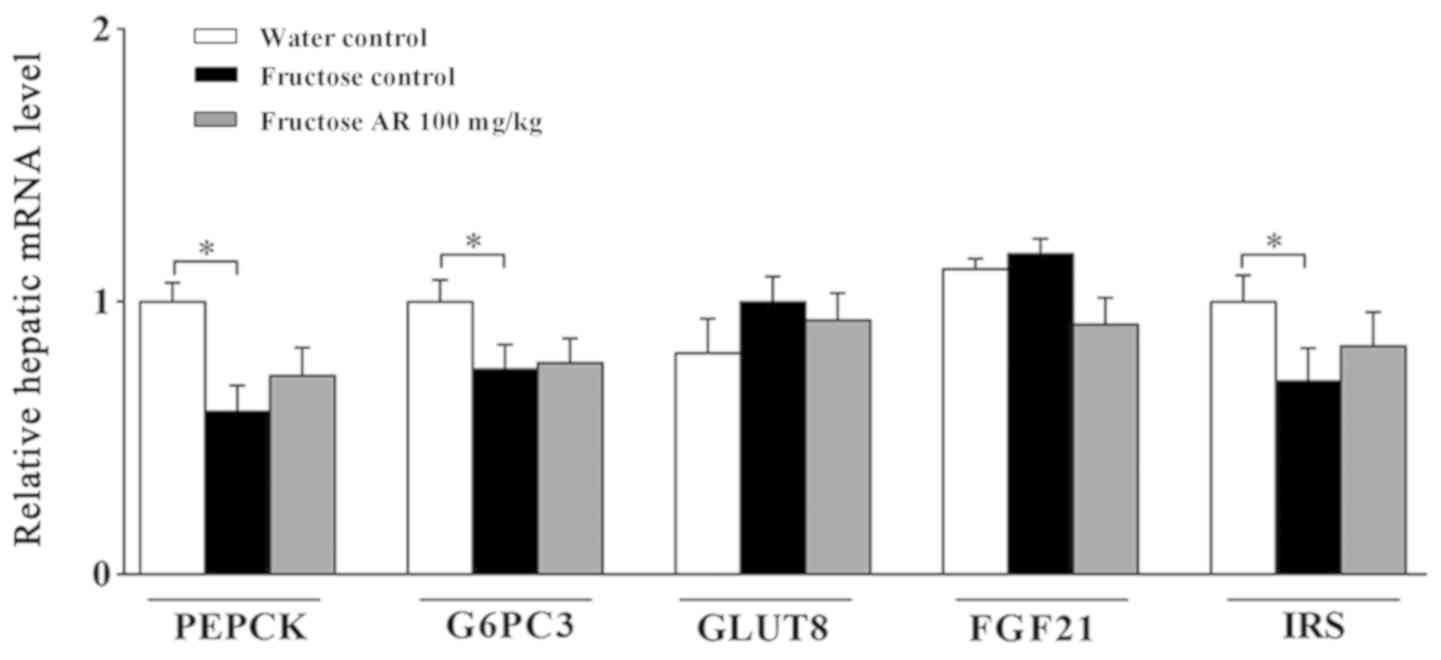
Expression of genes responsible for
gluconeogenesis and insulin signaling
Gluconeogenesis occurs mainly in the liver and is
mediated by glucagon, and can be counteracted by insulin; insulin
triggers the phosphorylation of enzymes and regulatory proteins via
protein kinase A, resulting in the inhibition of glycolysis
(47). Insulin can also induce the
cascade of genes required for the synthesis of fatty acids
(11,48). Next, the expression of the key
gluconeogenic enzymes phosphoenolpyruvate carboxykinase (PEPCK) and
genes associated with the insulin signaling pathway were examined.
It was identified that PEPCK, glucose-6-phosphatase 3 and insulin
receptor substrate mRNA expression levels were significantly
decreased after fructose alone compared with those in the water
control group, but AR treatment had a minimal effect on these genes
compared with the fructose control group (Fig. 6). In addition, the expression levels
of relevant genes, such as GLUT 8 and fibroblast growth factor 21,
exhibited no significant differences under these conditions
(Fig. 6).
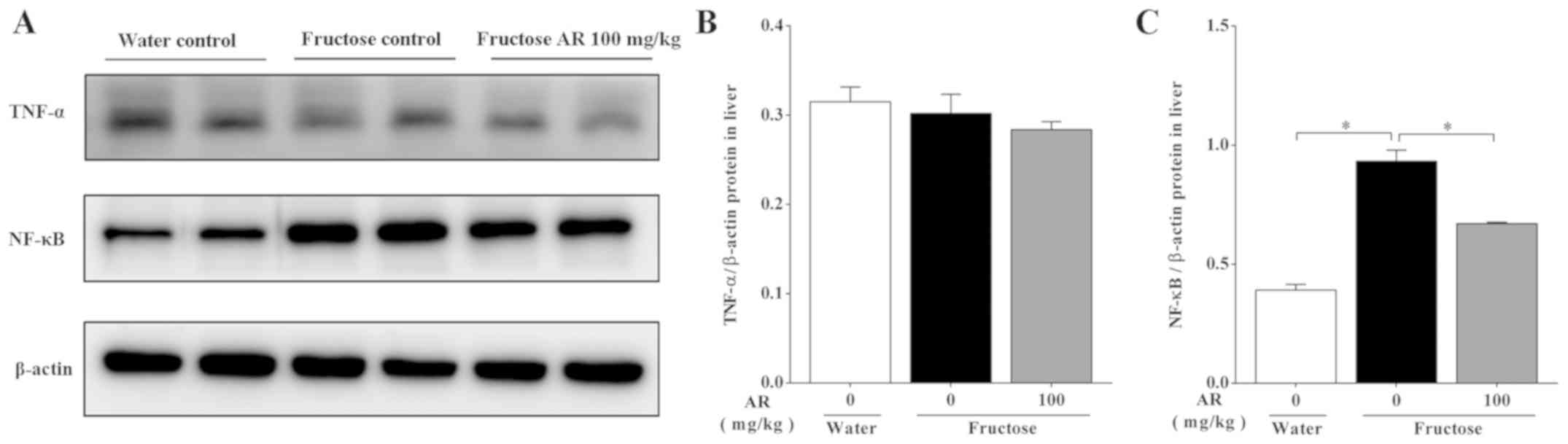
Expression of proteins associated with
inflammation
The overconsumption of fructose provokes metabolic
changes that result in a chronic low-grade inflammation (49). The expression levels of
inflammation-related proteins were assessed, and the results
demonstrated that fructose induced NF-κB p65 upregulation compared
with the water control group; in addition, rats treated with AR
exhibited lower expression of NF-κB p65 compared with fructose
group (Fig. 7). The expression of
TNF-α showed no significant differences under all the conditions.
Collectively, the result indicated that AR-mediated amelioration of
fructose-induced hepatic lipid accumulation may be associated with
the inflammatory pathway.
Discussion
It has been revealed that apple pomace improves
metabolic abnormalities (28,31).
Furthermore, our previous study reported that AR suppressed insulin
resistance via modulation of sarcolemmal CD36 and GLUT-4 in
fructose-fed rats (38). In the
present study, it was indicated that AR decreased chronic liquid
fructose consumption-induced hepatic steatosis, as demonstrated by
the decrease in liver triglyceride concentrations and attenuation
in Oil Red O staining area in the livers of rats. However, AR
treatment did not affect body or liver weight and had minimal
effects on plasma and liver concentrations of total cholesterol.
Therefore, these findings suggested a specific anti-steatosis
effect of AR in rats.
The present study investigated the expression of
genes involved in de novo lipogenesis, fatty acid lipolysis
and mobilization, gluconeogenesis and the insulin signaling
pathway; however, none of these were affected by AR treatment.
Thus, it was speculated that AR treatment diminished
fructose-induced hepatic lipid deposition independently of any of
the mechanisms listed above.
The results of the present study also suggested that
AR treatment resulted in reduced protein expression levels of
hepatic PPARα and CPT1α in fructose-fed rats compared with those in
the untreated rats. Furthermore, via the stimulation of the nuclear
transcription factor PPARα receptor, AR treatment activated PGC1α
signaling for transcriptional regulation of a number of proteins
that alter metabolism within mitochondria. PGC-1a can be
deacetylated by SIRT1, and form a complex with PPARa, leading to
increased FA oxidation (50).
Moreover, when FAO increases, the activity of SIRT1 also increases
(22). The direct SIRT1 substrate
PGC1α, which is deacetylated and hyperactived by SIRT1, cooperates
with PPARα to induce mitochondrial biogenesis and β-oxidation
reactions (51). The results of the
present study indicated that the expression levels of SIRT1, PGC1α
and PPARα were increased by AR treatment, suggesting that
AR-induced amelioration of hepatic steatosis may be associated with
enhanced mitochondrial biogenesis and β-oxidation. However, AR
treatment had no effect on the factors associated with oxidative
stress such as MDA, SOD, GSH and SDH, which may be due to the short
duration of the experimental period.
There are seven known SIRT enzymes, SIRT1-SIRT7, in
mammals (52-54).
SIRT3-SIRT5 are located in the mitochondria, whereas SIRT1, SIRT6
and SIRT7 are principally located in the nucleus, and the majority
of SIRT2 is located in the cytoplasm (55,56). The
homeostasis of NAD+ has been reported to serve a vital
role in the inception of fatty liver by modulating mitochondrial
efficiency (57). Furthermore, SIRTs
utilize NAD+ as a co-substrate to eliminate acetyl
moieties from lysines on histones and proteins (54). Among them, SIRT1 fulfills the key
criteria to be considered a NAD+ sensor (58). Moreover, through the modulation of
PPAR activity, SIRT1 controls hepatic lipid metabolism (21,22). In
line with the co-regulation of NAD+ and SIRT1, a
sufficient NAD+ pool maintains metabolic health by
restoring SIRT1 activity and signaling (58). Thus, this may be a mechanism that
explains how AR elevates intracellular SIRT1 levels and activates
NAD+-dependent SIRT-mediated signaling pathways in
metabolism-related chronic diseases (59). Therefore, it was speculated in the
present study that AR may restore the expression of SIRT1 by
regulating the NAD+ homeostasis pathway.
Previous studies have reported that PGC1α is a key
factor coordinating the gene expression that activates the
mitochondrial oxidative metabolism (60). Activated AMPK binds to the promoter
of PGC1α and is subsequently activated by SIRT1 to increase the
expression of genes that are critical to FAO (61). In addition, SIRT1 deacetylates its
substrate PGC1α to become hyperactive (52), and associates with PPARα on target
promoters to facilitate gene transcription in order to control
hepatic lipid metabolism (21-22,52). PPARα is the principal regulator of
FAO metabolism via the transcriptional induction of several
enzymes, such as acyl-CoA dehydrogenase medium chain, CPT1α and
CPT2(62). CPT1α, which catalyze the
rate-limiting step in mitochondrial FAO (63). Therefore, it was speculated in the
present study that SIRT1 may directly regulate PGC1α, thus
restoring the expression of CPT1α and exerting hepatoprotective
effects, which may be partially dependent on the antioxidant
capacity of AR. In addition, moderate expression of SIRT1 induces
resistance to oxidative stress (63), and agonist-dependent SIRT1 activation
can suppress the NF-κB transcriptional activity, which results in
subdued oxidative and inflammatory pathology and enhanced
antioxidant status in chronic chagasic cardiomyopathy (64). In line with these findings, the
present results suggested that AR treatment reversed the decreased
SIRT1 expression in the liver of fructose-fed rats and
downregulated the expression of NF-κB. A previous study has
provided genetic evidence for a functional crosstalk of nuclear
factor erythroid 2-related factor (Nrf2) and NF-κB p65 in
hepatocytes, which protects the liver from inflammation (65). Another study has reported crosstalk
between SIRT1 and Nrf2 as SIRT1 is reduced in
Nrf2-/- murine fibroblasts, and the increase
in Nrf2 and Nrf2-dependent gene expression is associated with a
significant increase in heart SIRT1 in senescence-accelerated
mouse-prone 8 mice (66). However,
further investigation is required to assess the expression of Nrf2
and to further elucidate the function of SIRT1 as well as the
underlying mechanisms of the metabolic action of AR.
In conclusion, the results of the present study
suggested that treatment with a mixture of AR improves chronic
fructose consumption-induced hepatic steatosis in rats by enhancing
FAO and suppressing inflammation. Moreover, there are some
limitations in the present study. Firstly, although the mixture of
apple pomace and rosemary extract could ameliorate hepatic
steatosis in fructose-induced NAFLD, additional studies are
required to investigate the individual contributions of the two
components during this process. Additionally, it is necessary to
consider the potentially important sex-specific effects in
metabolic improvement. However, the present study provided novel
evidence regarding the use of AR as a functional food and drug for
the prevention and treatment of metabolism-associated
disorders.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant nos. 81374033 and 81673659), the
Foundation of Chongqing Health and Family Planning Commission
(grant no. ZY201702133), the Science and Technology Research
Program of Chongqing Municipal Education Commission (grant no.
KJQN201800432) and the Chongqing Science and Technology Bureau
(grant no. cstc2017jcyjAX0374).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JWW, JY and LY designed the study. RB, JWW and LY
wrote the manuscript. RB, CY, TW, LL, JL, YL and HL performed the
experiments. RB, CL, ZC, TW, DK, JY, LY and JWW acquired and
analyzed the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study protocol was approved by the
Animal Ethics Committee of Chongqing Medical University (Chongqing,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hassan K, Bhalla V, El Regal ME and
A-Kader HH: Nonalcoholic fatty liver disease: A comprehensive
review of a growing epidemic. World J Gastroenterol.
20:12082–12101. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bray GA, Nielsen SJ and Popkin BM:
Consumption of high-fructose corn syrup in beverages may play a
role in the epidemic of obesity. Am J Clin Nutr. 79:537–543.
2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Trindade CT, Kurokawa , Cilmery
Barreiros RC, Bossolan and Grasiela : High-fructose
consumption and metabolic diseases. Fructose: Synthesis, Functions
and Health Implications. 37–60. 2012.
|
|
4
|
Kuzma JN, Cromer G, Hagman DK, Breymeyer
KL, Roth CL, Foster-Schubert KE, Holte SE, Weigle DS and Kratz M:
Consuming glucose-sweetened, not fructose-sweetened, beverages
increases fasting insulin in healthy humans. Eur J Clin Nutr.
73:487–490. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Aeberli I, Hochuli M, Gerber PA, Sze L,
Murer SB, Tappy L, Spinas GA and Berneis K: Moderate amounts of
fructose consumption impair insulin sensitivity in healthy young
men: A randomized controlled trial. Diabetes Care. 36:150–156.
2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tappy L and Lê KA: Metabolic effects of
fructose and the worldwide increase in obesity. Physiol Rev.
90:23–46. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
DiNicolantonio JJ, Mehta V, Onkaramurthy N
and O'Keefe JH: Fructose-induced inflammation and increased
cortisol: A new mechanism for how sugar induces visceral adiposity.
Prog Cardiovasc Dis. 61:3–9. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhu L, Baker SS, Liu W, Tao MH, Patel R,
Nowak NJ and Baker RD: Lipid in the livers of adolescents with
nonalcoholic steatohepatitis: Combined effects of pathways on
steatosis. Metabolism. 60:1001–1011. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kohjima M, Enjoji M, Higuchi N, Kato M,
Kotoh K, Yoshimoto T, Fujino T, Yada M, Yada R, Harada N, et al:
Re-evaluation of fatty acid metabolism-related gene expression in
nonalcoholic fatty liver disease. Int J Mol Med. 20:351–358.
2007.PubMed/NCBI
|
|
10
|
Kasper W, ter Horst ID and Mireille J:
Serlie: Fructose consumption, lipogenesis, and non-alcoholic fatty
liver disease. Nutrients. 9(981)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shimomura I, Bashmakov Y, Ikemoto S,
Horton JD, Brown MS and Goldstein JL: Insulin selectively increases
SREBP-1c mRNA in the livers of rats with streptozotocin-induced
diabetes. Proc Natl Acad Sci USA. 96:13656–13661. 1999.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Filhoulaud G, Guilmeau S, Dentin R, Girard
J and Postic C: Novel insights into ChREBP regulation and function.
Trends Endocrinol Metab. 24:257–268. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shimano H, Horton JD, Shimomura I, Hammer
RE, Brown MS and Goldstein JL: Isoform 1c of sterol regulatory
element binding protein is less active than isoform 1a in livers of
transgenic mice and in cultured cells. J Clin Invest. 99:846–854.
1997.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Higuchi N, Kato M, Shundo Y, Tajiri H,
Tanaka M, Yamashita N, Kohjima M, Kotoh K, Nakamuta M, Takayanagi
R, et al: Liver X receptor in cooperation with SREBP-1c is a major
lipid synthesis regulator in nonalcoholic fatty liver disease.
Hepatol Res. 38:1122–1129. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xie Z, Li H, Wang K, Lin J, Wang Q, Zhao
G, Jia W and Zhang Q: Analysis of transcriptome and metabolome
profiles alterations in fatty liver induced by high-fat diet in
rat. Metabolism. 59:554–560. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Postic C and Girard J: Contribution of de
novo fatty acid synthesis to hepatic steatosis and insulin
resistance: Lessons from genetically engineered mice. J Clin
Invest. 118:829–838. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Ipsen DH, Lykkesfeldt J and Tveden-Nyborg
P: Molecular mechanisms of hepatic lipid accumulation in
non-alcoholic fatty liver disease. Cell Mol Life Sci. 75:3313–3327.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yamaguchi T, Omatsu N, Matsushita S and
Osumi T: CGI-58 interacts with perilipin and is localized to lipid
droplets. Possible involvement of CGI-58 mislocalization in
Chanarin-Dorfman syndrome. J Biol Chem. 279:30490–30497.
2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ding J, Li M, Wan X, Jin X, Chen S, Yu C
and Li Y: Effect of miR-34a in regulating steatosis by targeting
PPARα expression in nonalcoholic fatty liver disease. Sci Rep.
5(13729)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Reddy JK and Hashimoto T: Peroxisomal
beta-oxidation and peroxisome proliferator-activated receptor
alpha: An adaptive metabolic system. Annu Rev Nutr. 21:193–230.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cao Y, Xue Y, Xue L, Jiang X, Wang X,
Zhang Z, Yang J, Lu J, Zhang C, Wang W, et al: Hepatic menin
recruits SIRT1 to control liver steatosis through histone
deacetylation. J Hepatol. 59:1299–1306. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xu F, Gao Z, Zhang J, Rivera CA, Yin J,
Weng J and Ye J: Lack of SIRT1 (Mammalian Sirtuin 1) activity leads
to liver steatosis in the SIRT1+/- mice: A role of lipid
mobilization and inflammation. Endocrinology. 151:2504–2514.
2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hernández-Rodas MC, Valenzuela R,
Echeverría F, Rincón-Cervera MÁ, Espinosa A, Illesca P, Muñoz P,
Corbari A, Romero N, Gonzalez-Mañan D, et al: Supplementation with
docosahexaenoic acid and extra virgin olive oil prevents liver
steatosis induced by a high-fat diet in mice through PPAR-α and
Nrf2 upregulation with concomitant SREBP-1c and NF-κB
downregulation. Mol Nutr Food Res. 61(61)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Valenzuela R, Illesca P, Echeverría F,
Espinosa A, Rincón-Cervera MÁ, Ortiz M, Hernandez-Rodas MC,
Valenzuela A and Videla LA: Molecular adaptations underlying the
beneficial effects of hydroxytyrosol in the pathogenic alterations
induced by a high-fat diet in mouse liver: PPAR-α and Nrf2
activation, and NF-κB down-regulation. Food Funct. 8:1526–1537.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Munteanu MA, Nagy GA and Mircea PA:
Current management of NAFLD. Clujul Med. 89:19–23. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li S, Xu Y, Guo W, Chen F, Zhang C, Tan
HY, Wang N and Feng Y: The impacts of herbal medicines and natural
products on regulating the hepatic lipid metabolism. Front
Pharmacol. 11(351)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kammerer DR, Kammerer J, Valet R and Carle
R: Recovery of polyphenols from the by-products of plant food
processing and application as valuable food ingredients. Food Res
Int. 65:2–12. 2014. View Article : Google Scholar
|
|
28
|
Hyson DA: A comprehensive review of apples
and apple components and their relationship to human health. Adv
Nutr. 2:408–420. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bhushan S, Kalia K, Sharma M, Singh B and
Ahuja PS: Processing of apple pomace for bioactive molecules. Crit
Rev Biotechnol. 28:285–296. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Skinner RC, Warren DC, Lateef SN, Benedito
VA and Tou JC: Apple pomace consumption favorably alters hepatic
lipid metabolism in young female Sprague-Dawley rats fed a western
diet. Nutrients. 10(10)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cho KD, Han CK and Lee BH: Loss of body
weight and fat and improved lipid profiles in obese rats fed apple
pomace or apple juice concentrate. J Med Food. 16:823–830.
2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jeong JW, Shim JJ, Choi ID, Kim SH, Ra J,
Ku HK, Lee DE, Kim TY, Jeung W, Lee JH, et al: Apple pomace extract
improves endurance in exercise performance by increasing strength
and weight of skeletal muscle. J Med Food. 18:1380–1386.
2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Makarova E, Górnaś P, Konrade I, Tirzite
D, Cirule H, Gulbe A, Pugajeva I, Seglina D and Dambrova M: Acute
anti-hyperglycaemic effects of an unripe apple preparation
containing phlorizin in healthy volunteers: A preliminary study. J
Sci Food Agric. 95:560–568. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bakirel T, Bakirel U, Keleş OU, Ulgen SG
and Yardibi H: In vivo assessment of antidiabetic and antioxidant
activities of rosemary (Rosmarinus officinalis) in
alloxan-diabetic rabbits. J Ethnopharmacol. 116:64–73. 2008.
View Article : Google Scholar
|
|
35
|
Nazem F, Farhangi N and Neshat-Gharamaleki
M: Beneficial effects of endurance exercise with Rosmarinus
officinalis Labiatae leaves extract on blood antioxidant enzyme
activities and lipid peroxidation in streptozotocin-induced
diabetic rats. Can J Diabetes. 39:229–234. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ramadan KS, Khalil OA, Danial EN, Alnahdi
HS and Ayaz NO: Hypoglycemic and hepatoprotective activity of
Rosmarinus officinalis extract in diabetic rats. J Physiol
Biochem. 69:779–783. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jäger S, Trojan H, Kopp T, Laszczyk MN and
Scheffler A: Pentacyclic triterpene distribution in various plants
- rich sources for a new group of multi-potent plant extracts.
Molecules. 14:2016–2031. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ma P, Yao L, Lin X, Gu T, Rong X, Batey R,
Yamahara J, Wang J and Li Y: A mixture of apple pomace and rosemary
extract improves fructose consumption-induced insulin resistance in
rats: Modulation of sarcolemmal CD36 and glucose transporter-4. Am
J Transl Res. 8:3791–3801. 2016.PubMed/NCBI
|
|
39
|
Gao H, Guan T, Li C, Zuo G, Yamahara J,
Wang J and Li Y: Treatment with ginger ameliorates fructose-induced
fatty liver and hypertriglyceridemia in rats: Modulation of the
hepatic carbohydrate response element-binding pro-tein-mediated
pathway. Evid Based Complement Alternat Med.
2012(570948)2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Liu C and Li Y, Zuo G, Xu W, Gao H, Yang
Y, Yamahara J, Wang J and Li Y: Oleanolic acid diminishes liquid
fructose-induced fatty liver in rats: role of modulation of hepatic
sterol regulatory element-binding protein-1c-mediated expression of
genes responsible for de novo fatty acid synthesis. Evid Based
Complement Alternat Med. 2013(534084)2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang J, Gao H, Ke D, Zuo G, Yang Y,
Yamahara J and Li Y: Improvement of liquid fructose-induced adipose
tissue insulin resistance by ginger treatment in rats is associated
with suppression of adipose macrophage-related proinflammatory
cytokines. Evid Based Complement Alternat Med.
2013(590376)2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Li Y, Wang J, Gu T, Yamahara J and Li Y:
Oleanolic acid supplement attenuates liquid fructose-induced
adipose tissue insulin resistance through the insulin receptor
substrate-1/phosphatidylinositol 3-kinase/Akt signaling pathway in
rats. Toxicol Appl Pharmacol. 277:155–163. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Xing X, Li D, Chen D, Zhou L, Chonan R,
Yamahara J, Wang J and Li Y: Mangiferin treatment inhibits hepatic
expression of acyl-coenzyme A: Diacylglycerol acyltransferase-2 in
fructose-fed spontaneously hypertensive rats: a link to
amelioration of fatty liver. Toxicol Appl Pharmacol. 280:207–215.
2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Villena JA, Roy S, Sarkadi-Nagy E, Kim KH
and Sul HS: Desnutrin, an adipocyte gene encoding a novel patatin
domain-containing protein, is induced by fasting and
glucocorticoids: Ectopic expression of desnutrin increases
triglyceride hydrolysis. J Biol Chem. 279:47066–47075.
2004.PubMed/NCBI View Article : Google Scholar
|
|
46
|
van Rijn JM, Ardy RC, Kuloğlu Z, Härter B,
van Haaften-Visser DY, van der Doef HP, van Hoesel M, Kansu A, van
Vugt AH, Thian M, et al: Intestinal failure and aberrant lipid
metabolism in patients with DGAT1 deficiency. Gastroenterology.
155(130-143.e15)2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kraus-Friedmann N and Feng L: The role of
intracellular Ca2+ in the regulation of gluconeogenesis.
Metabolism. 45:389–403. 1996.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Horton JD, Bashmakov Y, Shimomura I and
Shimano H: Regulation of sterol regulatory element binding proteins
in livers of fasted and refed mice. Proc Natl Acad Sci USA.
95:5987–5992. 1998.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Vasiljević A, Bursać B, Djordjevic A,
Milutinović DV, Nikolić M, Matić G and Veličković N: Hepatic
inflammation induced by high-fructose diet is associated with
altered 11βHSD1 expression in the liver of Wistar rats. Eur J Nutr.
53:1393–1402. 2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Mary C Sugden, Paul W Caton and Mark J
Holness: PPAR control: It's SIRTainly as easy as PGC. J Endocrinol.
204:93–104. 2010.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lee WJ, Kim M, Park HS, Kim HS, Jeon MJ,
Oh KS, Koh EH, Won JC, Kim MS, Oh GT, et al: AMPK activation
increases fatty acid oxidation in skeletal muscle by activating
PPARalpha and PGC-1. Biochem Biophys Res Commun. 340:291–295.
2006.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Haigis MC and Sinclair DA: Mammalian
sirtuins: Biological insights and disease relevance. Annu Rev
Pathol. 5:253–295. 2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Hall JA, Dominy JE, Lee Y and Puigserver
P: The sirtuin family's role in aging and age-associated
pathologies. J Clin Invest. 123:973–979. 2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Houtkooper RH, Cantó C, Wanders RJ and
Auwerx J: The secret life of NAD+: An old metabolite
controlling new metabolic signaling pathways. Endocr Rev.
31:194–223. 2010.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Michishita E, Park JY, Burneskis JM,
Barrett JC and Horikawa I: Evolutionarily conserved and
nonconserved cellular localizations and functions of human SIRT
proteins. Mol Biol Cell. 16:4623–4635. 2005.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Verdin E, Hirschey MD, Finley LW and
Haigis MC: Sirtuin regulation of mitochondria: Energy production,
apoptosis, and signaling. Trends Biochem Sci. 35:669–675.
2010.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Gariani K, Menzies KJ, Ryu D, Wegner CJ,
Wang X, Ropelle ER, Moullan N, Zhang H, Perino A, Lemos V, et al:
Eliciting the mitochondrial unfolded protein response by
nicotinamide adenine dinucleotide repletion reverses fatty liver
disease in mice. Hepatology. 63:1190–1204. 2016.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Cantó C, Menzies KJ and Auwerx J: NAD(+)
metabolism and the control of energy homeostasis: A balancing act
between mitochondria and the nucleus. Cell Metab. 22:31–53. 2015.
View Article : Google Scholar
|
|
59
|
Ruan Q, Ruan J, Zhang W, Qian F and Yu Z:
Targeting NAD+ degradation: The therapeutic potential of
flavonoids for Alzheimer's disease and cognitive frailty. Pharmacol
Res. 128:345–358. 2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Echeverría F, Valenzuela R, Bustamante A,
Álvarez D, Ortiz M, Espinosa A, Illesca P, Gonzalez-Mañan D and
Videla LA: High-fat diet induces mouse liver steatosis with a
concomitant decline in energy metabolism: Attenuation by
eicosapentaenoic acid (EPA) or hydroxytyrosol (HT) supplementation
and the additive effects upon EPA and HT co-administration. Food
Funct. 10:6170–6183. 2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Jäger S, Handschin C, St-Pierre J and
Spiegelman BM: AMP-activated protein kinase (AMPK) action in
skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl
Acad Sci USA. 104:12017–12022. 2007.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Wahli W and Michalik L: PPARs at the
crossroads of lipid signaling and inflammation. Trends Endocrinol
Metab. 23:351–363. 2012.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Alcendor RR, Gao S, Zhai P, Zablocki D,
Holle E, Yu X, Tian B, Wagner T, Vatner SF and Sadoshima J: Sirt1
regulates aging and resistance to oxidative stress in the heart.
Circ Res. 100:1512–1521. 2007.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Wan X, Wen JJ, Koo SJ, Liang LY and Garg
NJ: SIRT1-PGC1α-NFκB Pathway of oxidative and inflammatory stress
during Trypanosoma cruzi infection: Benefits of
SIRT1-targeted therapy in improving heart function in chagas
disease. PLoS Pathog. 12(e1005954)2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Köhler UA, Böhm F, Rolfs F, Egger M,
Hornemann T, Pasparakis M, Weber A and Werner S: NF-κB/RelA and
Nrf2 cooperate to maintain hepatocyte integrity and to prevent
development of hepatocellular adenoma. J Hepatol. 64:94–102.
2016.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Bayram B, Ozcelik B, Grimm S, Roeder T,
Schrader C, Ernst IM, Wagner AE, Grune T, Frank J and Rimbach G: A
diet rich in olive oil phenolics reduces oxidative stress in the
heart of SAMP8 mice by induction of Nrf2-dependent gene expression.
Rejuvenation Res. 15:71–81. 2012.PubMed/NCBI View Article : Google Scholar
|