Introduction
Type 2 diabetic osteoporosis (T2DOP) is a severe
chronic complication that affects the skeletal system and is caused
by diabetes (1). T2DOP has become a
common secondary cause of osteoporosis that accelerates bone loss
and leads to bone fractures (1). The
incidence of T2DOP fracture has been reported to be as high as 50%
worldwide in 2015 and continues to rise with the increasing age of
patients with islet function failure, resulting in major
socioeconomic burden and a serious decline in quality of life
(2). Chronically poor control of
blood glucose and lipid levels are important factors that lead to
osteoporosis in patients with type 2 diabetes mellitus (T2DM)
(3). Long-term hyperglycemia affects
bone formation and bone resorption, leading to osteoporosis
(3). Furthermore, high triglyceride
(TG) and total cholesterol (TC) levels reduce bone density in
patients with diabetes (3). Active
control of blood glucose and blood lipid is conducive to early
prevention of osteoporosis (3).
There are currently no specific drugs for the
clinical treatment of T2DOP (4).
However, basic treatment principles are comprised of primary
disease control, calcium supplements and vitamin D (5). Currently, osteoporosis treatments,
including bisphosphonates and calcium supplements, are not
considered as the first choice of treatment for secondary
osteoporosis and may have severe side effects (6).
Extensive research has revealed that certain Chinese
herbs may help preserve bone structure and regulate lipid levels
(7,8). Curcumin (Cur) is a natural polyphenolic
compound extracted from the roots of the genus Curcuma in
the dry ginger family (9). Cur has
been reported to exhibit bone-protective properties in
post-menopausal osteoporosis (10,11) and
to prevent bone loss by inhibiting osteoclasts in a diabetic and
osteoporotic animal model (12).
However, the effect of Cur on T2DOP remains unclear. Furthermore,
the effect of Cur on bone microstructure requires further
investigation. A previous study demonstrated that Cur can inhibit
Smad2/3 phosphorylation caused by transforming growth factor
(TGF)β1 signaling by upregulating Smad7 in hepatic stellate cells,
thus exerting an anti-liver fibrosis effect (13). However, whether the anti-osteoporotic
effect of Cur is associated with the TGFβ/Smad signaling regulation
pathway has not been previously reported.
Therefore, the aim of the current study was to
comprehensively investigate the effect of Cur on osteoporosis in
T2DM rats by observing the 3D structure of bone microstructure and
by evaluating bone microstructure, bone biomechanics, serum bone
conversion metabolism, blood glucose and blood lipid indicators.
Furthermore, the effect of Cur on TGFβ1, type I TGFβ receptor
(TβRI), TβRII and Smad2/3 expression in T2DOP rats was observed,
and the association between expression changes and their
anti-osteoporotic effects was assessed.
Materials and methods
Drugs and reagents
Cur was purchased from Vientiane Tianjin HengYuan
Technology Co., Ltd., calcitriol (Cal) from Roche Diagnostics
(Shanghai) Co., Ltd. and streptozotocin (STZ) from Sigma-Aldrich
(Merck KGaA). High-sugar and high-fat fodder (57.3% carbohydrate,
20% fructose, 10% lard, 2.5% cholesterol, 10% egg yolk and 0.2%
sodium cholate) was purchased from the Animal Experimental Center
of Southern Medical University (Guangzhou, China).
TC (cat. no. A111-1-1), TG (cat. no. F001-1-1) and
low-density lipoprotein cholesterol (LDL-C; cat. no. A113-2-1)
assay kits and serum osteocalcin (OCN; cat. no. H152) and
C-terminal type-I peptide (CTX-I; cat. no. H287) ELISA kits were
purchased from Nanjing Jiancheng Bioengineering Institute. Smad2/3
(cat. no. TA347074; 1:1,000) and phosphorylated (p)-Smad2/3 (cat.
no. TA501728; dilution, 1,000) antibodies were purchased from
Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.
Experimental animals
The animal experiments were approved by the Ethics
Committee of Zhaoqing Medical College. A total of 50 male
Sprague-Dawley rats (age, 8 weeks; weight; 180±20 g) were obtained
from the Southern Medical Experimental Animal Center (certification
no. SCXK 2017-0012). The animals were allowed to acclimatize to the
laboratory conditions for 7 days before experiments and were housed
at 25±2˚C and a relative humidity of 60-70% with 12 h light/dark
cycles. The animals had access to dry pellet food and water ad
libitum.
Induction of diabetes with high-sugar,
high-fat diet and STZ
A total of ten rats were assigned to the control
group and were administered a high-sugar, high-fat diet only for 12
weeks. A total of 40 rats were used to establish the T2DM model.
According to previous literature (14), these rats were administered a
high-sugar, high-fat diet for 4 weeks. After 4 weeks, each rat
received an intraperitoneal injection of 3% STZ (40 mg/kg)
(15). Rats with fasting blood
glucose (FBG) ≥7.0 mmol/l were selected for further study.
Experimental design
The rats continued to receive the high-sugar,
high-fat diet throughout the course of the present study. The
animals were divided into five groups (n=10/group) and received the
following treatments for 8 weeks (Table
I): Control, T2DM, T2DM treated with 110 mg/kg/day Cur (T2DM +
Cur), T2DM treated with 0.045 µg/kg/day Cal (T2DM + Cal) and T2DM
treated with 200 mg/kg/day metformin (T2DM + Met; cat. no. 317240;
Sigma-Aldrich; Merck KGaA). As Cur and Cal are insoluble in water
(16,17), their suspension was prepared with 1%
sodium carboxymethyl cellulose(cat. no. 419273; Sigma-Aldrich;
Merck KGaA). The control and T2DM groups were given 1% sodium
carboxymethyl cellulose (5 ml/kg) daily by oral gavage and the T2DM
+ Cur, T2DM + Cal and T2DM + Met groups were given their respective
drugs (5 ml/kg) by oral gavage. FBG and body weight were measured
once weekly for 8 weeks.
 | Table ITreatments and dosages use for the
animal experimental design. |
Table I
Treatments and dosages use for the
animal experimental design.
| Group | Treatment and
dosage |
|---|
| Control | Normal control rats
were administered a high-sugar high-fat diet +1% sodium
carboxymethyl cellulose (5 ml/kg) daily by oral gavage |
| T2DM | Diabetic control
rats were administered a high-sugar high-fat diet +1% sodium
carboxymethyl cellulose (5 ml/kg) daily by oral gavage +3% STZ (40
mg/kg) by intraperitoneal injection |
| T2DM + Cur | Diabetic rats were
administered a high-sugar high-fat diet +3% STZ (40 mg/kg) by
intraperitoneal injection + Cur (110 mg/kg/day) orally |
| T2DM + Cal | Diabetic rats were
administered a high-sugar high-fat diet +3% STZ (40 mg/kg) by
intraperitoneal injection + Cal (0.045 µg/kg/day) orally |
| T2DM + Met | Diabetic rats were
administered a high-sugar high-fat diet +3% STZ (40 mg/kg) by
intraperitoneal injection + Met (200 mg/kg/day) orally |
Sample collection and application
At the end of the experiment, all rats were
euthanized via cardiac puncture under anesthesia with
intraperitoneal injection of 45 mg/kg sodium pentobarbitone. A
total of 2 ml of serum was collected for biomarker assays. The left
and right femora were dissected for bone biomechanical analysis and
micro-CT analysis, respectively. The left proximal tibial
metaphysis (PTM) was cut into decalcified 5 µm sections for
immunohistochemistry (IHC) analysis. The right PTM was used for
reverse transcription-quantitative PCR (RT-qPCR) analysis.
Serum biomarker assay
Blood samples (5 ml) were processed as previously
described (12). Briefly, the serum
was separated by centrifugation at 1,000 x g at room temperature
for 15 min and stored at -80˚C. The serum lipids (TC, TG and LDL-C)
and serum bone formation markers (OCN and CTX-I) were determined
using ELISA kits (Nanjing Jiangcheng Biological Bioengineering),
according to the manufacturer's protocol.
Biomechanical analysis of rat
femora
In the biomechanical bone test, the left femora from
each group of rats was placed in 70% ethanol for preservation at
room temperature. The liquid attached to the femur was blotted with
medical gauze, followed by the experiment of bone biomechanical
measurement. The Lloyd LR5K Plus Bone Biomechanical Detection
system (LLOYD Instruments Ltd.; AMETEK Test & Calibration
Instruments) was used to perform a three-point bending test to
analyze the biomechanical properties of the femora. The left femur
of each group of rats was placed into the machine at a loading
speed of 2 mm/min and a span load of 20 mm. Each biomechanical
property, including maximum load, breaking load, elastic load and
the bone rigidity coefficient, was plotted corresponding to the
load-displacement curve and calculated according to the respective
equations that reflect bone stiffness (18).
Determination of bone microstructure
in rat femora
The metaphyses of the right femurs were measured
using a micro-CT (CT-40; Scanco Medical). The specific parameters
to test micro-CT parameters were as follows: X-line energy 70 kVP,
114 µA, 500 sheet of 700-nm slices and 30 min scan time. Following
the scan, 1.0 mm growth plates were selected from left distal
femora. The 3D reconstruction of the femora were constructed
according to the following conditions: A thickness of 3.0 mm bone
was considered as the cancellous bone region of interest to draw a
reconstruction line (19) for bone
reconstruction and quantitative image analysis was performed to
extract information using the software supplied with the micro-CT
(version 4; SCANCO Medical AG). The physical parameters examined
were as follows: Bone volume fraction (bone volume/total volume;
BV/TV), connection density (Conn.D), trabecular number, (Tb.N),
trabecular separation (Tb.Sp) and trabecular thickness (Tb.Th).
RT-qPCR analysis
Gene expression was analyzed using RT-qPCR. Bone
tissue from the right tibia (100 mg) was weighed and RNA was
extracted using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
RT was performed to synthesize cDNA using a kit (cat. no. 6110A;
Takara Bio, Inc.), according to the manufacturer's protocol.
RT-qPCR was conducted using a SYBR green kit (cat. no. RR420A;
Takara Bio, Inc.) on a QuantStudio 12K Flex RT PCR System (Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
The thermocycling conditions were as follows: Initial denaturation
at 95˚C for 30 sec; 40 cycles of 95˚C for 5 sec, 60˚C for 45 sec,
and the melting curve analysis at 95˚C for 5 sec and 60˚C for 60
sec. The primers used are listed in Table II. β-actin was used as the reference
gene. The 2-ΔΔCq method was used to quantify the mRNA
expression. All data are presented as relative to the control
group.
 | Table IIPrimers used in reverse
transcription-quantitative PCR. |
Table II
Primers used in reverse
transcription-quantitative PCR.
| Gene | Primer sequences
(5'-3') |
|---|
| TGFβ1 | Forward:
CCATGACATGAACCGACCCT |
| | Reverse:
CCGGGTTGTGTTGGTTGTAG |
| TβRI | Forward:
GGCTCTGCTTTGTCTCTGTCAC |
| | Reverse:
GGTCCTCTTCATTTGGCACTC |
| TβRII | Forward:
GAAGTCTGTGTGGCTGTATGGAG |
| | Reverse:
GCGGTAGCAGTAGAAGATGATG |
| Smad2 | Forward:
GCAGGTGGTGGAGAACAGAAT |
| | Reverse:
CCGTATTTGCTGTACTCAGTCCC |
| Smad3 | Forward: CAG
GAGGAGAAGTGGTGCGA |
| | Reverse:
TGGTGTTCACGTTCTGCGTG |
| β-actin | Forward:
GACCGCAACAACGCAATCTATGAC |
| | Reverse:
TGCTCCACAGTTGACTTGAATCTCTG |
IHC
Left tibia specimens were fixed in 4%
paraformaldehyde for 12 h at room temperature and decalcified for 4
weeks and embedded in paraffin. Sections (5 µm) were placed in a
drying oven at 60˚C for 3 h, dewaxed and hydrated, and then washed
with 1X PBS solution three times. After 5 min, the tissue specimens
were placed in citrate buffer (pH 6.0) at 60˚C for 30 min for
antigen retrieval, followed by washing with 1X PBS solution three
times for 5 min. Specimens were then incubated with smad2/3 and
p-smad2/3 (dilution, 1:200) at 4˚C overnight, followed by
incubation with the corresponding secondary antibody (Goat
anti-mouse; 1:20,000; cat. no. sc-2005; Santa Cruz Biotechnology,
Inc.) at room temperature for 2 h. DAB staining was applied for 30
sec at room temperature and hematoxylin counterstaining for 2 min
at room temperature, followed by drying and mounting. The stained
sections were observed under a light microscope at a magnification
of x200 and three fields of view from each bone tissue section were
imaged with a Nikon Eclipse E400 camera (Nikon Corporation) with
Picture Frame software (E400; Nikon Corporation). The images were
analyzed and measured with Image-ProPlus software (version 6.0;
Media Cybernetics, Inc.) to determine the mean optical density of
the positive area of each specimen, as previously described
(20).
Statistical analysis
Data are presented as mean ± standard deviation and
were analyzed using SPSS software for Windows (version 16.0; SPSS,
Inc.). All experiments were performed in triplicate. Statistical
differences between groups were analyzed using one-way ANOVA and
Tukey's honest significant difference post-hoc test. Mixed ANOVA
was used to compare body weight and glucose level. P<0.05 was
considered to indicate a statistically significant difference.
Results
Cur increases body weight and
decreases lipid and blood glucose levels in T2DM rats
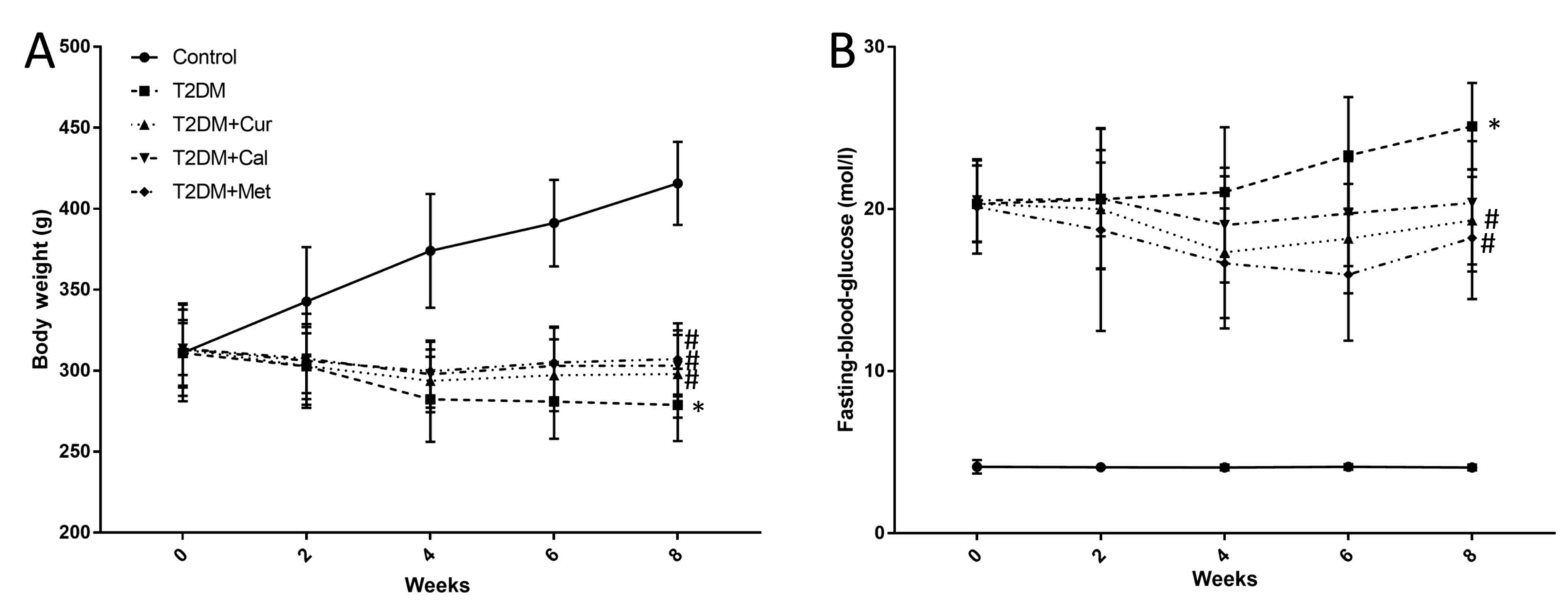
After the T2DM rat model was established, weight
were measured once weekly every 8 weeks. Success of the model was
determined by analyzing the body weight and FBG. According to the
results, the weight of the rats in the T2DM group was significantly
decreased compared with controls (P<0.05) and Cur, Cal and Met
increased the body weight of T2DM rats compared with the T2DM
group; however, there was no significance between these groups
(Fig. 1A). FBG levels in T2DM rats
were increased compared with controls (P<0.05), while Cur and
Met significantly decreased FBG level compared with the T2DM group.
However, there was no significant difference in the levels of of
Cal on FBG compared with the T2DM group (P<0.05; Fig. 1B).
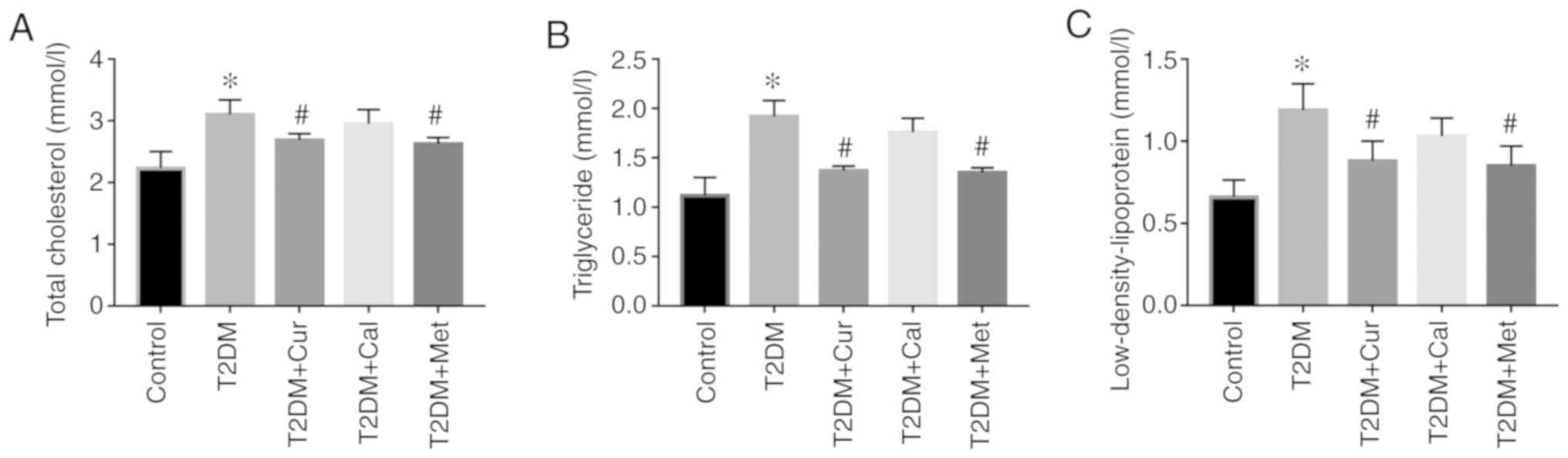
At the end of the experiments, serum lipid levels
were determined and the results demonstrated that TC, TG and LDL-C
were significantly increased following a high-fat, high-sugar diet
in T2DM rats compared with controls (P<0.05; Fig. 2). Following treatment with Cur and
Met, lipid levels significantly decreased compared with the T2DM
group (P<0.05). Furthermore, no significant difference was
reported following Cal treatment. These results indicated that Cur
may regulate dysregulated lipid and glucose levels and that Met had
similar effects.
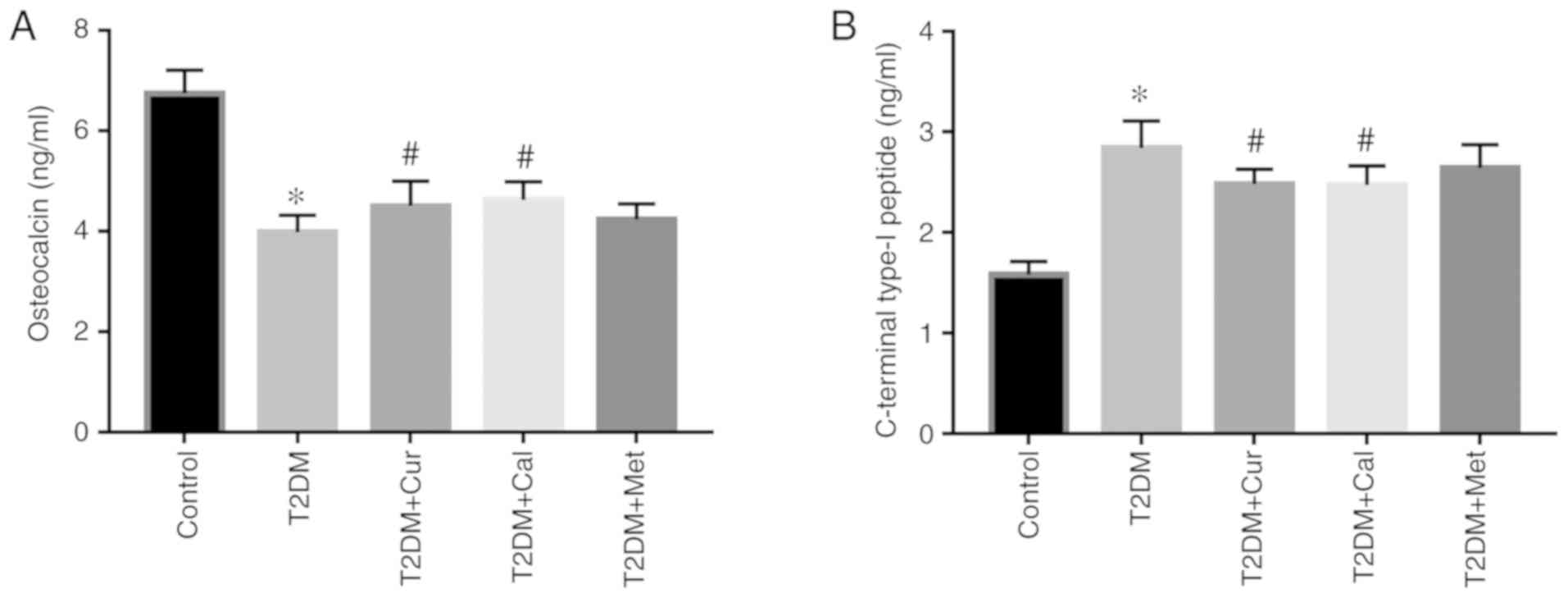
Cur increases OCN and decreases CTX-I
levels
Bone formation marker OCN and bone turnover marker
CTX-I levels in serum were measured using respective ELISA kits.
The results indicated lower bone formation and higher bone
absorption activity in T2DM rats compared with controls
(P<0.05). However, Cur and Cal reversed this effect by
significantly increasing OCN and decreasing CTX-I levels compared
with the T2DM group (P<0.05; Fig.
3). There was no significant effect of Met on OCN and CTX-I
level compared with the T2DM group.
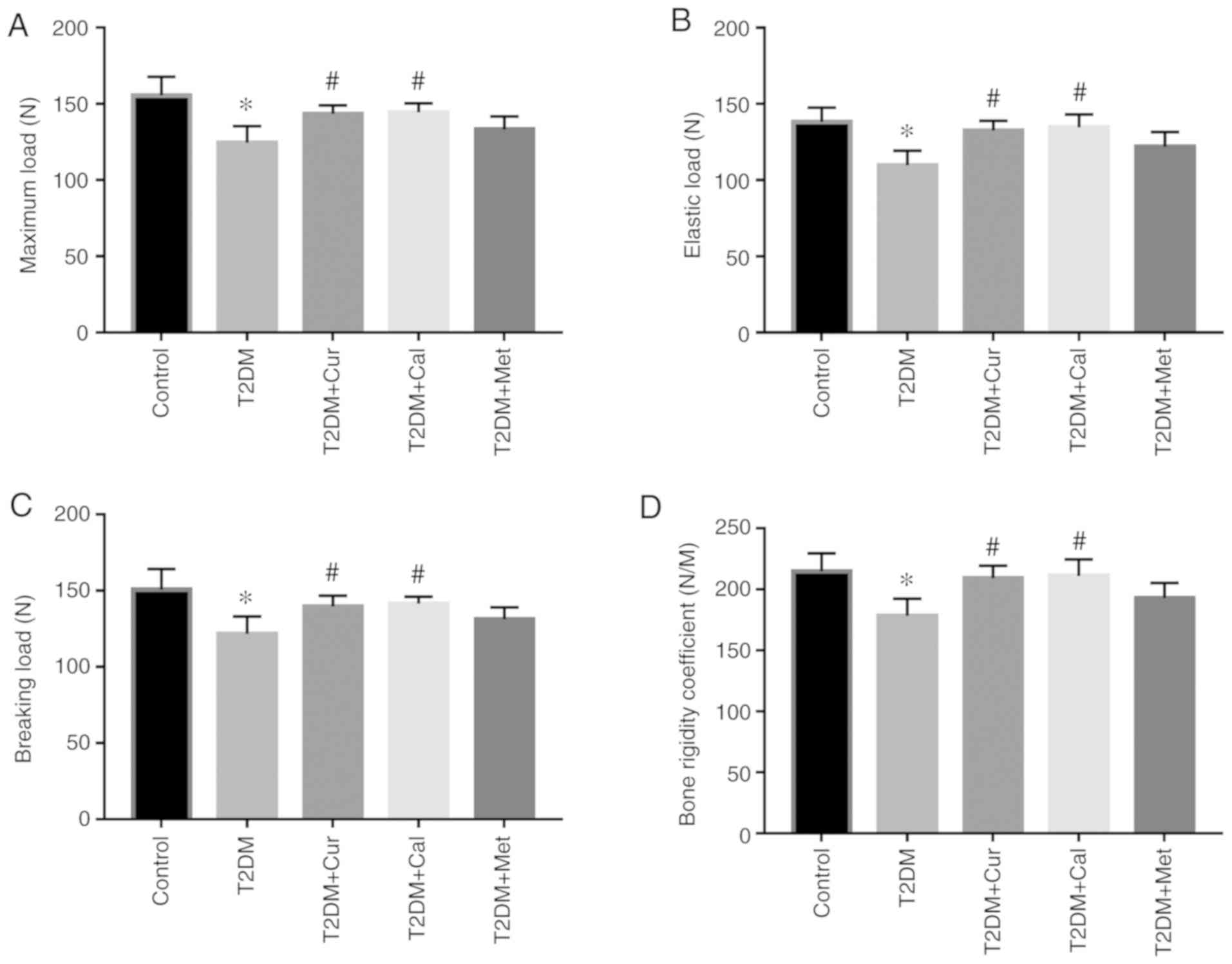
Cur improves bone biomechanical
properties and preserves bone microarchitecture in T2DM rats
To assess the role of Cur in T2DM rat bones, the
biomechanical properties of the femur were examined. The results
demonstrated that elastic load, breaking load, maximum load and the
bone rigidity coefficient of the T2DM group were significantly
lower compared with controls (P<0.05; Fig. 4). Moreover, Cur- and Cal-treated
groups had significantly improved biomechanical properties compared
with the T2DM group. There was no significant effect of Met on
biomechanical properties compared with the T2DM group.
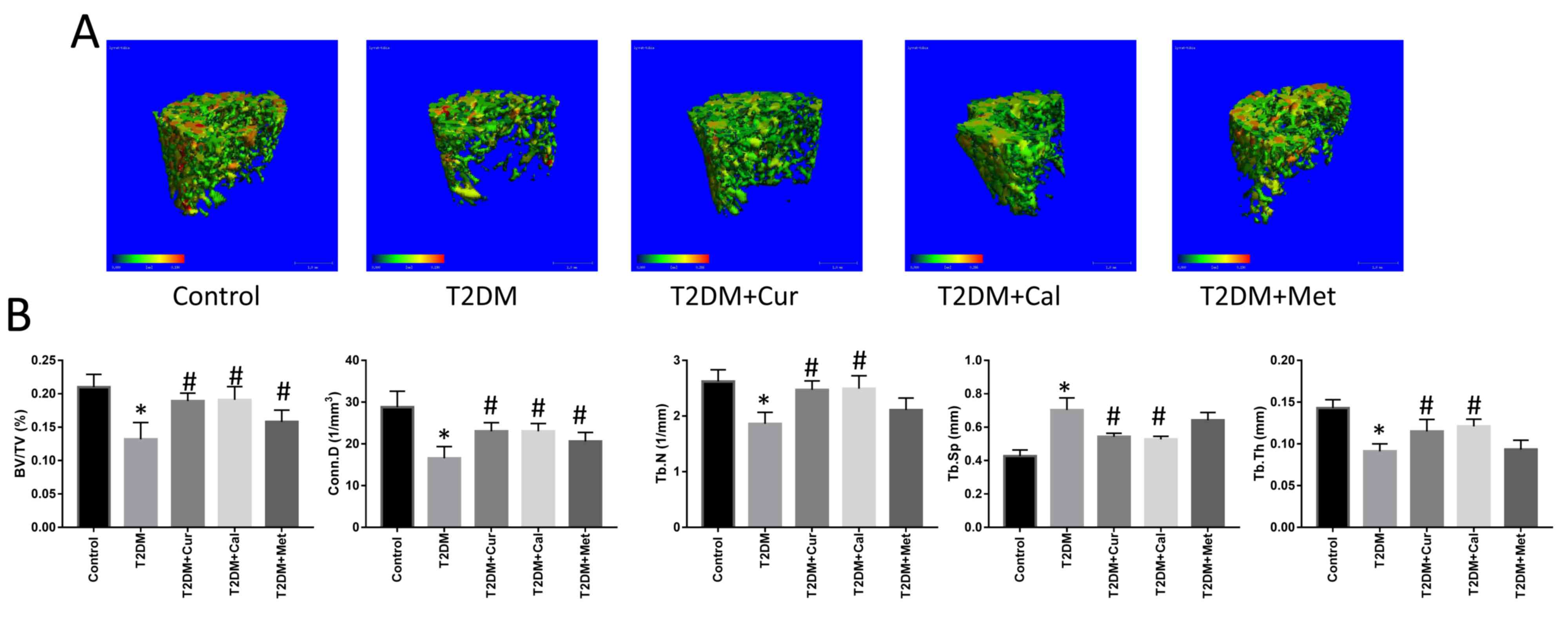
Subsequently, micro-CT was used to create
representative 3D reconstruction images to examine the trabecular
microarchitecture of rat femora (Fig.
5A). Microarchitecture parameters (BV/TV, Conn.D, Tb. N, Tb and
Th) in T2DM rats were significantly decreased compared with
controls (P<0.05; Fig. 5B),
indicating that the high-fat, high-sugar diet was detrimental to
rat bone and that the T2DM rat model was successfully established.
Following treatment with Cur, the parameters BV/TV, Conn.D, Tb.N
and Tb.Th were significantly increased, while Tb.Sp was decreased
compared with the T2DM group (P<0.05). Cal exerted similar
effects to Cur in terms of bone biomechanical properties and bone
microarchitecture. However, Met did not exhibit any significance
between groups.
Bone-protective effects of Cur are
mediated via the TGFβ/Smad2/3 pathway
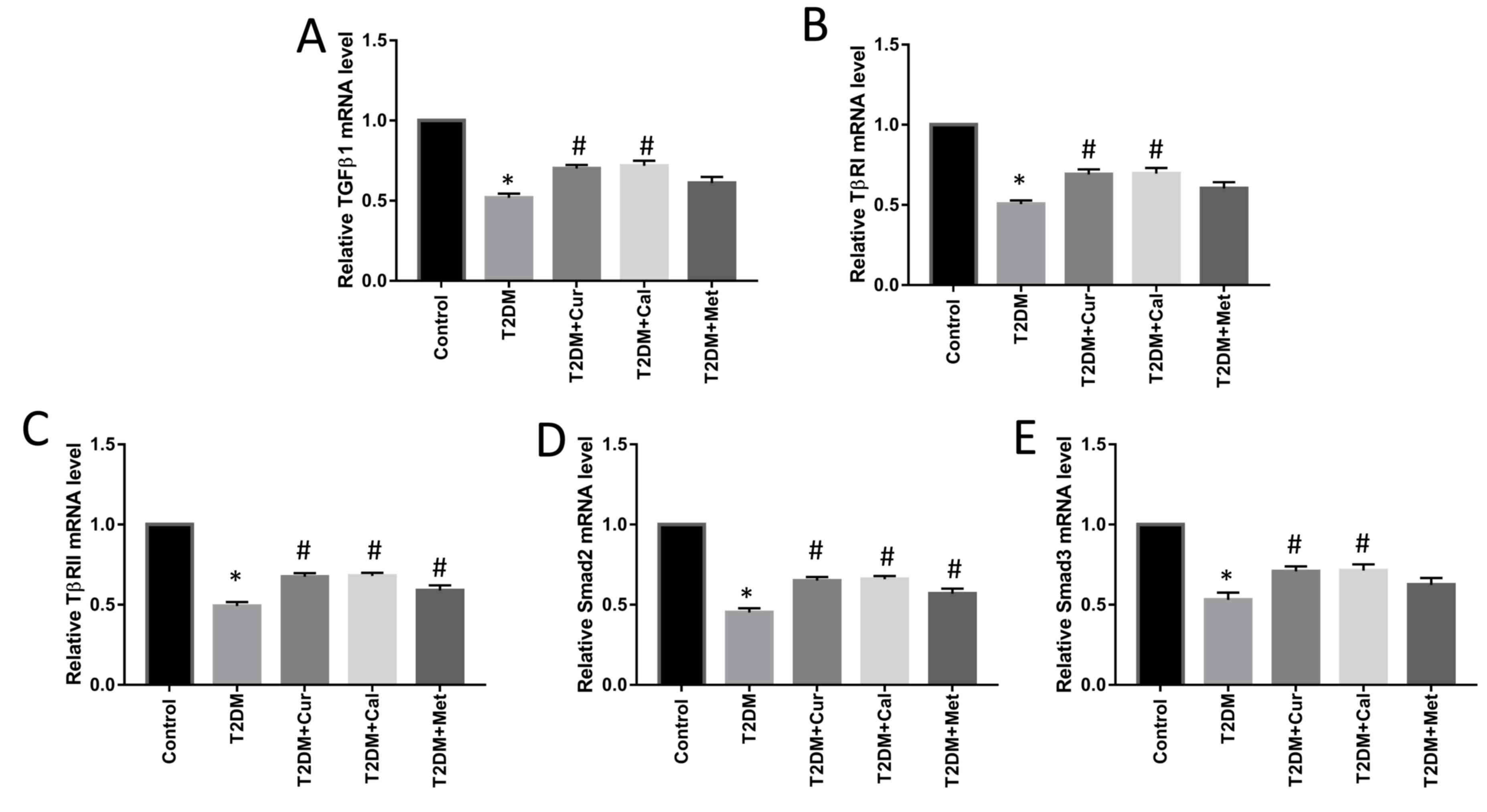
mRNA levels of TGFβ1, TβRI, TβRII and Smad2/3 were
determined using RT-qPCR to examine the mechanism of action of Cur.
The mRNA levels of TGFβ1, TβRI, TβRII and Smad2/3 in T2DM group
were significantly decreased compared with controls (P<0.05) and
Cur and Cal significantly increased TGFβ1, TβRI, TβRII and Smad2/3
mRNA expression levels in the right PTM compared with the T2DM
group (P<0.05; Fig. 6) and Met
significantly increased TβRII and Smad2 mRNA expression levels
compared with the T2DM group (P<0.05; Fig. 6)
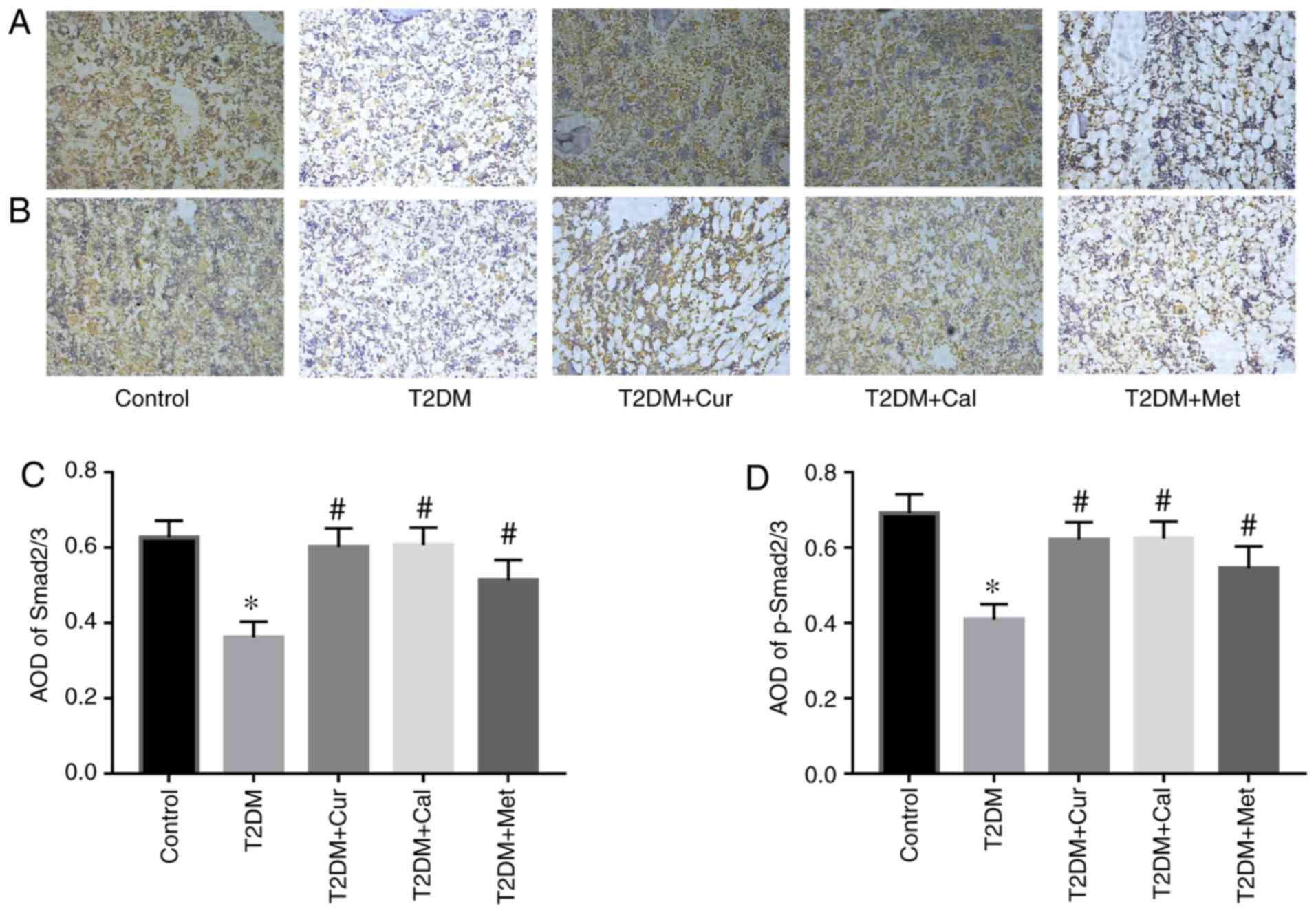
Furthermore, IHC was performed to evaluate Smad2/3
and p-Smad2/3 protein expression levels. The results indicated that
Cur significantly increased Smad2/3 expression and promoted Smad2/3
phosphorylation (Fig. 7). These
results suggested that may Cur protect bone via TGFβ/Smad2/3
signaling pathway activation.
Discussion
The aim of the current study was to comprehensively
assess the effect of Cur on osteoporosis in T2DM rats by observing
3D structural diagrams of bone microstructures, and evaluating bone
microstructure, bone biomechanics, serum bone conversion
metabolism, blood glucose and blood lipid indicators. Additionally,
the TGFβ/Smads signaling pathway was used to provide a theoretical
basis for the mechanism of action via which Cur acts in the
prevention and treatment of osteoporosis in T2DM.
Recently, it has been reported that certain herbal
extracts may prevent bone loss and protect against diabetes-induced
bone microarchitecture disruption (21,22). Cur
is an extract from the roots of the genus Curcuma and previous
studies have revealed that it can reduce inflammation and oxidative
stress in a diabetic rat model, improve islet cell function and
reduce blood glucose levels (23,24). In
addition, Cur may improve diabetes-induced damage of the retina,
heart, kidney and other organs, thus demonstrating promising
therapeutic effects against diabetic complications (25,26).
Furthermore, Cur serves a key role in the metabolism of bone
reconstruction (27).
In the present study, the role of Cur in a T2DM rat
model and its underlying mechanism of action were investigated. The
T2DM rat model was successfully established using a high-sugar,
high-fat diet combined with low dose STZ (28). After establishing a successful model,
differences in body mass were observed among the five groups of
rats. The body mass of the T2DM, T2DM + Cur, T2DM + Cal and T2DM +
Met groups was lower compared with control group, and this
difference persisted until the end of the experiment. After week 8,
the body mass of the T2DM + Cur, T2DM + Cal and T2DM + Met groups
was higher compared with the T2DM group. Therefore, it was
speculated that body mass notably affected bone density and bone
mass in the present study.
Epidemiological studies have reported that patients
with diabetes often exhibit increased TC, TG and LDL-C levels,
which may lead to abnormal lipid metabolism (29). The present results of serum lipid
measurement in the T2DM group were consistent with those of
previous studies (29,30). Furthermore, the present results
demonstrated that dyslipidemia in T2DM rats was alleviated by Cur
or Met. This effect may be associated with the ability of Met to
activate the 5'AMP-activated protein kinase pathway, inhibit
endogenous liver X receptor, reduce the expression of sterol
regulatory element-binding protein 1, improve fatty acid
metabolism, improve hyperlipidemia and reduce the risk of
atherosclerosis (31).
Rat femoral bone mass was evaluated by micro-CT and
the parameters of bone morphometry examined included BV/TV, Conn.D,
Tb.Sp, Tb.N and Tb.Th. The results demonstrated that BV/TV, Conn.D,
Tb.N and Tb.Th were lower in the T2DM group compared with controls,
while Tb.Sp was increased; this indicated that a high-sugar,
high-fat diet combined with low doses of STZ damaged bone
microarchitecture. The 3D reconstruction was also in line with this
finding, as the results indicated trabecular bone degeneration and
cancellous bone loss, which lead to decreased bone mass.
Additionally, the biomechanical properties of femora were lower in
the T2DM group compared with controls. Therefore, T2DM rats
exhibited dysregulated lipid and glucose levels, and disrupted bone
biomechanical properties and microarchitecture. Bone mineral
density was not evaluated in the present study as the research was
focused on the bone microarchitectures.
Insulin can directly stimulate osteoblasts, and
promote amino acid accumulation and bone collagen and matrix
synthesis (32). In patients with
diabetes, insulin secretion is maladjusted or dysfunctional, and
insulin resistance may lead to osteoporosis (33). CTX-I and OCN levels are
representative markers of osteoclast and osteoblast
differentiation, respectively (34).
When insulin resistance occurs, CTX-I levels increase, leading to
bone resorption (35). In the
present study, the bone formation marker OCN was decreased and the
bone turnover marker CTX-I was increased in the serum of T2DM rats,
indicating that bone formation was inhibited and bone resorption
was activated, respectively. The results demonstrated that CTX-I
levels in the T2DM + Cur group were lower compared with the T2DM
group, indicating that Cur suppressed the function of osteoclasts
and inhibited bone resorption in diabetic rats. It has been
previously reported that Cur improved tartrate-resistant acid
phosphatase activity and mRNA expression in diabetic rats,
indicating that Cur treatment decreases osteoclast activity and
thus inhibits bone resorption and protects bone microstructure
(36).
In the present study, following treatment with Cur,
lipid and glucose levels improved and Met treatment demonstrated
similar results. The hypoglycemic mechanism involved may be
associated with the enhancement of antioxidant capacity, immunity
and hepatic glucokinase activity, and Cur may serve a role in
decreasing lipid levels by increasing the reductase activity of
β-hydroxy β-methylglutaryl-coenzyme A, thus increasing the number
of LDL receptors in the liver and removing cholesterol from tissues
(37). However, the mechanism
underlying its hypoglycemic and lipid-lowering properties has not
been fully elucidated. In the present study, abnormal glucose and
lipid metabolism in T2DM was accompanied by changes in bone
ultrastructure. The underlying mechanism may involve the disruption
of the dynamic balance of bone remodeling due to disordered glucose
and lipid metabolism, and hyperglycemia, resulting in bone
absorption that overrides bone formation and leads to bone loss
(38). Cur treatment improved bone
metabolism and structure, which may be due to the reduction of
blood glucose and lipid levels and enhanced bone formation. In
addition, the results demonstrated that Cal was unable to regulate
lipid and glucose levels. It was also found that the bone formation
marker OCN was increased and the bone turnover marker CTX-I
decreased by Cur; Cal exerted a similar effect. However, effect of
Met on bone formation marker did not significantly increase OCN
expression, indicating that the effect of Met on osteoporosis was
limited.
Numerous cytokines are involved in bone remodeling,
including TGFβ1, which is the primary cytokine associated with this
process (39,40). TGFβ1 is secreted by osteoblasts and
promotes osteoblast differentiation and inhibits osteoclast
activity (41). A previous study
reported that T2DM rats exhibited lower TGFβ1 expression when the
bone was damaged compared with that at baseline (42). However, increasing TGFβ1 level
protects bone as it slows bone loss and delays diabetic damage and
may therefore be a promising therapeutic target for the management
of T2DOP (43). The current study
demonstrated that Cur increased TGFβ1 mRNA expression and promoted
Smad2/3 phosphorylation, indicating that the effects of Cur may be
mediated via the TGFβ1/Smad2/3 pathway.
The aim of the current study was to identify a
natural drug that can simultaneously be used in the treatment of
diabetes and osteoporosis. The present study investigated the
effect of Cur on lipid and glucose levels, bone biomechanical
properties and bone microarchitecture in T2DM rats. Collectively,
it was demonstrated that serum lipid and blood glucose
dysregulation was ameliorated by Cur. Moreover, the loss of bone
mass and the disruption of bone microstructure and biomechanical
properties were reversed by Cur. To the best of our knowledge, the
present study was the first to demonstrate that Cur protects the
bone in T2MD rats via the TGFβ/Smad2/3 pathway in vivo.
However, the current study had certain limitations. First, the
underlying mechanism of Cur requires further investigation. Second,
the effect of Cur on the bone mineral density remains unclear.
Overall, it was hypothesized that Cur may have a potential clinical
application in secondary osteoporosis. However, its poor
bioavailability remains the main limitation restricting its
application.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Zhaoqing
Medical College 2017 Annual University-Level Research Project
(grant no. 2017K12), the 2019 Guangdong Medical Research Fund
Project (grant no. B2019229), the Science and Technology Million
Project Joint Project of Inner Mongolia Medical University [grant
no. YKD2018KJBW(LH)082] and the Medical Health Science and
Technology Project of Baotou Inner Mongolia (grant no.
WSJJ2019029).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL, BZ and SL performed the experiments, analyzed
data, participated in study design and wrote the manuscript. YZh,
YY and ZB conducted data analysis. YZe and DL designed the study
and participated in development and coordination. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the Ethics
Committee of Zhaoqing Medical College.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Leslie WD, Rubin MR, Schwartz AV and Kanis
JA: Type 2 diabetes and bone. J Bone and Miner Res. 27:2231–2237.
2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Goldshtein I, Nguyen AM, Depapp AE,
Ish-Shalom S, Chandler JM, Chodick G and Shalev V: Epidemiology and
correlates of osteoporotic fractures among type 2 diabetic
patients. Arch Osteoporos. 13(15)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen Z, Zhao GH, Zhang YK, Shen GS, Xu YJ
and Xu NW: Research on the correlation of diabetes mellitus
complicated with osteoporosis with lipid metabolism, adipokines and
inflammatory factors and its regression analysis. Eur Rev Med
Pharmacol Sci. 21:3900–3905. 2017.PubMed/NCBI
|
|
4
|
Martínez-Laguna D, Reyes C,
Carbonell-Abella C, Losada Grande E, Soldevila Madorell B, Mauricio
D, Díez-Pérez A, Nogués X and Prieto-Alhambra D: Use of drugs for
osteoporosis treatment in patients with type 2 diabetes mellitus:
Population-based cohort study. Rev Osteoporos Metab Miner.
9:107–113. 2017.
|
|
5
|
Parsons V, Mitchell CJ, Reeve J and Hesp
R: The use of sodium fluoride, vitamin D and calcium supplements in
the treatment of patients with axial osteoporosis. Calcif Tissue
Res. 22 (Suppl):S236–S240. 1977.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Janovská Z: Bisphosphonate-related
osteonecrosis of the jaws. A severe side effect of bisphosphonate
therapy. Acta Medica (Hradec Kralove). 55:111–115. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tao W, Deqin Z, Yuhong L, Hong L, Zhanbiao
L, Chunfeng Z, Limin H and Xiumei G: Regulation effects on abnormal
glucose and lipid metabolism of TZQ-F, a new kind of traditional
Chinese medicine. J Ethnopharmacol. 128:575–582. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xu H, Xu HE and Ryan D: A study of the
comparative effects of hawthorn fruit compound and simvastatin on
lowering blood lipid levels. Am J Chin Med. 37:903–908.
2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sharma R, Gescher A and Steward W:
Curcumin: The story so far. Eur J Cancer. 41:1955–1968.
2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yang MW, Wang TH, Yan PP, Chu LW, Yu J,
Gao ZD, Li YZ and Guo BL: Curcumin improves bone microarchitecture
and enhances mineral density in APP/PS1 transgenic mice.
Phytomedicine. 18:205–213. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li G, Bu J, Zhu Y, Xiao X, Liang Z and
Zhang R: Curcumin improves bone microarchitecture in
glucocorticoid-induced secondary osteoporosis mice through the
activation of microRNA-365 via regulating MMP-9. Int J Clin Exp
Pathol. 8:15684–15695. 2015.PubMed/NCBI
|
|
12
|
Folwarczna J, Zych M and Trzeciak HI:
Effects of curcumin on the skeletal system in rats. Pharmacol Rep.
62:900–909. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen NZ, Geng QQ, Zheng JB, He S, Huo XG
and Sun XJ: Suppression of the TGF-β/Smad signaling pathway and
inhibition of hepatic stellate cell proliferation paly a role in
the hepatoprotective effects of curcumin against alcohol-induced
hepatic fibrosis. Int J Mol Med. 34:1110–1116. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wu K, Gong Z, Zou L, Ye H, Wang C and Liu
Y, Liang Y, Li Y, Ren J, Cui L and Liu Y: Sargassum integerrimum
inhibits oestrogen deficiency and hyperlipidaemia-induced bone loss
by upregulating nuclear factor (erythroid-derived 2)-like 2 in
female rats. J Orthop Translat. 19:106–117. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gundala NKV, Naidu VGM and Das UN:
Amelioration of streptozotocin-induced type 2 diabetes mellitus in
Wistar rats by arachidonic acid. Biochem Biophys Res Commun.
496:105–113. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Eriksen SP, Irwin GM and Swintosky JV:
Antacid properties of calcium, magnesium, and aluminum salts of
water-insoluble aliphatic acids. J Pharmaceutical Sci. 52:552–556.
1963.
|
|
17
|
Parvathy KS: Chemical approaches toward
preparation of water-soluble curcumin derivatives. PhD thesis,
University of Mysore, India, 2009.
|
|
18
|
Mchorney CA, Schousboe JT, Cline RR and
Weiss TW: The impact of osteoporosis medication beliefs and
side-effect experiences on non-adherence to oral bisphosphonates.
Curr Med Res Opin. 23:3137–3152. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cao L, Liu J and Tang X: What the back of
the object looks like: 3D reconstruction from line drawings without
hidden lines. IEEE Trans Pattern Anal Mach Intell. 30:507–517.
2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Francisco JS, de Moraes HP and Dias EP:
Evaluation of the image-pro plus 4.5 software for automatic
counting of labeled nuclei by PCNA immunohistochemistry. Braz Oral
Res. 18:100–104. 2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang T, Cai L, Wang Y, Wang Q, Lu D, Chen
H and Ying X: The protective effects of silibinin in the treatment
of streptozotocin-induced diabetic osteoporosis in rats. Biomed
Pharmacother. 89:681–688. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li H, Chu S, Zhao H, Liu D, Liu X, Qu X,
Chen J, Li Z and Li J: Effect of Zishen Jiangtang pill, a Chinese
herbal product, on rats with diabetic osteoporosis. Evid Based
Complement Alternat Med. 2018(7201914)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Aggarwal BB and Harikumar KB: Potential
therapeutic effects of curcumin, the anti-inflammatory agent,
against neurodegenerative, cardiovascular, pulmonary, metabolic,
autoimmune and neoplastic diseases. Int J Biochem Cell Biol.
41:40–59. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gutierres VO, Assis RP, Arcaro CA,
Oliveira JO, Oliveira Lima TF, Remédio Zeni Beretta AL, Costa PI,
Baviera AM and Brunetti IL: Curcumin improves the effect of a
reduced insulin dose on glycemic control and oxidative stress in
streptozotocin-diabetic rats. Phytother Res. 33:976–988.
2019.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Stanić Z: Curcumin, a compound from
natural sources, a true scientific challenge-a review. Plant Foods
Hum Nutr. 72:1–12. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jiménez-Osorio AS, Monroy A and Alavez S:
Curcumin and insulin resistance-molecular targets and clinical
evidences. Biofactors. 42:561–580. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hussan F, Ibraheem NG, Kamarudin TA, Shuid
AN, Soelaiman IN and Othman F: Curcumin protects against
ovariectomy-induced bone changes in rat model. Evid Based
Complement Alternat Med. 2012(174916)2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Rastmanesh R: Effects of fish oil on
cytokines, glycemic control, blood pressure, and serum lipids in
patients with type 2 diabetes mellitus. J Obesity Weight Loss Ther
S3, 2013.
|
|
29
|
Li L, Liao G, Yang G, Lu Y, Du X, Liu J,
Li L, Wang C, Li L, Ren Y, et al: High-fat diet combined with
low-dose streptozotocin injections induces metabolic syndrome in
macaca mulatta. Endocrine. 49:659–668. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Eid S, Sas KM, Abcouwer SF, Feldman EL,
Gardner TW, Pennathur S and Fort PE: New insights into the
mechanisms of diabetic complications: Role of lipids and lipid
metabolism. Diabetologia. 62:1539–1549. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zou MH, Kirkpatrick SS, Davis BJ, Nelson
JS, Wiles WG IV, Schlattner U, Neumann D, Brownlee M, Freeman MB
and Goldman MH: Activation of the AMP-activated protein kinase by
the anti-diabetic drug metformin in vivo. Role of mitochondrial
reactive nitrogen species. J Biol Chem. 279:43940–43951.
2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Riddle RC and Clemens TL: Insulin,
osteoblasts, and energy metabolism: Why bone counts calories. J
Clin Invest. 124:1465–1467. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Arikan S, Tuzcu A, Bahceci M, Ozmen S and
Gokalp D: Insulin resistance in type 2 diabetes mellitus may be
related to bone mineral density. J Clin Densitom. 15:186–190.
2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lei T, Liang Z, Li F, Tang C, Xie K, Wang
P, Dong X, Shan S, Jiang M, Xu Q, et al: Pulsed electromagnetic
fields (PEMF) attenuate changes in vertebral bone mass,
architecture and strength in ovariectomized mice. Bone. 108:10–19.
2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ables GP, Perrone CE, Orentreich D and
Orentreich N: Methionine-restricted C57BL/6J mice are resistant to
diet-induced obesity and insulin resistance but have low bone
density. PLoS One. 7(e51357)2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hie M, Yamazaki M and Tsukamoto I:
Curcumin suppresses increased bone resorption by inhibiting
osteoclastogenesis in rats with streptozotocin-induced diabetes.
Eur J Pharmacol. 621:1–9. 2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jang EM, Choi MS, Jung UJ, Kim MJ, Kim HJ,
Jeon SM, Shin SK, Seong CN and Lee MK: Beneficial effects of
curcumin on hyperlipidemia and insulin resistance in high-fat-fed
hamsters. Metabolism. 57:1576–1583. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kanazawa I and Sugimoto T: The
relationship between bone and glucose/lipid metabolism. Clin
Calcium. 23:181–188. 2013.PubMed/NCBI(In Japanese).
|
|
39
|
Filvaroff E, Erlebacher A, Ye J, Gitelman
SE, Lotz J, Heillman M and Derynck R: Inhibition of TGF-beta
receptor signaling in osteoblasts leads to decreased bone
remodeling and increased trabecular bone mass. Development.
126:4267–4279. 1999.PubMed/NCBI
|
|
40
|
Zimmermann G, Henle P, Küsswetter M,
Moghaddam A, Wentzensen A, Richter W and Weiss S: TGF-beta1 as a
marker of delayed fracture healing. Bone. 36:779–785.
2005.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z,
Zhao L, Nagy TR, Peng X, Hu J, et al: TGF-beta1-induced migration
of bone mesenchymal stem cells couples bone resorption with
formation. Nat Med. 15:757–765. 2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ziyadeh FN, Hoffman BB, Han DC,
Iglesias-De la Cruz MC, Hong SW, Isono M, Chen S, McGowan TA and
Sharma K: Long-term prevention of renal insufficiency, excess
matrix gene expression, and glomerular mesangial matrix expansion
by treatment with monoclonal antitransforming growth factor-beta
antibody in db/db diabetic mice. Proc Natl Acad Sci USA.
97:8015–8020. 2000.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wang CJ, Yang KD, Ko JY, Huang CC, Huang
HY and Wang FS: The effects of shockwave on bone healing and
systemic concentrations of nitric oxide (NO), TGF-beta1, VEGF and
BMP-2 in long bone non-unions. Nitric Oxide. 20:298–303.
2009.PubMed/NCBI View Article : Google Scholar
|