Introduction
Diffuse large B-cell lymphoma (DLBCL) is highly
invasive and is the most common subtype of adult non-Hodgkin's
lymphoma (NHL), accounting for 30-40% of all NHL (1). Rituximab combined with CHOP-based
chemotherapy is considered a curative treatment, and 60-70% of
patients can be treated following first-line immune-chemotherapy,
while 30-40% of patients relapse at a certain point during the
disease (2-4).
Therefore, from the perspective of developing a comprehensive
treatment, it is crucial to identify novel therapeutics for DLBCL.
As important components of complementary and alternative medicines,
Traditional Chinese Medicines (TCM) have long been practiced in
China and are gaining popularity in the western countries, such as
the USA, UK and Germany (5-7).
According to TCM, factors impacting DLBCL development include
deficiencies in the internal organs and the accumulation of phlegm
(8). Previous studies have shown
that Yiqichutan as a decoction, which was developed to treat NHL,
can inhibit DLBCL cell growth and significantly reduce
phosphorylated-AKT expression level in DLBCL cells (8,9).
However, the underlying mechanism of Yiqichutan for treating DLBCL
remains elusive.
Expression profile analysis is widely used to
identify genetic variations in oncological research (10), thus, the present study use microarray
analysis to investigate the effects of Yiqichutan treatment on
DLBCL mRNAs. Previous studies have shown that the interaction
between microRNAs (miRNAs/miRs) and transcription factors may be
associated with the progression from low-grade to a highly
aggressive lymphoma, such as in DLBCL (11). Furthermore, previous studies have
shown that miR-34a acts as a strong tumor suppressor in solid
cancer types, including lung (12,13),
prostate (14), pancreatic (15), renal (16) and metastatic bone cancer (17). Moreover, epigenetic inactivation of
miR-34a by aberrant expression levels can be found in 18% of NHLs
(18). Bioinformatic target
prediction combined with functional analyses has revealed that the
oncogene C-MYC mRNA can be regulated by miR-34a (19), and acts via post-transcriptional
control of the transcription factor forkhead box (Fox) protein
family, especially Foxp1 which is a hematopoietic oncoprotein
overexpressed in DLBCL (20,21). Therefore, the C-MYC/miR-34a pathway
may be closely related to the occurrence and development of DLBCL.
Thus, the present study investigated the impact of Yiqichutan
treatment on the regulation of the C-MYC/miR-34a signaling pathway.
It was found that, Yiqichutan treatment affected DLBCL cells via
several signaling pathways and that Yiqichutan may inhibit the
proliferation of DLBCL cells by blocking the C-MYC/miR-34a
signaling pathway.
Materials and methods
Cell culture
Human DLBCL SUDHL-6 cells were gifted from The
Cancer Hospital of Sun Yat-sen University Cancer Center, and have
been authenticated using short tandem repeat matching analysis. The
cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) and 100 U/ml penicillin/streptomycin (Gibco; Thermo Fisher
Scientific, Inc.) at 37˚C with 5% CO2, and saturated
humidity. The medium was replaced every other day.
Yiqichutan
Yiqichutan treatment was composed of a decoction
prepared with 15 g American ginseng, 15 g Pinellia ternata,
15 g Cremastra appendiculata, 30 g Ranunculus
ternatus, 15 g Fritillaria thunbergii, 15 g Herba
sarcandrae, 10 g fried batryticated silkworm and 30 g
Ganoderma lucidum (http://www.theplantlist.org). The raw materials were
purchased from The First Affiliated Hospital of Guangzhou
University of Chinese Medicine, and cooled with purified water
three times for 30 min each, at 10, 8 and 8 times the weight of the
raw materials (w/v), sequentially. Pinellia ternate was
boiled (100˚C) for 30 min prior to use. The boiled water was
collected, filtered and condensed to 2 g of raw materials per ml in
the final preparation, then sealed in bags with a sealing machine
and stored at 4˚C for subsequent use.
mRNA microarray expression profiling,
Gene Ontology (GO) analysis and pathway enrichment analysis
Microarray hybridization was carried out by Shanghai
Genechem Co., Ltd. for the present study. After treatment for 48 h
(37˚C) with 14.65 mg/ml Yiqichutan or a control (sterile water) for
SUDHL-6 cells, three cell samples were collected from the
Yiqichutan treatment group and the control group. For each
treatment group of cells, the total RNA extracted with
TRlzol® (Invitrogen; Thermo Fisher Scientific, Inc.) was
purified according to the manufacturer's instructions and reverse
transcribed into cDNA using M-MLV reverse transcriptase (cat. no.
M1705; Promega Corporation) according to the manufacturer's
instructions. The purified, sense-strand cDNA was fragmented and
labelled by terminal deoxynucleotidyl transferase using a GeneChip
WT Pico kit (cat. no. 902623; Applied Biosystems; Thermo Fisher
Scientific, Inc.). The purified labeled cDNA was then subjected to
hybridization using Affymetrix Clariom S Genechip WT Pico reagent
kit (Clariom S Assay; Affymetrix; Thermo Fisher Scientific, Inc.)
following the manufacturer's instructions. An Affymetrix Gene ChIP
scanner was used to scan the microarrays, and the CEL files
generated were analyzed using Affymetrix Expression Console
software (version 4.0; Affymetrix; Thermo Fisher Scientific, Inc.).
The data were normalized using logarithmic transformation. The low
expressed genes detected in all the samples were selected for
elimination and further data analysis. Only genes with an ANOVA
P≤0.05 were considered as differentially expressed between the
experimental and control groups. GO analysis provides a controlled
vocabulary to describe gene and gene product attributes in any
organism (http://www.geneontology.org).
Fisher's exact test was used to detect overlap, which would be
expected by chance, between the differentially expressed list and
the GO annotation list. The P-value denotes the significance of GO
term enrichment among differentially expressed genes (P≤0.05). The
Kyoto Encyclopedia of Genes and Genomes (KEGG) is a set of
high-throughput genes and protein pathways (22). KEGG analyses can be found in the
following database: http://www.genome.jp/kegg. P≤0.05 (EASE-score, Fisher
P-value or hypergeometric P-value) denotes the significance of the
pathway correlations.
Extraction of total RNA and reverse
transcription-quantitative PCR (RT-qPCR)
At 48 h after treating with Yiqichutan,
5-10x106 cells per group were collected and total RNA
was isolated using TRIzol® reagent (cat. no. TR118-500;
Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. A total of 1 µg l extracted RNA was used
to synthesize cDNA in a reaction using oligo (dT) primers and M-MLV
reverse transcriptase (cat. no. M1705; Promega Corporation)
according to the manufacturer's instructions. PCR amplification of
mRNA and miRNA was performed with a GoTaq® qPCR Master
mix (Promega Corporation; cat. no. A6002) on an RT-qPCR instrument
(Bio-Rad Laboratories, Inc.; MiniOpticon). The following
thermocycling conditions used were: Initial denaturation at 95˚C
for 120 sec; 40 cycles of 95˚C for 15 sec, and a final extension at
60˚C for 30 sec. The PCR products were calculated with the
2-ΔΔCq method (23), using GAPDH as an mRNA internal
control and U6 as a miRNA internal control. The primers used for
amplifying specific genes are shown in Table SI.
Cell transfection
A total of 50 nM miRNA mimics (Shanghai GenePharma
Co., Ltd) and 200 nM small interfering RNAs (siRNAs; Shanghai
GenePharma Co., Ltd.) were used for transfection. The siRNA
targeting C-MYC sequences were as follows: C-MYC siRNA forward,
5'-GAACACACAACGUCU UGGATT-3' and reverse, 5'-UCCAAGACGUUGUGUGUU
CTT-3'; and siC-MYC-2 forward, 5'-AACGUUAGCUUCAC CAACATT-3' and
reverse, 5'-UGUUGGUGAAGCUAACGU UTT-3'. The hsa-miR-34a-5p mimics
sequences were as follows: Forward, 5'-UGGCAGUGUCUUAGCUGGUUGU-3'
and reverse, 5'-AACCAGCUAAGACACUGCCAUU-3'. Lipofectamine 2000
transfection reagent (Thermo Fisher Scientific, Inc.) was used for
cell transfection, following the manufacturer's instructions. Cells
were collected 48 h after transfection and the C-MYC expression
levels were determined by RT-qPCR as described above.
Western blot analysis
After being treated and cultured, the cells were
harvested and protein extractions were prepared with a modified
RIPA buffer (Beyotime Institute of Biotechnology) with 0.5% SDS in
the presence of a proteinase inhibitor cocktail (Beyotime Institute
of Biotechnology). A bicinchoninic acid protein concentration kit
(cat. no. P0011; Beyotime Institute of Biotechnology) was used to
determine protein concentration. A total of 20 µg protein/lane was
separated by 6% or 12% SDS-PAGE. Proteins were then transferred
onto a PVDF membrane. The membranes were then blocked with 5% BSA
containing TBS-0.05% Tween-20 for ~2 h at room temperature and
incubated overnight at 4˚C with the following primary antibodies:
Anti-C-MYC (1:1,000; cat. no. ab32072; Abcam), anti-Foxp1 (1:1,000;
cat. no. 4402; Cell Signaling Technology, Inc.) and GAPDH (1:1,000;
cat. no. ab8245; Abcam). After washing with PBS containing 0.1%
Tween-20 three times for 5 min each, the membranes were incubated
with horseradish peroxidase-linked immunoglobulin G (1:1,000; cat.
no. 7074; Cell Signaling Technology, Inc.) secondary antibody for 2
h at room temperature. The membranes were developed using an
enhanced chemiluminescence system (Guangzhou Forevergreen
Biosciences Co., Ltd.).
MTS assay
The viability of SUDHL-6 cells was evaluated by cell
proliferation rates measured with an MTS Cell Proliferation assay
kit (Promega Corporation; cat. no. G3580) following the
manufacturer's protocols. SUDHL-6 cells were incubated in 5%
CO2 and at 37˚C in 96-well microtiter plates at ~80%
confluency and treated with different concentrations of 4, 8, 12,
16 and 20 mg/ml of Yiqichutan. After 24, 48 and 72 h, SUDHL-6 cells
were incubated with 20 g of MTS reagent for 4 h at 37˚C in a
humidified culture chamber supplied with 5% CO2. The
optical density 490 nm values were measured using a plate reader
(Diatek DR-200Bs), and were used to evaluate cell proliferation and
viability. For statistical analysis, three replicates were carried
out.
Cell apoptosis analysis
Flow cytometry was used to perform cell apoptosis
analysis. The cells were incubated at 37˚C in six-well plates and
treated with 200 nM siRNAs or 50 nM miRNA mimics, or with different
concentrations of Yiqichutan (4, 8, 12, 16 and 20 mg/ml) for 48 h.
Cells were collected by centrifugation at 800 x g and 4˚C for 5 min
and washed with PBS buffer. The cells were stained with 5 µl APC
Annexin V (BD Biosciences) and 10 µl 7-AAD (BD Biosciences) for 15
min in the dark at room temperature, then analyzed by flow
cytometry (FACSAria III; BD Biosciences). A FACScalibur flow
cytometer (BD Biosciences) was used for analysis.
Statistical analysis
Data are presented as the mean ± SD. SPSS 18.0
statistical software (SPSS, Inc.) and GraphPad Prism 7.0 (GraphPad
Software, Inc.) were used for analysis. A Student's t-test was
performed to test the difference between two groups and one-way
ANOVA tests with Tukey's test were used to analyze differences
among groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
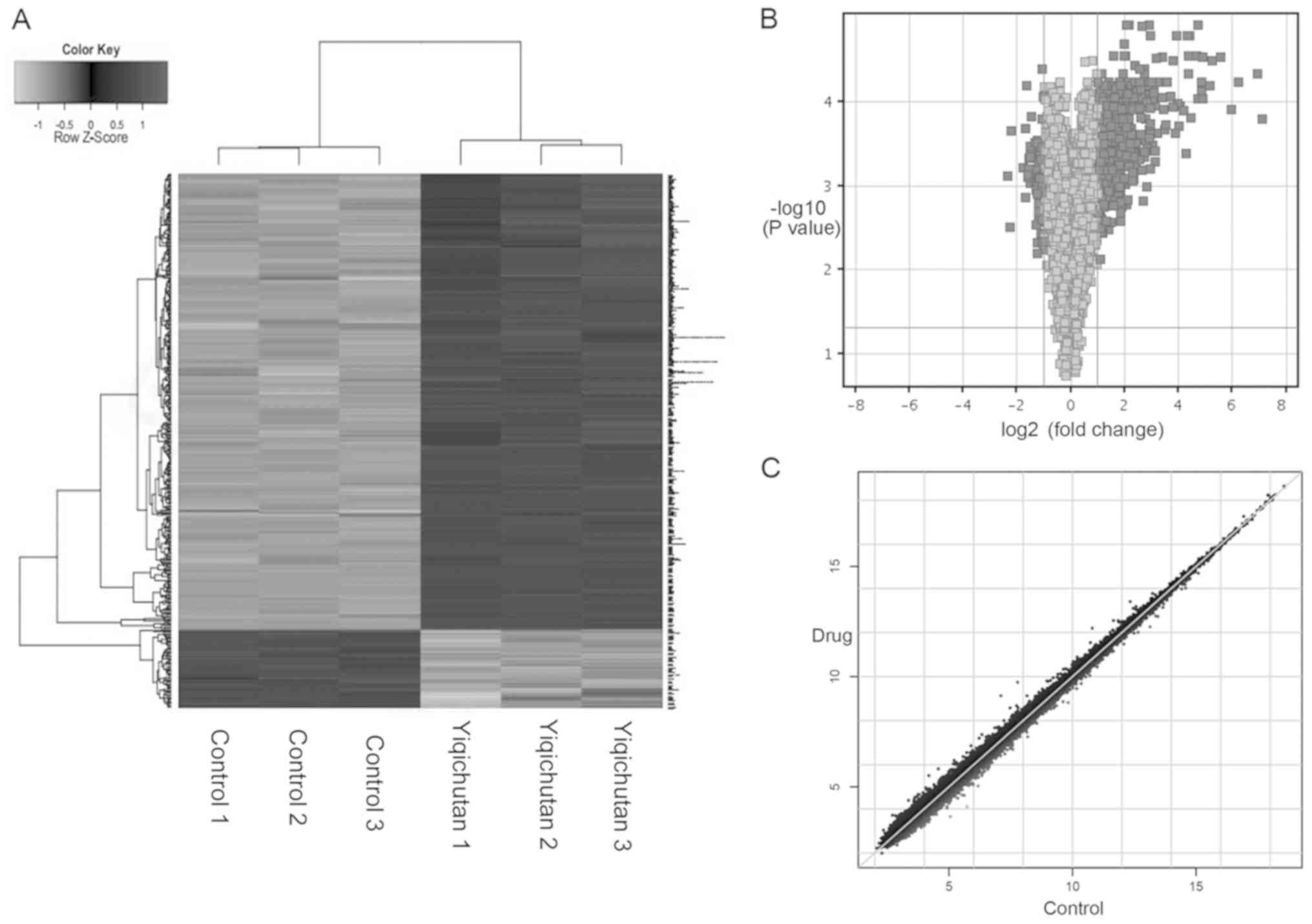
Comparison of gene expression profiles
between the two groups
The expression profiles of 21,448 common genes were
found in all the samples, and a total of 991 differentially
expressed genes were identified in SUDHL-6 cells treated with
Yiqichutan. Of these genes, 498 were upregulated and 493 genes were
downregulated, and a hierarchical clustering of mRNA expressions is
shown Fig. 1A. Furthermore, the
volcano and scatter plots indicated a significant variation of mRNA
expression level between the control group and the treatment group
(Fig. 1B and C).
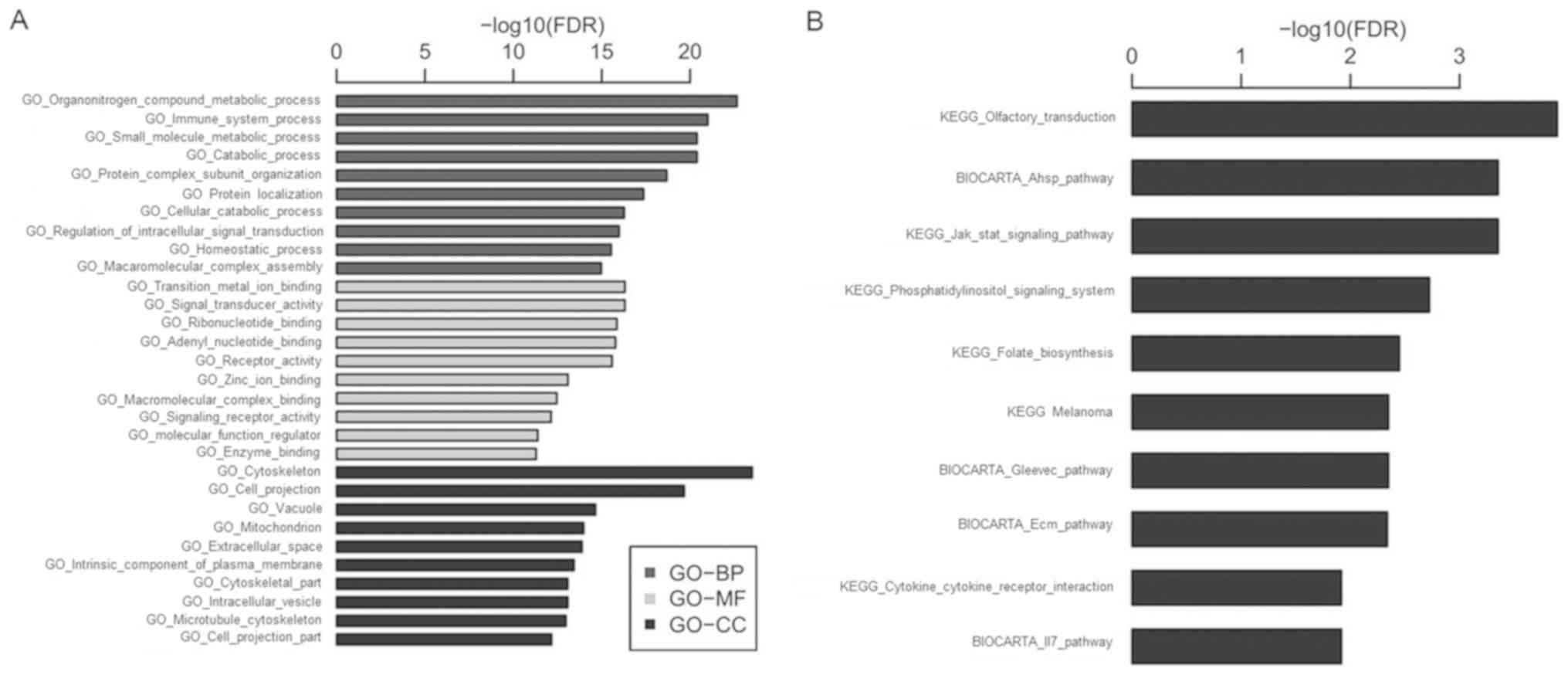
GO and KEGG pathway analysis
To investigate potential gene and gene product
enrichments in molecular functions, biological processes and
cellular components, GO analysis was performed with the
differentially expressed mRNAs (Fig.
2A). The differentially expressed genes were subjected to
pathway analysis based on the KEGG database and the associated 10
pathways are shown in Fig. 2B, which
included the Jak/Stat and PI3K signaling pathways. Thus, the
present results suggested that these pathways may contribute to the
pathogenesis and biochemical characteristics of DLBCL, and that
Yiqichutan may play a role in treating DLBCL via these
pathways.
Confirmation of the microarray data by
RT-qPCR analysis
While 991 mRNAs were found to have significant
changes in SUDHL-6 cells after treating with Yiqichutan in
microarray assays, to assess the reliability of the microarray data
several significantly differentially expressed genes were selected
for RT-qPCR analysis, including C-MYC, Mouse double minute 2
(MDM2), fibroblast growth factor 2 (FGF2), Dual specificity protein
phosphatase 16, suppressor of cytokine signaling 2 (SOCS2) and bone
morphogenetic protein receptor type 2. The RT-qPCR results were in
line with the microarray data as both showed the same trends
(P<0.05; Fig. 3). The oncogene
C-MYC encodes the nuclear transcription factor C-MYC, which is
involved in both the Jak/Stat and PI3K signaling pathways (24), which were the molecular pathways
identified in the KEGG analysis. Therefore, the C-MYC pathway was
selected for further analysis.
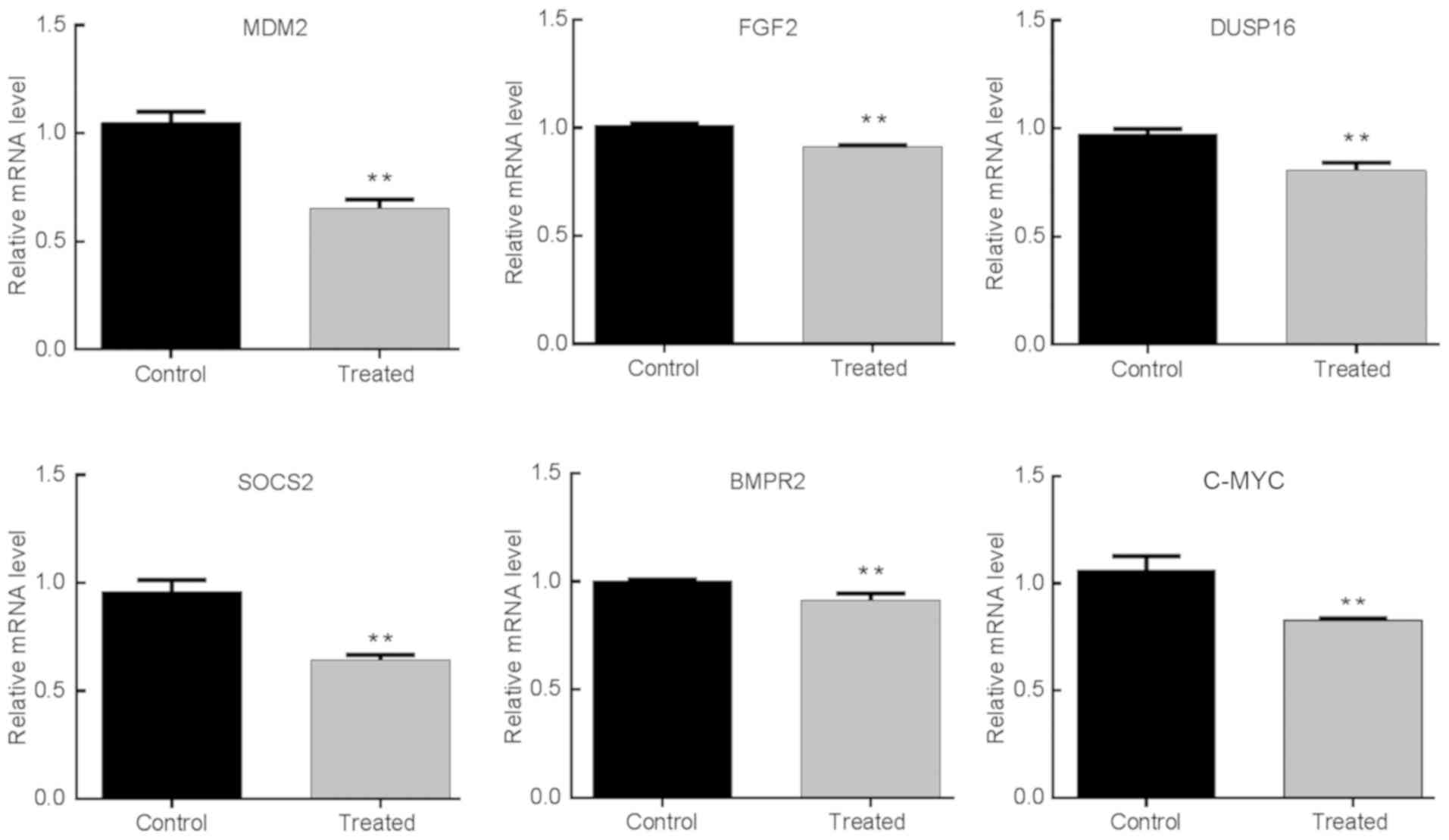 | Figure 3Confirmation of the microarray data
by RT-qPCR analysis. In total, six representative mRNAs expressions
in DLBCL SUDHL-6 cells were analyzed by RT-qPCR, including C-MYC,
MDM2, FGF2, DUSP16, SOCS2 and BMPR2. **P<0.01.
RT-qPCR, reverse transcription-quantitative PCR; MDM2, mouse double
minute 2; FGF2, fibroblast growth factor 2; DUSP16, Dual
specificity protein phosphatase 16; SOCS2, suppressor of cytokine
signaling 2; BMPR2, bone morphogenetic protein receptor type 2. |
Knockdown of C-MYC increases miR-34a
expression level and targets Foxp1
In order to identify whether the transcription
factor C-MYC was involved in the regulation of miR-34a to affect
the progression of DLBCL, SUDHL-6 cells were transfected with C-MYC
siRNAs and miR-34a mimics. It was found that the expression levels
of C-MYC and miR-34a in SUDHL-6 cells were significantly suppressed
and increased after treatment with C-MYC and miR-34a mimic,
respectively (Fig. 4A-C). Moreover,
C-MYC inhibition may increase the expression level of miR-34a and
reduce Foxp1 expression level. In addition, miR-34a overexpression
and decreased Foxp1 expression levels were more significant after
transfection with both C-MYC siRNAs and miR-34a mimics (Fig. 4D-F). Thus, the present results
suggested that C-MYC may be a key upstream mediator, and Foxp1 is a
key downstream mediator of miR-34a in SUDHL-6 cells. Therefore, the
effect of C-MYC knockdown on cell proliferation and apoptosis were
analyzed. The RT-qPCR results indicated that Bax and poly
(ADP-ribose) polymerase (PARP), key players in apoptosis, were
significantly upregulated with the depletion of C-MYC, with or
without miR-34a, while Bcl2 expression was significantly reduced.
Moreover, proliferating cell nuclear antigen (PCNA) expression
levels were significantly suppressed by C-MYC knockdown or miR-34a
mimics transfection in SUDHL-6 cells (Fig. 4G). Therefore, significantly altered
expression levels of these key markers suggested that the
regulation of C-MYC and miR-34a expression levels promoted DCBCL
progression via the apoptotic signaling pathway.
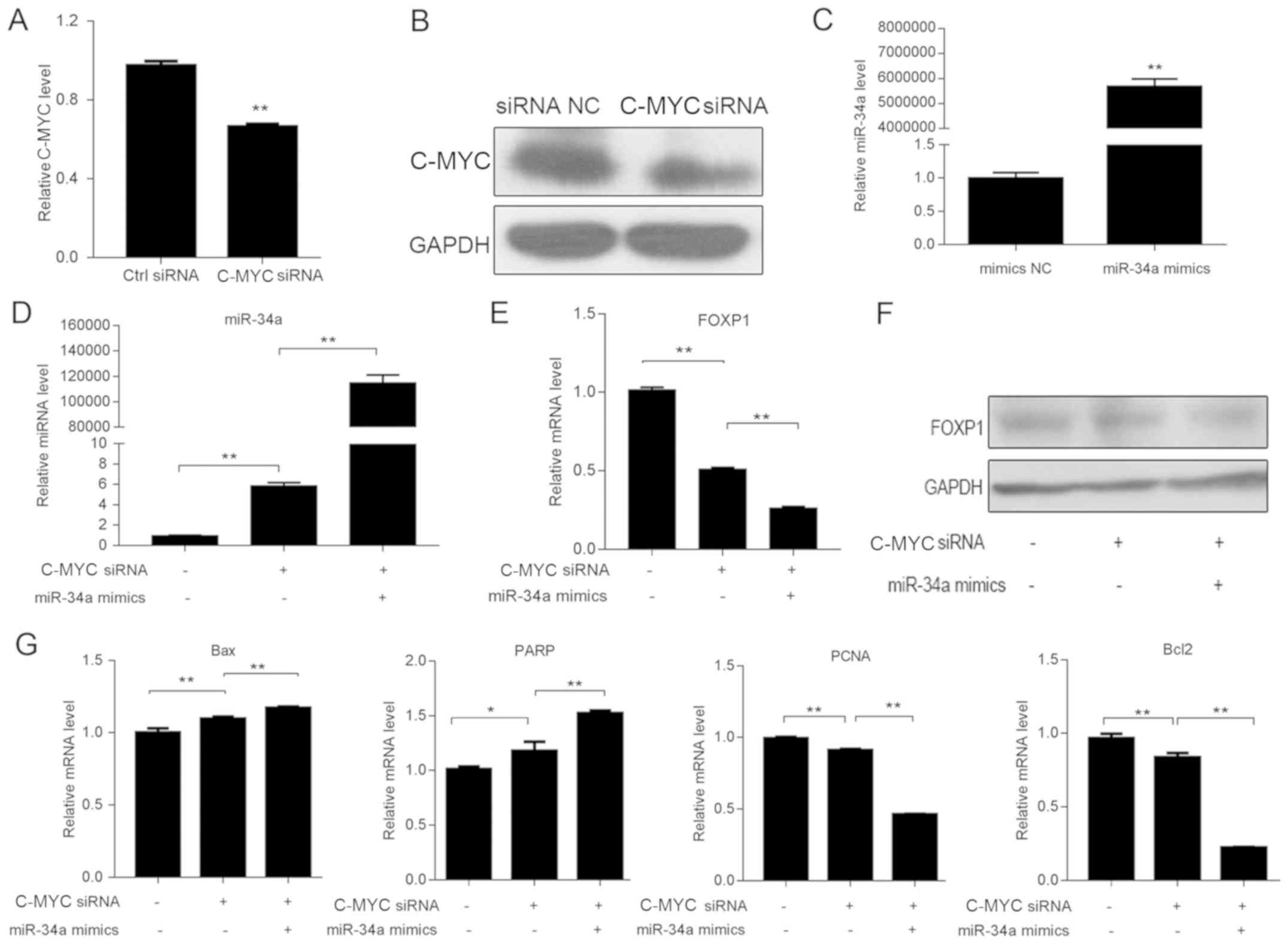 | Figure 4Knockdown of C-MYC increases miR-34a
expression level. Expression level of C-MYC in SUDHL-6 cells
transfected with siRNAs was determined by (A) RT-qPCR and (B)
western blotting. C-MYC siRNA could decrease the expression level
of C-MYC. (C) Expression level of miR-34a in SUDHL-6 cells
transfected with miR-34a NC and mimics was measured by RT-qPCR.
Relative mRNA expression levels of (D) miR-34a and (E) Foxp1 in
SUDHL-6 cells transfected with C-MYC siRNAs, with or without
miR-34a mimics, were analyzed by RT-qPCR. (F) Protein expression
level of Foxp1 was determined by western blotting. Data are
presented as the mean ± SD of three separate experiments. (G)
RT-qPCR results indicated that the expression levels of key factors
in proliferation and apoptosis, including Bax, Bcl2, PCNA and PARP,
were significantly altered with the depletion of C-MYC, with or
without miR-34a. *P<0.05, **P<0.01.
RT-qPCR, reverse transcription-quantitative PCR; NC, negative
control; miR, microRNA; siRNA, small interfering RNA; Foxp1,
forkhead box 1; PCNA, proliferating cell nuclear antigen; PARP,
poly (ADP-ribose) polymerase. |
Yiqichutan inhibits the growth of
DLBCL cells
To investigate the effect of Yiqichutan on DLBCL
cells, SUDHL-6 cells were treated with Yiqichutan and viability was
measured by MTS (Fig. 5A). It was
found that cell viability was inhibited after 4-20 mg/ml Yiqichutan
treatment in a concentration- and time-dependent manner compared
with the control group. The IC50 was 14.65 mg/ml for
SUDHL-6 cells 48 h after treatment.
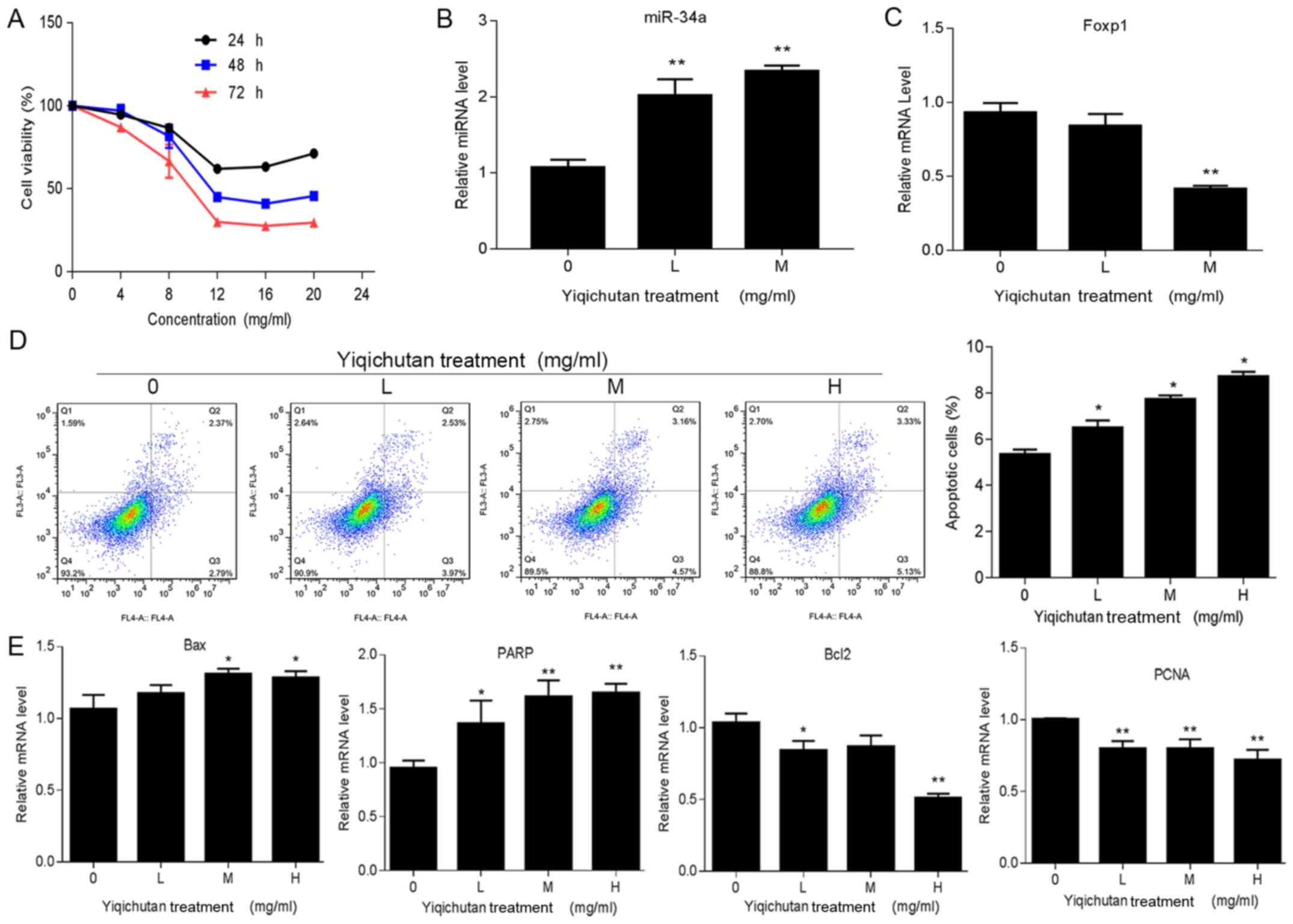 | Figure 5Effect of Yiqichutan on SUDHL-6
cells. (A) SUDHL-6 cells were treated with different concentrations
of Yiqichutan. The viability of SUDHL-6 cells was measured with an
MTS assay at 24, 48 and 72 h. (B) Cell apoptosis was detected by
flow cytometry. (C) Genes associated with proliferation, such as
PCNA, and apoptosis, including Bax, Bcl2 and PARP, signaling
pathways were measured by RT-qPCR. (D) Expressions levels of
miR-34a and Foxp1 in SUDHL-6 cells treated with Yiqichutan were
detected by RT-qPCR. (E) RT-qPCR results indicated that the
expression levels of Bax, Bcl2, PCNA and PARP, were significantly
altered after Yiqichutan treatment. IC50=14.65 mg/ml.
Data are presented as the mean ± SD of three separate experiments.
*P<0.05, **P<0.01. L, low concentration
of Yiqichutan 1/3 IC50; M, middle concentration, 1/2
IC50; H, high concentration, IC50. RT-qPCR,
reverse transcription-quantitative PCR; miR, microRNA; Foxp1,
forkhead box 1; PCNA, proliferating cell nuclear antigen; PARP,
poly (ADP-ribose) polymerase. |
Yiqichutan promotes apoptosis of tumor
cells
SUDHL-6 cells were treated with different
concentrations of Yiqichutan. Flow cytometric analysis results
suggested that Yiqichutan treatment promoted cell apoptosis in a
concentration-dependent manner (Fig.
5D)
Subsequently, a number of markers associated with
cell proliferation and apoptosis were measured by RT-qPCR (Fig. 5E). It was demonstrated that the
expression levels of Bax and PARP increased after Yiqichutan
treatment, while the Bcl2 expression level was significantly
reduced. Furthermore, the PCNA gene, which is associated with cell
proliferation (25), was also
downregulated in SUDHL-6 cells treated with Yiqichutan.
Collectively, the present results indicated that Yiqichutan
treatment promoted SUDHL-6 cell apoptosis.
Yiqichutan promotes miR-34a and
reduces Foxp1 expression levels in DLBCL cells
To assess whether Yiqichutan could regulate the
expression levels of miR-34a and Foxp1, RT-qPCR was performed. It
was found that the higher the concentration of Yiqichutan, the
higher the expression level of miR-34a (Fig. 5B). However, the expression level of
the Foxp1 was reduced, particularly after treatment with the 1/2
IC50 Yiqichutan (Fig 5C).
Therefore, the present results supported the conclusion that
Yiqichutan may upregulate miR-34a to promote cell apoptosis and
suppress DCBCL progression by reducing the expression level of
Foxp1.
Discussion
The present study use microarrays to investigate
possible positive effects of Yiqichutan treatment on DLBCL. The
present results suggested that Yiqichutan inhibited DLBCL by acting
on several pathways including the Jak/Stat and PI3K signaling
pathways, which act as regulators of cell differentiation,
migration and proliferation (26).
Furthermore, the activation of these pathways can also cause
oncogenic transformation and tumor development (26). Several key genes in these pathways
such as SOCS2 of the Jak/Stat pathway, MDM2 and FGF2 of the PI3K
pathway, and C-MYC of both the Jak/Stat and PI3K pathways are
related to oncogenesis and tumor promotion in NHL (27-29).
In the present study, it was found that the expression levels of
these genes were downregulated after Yiqichutan treatment in vitro,
indicating a multi-target antitumor role of Yiqichutan in
DLBCL.
miRNAs are an abundant class of small non-coding
RNAs that modulate the expression of their target genes at the
post-transcriptional level (30).
miR-34a is located on chromosome 1p36.22 in a region associated
with various malignancies (31).
miR-34a overexpression inhibits the growth of various cancer types
in vitro and acts as a tumor suppressor in DLBCL (12,14,15,18).
Moreover, high miR-34a expression level improves the host response
to doxorubicin in DLBCL (32), and
its aberrant expression indicates poor prognosis in gastric MALT
lymphoma and DLBCL (33). However,
investigating miR-34a function is complicated. Contrary to
expectation, the knockdown of endogenous miR-34a results in the
inhibition of cell proliferation in chronic lymphocytic leukemia
(34). Furthermore, miR-34a
overexpression can also show anti-apoptotic effects by compromising
the MYC/ADP ribosylation factors/MDM2/p53 axis in MYC-driven
lymphomas (35). In the present
study, miR-34a was transcriptionally repressed by C-MYC, and
knockdown of C-MYC increased the expression level of miR-34a and
inhibited cell proliferation in DLBCL. As the upregulation of
miR-34a can repress C-MYC, the present results suggested a negative
feedback loop between C-MYC and miR-34a, where miR-34a suppresses
C-MYC and vice versa. The present results are consistent with those
from Craig et al (11) in
which epigenetic silencing, both MYC-dependent and -independent,
may contribute to miR-34a dysregulation in gastric DLBCL.
Foxp1 functions as an oncogene in controlling tumor
development in several malignancies, but its prognostic value in
tumors is not fully understood (36). The aberrant expression of Foxp1 is a
common feature of DLBCL, indicating a strong negative prognosis
indicator of patient survival (37,38).
Bioinformatically predicted targets of miR-34a include Foxp1, which
harbors putative miR-34a seed regions in its 3' untranslated region
(21). To assess a possible causal
link between miRNA expression and Foxp1 downregulation in DLBCL,
the present study knocked down C-MYC and induced miRNA expression
in the SUDHL-6 cells. It was found that the transient knockdown
decreased the expression level of Foxp1, and knockdown of C-MYC and
miR-34a overexpression reduced Foxp1 expression level to a greater
extent. Moreover, DLBCL proliferation was significantly reduced
after C-MYC knockdown, and the apoptotic rate increased with the
change in expression level of the apoptotic factors Bax, Bcl-2 and
PARP. In line with results from a previous study, the present
results indicated that the C-MYC/miR-34a pathway may have an
important role in the apoptotic process of DLBCL (11).
The present study found that Yiqichutan treatment
significantly inhibited the growth of SUDHL-6 cells in a
concentration- and time-dependent manner. Furthermore, increased
apoptosis was identified in treated cells. The mechanism of this
inhibition may be related to the miR-34a/Foxp1 pathway, as
Yiqichutan increased the expression level of miR-34a and inhibited
that of Foxp1. Our previous study showed that Yiqichutan inhibited
the growth of DLBCL cells via the regulation of the PI3K/AKT
pathway (8). Furthermore, PI3K/AKT
inhibition may induce downregulation of the transcription factor
C-MYC, as re-expression of C-MYC rescues DLBCL cells from
PTEN-induced toxicity (39).
Therefore, regulation of the C-MYC/miR-34a pathway by Yiqichutan
may be related to its effect on the upstream PI3K/AKT signaling
pathway, involving the transcription factor C-MYC.
miR-targeting oligonucleotides have certain
advantages as therapeutic tools, most notably the ease with which
oligonucleotides can be chemically modified to enhance their
pharmacokinetic/pharmacodynamic profiles and the ability of miRNAs
to target multiple genes simultaneously (40). A promising strategy to achieve a
therapeutic effect by targeting the miRNA regulatory network is to
induce the expression of miRNAs that act as tumor suppressor genes
(40), such as miR-34a. The first
evidence for the success of chemically synthesized miR-34a
‘replacement therapy' in cancer treatment was reported using a
preclinical model of non-small cell lung cancer (13). In addition, miR-34a administration in
a xenotransplant DLBCL and multiple myeloma model resulted in a
significant reduction in tumor growth (41,42). The
present results indicated the role of Yiqichutan in regulating the
C-MYC/miR-34a pathway and increasing the expression levels of
miR-34a, which was in accordance with the function of formulated
synthetic miR-34a. Thus, the present study identified the potential
to develop Yiqichutan-based treatment strategies in patients with
DLBCL.
In conclusion, the present study investigated the
expression profiles of mRNA in DLBCL cells exposed to Yiqichutan,
and found that the C-MYC/miR-34a pathway was associated with the
growth of DLBCL cells. Furthermore, Yiqichutan treatment may
inhibit the proliferation of DLBCL cells by inhibiting the
C-MYC/miR-34a pathway. However, the main limitation of the present
study was the lack of in vivo experiments. Thus, further
in vivo studies and component analysis of Yiqichutan are
required to investigate the mechanism of Yiqichutan treatment in
DLBCL cells.
Supplementary Material
Table S1. Primers used in PCR
validation.
Acknowledgements
The authors would like to thank Dr Zhuo Lv from
people’s hospital of Huadu, who provided suggestions for the
experimen
Funding
The current study was supported by grants from the
National Natural Science Foundation of China (grant no. 81873147),
Guangzhou Municipal Science and Technology Project (grant no.
201607010384), and High-Level University Project of Guangzhou
University of Chinese Medicine (grant no. A1-AFD018171Z11069).
Availability of data and materials
The data that support the results of this study are
available from Shanghai GenePharma Co., Ltd., but restrictions
apply to the availability of these data, which were used under
license for the current study and therefore are not publicly
available. Data are, however, available from the authors upon
reasonable request and with permission of Shanghai GenePharma Co.,
Ltd.
Authors' contributions
LZ designed and performed experiments and wrote the
manuscript. YZ performed experiments and wrote the manuscript. JW
performed experiments. ZL analyzed the data. LL analyzed the data,
revised the manuscript and gave final approval of the version to be
published. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Swerdlow SH, Campo E, Pileri SA, Harris
NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz
AD, et al: The 2016 revision of the World Health Organization
classification of lymphoid neoplasms. Blood. 127:2375–2390.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Coiffier B: Rituximab in the treatment of
diffuse large B-cell lymphomas. Semin Oncol. 29:30–35.
2002.PubMed/NCBI
|
|
3
|
Habermann TM, Weller EA, Morrison VA,
Gascoyne RD, Cassileth PA, Cohn JB, Dakhil SR, Woda B, Fisher RI,
Peterson BA, et al: Rituximab-CHOP versus CHOP alone or with
maintenance rituximab in older patients with diffuse large B-cell
lymphoma. J Clin Oncol. 24:3121–3127. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sehn LH, Donaldson J, Chhanabhai M,
Fitzgerald C, Gill K, Klasa R, MacPherson N, O'Reilly S, Spinelli
JJ, Sutherland J, et al: Introduction of combined CHOP plus
rituximab therapy dramatically improved outcome of diffuse large
B-cell lymphoma in British Columbia. J Clin Oncol. 23:5027–5033.
2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Park JJ, Beckman-Harned S, Cho G, Kim D
and Kim H: The current acceptance, accessibility and recognition of
Chinese and Ayurvedic medicine in the United States in the public,
governmental, and industrial sectors. Chin J Integr Med.
18:405–408. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rashrash M, Schommer JC and Brown LM:
Prevalence and Predictors of Herbal Medicine Use Among Adults in
the United States. J Patient Exp. 4:108–113. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Scheid V, Tuffrey V, Weijburg T, Bovey M
and Ward T: Chinese medicine treatment for menopausal symptoms in
the UK health service: Is a clinical trial warranted? Maturitas.
80:179–186. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhai L, Chen C, Zhao Y and Lin L: Effect
of Qi-benefiting and Phlegm-eliminating Recipe on Proliferation and
Serine/threonine-protein Kinase AKT Expression of Diffused Large
B-cell Lymphoma Cells. J Guangzhou Univ Tradit Chin Med.
33:556–560. 2016.(In Chinese).
|
|
9
|
Gao T, Chen Z and Zhang E: Theoretical
Basis and Evidence-based Basis of Invigorating Qi and Expelling
Phlegm Method in Preventing and Treating Lung Cancer. Liaoning J
Tradit Chin Med. 45:43–46. 2018.(In Chinese).
|
|
10
|
Kim K, Zakharkin SO and Allison DB:
Expectations, validity, and reality in gene expression profiling. J
Clin Epidemiol. 63:950–959. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Craig VJ, Cogliatti SB, Imig J, Renner C,
Neuenschwander S, Rehrauer H, Schlapbach R, Dirnhofer S, Tzankov A
and Müller A: Myc-mediated repression of microRNA-34a promotes
high-grade transformation of B-cell lymphoma by dysregulation of
FoxP1. Blood. 117:6227–6236. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yanaihara N, Caplen N, Bowman E, Seike M,
Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, et
al: Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wiggins JF, Ruffino L, Kelnar K, Omotola
M, Patrawala L, Brown D and Bader AG: Development of a lung cancer
therapeutic based on the tumor suppressor microRNA-34. Cancer Res.
70:5923–5930. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu C, Kelnar K, Liu B, Chen X,
Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, et
al: The microRNA miR-34a inhibits prostate cancer stem cells and
metastasis by directly repressing CD44. Nat Med. 17:211–215.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Tang Y, Tang Y and Cheng YS: miR-34a
inhibits pancreatic cancer progression through Snail1-mediated
epithelial-mesenchymal transition and the Notch signaling pathway.
Sci Rep. 7:38232:2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lodygin D, Tarasov V, Epanchintsev A,
Berking C, Knyazeva T, Körner H, Knyazev P, Diebold J and Hermeking
H: Inactivation of miR-34a by aberrant CpG methylation in multiple
types of cancer. Cell Cycle. 7:2591–2600. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Krzeszinski JY, Wei W, Huynh H, Jin Z,
Wang X, Chang TC, Xie XJ, He L, Mangala LS, Lopez-Berestein G, et
al: miR-34a blocks osteoporosis and bone metastasis by inhibiting
osteoclastogenesis and Tgif2. Nature. 512:431–435. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chim CS, Wong KY, Qi Y, Loong F, Lam WL,
Wong LG, Jin DY, Costello JF and Liang R: Epigenetic inactivation
of the miR-34a in hematological malignancies. Carcinogenesis.
31:745–750. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Christoffersen NR, Shalgi R, Frankel LB,
Leucci E, Lees M, Klausen M, Pilpel Y, Nielsen FC, Oren M and Lund
AH: p53-independent upregulation of miR-34a during oncogene-induced
senescence represses MYC. Cell Death Differ. 17:236–245.
2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jia L, Chopp M, Wang L, Lu X, Zhang Y,
Szalad A and Zhang ZG: miR-34a Regulates Axonal Growth of Dorsal
Root Ganglia Neurons by Targeting FOXP2 and VAT1 in Postnatal and
Adult Mouse. Mol Neurobiol. 55:9089–9099. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rao DS, O'Connell RM, Chaudhuri AA,
Garcia-Flores Y, Geiger TL and Baltimore D: MicroRNA-34a perturbs B
lymphocyte development by repressing the forkhead box transcription
factor Foxp1. Immunity. 33:48–59. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kanehisa M, Furumichi M, Tanabe M, Sato Y
and Morishima K: KEGG: New perspectives on genomes, pathways,
diseases and drugs. Nucleic Acids Res. 45 D:D353–D361.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yamada O and Kawauchi K: The role of the
JAK-STAT pathway and related signal cascades in telomerase
activation during the development of hematologic malignancies.
JAK-STAT. 2(e25256)2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dietrich DR: Toxicological and
pathological applications of proliferating cell nuclear antigen
(PCNA), a novel endogenous marker for cell proliferation. Crit Rev
Toxicol. 23:77–109. 1993.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Steelman LS, Pohnert SC, Shelton JG,
Franklin RA, Bertrand FE and McCubrey JA: JAK/STAT, Raf/MEK/ERK,
PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis.
Leukemia. 18:189–218. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gupta A, Shah K, Oza MJ and Behl T:
Reactivation of p53 gene by MDM2 inhibitors: A novel therapy for
cancer treatment. Biomed Pharmacother. 109:484–492. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Song W, Liu MG, Zhang JB, Zhang JJ, Sun MM
and Yu QK: Mechanism of action of EBV, Bcl-2, p53, c-Myc and Rb in
non-Hodgkin's lymphoma. Eur Rev Med Pharmacol Sci. 20:1093–1097.
2016.PubMed/NCBI
|
|
29
|
Letellier E and Haan S: SOCS2:
Physiological and pathological functions. Front Biosci (Elite Ed).
8:189–204. 2016.PubMed/NCBI
|
|
30
|
Michlewski G and Cáceres JF:
Post-transcriptional control of miRNA biogenesis. RNA. 25:1–16.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M,
et al: Human microRNA genes are frequently located at fragile sites
and genomic regions involved in cancers. Proc Natl Acad Sci USA.
101:2999–3004. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Marques SC, Ranjbar B, Laursen MB,
Falgreen S, Bilgrau AE, Bødker JS, Jørgensen LK, Primo MN, Schmitz
A, Ettrup MS, et al: High miR-34a expression improves response to
doxorubicin in diffuse large B-cell lymphoma. Exp Hematol.
44:238–246 e232. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
He M, Gao L, Zhang S, Tao L, Wang J, Yang
J and Zhu M: Prognostic significance of miR-34a and its target
proteins of FOXP1, p53, and BCL2 in gastric MALT lymphoma and
DLBCL. Gastric Cancer. 17:431–441. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Saleh LM, Wang W, Herman SE, Saba NS,
Anastas V, Barber E, Corrigan-Cummins M, Farooqui M, Sun C, Sarasua
SM, et al: Ibrutinib downregulates a subset of miRNA leading to
upregulation of tumor suppressors and inhibition of cell
proliferation in chronic lymphocytic leukemia. Leukemia.
31:340–349. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Musilova K and Mraz M: MicroRNAs in B-cell
lymphomas: How a complex biology gets more complex. Leukemia.
29:1004–1017. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Xiao J, He B, Zou Y, Chen X, Lu X, Xie M,
Li W, He S, You S and Chen Q: Prognostic value of decreased FOXP1
protein expression in various tumors: A systematic review and
meta-analysis. Sci Rep. 6(30437)2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Flori M, Schmid CA, Sumrall ET, Tzankov A,
Law CW, Robinson MD and Müller A: The hematopoietic oncoprotein
FOXP1 promotes tumor cell survival in diffuse large B-cell lymphoma
by repressing S1PR2 signaling. Blood. 127:1438–1448.
2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gascoyne DM and Banham AH: The
significance of FOXP1 in diffuse large B-cell lymphoma. Leuk
Lymphoma. 58:1037–1051. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Pfeifer M, Grau M, Lenze D, Wenzel SS,
Wolf A, Wollert-Wulf B, Dietze K, Nogai H, Storek B, Madle H, et
al: PTEN loss defines a PI3K/AKT pathway-dependent germinal center
subtype of diffuse large B-cell lymphoma. Proc Natl Acad Sci USA.
110:12420–12425. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Li Z and Rana TM: Therapeutic targeting of
microRNAs: Current status and future challenges. Nat Rev Drug
Discov. 13:622–638. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Craig VJ, Tzankov A, Flori M, Schmid CA,
Bader AG and Müller A: Systemic microRNA-34a delivery induces
apoptosis and abrogates growth of diffuse large B-cell lymphoma in
vivo. Leukemia. 26:2421–2424. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Di Martino MT, Leone E, Amodio N, Foresta
U, Lionetti M, Pitari MR, Cantafio ME, Gullà A, Conforti F, Morelli
E, et al: Synthetic miR-34a mimics as a novel therapeutic agent for
multiple myeloma: in vitro and in vivo evidence. Clin Cancer Res.
18:6260–6270. 2012.PubMed/NCBI View Article : Google Scholar
|