Introduction
Pancreaticoduodenectomy (PD) is an operative method
for treating patients with pancreatic, biliary tract or duodenal
neoplasm. However, this surgical procedure is strongly associated
with perioperative high morbidity and mortality rates, ranging from
35-60 and 0-4%, respectively (1-6).
Predominant serious postoperative complications after PD involve
pancreatic fistula (PF), intraperitoneal bleeding, intra-abdominal
abscess, delayed gastric emptying and bile leakage. Of these, PF is
observed particularly frequently after PD, consequently being
associated with prolonged hospitalization, increased medical costs
and delayed introduction of postoperative chemotherapy, resulting
in an increased mortality rate if bacterial infection,
intra-abdominal abscess or bleeding follow PF. As the incidence of
clinically relevant postoperative pancreatic fistula (CR-POPF)
after PD reaches approximately 12-30% (2,4-10),
the early detection of this condition is very important for
managing patients who have undergone PD.
Presepsin has been used as a novel biomarker for
detecting bacterial infection (11,12).
Presepsin is also known as soluble CD14 subtype that is released
into the circulation after the activation of the pro-inflammatory
signal cascade on contact with infectious agents (12,13).
Previous studies have shown that serum presepsin is useful for the
early detection of bacterial infection and sepsis (11,12).
POPF is considered to be closely associated with bacterial
infection (8,14). Indeed, surgical site bacterial
infection involves the secretion of certain protease activators in
the pancreatic juice that convert trypsinogen to trypsin,
subsequently inducing CR-POPF (14).
However, no study has focused on the utility of presepsin as a
predictive marker for CR-POPF.
We hypothesized that presepsin might be a useful
biomarker for the early detection of CR-POPF after PD.
Materials and methods
Patients
A prospective pilot study was conducted in a single
institute (Department of Surgery, Saga Medical Center Koseikan) for
30 consecutive patients who underwent PD for various primary
diseases between May 2017 and March 2019. All patients and their
families were fully informed about the surgical procedures in order
to obtain their written consent, including broad consent to
participate in this study. The medical ethics committee of Saga
Medical Center Koseikan reviewed and approved this study design
(permission nos. 17-01-01-04, 19-05-01-01).
The primary diseases of the pancreatic, biliary
tract or duodenal neoplasms were diagnosed preoperatively by
various imaging modalities and/or a pathological examination.
Biliary stent drainage was performed during endoscopic retrograde
cholangiopancreatography for patients manifesting obstructive
jaundice.
Surgical procedure and postoperative
management
All operations were performed by experienced
surgeons whose quality was certified by the Japanese Society of
Hepato-Biliary-Pancreatic Surgery or supervised by such expert
surgeons. All patients were subjected to the same surgical
procedure as follows: Patients received subtotal stomach-preserving
PD with regional lymph node dissection according to the presence of
malignant disease or potential malignancy. The digestive tract was
reconstructed by the modified Child's method with ante-colic
gastro-jejunostomy. Pancreatico-jejunostomy was performed by
duct-to-mucosa anastomosis in addition to pull-through adhesive
anastomosis using the modified Blumgard procedure (15). Duct-to-mucosa anastomosis was
performed with double-layer anastomosis using 3-0 non-absorbable
monofilament sutures and 4-0 absorbable sutures. A 4- to 6-Fr
polyvinyl chloride tube in the pancreatic duct and 3-mm polyvinyl
chloride tube in the hepatic bile duct were inserted individually
for external drainage. In addition, a couple of drainage tubes were
placed around the pancreaticojejunostomy and hepaticojejunostomy
for intra-abdominal drainage. The drainage fluid was continuously
suctioned and collected for a later analysis. Fresh sample of the
drainage fluid were collected in the morning and analyzed. Tube
gastrostomy was not used in any case.
All of the patients received an intravenous drip
infusion of antibiotics with 1 g of cefmetazole sodium every 3 h
during the operation and 1 g of cefmetazole sodium twice a day on
postoperative day (POD) 1 and 2. The intra-abdominal drainage tubes
were removed or gradually pull out when CR-POPF and intra-abdominal
infection were deemed negligible. It is considered as below:
Inflammatory markers are improved and/or the volume of the
drainage fluid is reduced without bacterial detection. After
the removal of the intra-abdominal drainage tubes, the external
stent tubes in the pancreatic duct and hepatic bile duct were
removed.
Statistical analyses
The following data were collected from the
prospectively maintained comprehensive database or medical records:
Gender, age, body mass index, American Society of Anesthesiologists
(ASA) physical status score, history of diabetes mellitus, current
smoking habit, prior abdominal operations, presence of preoperative
biliary drainage, diameter of the main pancreatic duct, origin of
the primary disease, TNM-staging, presence of extended vascular
resection, operative time, intra- and post-operative blood
transfusion, pancreatic texture, presence of postoperative
complications, length until removal of the surgical drainage tube,
length of the post-operative hospital stay and pre- and
post-operative laboratory data.
PF was graded according to the guideline of the
International Study Group on PF (ISGPF) in 2016(16), and grades B and C were defined as
indicating the presence of CR-POPF in this study. In brief,
biochemical leak (BL) is clinically unimportant and is not referred
to a true PF. A PF grade B requires a change in the postoperative
management, namely the drainage tubes are either left in place
>3 weeks or repositioned through endoscopic or percutaneous
procedures. A PF Grade C requires reoperation since it can lead to
organ failure and/or mortality as a result of the PF.
The summary data for continuous variables were
expressed as the median and range (minimum, maximum). In the
univariate analysis, patient subgroups were compared in terms of
continuous variables by Wilcoxon's rank sum test for continuous
variables and Fisher's exact test for binary variables. Receiver
operation characteristics (ROC) analyses and the calculation of the
area under the curve (AUC) were used to examine the capability of
markers to diagnose PF. Continuous variables were converted into
binary variables based on the optimal cut-off values using the ROC
analyses. A multiple logistic regression analysis was performed to
identify significant independent markers using two markers which
showed a high level of AUC (>0.8) in the ROC. The data were
expressed as the odds ratios (ORs) with 95% confidence intervals
(CIs). A P-value of less than 0.05 was considered statistically
significant. All analyses were conducted using the SPSS software
program, version 25.0. (IBM Corp).
Results
The characteristics of the patients are summarized
in Table I. The median age of the
patients was 72 years old (range 37-84), and 43.3 and 56.7% of the
patients were male and female, respectively. Primary diseases were
pancreatic cancer in 10 patients (33.3%), bile duct cancer (33.3%)
in 10, duodenal cancer (23.3%) in 7 and benign duodenal tumor in 3
(10%). Regarding CR-POPF (grade B and C), grade B POPF occurred in
15 patients (50%), and grade C POPF was not found in this series.
Among the 15 patients with non-CR-POPF, 10 (33.3%) did not show
increased amylase in the drainage fluid, while the remaining 5
(16.7%) exhibited evidence of BL.
 | Table IPatients characteristics. |
Table I
Patients characteristics.
| Characteristic | Patients (n=30) |
|---|
| Sex
(males:females) | 13:17 |
| Age [years, median
(min, max)] | 72 (37, 84) |
| Body mass index,
kg/m2; median (min, max) | 22.6 (18.7,
30.7) |
| ASA (PS1,2:
PS3,4) | 27:3 |
| Diabetes mellitus
(yes:no) | 9:21 |
| Currently smoking
(yes:no) | 4:26 |
| Prior abdominal
surgery (yes:no) | 3:27 |
| Preoperative biliary
drainage (yes:no) | 17:13 |
| White blood cells,
µl; median (min, max) | 5,300 (3,300,
9,700) |
| Hemoglobin, g/dl;
median (min, max) | 13.0 (9.7, 15.6) |
| Albumin, g/dl; median
(min, max) | 4.1 (3.1, 5.0) |
| Total bilirubin,
mg/dl; median (min, max) | 0.9 (0.3, 2.7) |
| C-reactive protein,
mg/dl; median (min, max) | 0.08 (0.01,
1.28) |
| Tumor kind
(benign:malignant) | 3:27 |
|
Pancreatic
cancer | 10 (33.3%) |
|
Bile duct
cancer | 10 (33.3%) |
|
Duodenal
cancer | 7 (23.3%) |
|
Benign
duodenal tumor | 3 (10.0%) |
| Clinically relevant
pancreatic fistula (yes:no) | 15:15 |
|
No amylase
detected | 10 (33.3%) |
|
Biochemical
leak | 5 (16.7%) |
|
Grade B | 15 (50.0%) |
|
Grade C | 0 (0%) |
| Biliary leak
(yes:no) | 0:30 |
| Abdominal abscess
(yes:no) | 6:24 |
| Wound infection
(yes:no) | 0:30 |
| Postoperative
bleeding (yes:no) | 0:30 |
| Delayed gastric
emptying (yes:no) | 0:30 |
| Gastrointestinal
leakage (yes:no) | 0:30 |
| Re-operation
(yes:no) | 0:30 |
| Morality
(yes:no) | 0:30 |
| Post-operative
hospital stay, days; median (min, max) | 28 (19, 61) |
Table II shows the
results of the univariate analyses of the patient-related factors
with CR-POPF. Patients with non-pancreatic cancers, a smaller
pancreatic duct and soft pancreas texture experienced CR-POPF
significantly more frequently than others (P=0.005, P=0.004 and
P=0.014, respectively). Among the laboratory data obtained on POD1,
a higher white blood cell count, higher levels of serum amylase and
serum presepsin and higher concentration of presepsin in the
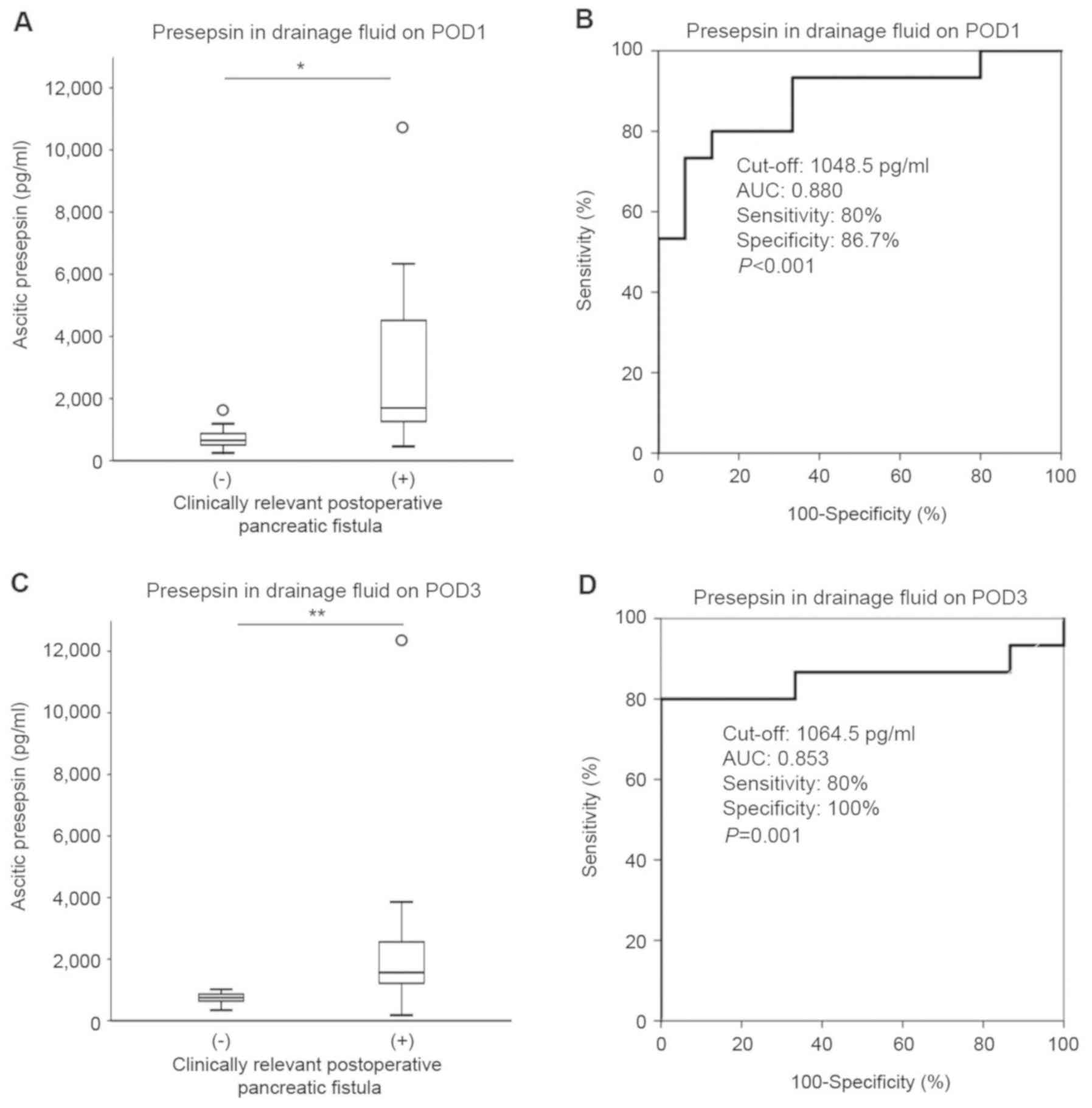
drainage fluid (Fig. 1A) were
significantly more common in patients with CR-POPF than in those
without CR-POPF (P=0.040, P=0.002, P=0.012 and P<0.001,
respectively). The postoperative hospital stay was also
significantly correlated with CR-POPF (P=0.004).
 | Table IIUnivariate analysis of patients. |
Table II
Univariate analysis of patients.
| | Clinically relevant
postoperative pancreatic fistula |
|---|
| Characteristic | (-) n=15 | (+) n=15 | P-value |
|---|
| A, Patient
characteristic |
|---|
| Sex
(male:female) | 5:10 | 8:7 | 0.462 |
| Age, years; median
(min, max) | 74 (37, 84) | 72 (54, 82) | 0.663 |
| Body mass index,
kg/m2; median (min, max) | 22.4 (18.7,
27.3) | 23.5 (19.6,
30.7) | 0.198 |
| ASA (PS1,2:
PS3,4) | 12:3 | 15:0 | 0.224 |
| Diabetes mellitus
(yes:no) | 5:10 | 4:11 | >0.999 |
| Current Smoking
(yes:no) | 0:15 | 4:11 | 0.100 |
| Prior abdominal
surgery (yes:no) | 2:13 | 1:14 | >0.999 |
| Preoperative biliary
drainage (yes:no) | 6:9 | 11:4 | 0.139 |
| B, Preoperative
laboratory data |
| White blood cells,
µl; median (min, max) | 4,700 (3,500,
8,600) | 6,200 (3,300,
9,700) | 0.237 |
| Hemoglobin, g/dl;
median (min, max)) | 12.7 (9.7,
14.9) | 13.0 (10.4,
15.6) | 0.281 |
| Albumin, g/dl;
median (min, max) | 4.1 (3.2, 5.0) | 4.0 (3.1, 4.7) | 0.348 |
| Total bilirubin,
mg/dl; median (min, max) | 0.8 (0.3, 2.3) | 1.2 (0.4, 2.7) | 0.140 |
| C-reactive protein,
mg/dl; median (min, max) | 0.06 (0.02,
1.28) | 0.10 (0.01,
1.04) | 0.775 |
| Tumor kind
(benign:malignant) | 1:14 | 2:13 | >0.999 |
| Pancreatic cancer
(yes:no) | 9:6 | 1:14 | 0.005 |
| Diameter of
pancreatic duct, mm; median (min, max) | 5.49 (1.43,
12.36) | 1.83 (1.15,
4.42) | 0.004 |
| Tumor invasion
(tumor limited to organ/tumor extends beyond organ) | 6:9 | 7:8 | >0.999 |
| Lymph node
metastasis (negative:positive) | 8:7 | 10:5 | 0.710 |
| Distant organ
metastasis (negative:positive) | 15:0 | 14:1 | >0.999 |
| Vascular resection
(yes:no) | 4: 11 | 1:14 | 0.330 |
| Operative time,
min; median (min, max) | 319 (201, 513) | 358 (217, 701) | 0.071 |
| Intraoperative
bleeding, ml; median (min, max) | 427 (71,
1,100) | 400 (170,
1,194) | 0.740 |
| Pancreas texture
(hard:soft) | 8:7 | 1:14 | 0.014 |
| Intraoperative
blood transfusion (yes:no) | 1:14 | 2:13 | >0.999 |
| Postoperative blood
transfusion (yes:no) | 0:15 | 1:14 | >0.999 |
| C, Postoperative
laboratory data collected on POD1 |
| Bacteria detected
in the fluid collection (yes:no) | 1:14 | 3:12 | 0.598 |
| White blood cells,
µl; median (min, max) | 8,900 (5,550,
13,800) | 12,000 (5,700,
20,300) | 0.040 |
| C-reactive protein,
mg/dl; median (min, max) | 8.33 (5.27,
14.08) | 8.03 (5.29,
14.48) | 0.772 |
| Serum amylase,
IU/l; median (min, max) | 110 (57, 913) | 638 (179,
1,113) | 0.002 |
| Serum presepsin,
pg/ml; median (min, max) | 219 (141, 801) | 378 (215,
3,818) | 0.012 |
| Presepsin in
drainage fluid, pg/ml; median (min, max) | 653 (248,
1,609) | 1,695 (460,
10,730) | <0.001 |
| Post-operative
hospital stay, days; median (min, max) | 25 (22, 48) | 37 (19, 61) | 0.004 |
In addition, the white blood cell count, levels of
serum amylase and serum presepsin and concentration of presepsin in
the drainage fluid on POD1 were examined using ROC curves analyses
to predict POPF. The cut-off values of 11,200/µl for the white
blood cell count, 399 IU/l for the serum amylase level, 250 pg/ml
for the serum presepsin level and 1,048.5 pg/ml for the
concentration of presepsin in the drainage fluid were determined
using the AUCs of 0.720, 0.836, 0.769 and 0.880, respectively
(Table III, Fig. 1B). Furthermore, the serum amylase
level and presepsin concentration in the drainage fluid on POD1
were evaluated using a multivariate analysis to test whether or not
they were independent factors predicting CR-POPF. The multivariate
logistic regression analysis revealed that a higher level of
presepsin in the drainage fluid on POD1 was an independent
predictive marker for CR-POPF (OR: 14.503, 95% CI: 1.750-120.229,
P=0.013; Table IV).
 | Table IIIReceiver operating characteristic
analysis of variables. |
Table III
Receiver operating characteristic
analysis of variables.
| Variables | Cut-off value | AUC | Sensitivity | Specificity | P-value |
|---|
| White blood cells
on POD1, µl | 11,200 | 0.720 | 0.600 | 0.933 | 0.040 |
| Serum amylase on
POD1, IU/l | 399 | 0.836 | 0.800 | 0.800 | 0.002 |
| Serum presepsin on
POD1, pg/ml | 250 | 0.769 | 0.733 | 0.667 | 0.012 |
| Presepsin in
drainage fluid on POD1, pg/ml | 1,048.5 | 0.880 | 0.800 | 0.867 | <0.001 |
 | Table IVMultivariate analysis. |
Table IV
Multivariate analysis.
| | Multivariate
analysis |
|---|
| Parameters | OR | (95% CI) | P-value |
|---|
| Serum amylase on
POD1 | 7.817 | (0.969-63.052) | 0.054 |
| Presepsin in
drainage fluid on POD1 | 14.503 |
(1.750-120.229) | 0.013 |
The presepsin concentration in the drainage fluid on
POD3 was also significantly increased after revealing CR-POPF
(P=0.001; Fig. 1C). The ROC analysis
with a cut-off level of 1,064.5 pg/ml demonstrated 90% accuracy,
80% sensitivity and 100% specificity (Fig. 1D). The combined results of presepsin
in the drainage fluid in POD1 and POD3 accurately confirmed a
diagnosis of CR-POPF (Table V;
accuracy: 90%, sensitivity: 93.3%, specificity: 86.7%).
 | Table VCombined results of presepsin in
patient drainage fluid on POD1 and POD3. |
Table V
Combined results of presepsin in
patient drainage fluid on POD1 and POD3.
| | Clinically relevant
postoperative pancreatic fistula |
|---|
| Presepsin in
drainage fluid on POD1 and POD3 | (-) n=15 | (+) n=15 | P-value |
|---|
| Both negative | 13 | 1 | <0.001 |
| Either
positive | 2 | 14 | |
Discussion
In patients receiving PD, a number of perioperative
risk factors of POPF have been reported, including male gender,
high body mass index, intra-operative bleeding, soft pancreatic
texture, increased pancreatic fat, increased pancreatic parenchymal
remnant volume and small pancreatic duct diameter (1,2,4,7,17,18).
Many predictive markers, such as postoperative elevated serum
amylase, C-reactive protein, serum lipase and lipase in the
drainage fluid, have also been developed (5-7,9,10,19).
However, the utility of these risk factors and predictive markers
of CR-POPF remains controversial. In this study, we confirmed the
utility of a novel predictive marker for CR-POPF after PD by
focusing on presepsin as a bacterial infection and/or inflammation
marker. We demonstrated for the first time that the concentration
of presepsin in the drainage fluid was an accurate predictive maker
for the detection of CR-POPF.
A recent study suggested that CR-POPF is consistent
with PF accompanied by bacterial infection (8,14).
Pancreatic juice contains alkaline digestive enzyme and a high
concentration of bicarbonate ions. Enzymatic proteins constitute a
large component of the pancreatic juice; for example, trypsin is a
crucial digestive enzyme that activates other digestive enzymes
(14,20). PF develops after PD due to autolysis
caused by activated trypsin, leading to tissue damage. As a result,
PF induces intra-abdominal abscess and intra-peritoneal bleeding
due to vascular rupture (14).
Yamashita et al reported that infection with bacteria
involves the secretion of a protease activator of trypsinogen to
trypsin. Therefore, controlling bacterial infection in the
perioperative period of PD may be crucial for preventing the
development of CR-POPF (14). Based
on this rationale, we hypothesized that novel bacterial specific
inflammatory markers might be candidates for the earlier and more
accurate detection of CR-POPF after PD. In particular, we focused
on presepsin, which was identified as a predictive marker for
bacterial infection and has been reported to be more sensitive than
procalcitonin, a recently identified bacterial-specific
inflammatory marker (12). We
therefore selected presepsin as a candidate marker for detecting
CR-POPF.
We considered that the earlier and more accurate
diagnosis of CR-POPF would be closely associated with improving the
morbidity and mortality rate, resulting in a better quality of life
in patients. The usefulness of serum amylase (7,9,10), C-reactive protein (6), serum lipase (5) and lipase in the drainage fluid
(19) as diagnostic markers was
previously reported to diagnose CR-POPF as follows: AUC,
0.780-0.793; sensitivity, 81.5-91.7%; specificity, 55.5-72.7% for
serum amylase (9,10); AUC, 0.796; sensitivity, 94%;
specificity, 62% for C-reactive protein (6); AUC, 0.76; sensitivity, 92%;
specificity, 66% for serum lipase (5); AUC, 0.89; sensitivity, 88%;
specificity, 95% for lipase in drainage fluid (19). However, these diagnostic and
predictive markers are considered insufficient for the accurate
diagnosis of CR-POPF due to their relatively low specificity and
sensitivity values. We found that the measurement of presepsin in
the drainage fluid on POD1 and 3 was effective for predicting
CR-POPF. Furthermore, the combination of the concentrations of
presepsin in the drainage fluid on POD1 and POD3 showed even better
efficacy for this diagnosis (accuracy: 90.0%, sensitivity: 93.3%,
specificity: 86.7%). In this study, we excluded the amylase
concentration in the drainage fluid from the analysis, because the
definition of CR-POPF includes the concentration of amylase in the
drainage fluid itself. Indeed, the concentration of amylase in the
drainage fluid on POD1 was also significantly increased in the
CR-POPF group [median: 245 (range 33-3,894) vs. median: 5,009
(range 517-48,620), P<0.001] and was highly diagnostic
(accuracy: 90%, sensitivity: 93.3, specificity: 86.7%). We could
prospect that these factors such as presepsin, amylase and/or other
candidate factors in the different time point may have an important
role to predict the CR-POPF. In addition, multivariate analysis or
diagnostic model with combination these factor may improve the
prediction of CR-POPF in the future.
The delayed removal of abdominal drains was
suggested to be associated with retrograde infection and
intra-abdominal complications (21).
If true, then optimal drain management is crucial for the early
detection of CR-POPF. Conventional ways of detecting infections,
such as culture-based approaches, require sophisticated equipment
and highly proficient operators and timing. Therefore, we proposed
that if the presepsin level in the drain fluid exceeded 1,050 pg/ml
(based on our cut-off value of POD1 and POD3 for presepsin in
drainage fluid), continuous and additional anti-bacterial
therapeutics and/or prolonged drain management may need to be
considered.
Several limitations associated with the present
study warrant mention. First, the study was conducted at a single
institution with a relatively small number of subjects as a pilot
study. In addition, the incidence of CR-POPF was relatively high in
this study in comparison to the previous study (2,4-10).
Therefore, further studies will be needed with a large number of
the patients in order to confirm the utility of presepsin as a
novel predictive maker for CR-POPF after PD.
In conclusion, the presepsin level in the drainage
fluid is useful for detecting CR-POPF after PD.
Acknowledgements
The authors would like to thank Dr Naoya Kimura, Dr
Satomi Nakamura, Dr Hiroaki Nakamura, Dr Kohei Yamada, Dr Keiichiro
Okuyama, Dr Osamu Ikeda, Dr Tanaka Toshiya and Dr Seiji Sato (all,
Department of Surgery, Saga Medical Center Koseikan) for collecting
samples and useful discussions.
Funding
The current study was supported by Koseikan
Institutional Research Grant (grant no. 17-01-01-04).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MH and AM primarily designed the current study.
MH, AM and KK treated patients and collected samples and data. YS
and MY measured laboratory data, including presepsin. MH and ES
analyzed the data. MH, AM, KK, TM and HN interpreted the results
and wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All patients and their families provided informed
consent for participation after they were fully informed about the
surgical procedures of the current study. The Medical Ethics
Committee of Saga Medical Center Koseikan reviewed and approved the
present study (permission nos. 17-01-01-04 and 19-05-01-01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rosso E, Casnedi S, Pessaux P,
Oussoultzoglou E, Panaro F, Mahfud M, Jaeck D and Bachellier P: The
role of ‘fatty pancreas’ and of BMI in the occurrence of pancreatic
fistula after pancreaticoduodenectomy. J Gastrointest Surg.
13:1845–1851. 2009.
|
|
2
|
Kawai M, Kondo S, Yamaue H, Wada K, Sano
K, Motoi F, Unno M, Satoi S, Kwon AH, Hatori T, et al: Predictive
risk factors for clinically relevant pancreatic fistula analyzed in
1,239 patients with pancreaticoduodenectomy: Multicenter data
collection as a project study of pancreatic surgery by the Japanese
Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary
Pancreat Sci. 18:601–608. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kastenberg ZJ, Morton JM, Visser BC,
Norton JA and Poultsides GA: Hospital readmission after a
pancreaticoduodenectomy: An emerging quality metric? HPB (Oxford).
15:142–148. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kanda M, Fujii T, Suenaga M, Takami H,
Hattori M, Inokawa Y, Yamada S, Nakayama G, Sugimoto H, Koike M, et
al: Estimated pancreatic parenchymal remnant volume accurately
predicts clinically relevant pancreatic fistula after
pancreatoduodenectomy. Surgery. 156:601–610. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dalla Valle R, De Bellis M, Pedrazzi G,
Lamecchi L, Bianchi G, Pellegrino C and Iaria M: Can early serum
lipase measurement be routinely implemented to rule out clinically
significant pancreatic fistula after pancreaticoduodenectomy? Int J
Surg. 21 (Suppl 1):S50–S54. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Partelli S, Pecorelli N, Muffatti F,
Belfiori G, Crippa S, Piazzai F, Castoldi R, Marmorale C, Balzano G
and Falconi M: Early postoperative prediction of clinically
relevant pancreatic fistula after pancreaticoduodenectomy:
Usefulness of C-reactive Protein. HPB (Oxford). 19:580–586.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Okabayashi T, Maeda H, Nishimori I,
Sugimoto T, Ikeno T and Hanazaki K: Pancreatic fistula formation
after pancreaticooduodenectomy; for prevention of this deep
surgical site infection after pancreatic surgery.
Hepatogastroenterology. 56:519–523. 2009.PubMed/NCBI
|
|
8
|
Nagakawa Y, Matsudo T, Hijikata Y, Kikuchi
S, Bunso K, Suzuki Y, Kasuya K and Tsuchida A: Bacterial
contamination in ascitic fluid is associated with the development
of clinically relevant pancreatic fistula after
pancreatoduodenectomy. Pancreas. 42:701–706. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cloyd JM, Kastenberg ZJ, Visser BC,
Poultsides GA and Norton JA: Postoperative serum amylase predicts
pancreatic fistula formation following pancreaticoduodenectomy. J
Gastrointest Surg. 18:348–353. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Palani Velu LK, Chandrabalan VV, Jabbar S,
McMillan DC, McKay CJ, Carter CR, Jamieson NB and Dickson EJ: Serum
amylase on the night of surgery predicts clinically significant
pancreatic fistula after pancreaticoduodenectomy. HPB (Oxford).
16:610–619. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yaegashi Y, Shirakawa K, Sato N, Suzuki Y,
Kojika M, Imai S, Takahashi G, Miyata M, Furusako S and Endo S:
Evaluation of a newly identified soluble CD14 subtype as a marker
for sepsis. J Infect Chemother. 11:234–238. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chenevier-Gobeaux C, Borderie D, Weiss N,
Mallet-Coste T and Claessens YE: Presepsin (sCD14-ST), an innate
immune response marker in sepsis. Clin Chim Acta. 450:97–103.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wright SD, Ramos RA, Tobias PS, Ulevitch
RJ and Mathison JC: CD14, a receptor for complexes of
lipopolysaccharide (LPS) and LPS binding protein. Science.
249:1431–1433. 1990.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yamashita K, Sasaki T, Itoh R, Kato D,
Hatano N, Soejima T, Ishii K, Takenawa T, Hiromatsu K and Yamashita
Y: Pancreatic fistulae secondary to trypsinogen activation by
Pseudomonas aeruginosa infection after pancreatoduodenectomy. J
Hepatobiliary Pancreat Sci. 22:454–462. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Fujii T, Sugimoto H, Yamada S, Kanda M,
Suenaga M, Takami H, Hattori M, Inokawa Y, Nomoto S, Fujiwara M and
Kodera Y: Modified Blumgart anastomosis for pancreaticojejunostomy:
Technical improvement in matched historical control study. J
Gastrointest Surg. 18:1108–1115. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bassi C, Marchegiani G, Dervenis C, Sarr
M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink
MG, et al: The 2016 update of the International Study Group (ISGPS)
definition and grading of postoperative pancreatic fistula: 11
years after. Surgery. 161:584–591. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Del Chiaro M, Rangelova E, Ansorge C,
Blomberg J and Segersvard R: Impact of body mass index for patients
undergoing pancreaticoduodenectomy. World J Gastrointest
Pathophysiol. 4:37–42. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lee SE, Jang JY, Lim CS, Kang MJ, Kim SH,
Kim MA and Kim SW: Measurement of pancreatic fat by magnetic
resonance imaging: Predicting the occurrence of pancreatic fistula
after pancreatoduodenectomy. Ann Surg. 251:932–936. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Facy O, Chalumeau C, Poussier M, Binquet
C, Rat P and Ortega-Deballon P: Diagnosis of postoperative
pancreatic fistula. Br J Surg. 99:1072–1075. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Whitcomb DC and Lowe ME: Human pancreatic
digestive enzymes. Dig Dis Sci. 52:1–17. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kawai M, Tani M, Terasawa H, Ina S, Hirono
S, Nishioka R, Miyazawa M, Uchiyama K and Yamaue H: Early removal
of prophylactic drains reduces the risk of intra-abdominal
infections in patients with pancreatic head resection: Prospective
study for 104 consecutive patients. Ann Surg. 244:1–7.
2006.PubMed/NCBI View Article : Google Scholar
|