Introduction
Preeclampsia (PE) one of the main causes of maternal
and infant mortality during the perinatal period, with the
pathophysiological changes mainly occurring in early pregnancy
(1). However, the onset of PE occurs
after 20 weeks of pregnancy, with manifestations of hypertension,
proteinuria and edema (2). Under
serious circumstances, general convulsions and multiple organ
dysfunction can be observed (2,3). The
condition of PE is complex and difficult to predict as the etiology
and mechanism of pathogenesis are unclear.
Most of the symptoms associated with PE are rapidly
relieved following the delivery of the placenta, suggesting that
the placenta is the source of the disease (4). It is generally believed that PE is
caused by dysfunctional trophoblast invasion (5). Importantly, forkhead box M1 protein
(FOXM1) is expressed in cytotrophoblasts in the villi and decidual
tissue during early pregnancy, and possibly interacts with
placental trophoblasts during early placental development (6-8).
Therefore, it may be hypothesized that aberrant FOXM1 expression
could induce changes in intracellular signal transduction, leading
to trophoblastic dysfunction and PE. The main pathological feature
of the placenta in PE patients is insufficient trophoblast invasion
into the endometrium, with the depth only reaching the decidua
layer (9). This in turn results in
defective physiological ‘vascular recasting’ of the uterine spiral
arterioles and a reduction in villi area and density (10). Unlike the invasion of cancer cells,
trophoblast invasion is under strict paracrine regulation to
prevent excessive invasion (11,12).
Genes that regulate FOXM1 expression include microRNA (miRNA or
miR)-365-1 and miR-374b (13,14).
Furthermore, miR-21 has been reported to be upregulated in human
malignant tumors (15). However, the
potential relationship between miR-21 and FOXM1 in PE has not been
previously reported.
In the present study, the expression of miR-21 and
FOXM1 in placental tissues and blood samples from patients with PE
was assessed to investigate the potential regulatory relationship
between miR-21 and FOXM1.
Materials and methods
Patients
A total of 32 pregnant women with PE (age range,
22-39 years; median age, 32 years; mean age, 32±4.6 years) who
received regular birth examinations prior to cesarean section at
Huaian First People's Hospital between December 2013 and March 2018
were included in the present study. In addition, 28 normal pregnant
women with matched age, weeks of gestation and pre-pregnancy
indices (age range, 20-41 years; median age, 33 years; mean age,
33±3.9 years) were included into the control group. Inclusion
criteria were: Good health before pregnancy and no history of
induced abortion. Exclusion criteria included: Age over 45 years
old, history of immune diseases before pregnancy, history of
ovarian or uterine diseases, hormonal disorder and long-term use of
hormones or immunosuppressive drugs.
Peripheral blood samples (10-15 ml) were collected
from all subjects and centrifuged at 400 x g, 4˚C for 10 min before
the isolation of serum into 100-µl aliquots. During caesarean
section, placental tissues were collected from the center of the
placenta, 2.5 cm from the umbilical cord. All procedures performed
in the present study were approved by the Ethics Committee of
Nanjing Medical University. Written informed consent was obtained
from all patients or their families.
Cells
Human HTR8/SVneo cells (Wuhan PriCells Biomedical
Technology Co., Ltd.) in the logarithmic growth phase were seeded
(3x105/well) into 24-well plates 1 day before
transfection, and cultured in antibiotics-free F12/DMEM medium
(SH30023.01B; HyClone; GE Healthcare Life Sciences) supplemented
with 10% fetal bovine serum (SH30084.03; HyClone; GE Healthcare
Life Sciences) under 37˚C and 5% CO2 until 70%
confluency was reached. For transfection, 1.5 µl agomiR-negative
control (NC; agomiR-NC group; 5'-UUCUCCGAACGUGUCACGUTT-3' and
3'-TTAAGAGGCUUGCACAGUGCA-5') or agomiR-21 (miR-21 mimics group;
5'-UAGCUUAUCAGACUGAUGUUGA-3' and 3'-AUCGAAUAGUCUGACUACAACU-5'; both
20 pmol/µl; Sangon Biotech Co., Ltd.) was mixed with 50 µl Opti-Mem
medium (Thermo Fisher Scientific, Inc.) in one vial. In another
vial, 1 µl Lipofectamine® 2000 (Thermo Fisher
Scientific, Inc.) was mixed with 50 µl Opti-Mem medium. After 5
min, the contents of the two vials were combined, and the mixture
was incubated at room temperature for 20 min. This mixture was
subsequently added to the cells in their respective groups., The
medium was replaced with fresh F12/DMEM medium containing 10% fetal
bovine serum 6 h later. The cells were collected for downstream
assays following 48 h of further culture.
Reverse transcription-quantitative PCR
(RT-qPCR)
Tissue samples (100 mg) were ground into powder in
liquid nitrogen and lysed using 1 ml TRIzol® reagent
following the manufacturer's protocol (Thermo Fisher Scientific,
Inc.) for the extraction of total RNA. Serum (100 µl) or cells
(3x106) were directly lysed using 1 ml TRIzol reagent.
After assessing the concentration and quality of RNA using
ultraviolet spectrophotometry (NanoDrop™ ND2000; Thermo
Fisher Scientific, Inc.), cDNA was obtained by reverse
transcription from 1 µg RNA using miRcute miRNA cDNA First-chain
Synthesis kit according to the manufacturer's protocol (Tiangen
Biotech Co., Ltd.) and then stored at -20˚C. SuperReal PreMix (SYBR
Green) kit (Tiangen Biotech Co., Ltd.) was used to detect the
expression of FOXM1 mRNA using β-actin as an internal reference.
The sequences of the FOXM1 primers were forward,
5'-GGCTCCCGCAGCATCAAGCA-3' and reverse, 5'-TGTTCCGGCGGAGCTCTGGA-3'.
The sequences of the β-actin primers were forward,
5'-ATCTGGCACCACACCTTCACAATGAGCTGCG-3' and reverse,
5'-CGTCATACTCCTGCTTGCTGATCCACATCTGC-3'. The reaction system (25 µl)
was composed of 12.5 µl SYBR Premix Ex Taq, 0.5 µl upstream primer,
0.5 µl downstream primer, 1 µl cDNA and 10.5 µl ddH2O.
The thermocycling conditions were as follows: Initial denaturation
at 95˚C for 2 min, followed by 35 cycles of denaturation at 95˚C
for 30 sec, annealing at 56˚C for 30 sec and elongation at 72˚C for
1 min, final elongation at 72˚C for 2 min (iQ5 instrument; Bio-Rad
Laboratories, Inc.). The 2-ΔΔCq method (16) was used to calculate the relative
expression of FOXM1 mRNA against β-actin. Each sample was tested in
triplicate.
The expression of miR-21 was determined using
miRcute miRNA qPCR Detection kit (SYBR Green; Tiangen Biotech Co.,
Ltd.) with U6 as an internal reference. The sequences of the miR-21
primers were forward, 5'-GCCCGCTAGCTTATCAGACTGATG-3' and reverse,
5'-GTGCAGGGTCCGAGGT-3'. The sequences of the U6 primers were
forward, 5'-CTCGCTTCGGCAGCACA-3' and reverse,
5'-AACGCTTCACGAATTTGCGT-3'. The reaction system (20 µl) contained
10 µl miRcute miRNA pre-mix, 0.5 µl upstream primer, 0.5 µl
downstream primer, 2 µl cDNA and 7 µl ddH2O. The
thermocycling conditions were as follows: Initial denaturation at
95˚C for 5 min; followed by 40 cycles of denaturation at 95˚C for
15 sec, annealing at 56˚C for 25 sec and elongation at 72˚C for 40
sec (iQ5 instrument). The 2-ΔΔCq method (16) was used to calculate the relative
expression of miR-21 against U6. Each sample was tested in
triplicate.
Western blotting
Tissues (100 mg) were ground into powder, and cells
(1x106) were trypsinized and collected. The samples were
then lysed using radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology) for 30 min on ice. After
centrifugation at 1,500 x g and 4˚C for 10 min, the supernatant was
aspirated for the determination of protein concentration using a
bicinchoninic acid assay kit. The samples were then mixed with 5X
sodium dodecyl sulfate (SDS) loading buffer before denaturation in
boiling water for 5 min. The samples (50 µg/lane) were subjected to
10% SDS-polyacrylamide gel electrophoresis at 100 V. The resolved
proteins were transferred to polyvinylidene difluoride membranes on
ice (100 V, 2 h) and blocked with 5% skimmed milk (ddH2O
as diluent) at room temperature for 1 h. The membranes were
subsequently incubated with rabbit anti-human FOXM1 (1:2,000; cat.
no. ab175798; Abcam) or β-actin (1:5,000; cat. no. ab129348; Abcam)
polyclonal primary antibodies at 4˚C overnight. After extensive
washing with phosphate-buffered saline (PBS) supplemented with 0.1%
Tween-20 three times for 15 min, the membranes were incubated with
goat anti-rabbit horseradish peroxidase-conjugated secondary
antibody (1:3,000; cat. no. ab6721; Abcam) for 1 h at room
temperature before washing with PBS with Tween-20 three times for
15 min. The membranes were visualized using an enhanced
chemiluminescence detection kit (cat. no. ab65623; Abcam). Image
lab v3.0 software (Bio-Rad Laboratories, Inc.) was used to acquire
and analyze densitometry data. The relative expression of target
protein was normalized against β-actin expression.
Enzyme-linked immunosorbent assay
(ELISA)
FOXM1 ELISA kit (cat. no. CSB-EL008828HU; Cusabio
Biotech Co., Ltd.) was used to determine the concentration of
FOXM1. In microplates, standards (50 µl) and samples (10 µl serum
and 40 µl diluent) were added to certain wells whilst blank wells
were left empty. In the wells containing standards and samples,
horseradish peroxidase-labeled conjugates (100 µl) were added and
the plates were then sealed for incubation at 37˚C for 1 h. After
washing the plates five times, substrates A and B (50 µl of each)
were added to each well. After incubation at 37˚C for 15 min, stop
solution (50 µl) was added into each well, and the absorbance at
450 nm was measured within 15 min of stop solution being added.
Bioinformatics
To understand the regulatory mechanism of FOXM1,
miRanda (http://www.microrna.org/microrna/home.do) (17), TargetScan (http://www.targetscan.org) (18), PiTa (http://genie.weizmann.ac.il/pubs/mir07/mir07_data.html)
(19), RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/)
(20) and PICTA (http://pictar.mdc-berlin.de/) (21) were used to predict miRNAs that might
regulate FOXM1.
Dual-luciferase reporter assay
Potential wild-type (WT) and mutant seed regions of
miR-21 in the 3'-UTR of FOXM1 gene were first synthesized in
vitro (Sangon Biotech Co., Ltd.) and then cloned into
pMIR-REPORT luciferase reporter plasmids (Thermo Fisher Scientific,
Inc.) using SpeI and HindIII restriction sites.
Plasmids encoding either WT or mutant 3'-untranslated region (UTR)
sequences (0.8 µg) were co-transfected with agomiR-21 (100 nM;
Sangon Biotech Co., Ltd.) into 2x105 293T cells (The
Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences) at 37˚C for 24 h according to the manufacturer's manual.
As a control, 293T cells were transfected with empty plasmids and
agomiR-21 (NC group). After 24 h further incubation, the cells were
lysed using the Dual-Luciferase® reporter assay kit
(Promega Corporation) according to the manufacturer's protocol, and
luminescence intensity was measured using the GloMax®
20/20 luminometer (Promega Corporation). Using Renilla
luminescence activity as internal reference, the luminescence
values of each group of cells were measured.
MTT assay
Cells were first seeded into 96-well plates at a
density of 2x103 cells/well. Each condition was tested
in triplicate. At 24, 48 and 72 h after transfection, 20 µl MTT (5
g/l) solution was added to each well, followed by incubation for 4
h at 37˚C. After aspiration of medium, DMSO (150 µl/well) was added
to dissolve the formazan crystals. Absorbance at 490 nm was
measured in each well using a microplate reader (Bio-Rad
Laboratories, Inc.), and the results were used to plot cell
viability curves.
Statistical analysis
Results were analyzed using SPSS 20.0 statistical
software (IBM Corp.). Data are expressed as the mean ± standard
deviation. Data were tested for normality using the
Kolmogorov-Smirnov test. Differences among multiple groups were
analyzed using one-way ANOVA, and Student-Newman-Keuls test as
post-hoc test. Comparisons between two groups were carried out
using Student's t-test. The χ2 test was used to test the
association between PE and miR-21 expression. P<0.05 was
considered to indicate a statistically significant difference.
Results
Occurrence of PE is associated with
the reduced expression of FOXM1 mRNA
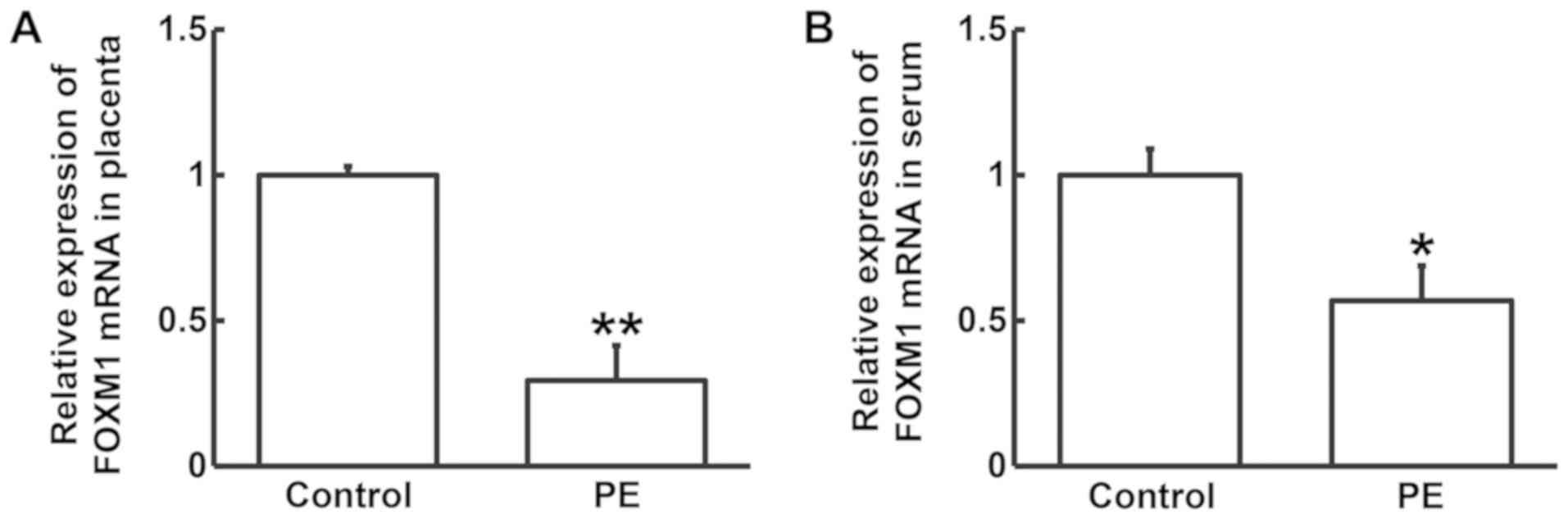
To measure FOXM1 mRNA expression in pregnant women
with and without PE, RT-qPCR analysis was performed. The expression
of FOXM1 mRNA in placental tissues and serum samples from PE
patients was found to be significantly lower compared with that in
the control group (P<0.05; Fig.
1A and B). This finding suggests
that the occurrence of PE is associated with FOXM1 mRNA
expression.
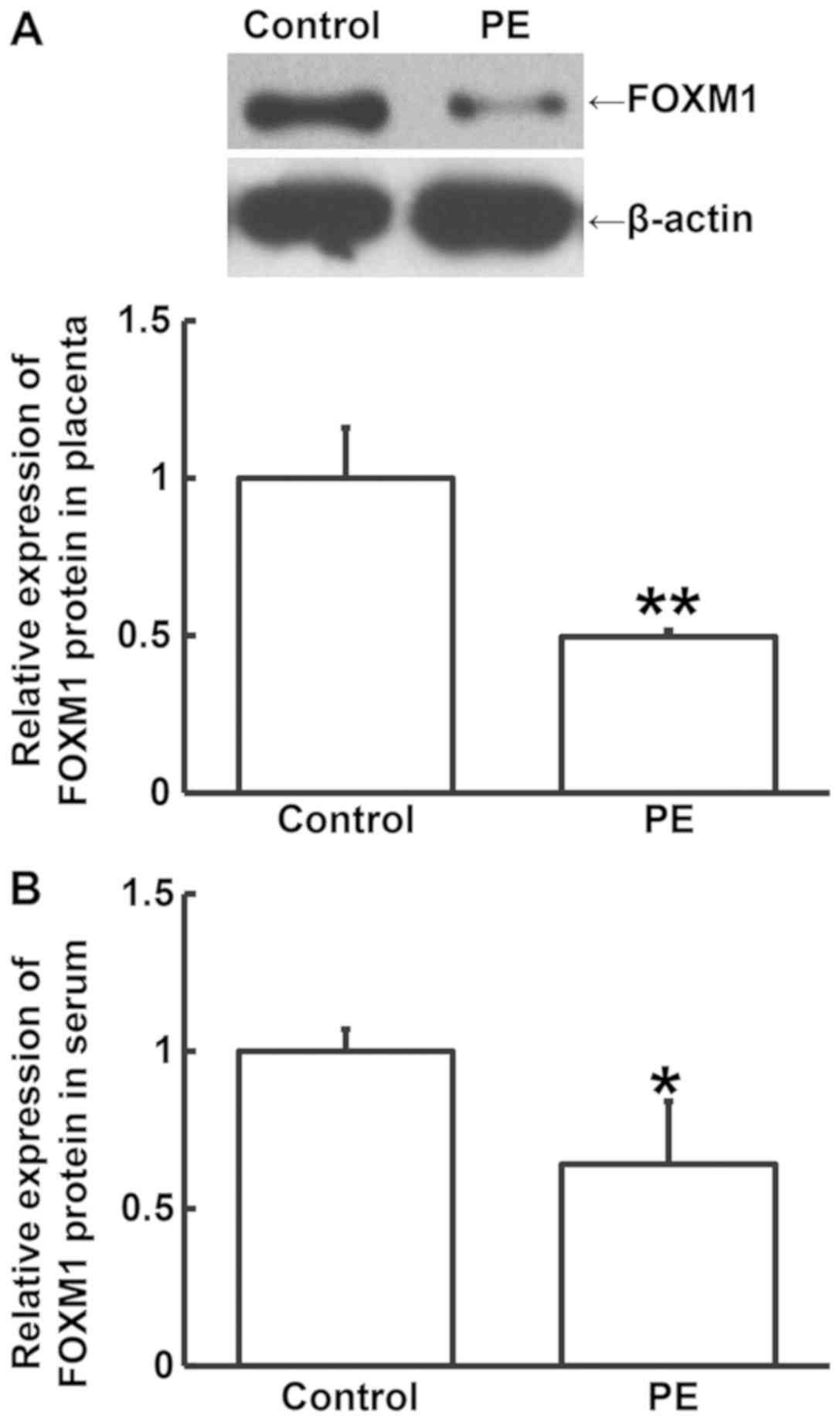
Decreased levels of FOXM1 protein may
serve a regulatory role in the occurrence of PE
Western blotting and ELISA were performed to measure
FOXM1 protein expression in placental tissues and serum samples,
respectively. FOXM1 protein expression in the placental tissues
from PE patients was significantly lower compared with that from
the control group (P<0.05; Fig.
2A). Similarly, the levels of circulating FOXM1 protein in
serum from PE patients was significantly lower compared with that
from the control group (P<0.05; Fig.
2B). These results suggest that decreased FOXM1 protein levels
may serve a regulatory role in the occurrence of PE.
miR-21 may serve a regulatory role in
the pathology of PE by affecting the expression of FOXM1 at the
transcriptional level
Using the listed bioinformatics tools, this present
study found 1,100 miRNAs that putatively target FOXM1. For example,
when using miRanda for prediction, the mirSVR score was -0.2837 and
the PhastCons score was 0.6586. Since the importance of miR-21 in
human diseases has been confirmed before, and the score obtained
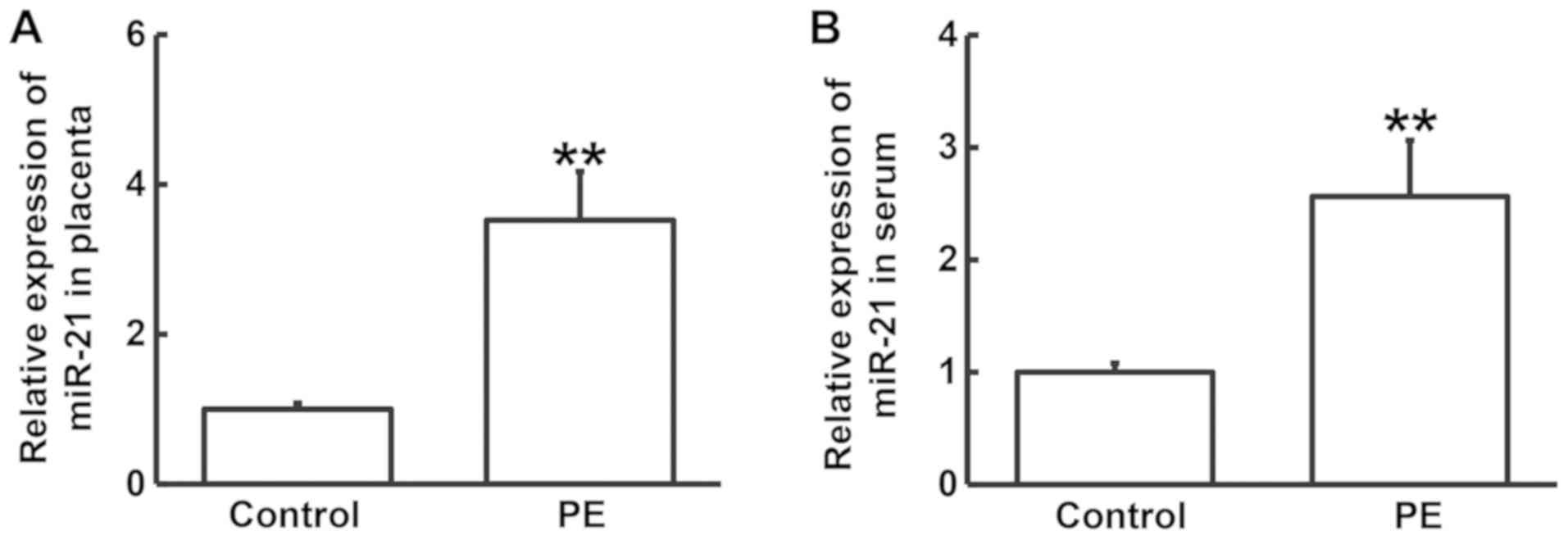
was relatively high, miR-21 was chosen for further study (Fig. 3). RT-qPCR was performed to determine
the levels of miR-21 in the placental tissues and serum samples of
PE patients and healthy controls. The expression of miR-21 in the
placental tissues and serum samples from PE patients was
significantly higher compared with those from the control group
(P<0.05; Fig. 4). For further
analysis, PE patients and control subjects were each divided into a
positive group and a negative group. If the relative expression of
miR-21, when normalized to the overall median expression levels of
miR-21, in the PE patients or control subjects was >1, they were
assigned to the positive group, whereas those with a relative
miR-21 expression of <1 were allocated to the negative group.
Analysis using the χ2 test did not indicate an
association between PE and miR-21 expression (P>0.05; Table I). However, these observations
suggest that miR-21 may serve a regulatory role in the pathology of
PE by regulating FOXM1 expression.
 | Table IAssociation analysis of miR-21
expression in PE patients and control subjects using the
χ2 test. |
Table I
Association analysis of miR-21
expression in PE patients and control subjects using the
χ2 test.
| Groups |
Positivea |
Negativeb | Total |
|---|
| Control | 18 | 10 | 28 |
| PE | 22 | 10 | 32 |
| P-value | | | 0.714393038 |
|
χ2-value | | | 0.133928571 |
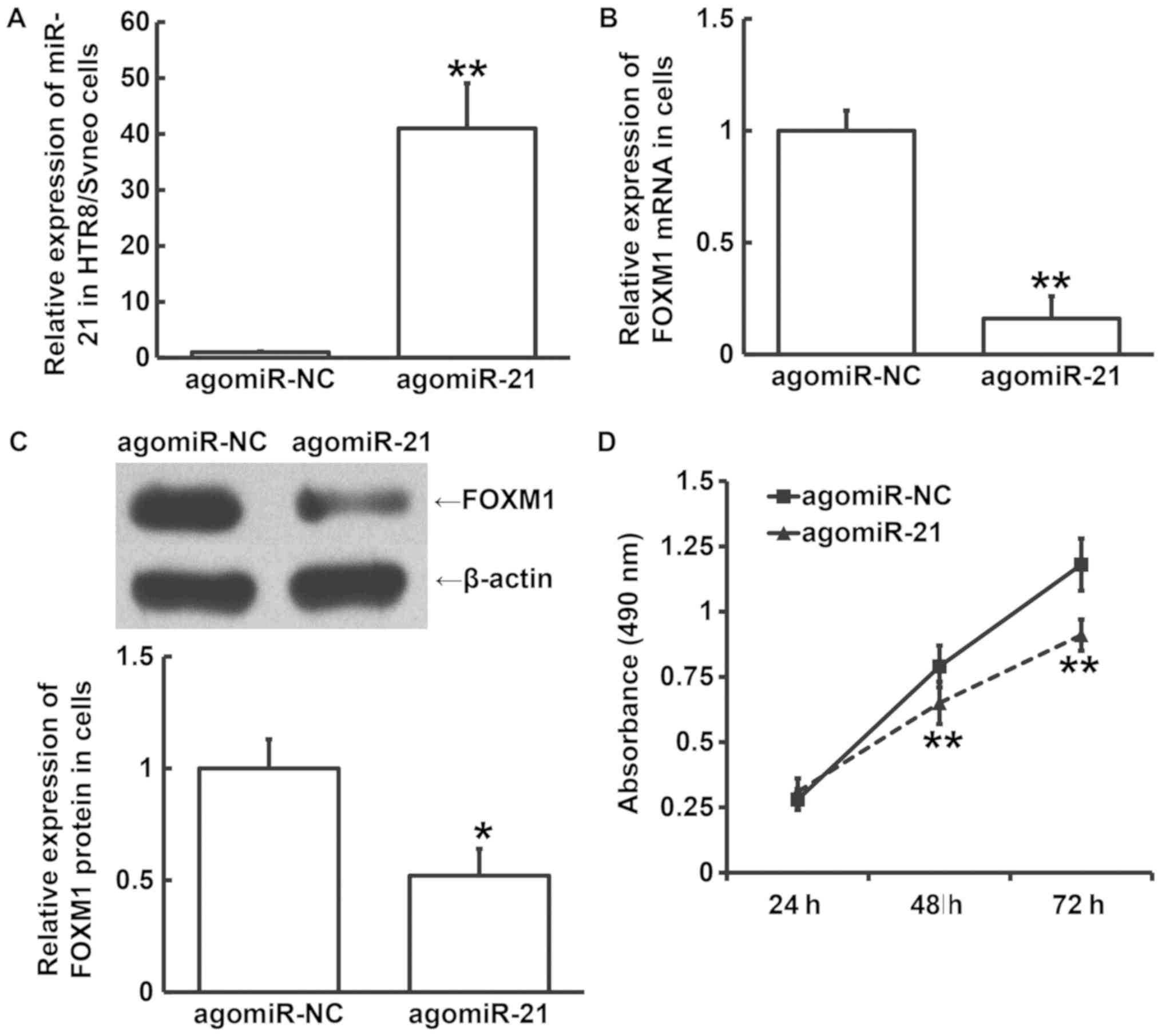
Overexpression of miR-21 may inhibit
the viability of HTR8/SVneo cells by reducing the expression of
FOXM1
To assess the effect of miR-21 on the expression of
FOXM1 and HTR8/SVneo cell viability, RT-qPCR, western blotting and
MTT assays were performed following transfection with agomiR-21.
The data showed that the expression of miR-21 in cells transfected
with agomiR-21 was significantly higher compared with that in the
agomiR-NC group (P<0.05; Fig.
5A). In addition, FOXM1 mRNA and protein expression in cells
transfected with agomiR-21 was significantly reduced compared with
that in the agomiR-NC group (P<0.05; Fig. 5B and C). The MTT assay results showed that the
absorbance values of cells transfected with agomiR-21 were
significantly lower compared with those in the agomiR-NC group
after 48 and 72 h (P<0.05; Fig.
5D). These findings indicate that miR-21 overexpression
inhibits HTR8/SVneo cell viability, possibly by reducing the
expression of FOXM1.
miR-21 directly binds to the 3'-UTR of
FOXM1 to regulate expression
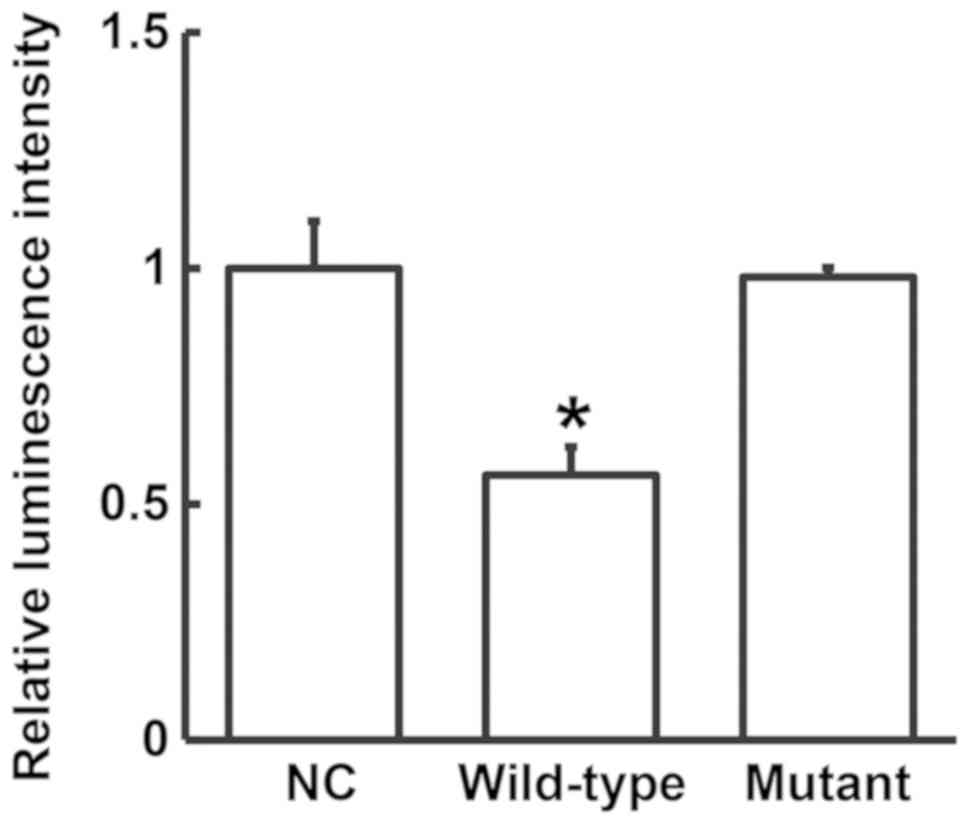
To test whether FOXM1 is a direct target of miR-21,
a dual-luciferase reporter assay was performed. The luminescence
intensity of the wild-type group was found to be significantly
lower compared with that in the NC group (P<0.05), whilst no
significant difference was observed between the mutant group and
the NC group (P>0.05; Fig. 6).
This suggests that miR-21 regulates FOXM1 expression by directly
binding to its 3'-UTR.
Discussion
Placental hypoperfusion leads to placental ischemia,
hypoxia and metabolic disorders, resulting in the production of
placenta-derived toxic factors, such as IL-6, IL-8 and TNF-α
(22,23). Additionally, vascular endothelial
injury and imbalance of vasoactive substances occur, resulting in a
series of severe pathological changes, extensive vascular
endothelial injury and finally PE (24). PE is reported to have two stages. The
first stage is characterized by placental hypoxia caused by
diminished villous trophoblast invasion and impaired vascular
remodeling. The second stage is characterized by an imbalance
between the reactive oxygen species (ROS) production system and the
antioxidant defense system, caused by the secretion of active
polypeptides into the maternal blood circulation and deposition of
ROS under the vascular endothelium (25,26).
This imbalance between the ROS production system and antioxidant
defense system aggravates maternal systemic arteriole injury,
leading to hypertension and proteinuria (27). The whole process may be associated
with reductions in placental trophoblast infiltration, ischemia,
hypoxia, increased apoptosis and abnormal lipid metabolism during
pregnancy (28-30).
FOXM1 is widely expressed in proliferating mammalian
cells and is an important mitosis-promoting factor (15,31). It
serves important roles in cell proliferation, differentiation,
senescence, organ formation, DNA damage repair, tumor formation and
invasion (32). FOXM1 is expressed
in trophoblasts and also in maternal decidual cells (12), whereby it indirectly regulates
trophoblast invasion through paracrine signaling to prevent
excessive invasion (11). As a
result, trophoblast invasion is a tightly regulated process
(12). In addition, PE is regulated
by various microRNAs. For example, miR-134 has previously been
found to inhibit trophoblast cell infiltration in the placenta of
patients with PE by reducing integrin subunit β1 expression
(33). These reports show that FOXM1
may be closely associated with the pathogenesis of PE.
The present study showed that FOXM1 mRNA and protein
expression in PE patients is reduced compared with that in healthy
pregnant women. Since FOXM1 may indirectly regulate trophoblast
invasion in a paracrine manner as aforementioned, reductions in
FOXM1 expression may reduce trophoblast invasion into the
endometrium.
As mRNA regulators, miRNAs are widely involved in a
number of pathophysiological processes, including tumor cell
proliferation, invasion and metastasis, hypertension, diabetes
mellitus and atherosclerosis (34).
As biomarkers, miRNAs are released into the blood by normal or
injured cells and participate in cell signaling transduction and
genetic transformation (35).
According to the bioinformatics analysis in the present study,
miR-21 is closely associated with FOXM1, implicating it as a
potential upstream regulator of FOXM1 expression. Previous studies
have shown that the expression of miR-21 is aberrantly upregulated
in esophageal cancer (36), liver
cancer (37), cervical cancer
(38), ovarian cancer (39), oral cancer (40), head and neck cancer (41), malignant glioma (42) and chronic lymphocytic leukemia
(43). In addition, miR-21 has also
been found to be upregulated in cell lines of lung cancer (44), colorectal cancer (45), Hodgkin lymphoma (46) and head and neck cancer (47). Therefore, the abnormal upregulation
of miR-21 usually suggests the existence of human diseases. In the
present study, it was found that miR-21 expression was elevated in
both placenta tissues and peripheral blood samples, consistent with
previous reports listed above (36-47).
Upregulation of miR-21 expression in HTR8/SVneo cells by agomiR-21
transfection demonstrated that FOXM1 mRNA and protein expression
was downregulated in cells overexpressing miR-21, and cell
viability was also reduced. Dual-luciferase reporter assay
demonstrated that FOXM1 is indeed a direct binding target of
miR-21. These observations suggest that the downregulation of FOXM1
by increased miR-21 expression reduces the proliferation of
trophoblasts, which attenuates their ability to infiltrate the
endometrium. Therefore, miR-21 is a potential target for PE
therapy, although its mechanism in trophoblast physiology requires
further and more refined research.
In conclusion, the present study demonstrates that
the human body regulates the viability of trophoblasts during PE by
upregulating miR-21 and thereby downregulating FOXM1 mRNA and
protein expression. In addition, miR-21 could possibly be a
therapeutic target for PE treatment. However, the exact effect and
mechanism of action of miR-21 in PE remain to be investigated
further at cellular, animal and clinical levels.
Acknowledgements
The authors thank Dr Muling Zhang of Huaian First
People's Hospital, Nanjing Medical University for her advice.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
The final version of the manuscript has been read
and approved by all authors. FZ, YS and HW collaborated to design
the study. FZ, YS and QG were responsible for performing
experiments. FZ, YS and QG analyzed the data. All authors
collaborated to interpret results and develop the manuscript.
Ethics approval and consent to
participate
All procedures performed in the present study were
approved by the Ethics Committee of Nanjing Medical University.
Written informed consent was obtained from all patients or their
families.
Patient consent for publication
Written informed consent for the publication of any
associated data and accompanying images were obtained from all
patients or their parents, guardians or next of kin.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cetin O, Guzel Ozdemir P, Kurdoglu Z and
Sahin HG: Investigation of maternal psychopathological symptoms,
dream anxiety and insomnia in preeclampsia. J Matern Fetal Neonatal
Med. 30:2510–2515. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sones JL and Davisson RL: Preeclampsia, of
mice and women. Physiol Genomics. 48:565–572. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Baumwell S and Karumanchi SA:
Pre-eclampsia: Clinical manifestations and molecular mechanisms.
Nephron Clin Pract. 106:c72–c81. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Brooks SA, Martin E, Smeester L, Grace MR,
Boggess K and Fry RC: miRNAs as common regulators of the
transforming growth factor (TGF)-β pathway in the preeclamptic
placenta and cadmium-treated trophoblasts: Links between the
environment, the epigenome and preeclampsia. Food Chem Toxicol.
98:50–57. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zuckerwise L, Li J, Lu L, Men Y, Geng T,
Buhimschi CS, Buhimschi IA, Bukowski R, Guller S, Paidas M and
Huang Y: H19 long noncoding RNA alters trophoblast cell migration
and invasion by regulating TβR3 in placentae with fetal growth
restriction. Oncotarget. 7:38398–38407. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gao F, Bian F, Ma X, Kalinichenko VV and
Das SK: Control of regional decidualization in implantation: Role
of FoxM1 downstream of Hoxa10 and cyclin D3. Sci Rep.
5(13863)2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xie Y, Cui D, Sui L, Xu Y, Zhang N, Ma Y,
Li Y and Kong Y: Induction of forkhead box M1 (FoxM1) by EGF
through ERK signaling pathway promotes trophoblast cell invasion.
Cell Tissue Res. 362:421–430. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Vaswani K, Hum MW, Chan HW, Ryan J,
Wood-Bradley RJ, Nitert MD, Mitchell MD, Armitage JA and Rice GE:
The effect of gestational age on angiogenic gene expression in the
rat placenta. PloS One. 8(e83762)2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Massimiani M, Salvi S, Piccirilli D and
Vecchione L: A4. EGFL7 in placenta trophoblast and endothelial
cells: Implications in the pathogenesis of pre-eclampsia. J
Maternal Fetal Med. 29:4. 2016.
|
|
10
|
Shah DA and Khalil RA: Bioactive factors
in uteroplacental and systemic circulation link placental ischemia
to generalized vascular dysfunction in hypertensive pregnancy and
preeclampsia. Biochem Pharmacol. 95:211–226. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Knofler M and Pollheimer J: IFPA Award in
Placentology lecture: molecular regulation of human trophoblast
invasion. Placenta. 33 (Suppl):S55–S62. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ji L, Brkic J, Liu M, Fu G, Peng C and
Wang YL: Placental trophoblast cell differentiation: physiological
regulation and pathological relevance to preeclampsia. Mol Aspects
Med. 34:981–1023. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gao F, Feng J, Yao H, Li Y, Xi J and Yang
J: LncRNA SBF2-AS1 promotes the progression of cervical cancer by
regulating miR-361-5p/FOXM1 axis. Artif Cells Nanomed Biotechnol.
47:776–782. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xia N, Tan WF, Peng QZ and Cai HN:
MiR-374b reduces cell proliferation and cell invasion of cervical
cancer through regulating FOXM1. Eur Rev Med Pharmacol Sci.
23:513–521. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhu H: Forkhead box transcription factors
in embryonic heart development and congenital heart disease. Life
Sci. 144:194–201. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Betel D, Wilson M, Gabow A, Marks DS and
Sander C: The microRNA.org resource: Targets and expression.
Nucleic Acids Res. 36:D149–D153. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 12(4)2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang Z, Tang D, Wang B, Wang Z and Liu M:
Analysis of miRNA-mRNA regulatory network revealed key genes
induced by aflatoxin B1 exposure in primary human hepatocytes. Mol
Genet Genomic Med. 7(e971)2019.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Rehmsmeier M, Steffen P, Hochsmann M and
Giegerich R: Fast and effective prediction of microRNA/target
duplexes. RNA. 10:1507–1517. 2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Krek A, Grün D, Poy MN, Wolf R, Rosenberg
L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M
and Rajewsky N: Combinatorial microRNA target predictions. Nat
Genet. 37:495–500. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Guibourdenche J, Leguy MC and Tsatsaris V:
Biology and markers of preeclampsia. Ann Biol Clin (Paris).
71:79–87. 2013.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
23
|
van Beek E and Peeters LL: The
pathogenesis of preeclampsia. Ned Tijdschr Geneeskd. 141:1379–1384.
1997.PubMed/NCBI(In Dutch).
|
|
24
|
Nissaisorakarn P, Sharif S and Jim B:
Hypertension in pregnancy: Defining blood pressure goals and the
value of biomarkers for preeclampsia. Curr Cardiol Rep.
18(131)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Goldman-Wohl DS and Yagel S: Examination
of distinct fetal and maternal molecular pathways suggests a
mechanism for the development of preeclampsia. J Reprod Immunol.
76:54–60. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Austgulen R: Recent knowledge on
mechanisms underlying development of pre-eclampsia. Tidsskr Nor
Laegeforen. 124:21–24. 2004.PubMed/NCBI(In Norwegian).
|
|
27
|
Xuan RR, Niu TT and Chen HM: Astaxanthin
blocks preeclampsia progression by suppressing oxidative stress and
inflammation. Mol Med Rep. 14:2697–2704. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bokslag A, van Weissenbruch M, Mol BW and
de Groot CJ: Preeclampsia; Short and long-term consequences for
mother and neonate. Early Hum Dev. 102:47–50. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gilani SI, Weissgerber TL, Garovic VD and
Jayachandran M: Preeclampsia and extracellular vesicles. Curr
Hypertens Rep. 18(68)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Karumanchi SA and Granger JP: Preeclampsia
and pregnancy-related hypertensive disorders. Hypertension.
67:238–242. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nestal de Moraes G, Bella L, Zona S,
Burton MJ and Lam EW: Insights into a critical role of the
FOXO3a-FOXM1 Axis in DNA damage response and genotoxic drug
resistance. Curr Drug Targets. 17:164–177. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zona S, Bella L, Burton MJ, Nestal de
Moraes G and Lam EW: FOXM1: An emerging master regulator of DNA
damage response and genotoxic agent resistance. Biochim Biophys
Acta. 1839:1316–1322. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zou AX, Chen B, Li QX and Liang YC:
MiR-134 inhibits infiltration of trophoblast cells in placenta of
patients with preeclampsia by decreasing ITGB1 expression. Eur Rev
Med Pharmacol Sci. 22:2199–2206. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cantini L, Isella C, Petti C, Picco G,
Chiola S, Ficarra E, Caselle M and Medico E: MicroRNA-mRNA
interactions underlying colorectal cancer molecular subtypes. Nat
Commun. 6(8878)2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nakamura T, Canaani E and Croce CM:
Oncogenic All1 fusion proteins target Drosha-mediated microRNA
processing. Proc Natl Acad Sci USA. 104:10980–10985.
2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Feber A, Xi L, Luketich JD, Pennathur A,
Landreneau RJ, Wu M, Swanson SJ, Godfrey TE and Litle VR: MicroRNA
expression profiles of esophageal cancer. J Thorac Cardiovasc Surg.
135:D255–D260. 2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu C, Yu J, Yu S, Lavker RM, Cai L, Liu
W, Yang K, He X and Chen S: MicroRNA-21 acts as an oncomir through
multiple targets in human hepatocellular carcinoma. J Hepatol.
53:98–107. 2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lui WO, Pourmand N, Patterson BK and Fire
A: Patterns of known and novel small RNAs in human cervical cancer.
Cancer Res. 67:6031–6043. 2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Iorio MV, Visone R, Di Leva G, Donati V,
Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, et
al: MicroRNA signatures in human ovarian cancer. Cancer Res.
67:8699–8707. 2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Gombos K, Horvath R, Szele E, Juhász K,
Gocze K, Somlai K, Pajkos G, Ember I and Olasz L: miRNA expression
profiles of oral squamous cell carcinomas. Anticancer Res.
33:1511–1517. 2013.PubMed/NCBI
|
|
41
|
Odar K, Boštjančič E, Gale N, Glavač D and
Zidar N: Differential expression of microRNAs miR-21, miR-31,
miR-203, miR-125a-5p and miR-125b and proteins PTEN and p63 in
verrucous carcinoma of the head and neck. Histopathology.
61:257–265. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ciafre SA, Galardi S, Mangiola A, Ferracin
M, Liu CG, Sabatino G, Negrini M, Maira G, Croce CM and Farace MG:
Extensive modulation of a set of microRNAs in primary glioblastoma.
Biochem Biophys Res Commun. 334:1351–1358. 2005.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Fulci V, Chiaretti S, Goldoni M, Azzalin
G, Carucci N, Tavolaro S, Castellano L, Magrelli A, Citarella F,
Messina M, et al: Quantitative technologies establish a novel
microRNA profile of chronic lymphocytic leukemia. Blood.
109:4944–4951. 2007.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K
and Yang GH: MicroRNA-21 (miR-21) represses tumor suppressor PTEN
and promotes growth and invasion in non-small cell lung cancer
(NSCLC). Clin Chim Acta. 411:846–852. 2010.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Asangani IA, Rasheed SA, Nikolova DA,
Leupold JH, Colburn NH, Post S and Allgayer H: MicroRNA-21 (miR-21)
post-transcriptionally downregulates tumor suppressor Pdcd4 and
stimulates invasion, intravasation and metastasis in colorectal
cancer. Oncogene. 27:2128–2136. 2008.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Gibcus JH, Tan LP, Harms G, Schakel RN, de
Jong D, Blokzijl T, Möller P, Poppema S, Kroesen BJ and van den
Berg A: Hodgkin lymphoma cell lines are characterized by a specific
miRNA expression profile. Neoplasia. 11:167–176. 2009.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Tran N, McLean T, Zhang X, Zhao CJ,
Thomson JM, O'Brien C and Rose B: MicroRNA expression profiles in
head and neck cancer cell lines. Biochem Biophys Res Commun.
358:12–17. 2007.PubMed/NCBI View Article : Google Scholar
|