Introduction
Fibroblast growth factors (FGFs) are autocrine and
paracrine growth factors that were initially identified as proteins
and are able to stimulate fibroblast proliferation (1). However, previous studies have reported
that, by binding to FGF receptors, FGFs are involved in multiple
biological processes, including cellular proliferation,
differentiation and tissue regeneration (2). Furthermore, FGFs have been applied to
wounded tissues to examine its regenerative capability and results
have revealed its healing potential (3). In this context, there have been several
studies identifying the effect of FGFs on different types of stem
cells (4,5); however, the role of FGFs remains
elusive due to varied and contradictive results. It is speculated
that FGFs have different effects depending on the developmental
stages of stem cells and their origins (6).
FGF-4 is a member of the FGF family and is a highly
mitogenic protein encoded by the FGF-4 gene. Similar to other
members of the FGF family, with a high affinity to its receptor,
FGF-4 affects the proliferation, differentiation and migration of
numerous types of cell (7).
Furthermore, FGF-4 has been tested for the clinical treatment of
angina (8). FGF-4 gene therapy using
adenoviral vector has also been applied for the treatment of
chronic ischemic heart disease (9).
In addition, FGF-4 has been reported to enhance cell survival
following ionization radiation (10). A previous study examining the effect
of FGF-4 on human bone marrow cells have indicated that it
stimulates cell proliferation in a dose-dependent manner (11). However, the precise effects of FGF-4
on different types of stem cells are yet to be established.
Dental stem cells, including gingiva-derived stem
cells, are considered to be promising candidates for restoring lost
periodontal tissue (12).
Furthermore, based on previous studies, FGF-4 may promote the
proliferation of mesenchymal stem cells (11,13). It
has also been reported that a 3-dimensional (3D) culture system may
enhance the understanding of cell proliferation and differentiation
in normal and pathologic environments (14). In addition, features of mesenchymal
stem cells under a 3D system may be different from the 2D culture
system (15), and the 3D spheroid
system may be applied as a tool for tissue regeneration (16).
Therefore, it was hypothesized that the addition of
FGF-4 may have specific effects on the viability and osteogenic
differentiation of mesenchymal stem cells. Thus, the aim of the
present study was to examine the effects of FGF-4 on the
morphology, viability and osteogenesis of stem cell spheroids
composed of gingiva-derived stem cells.
Materials and methods
Fabrication of stem cell
spheroids
To create stem cell spheroids, commercially
available concave microwells (cat. no. H389600; StemFIT 3D; Micro
FIT Co., Ltd.) with a 600-µm well diameter were used. A total of
4.5x105 stem cells were loaded into each well and
cultured to evaluate the cell response. Ethics approval was
obtained from the Institutional Review Board of Seoul St. Mary's
Hospital (approval no. KC17SESI0290) and the participant provided
written informed consent according to the Declaration of Helsinki.
All of the experiments were performed according to the relevant
guidelines, which are also specified in the Declaration of
Helsinki.
The tissue was obtained during the surgical
procedures of dental implant second-stage surgery from a
75-year-old healthy female on August 2013. The epithelium of the
gingival tissues was removed and cut into small pieces.
Subsequently, digestion of the tissues was performed with 2 mg/ml
collagenase IV (Sigma-Aldrich; Merck KGaA) and 1 mg/ml dispase
(Sigma-Aldrich; Merck KGaA) (17).
The cell suspension was filtered with a 70-µm cell strainer (Thermo
Fisher Scientific, Inc.) and seeded with α-minimum essential medium
(MEM; Gibco; Thermo Fisher Scientific, Inc.) containing 15% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 µg/ml streptomycin (Sigma-Aldrich; Merck KGaA).
Cell spheroids were generated with gingiva-derived stem cells and
were treated with FGF-4 (Prospec-Tany TechnoGene, Ltd.) at
concentrations of 0, 50, 100 and 200 ng/ml at 37˚C up to 14 days.
The morphology of the cell spheroids was evaluated using an
inverted light microscope (Leica DM IRM; Leica Microsystems GmbH).
The diameter of the spheroids was measured by comparing the
reference length on days 1, 3, 5 and 7(14).
Determination of cellular
viability
Stem cell spheroids were cultured in α-MEM (Gibco;
Thermo Fisher Scientific, Inc.) and the cellular viability was
qualitatively analyzed using a commercially available kit
(Live/Dead assay; Molecular Probes; Thermo Fisher Scientific, Inc.)
on day 3(18). The spheroids were
washed twice with the growth media and incubated at room
temperature for 30 min after applying 2 µl of 50 mM calcein
acetoxymethyl ester and 4 µl of 2 mM ethidium homodimer-1 (Thermo
Fisher Scientific, Inc.). Subsequently, stem cell spheroids were
observed using a fluorescence microscope at x200 magnification
(Axiovert 200; Zeiss AG). The assay was based on the principle that
the intact cells exhibit green fluorescence [excitation
(ex)/emission (em) ~495/~515 nm], while cells with a compromised
plasma membrane exhibit red fluorescence (ex/em ~495/~635 nm).
In addition, the number of viable cells was
quantitatively examined using a commercially available kit (Cell
Counting Kit-8; Dojindo Molecular Technologies, Inc.) on days 1, 3,
5 and 7 according to the manufacturer's instructions. The specific
time-points were selected for analysis according to a previous
study (19). Experiments were
carried out in triplicate.
Flow cytometric analysis
The spheroids were detached to obtain a single-cell
suspension prior to analysis. Stem cells were incubated with
specific FITC-conjugated mouse monoclonal antibodies to human CD90
(cat. no. 11-0909-42; eBioscience; Thermo Fisher Scientific, Inc.)
at 1 µg/ml concentration, which is considered a marker for a
variety of stem cells at day 1(20).
Quantification of stained cells was performed using a flow
cytometer (FACSCanto II; BD Biosciences) and the FACSDiva software
(v8.0.3; BD Biosciences).
Evaluation of osteogenic
differentiation
A total of 4.5x105 cells were grown in
each well with osteogenic media comprising α-MEM (Gibco; Thermo
Fisher Scientific, Inc.), 38 µg/ml dexamethasone, 2 mg/ml
glycerophosphate disodium salt hydrate, 10 mM ascorbic acid
2-phosphate and 200 mM L-glutamine on days 3, 7, 10 and 14.
Alkaline phosphatase activity was evaluated using a commercially
available assay kit (cat. no. K412-500; BioVision, Inc.). The
absorbance was measured at 405 nm after mixing a 5 mM
p-nitrophenylphosphate substrate with cell lysates using assay
buffer (cat. no. K412; BioVision, Inc.) and incubating it at 25˚C
for 40 min. Comparisons were made between the groups, as the same
number of cells was loaded in each group. The assays were performed
three times. Stem cell spheroids were stained with 2% Alizarin Red
S at room temperature for 30 min after fixing the cell spheroids
with 4% paraformaldehyde at room temperature for 15 min and washing
them with deionized water twice on day 14(18). The degree of osteogenesis was
evaluated by measuring the relative intensity of Alizarin red S
staining using an inverted light microscope at x100 magnification
(Leica DM IRM; Leica Microsystems GmbH).
mRNA quantification by reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from cell spheroids using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) at day 8 (21,22). SuperScript II RTase (Invitrogen;
Thermo Fisher Scientific, Inc.) was used for RT with total RNA
according to the manufacturer's instructions at 42˚C for 50 min.
Complementary DNA of mRNA was amplified using primer pairs as
follows: Runt-related transcription factor 2 (RUNX2) forward,
5'-CAGTTCCCAAGCATTTCATCC-3' and reverse, 5'-AGG
TGGCTGGATAGTGCATT-3'; bone γ-carboxyglutamate protein (BGLAP)
forward, 5'-AATCCGGACTGTGACGA GTT-3' and reverse,
5'-CAGCAGAGCGACACCCTAGA-3'; and β-actin forward,
5'-AATGCTTCTAGGCGGACTATGA-3' and reverse,
5'-TTTCTGCGCAAGTTAGGTTTT-3'. RT-qPCR was performed on the
StepOnePlus RT PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) using a SYBR-Green PCR kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. The thermocycling conditions were as follows: Initial
denaturation at 95˚C for 10 min, followed by 40 cycles of 95˚C for
15 sec and 30 sec at 59˚C. The data were analyzed using the StepOne
software v2.2.2 (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The expression of each RNA was normalized to an endogenous
control β-actin and was calculated using the 2-ΔΔCq
method (23). The experiments were
performed three times.
Western blot analysis
Cells were lysed and extracted using lysis and
extraction buffer (Pierce IP Lysis Buffer; cat. no. 87787; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocols
on day 7(24). Protein in the
whole-cell lysates was quantified using the bicinchoninic acid
assay (Thermo Fisher Scientific, Inc.). A total of 10 µg/lane of
protein samples were loaded on a 7.5% gel for collagen I and loaded
on a 10% gel for RUNX2 and GAPDH experiments, respectively and then
transferred to polyvinylidene difluoride membranes
(Immun-Blot®; Bio-Rad Laboratories, Inc.) for
immunoblotting. The membranes were blocked with 5% skim milk for 1
h at room temperature. The membranes were incubated with the
following primary antibodies overnight at 4˚C: Anti-collagen I
(1:500; cat. no. ab6308; Abcam), anti-RUNX2 antibody (1:200; cat.
no. ab76956; Abcam) and anti-GAPDH antibody (1:2,000; cat. no.
ab9485; Abcam). After washing with TBS-0.1% Tween-20, membranes
were incubated with horseradish peroxidase-conjugated secondary
antibodies, goat anti-mouse immunoglobulin G (IgG; cat. no.
ab205719; Abcam) and goat anti-rabbit IgG (cat. no. ab205718;
Abcam) at 1:10,000 dilution for 2 h at room temperature. The
immunoblot signals were visualized using horseradish peroxidase
substrate (cat. no. WBKLS0100; Merck KGaA).
Statistical analysis
Values are expressed as the mean ± standard
deviation. A test of normality was performed to confirm the
equality of variances in the samples. Differences among the groups
were analyzed using one-way analysis of variance with Tukey's
post-hoc test (SPSS 12 for Windows; IBM Corp.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Formation of cell spheroids with human
gingiva-derived stem cells
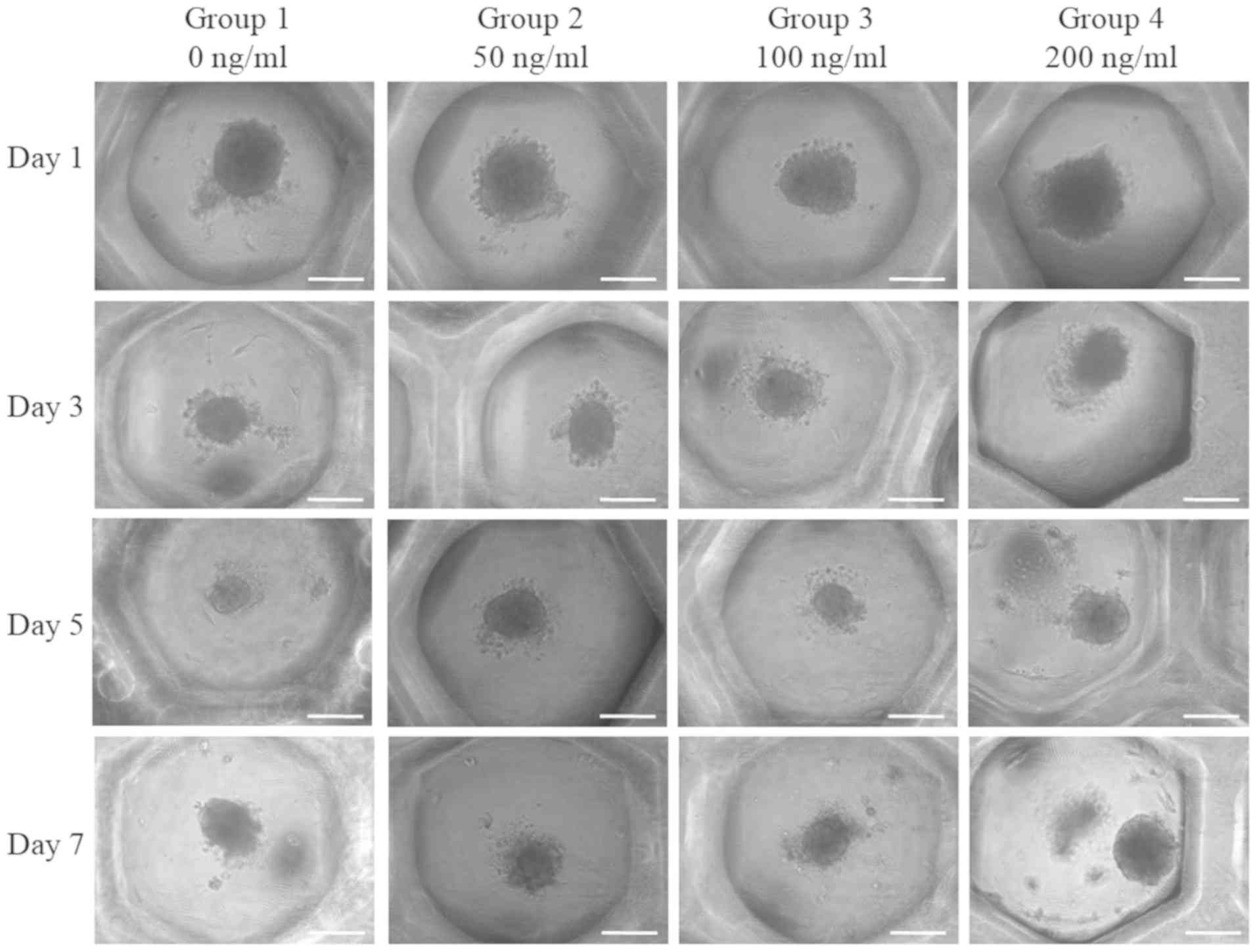
Spheroids were well formed in each microwell on day
1 (Fig. 1). Furthermore, no
noticeable morphological changes of the cell spheroids were
observed with the addition of FGF-4 at the concentrations of 50,
100 and 200 ng/ml. Images revealing the morphology of the spheres
of days 1, 3, 5 and 7 are presented in Fig. 1. It was indicated that there were no
noticeable changes at the longer incubation times.
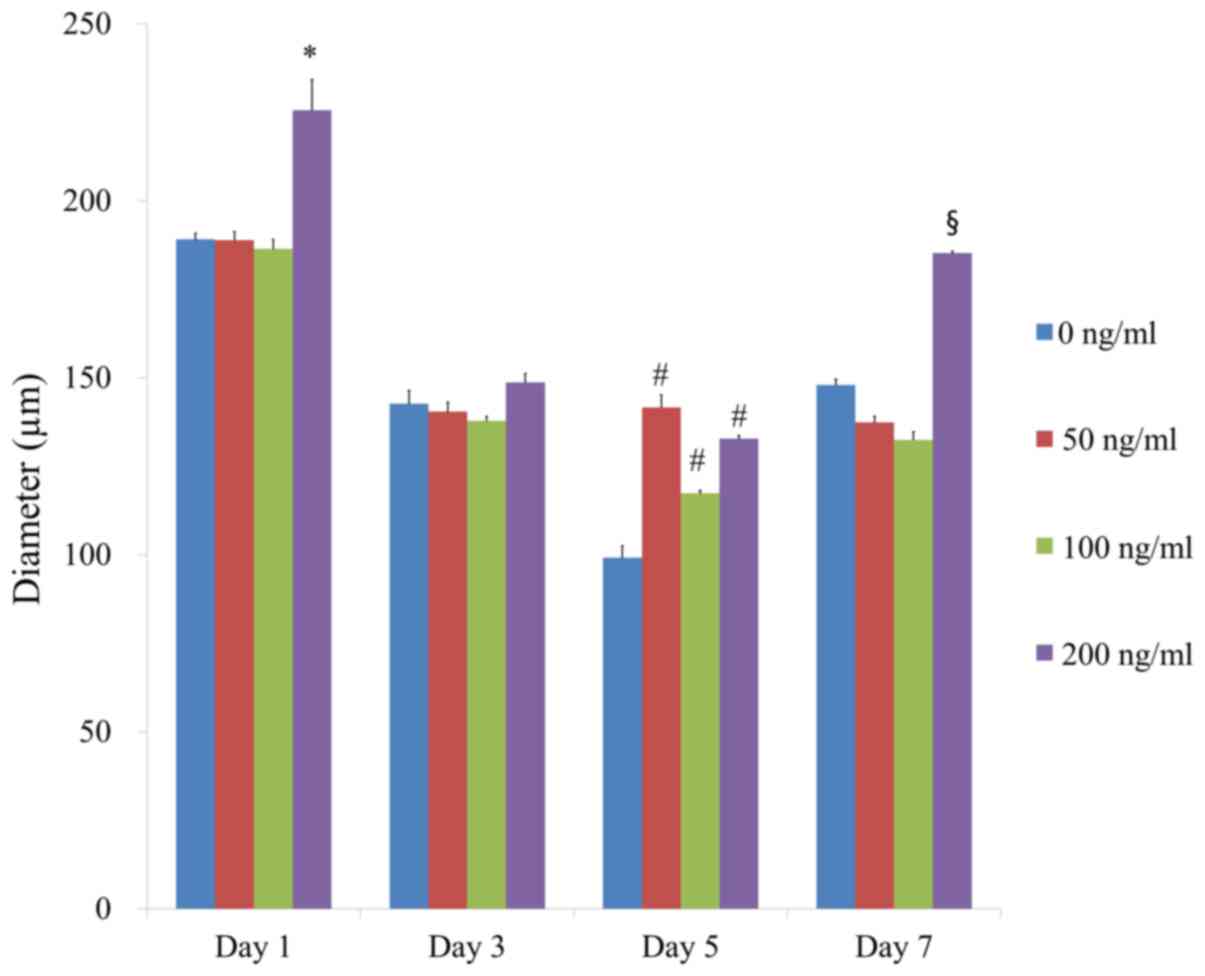
The average spheroid diameters at day 1, 3, 5 and 7
in the presence of FGF-4 at 0, 50, 100 and 200 ng/ml were presented
in Fig. 2. A statistically
significant increase was identified with FGF-4 at 200 ng/ml
compared with the control at day 1 (P<0.05). Addition of FGF-4
led to the increase of the diameter at 50, 100 and 200 ng/ml
compared with the control at day 5 (P<0.05). Furthermore, a
statistically significant increase was demonstrated in the group
treated with FGF-4 at 200 ng/ml compared with the control at day 7
(P<0.05).
Determination of cellular
viability
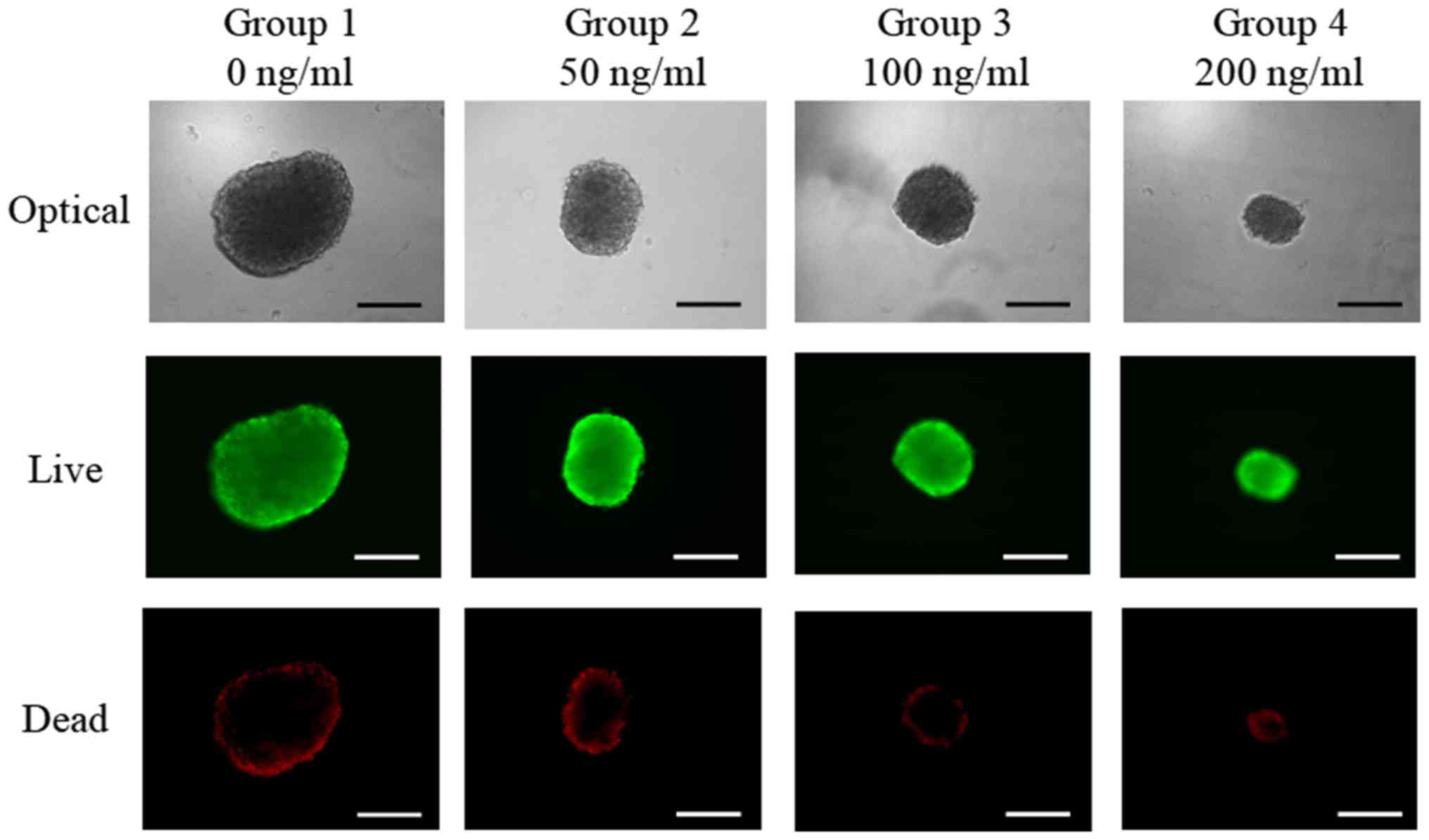
Qualitative results regarding the viability of cell
spheroids were obtained using a Live/Dead kit assay at day 3
(Fig. 3). In all cases, most of the
cells in the cell spheroids emitted green fluorescence. Red
fluorescence was partly seen on the boundary of the spheroids.
Furthermore, the quantitative results for cellular viability on
days 1, 3, 5 and 7 are provided in Fig.
4. The effect of FGF-4 at 0, 50, 100 and 200 ng/ml at day 1 on
the number of viable cells was quantified as 100.0±3.5, 98.0±2.5,
106.2±17.6 and 99.5±6.0%, respectively. The results indicated that
there were no significant differences among the groups on day 1
(P>0.05). Furthermore, no significant differences were
identified between the groups with longer incubation times
(P>0.05).
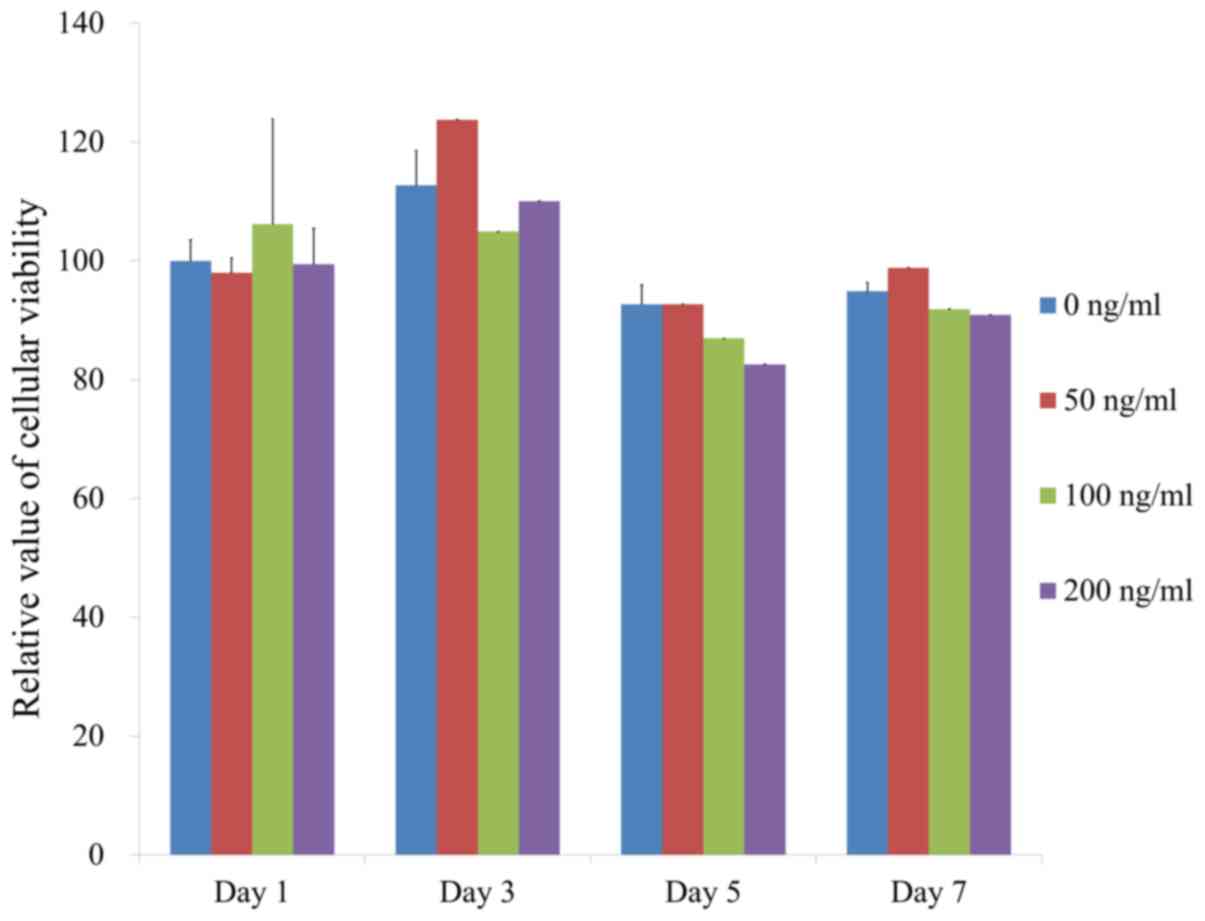 | Figure 4Cellular viability determined using a
Cell Counting Kit-8 assay on days 1, 3, 5 and 7. The effect of
FGF-4 at 0, 50, 100 and 200 ng/ml at day 1 on the number of viable
cells was quantified as 100.0±3.5, 98.0±2.5, 106.2±17.6 and
99.5±6.0%, respectively (P>0.05). Groups 1-4 were treated with
FGF-4 at 0, 50, 100 and 200 ng/ml, respectively. FGF-4, fibroblast
growth factor-4. |
Expression of stem cell markers
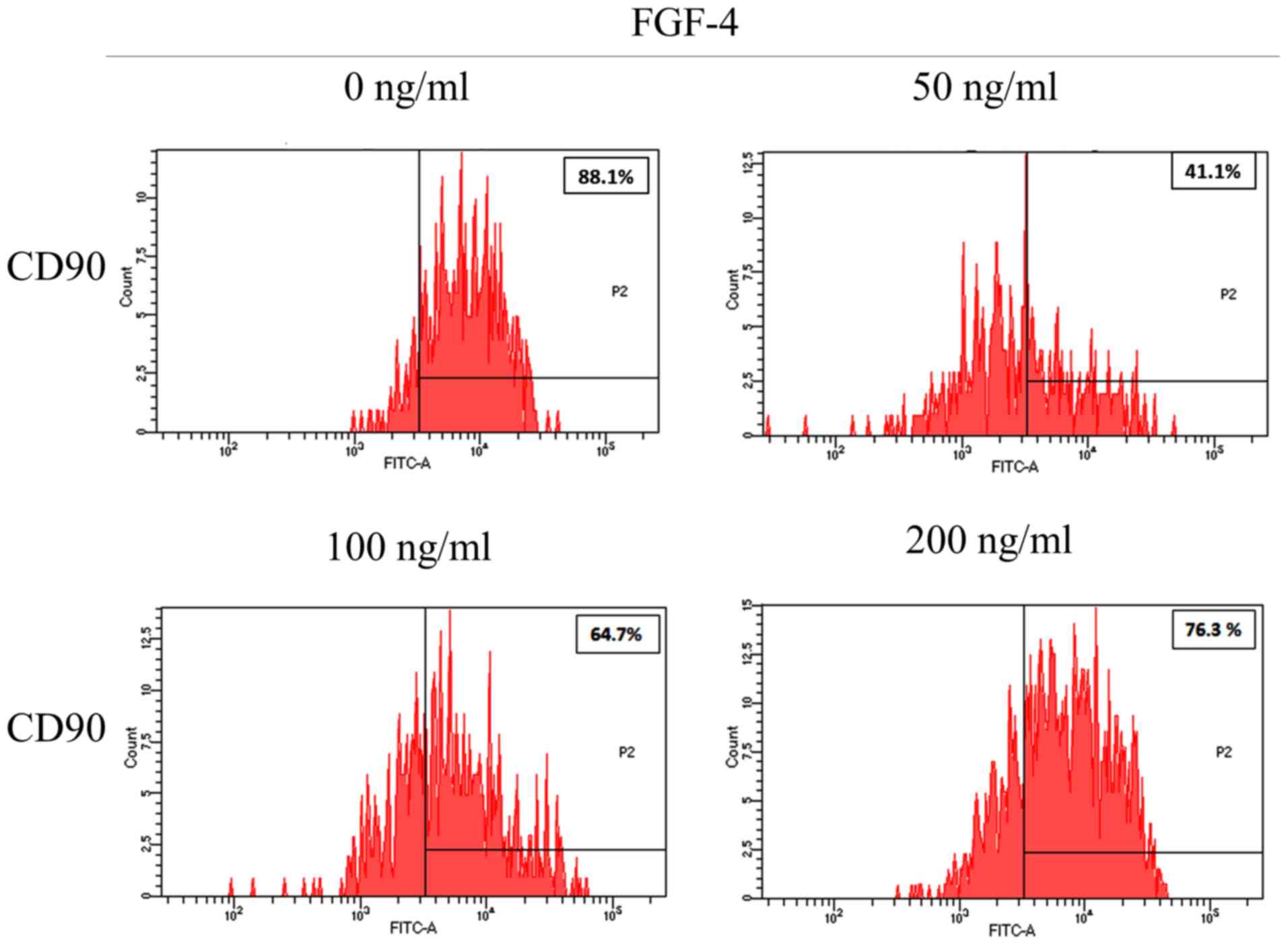
The expression of the CD90 surface marker was
observed on day 1 (Fig. 5); the
percentages of CD90 were 88.1% for the untreated control (0 ng/ml),
41.1% for the 50 ng/ml group, 64.7% for the 100 ng/ml group and
76.3% for the 200 ng/ml group.
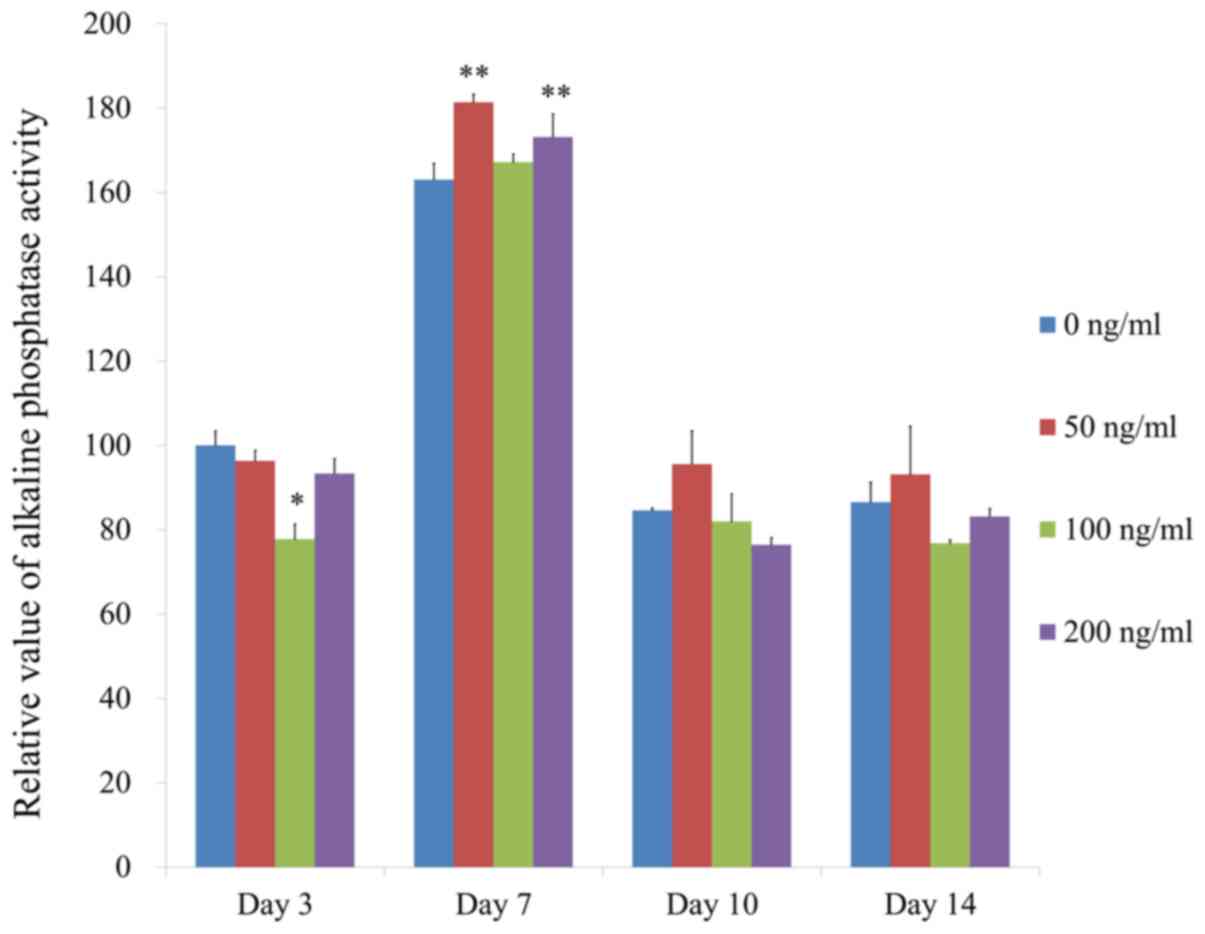
Increase of alkaline phosphatase
activity assay and Alizarin Red S staining with addition of
FGF-4
The results of the alkaline phosphatase activity
assay at days 3, 7, 10 and 14 are presented in Fig. 6. The relative value for alkaline
phosphatase activity at day 7 for the groups treated with FGF-4 at
50, 100 and 200 ng/ml were 111.3±1.1, 102.5±1.2 and 106.2±3.3%,
respectively, when the control group was considered 100%
(100.0±2.3%). In addition, the group treated with FGF-4 at 200
ng/ml had a significantly higher value compared with that of the
control group at day 7 (P<0.05).
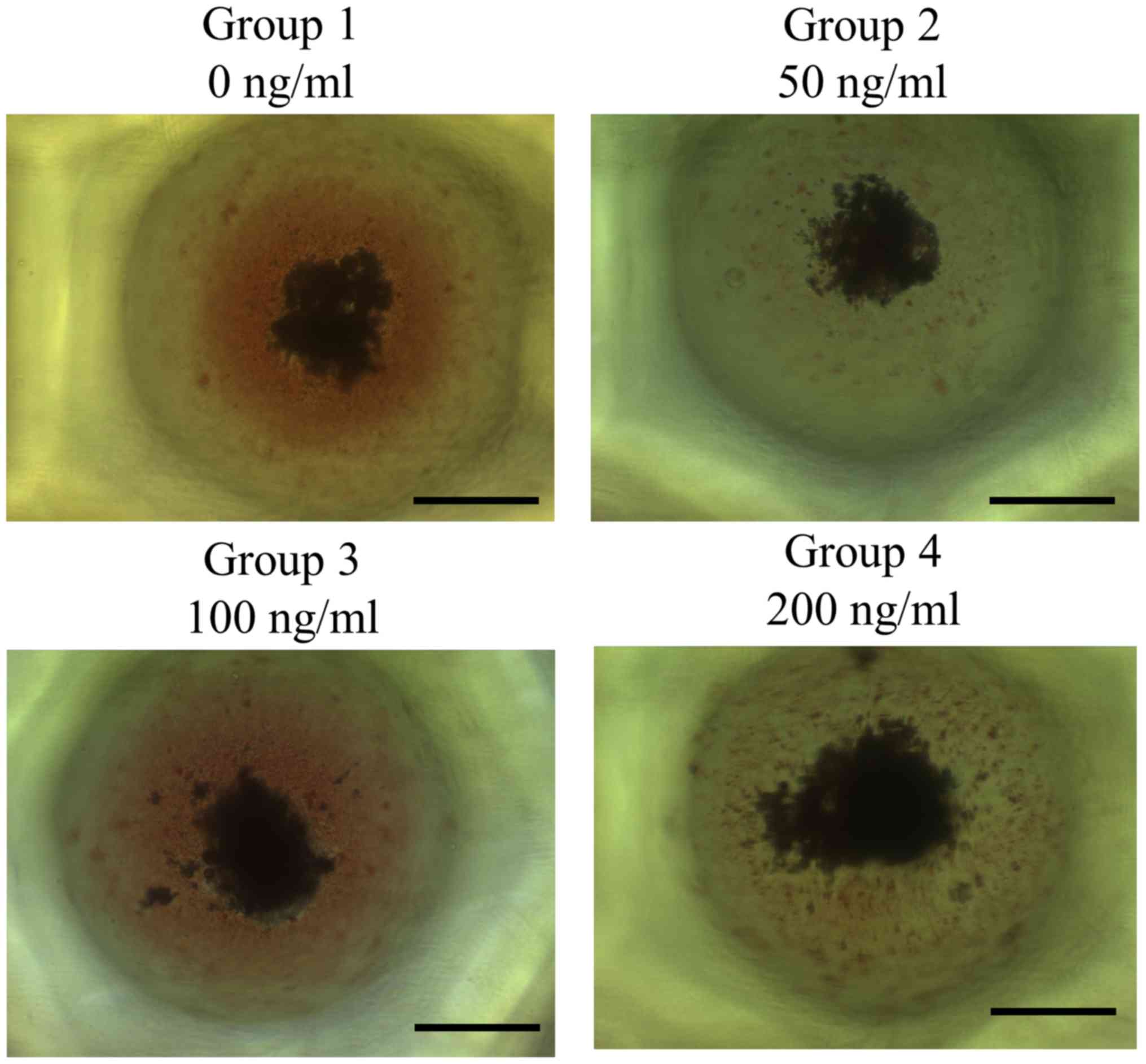
The results of the Alizarin Red S staining assay to
detect mineralization at day 14 are provided in Fig. 7A. It was observed that mineralized
extracellular deposits were present in each group. Furthermore, the
results of the quantitative analysis of Alizarin Red S staining
indicated increasing trends with increasing concentrations of
FGF-4, however, this was not statistically significant (P>0.05;
Fig. 7B).
Increaes of mRNA expression by RT-qPCR
and protein expression by Western blot analysis
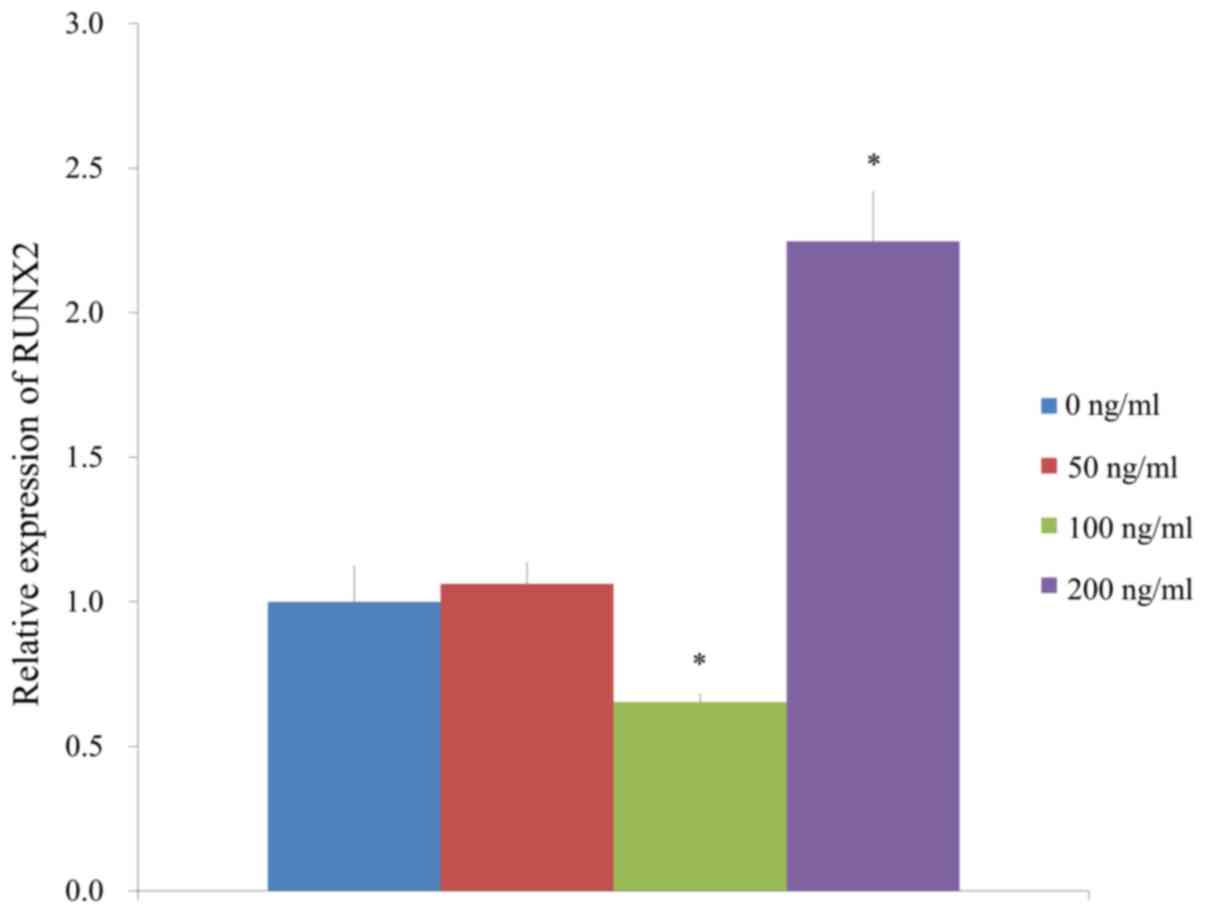
The results of the RT-qPCR analysis suggested that
the mRNA expression of RUNX2 was 100.0±12.0, 106.2±7.2, 65.3±2.7
and 224.6±17.1% for the groups treated with FGF-4 at 0, 50, 100 and
200 ng/ml, respectively. It was demonstrated that the application
of 100 ng/ml FGF-4 decreased RUNX2 expression but 200 ng/ml FGF-4
caused a significant increase in RUNX2 expression (Fig. 8A).
The RT-qPCR results also indicated that the mRNA
expression of BGLA was 100.0±4.8, 135.6±16.6, 86.8±21.2 and
293.3±43.7% in the groups treated with FGF-4 at 0, 50, 100 and 200
ng/ml, respectively. Of note, application of 200 ng/ml FGF-4
produced a significant increase in BGLA expression (Fig. 8B).
Western blot analysis was performed to detect the
expression of certain proteins following treatment with FGF-4 at
day 7 (Fig. 8C). The relative
expressions of collagen I expression of 0, 50, 100 and 200 ng/ml
groups after normalization were 100.0, 94.9, 98.7 and 152.1%,
respectively. It was indicated that the expression of collagen I
increased with the addition of FGF-4. Furthermore, the relative
expressions of RUNX2 expression of 0, 50, 100 and 200 ng/ml groups
after normalization were 100.0, 100.6, 101.0 and 118.3%,
respectively. Addition of FGF-4 enhanced the expression of
RUNX2.
Discussion
In the present study, the effects of FGF-4 on
cellular viability and osteogenic differentiation were investigated
using cell spheroids of stem cells. It was indicated that the
application of 200 ng/ml FGF-4 increased alkaline phosphatase
activity and the expression levels RUNX2 and BGLA.
Mesenchymal stem cells are well-known for their
pluripotent nature (25); these
cells are able to differentiate into tissues of mesodermal origin,
including tendons, bone, cartilage, ligaments, muscles and neurons
(26). It has also been reported
that mesenchymal stem cells may be isolated from human gingival
tissue (17). Gingiva may be a
desirable source of mesenchymal stem cell, as the harvesting
procedure is relatively less invasive and tissue may be harvested
during common dental treatments, including tooth extraction or
gingivectomy (27). Furthermore,
gingiva-derived mesenchymal stem cells grow faster than bone marrow
mesenchymal stem cells and exhibit a stable morphology without
losing their features of mesenchymal stem cells (28). Similar to other types of mesenchymal
stem cell, gingiva-derived stem cells have demonstrated a
significant osteogenic capability (29).
Mesenchymal stem cells have been reported to enhance
bone regeneration by exerting auto or paracrine effects via the
secretion of growth factors or direct differentiation into bone
cells (30). Previous studies have
revealed that mesenchymal stem cells are generally used with
scaffolds (31,32). Furthermore, due to its osteogenic
potential, a large number of dental studies have been performed
based on the mesenchymal stem cell-loaded
hydroxyapatite/β-tricalcium phosphate scaffold (31,33). If
it becomes possible to ensure that the actions of mesenchymal stem
cells are predictable and manageable, regeneration of alveolar bone
damaged by periodontal disease may become increasingly
feasible.
In recent years, 3D structures have gained increased
interest (34). Cellular features on
2D in vitro cultures have been improved to mimic
physiological conditions in vivo by applying 3D cultures
(35). Furthermore, 3D cultures of
adult human liver stem cells produced islet-like structures and
were able to reverse hyperglycemia in mice with severe diabetes
combined with immunodeficiency (34). In addition, 3D spheroid cultures
allow for the fabrication of bone marrow mesenchymal cells, which
retain osteogenic differentiation potential over a monolayer
culture of bone marrow mesenchymal cells without the requirement to
use chemicals or hormonal modulation (36). Spheroids of mesenchymal stem cells
also expressed higher transcription factors that regulate stemness
compared with monolayer cultures, along with higher alkaline
phosphatase activity and enhanced expression of
osteogenesis-associated genes (37).
In another previous study, encapsulation of stem cell
microspheroids was performed using gelatin-based hydrogels and it
was demonstrated to have promising potential for bone or cartilage
tissue engineering (38).
The present results suggested that significant
effects were achieved with 200 ng/ml FGF-4. The physiological
concentration of FGFs in humans may vary but the serum
concentration of FGF may be 10-100 pg/ml (39). In a previous study, FGF-4 was applied
at a range of concentrations ≥100 ng/ml for cell culture and at
0.03, 0.1 and 0.3 mg/kg for in vivo experiments (40). In another study, FGF-4 was prepared
at a concentration of 0.1 mg/ml, and it was subcutaneously injected
into rodent models at a dose of 0.1 mg/kg (41). Another study reported on injection of
10 µg FGF-4 in an altelocollagen carrier or the carrier alone into
the intended implant sites and it was revealed that a local single
injection of FGF-4 stimulates bone formation around titanium
implants in bone (42). Furthermore,
FGF-4 produces synergistic effects in ectopic bone formation, which
is induced by bone morphogenetic protein-2(41). However, it should be noted that the
optimal effective concentration of FGF-4 may differ due to
differences in cell types, stage and passage of the cells, system
model and duration of the culture (24,31).
Thus, the observations of the present study may apply only to cells
on the spheroid surface, but not for cells on the inside.
The present results indicated that cellular
viability was maintained in the presence of FGF-4, while osteogenic
differentiation of stem cell spheroids was enhanced, at least
partially by regulation of RUNX2 and BGLAP expression. In a
previous study, RUNX2 and BGLAP were selected as markers for
osteogenesis (38). RUNX2 is a
molecular biomarker for osteoblastic differentiation and is able to
induce the expression and synthesis of BGLAP (43). Furthermore, BGLAP is considered one
of the most specific markers of mature osteoblasts (22). Collagen I is considered as an
osteogenic marker and induction of osteogenic supplements led to
activation of collagen I expression (44). In a previous study, evaluation of
mesenchymal stem cells directed toward osteogenic differentiation
was performed by RNA extraction and PCR analysis of RUNX2 and BGLAP
(45). However, there are
limitations in the present study. The tissue was obtained from an
individual of old age and this may have influenced the results
(46). It appears that only the
cells on the surface of the spheroids were detectable using the
live/dead assay.
In conclusion, the present results suggested that
the application of FGF-4 maintained cellular viability while
enhancing the osteogenic differentiation of stem cell spheroids, at
least partially by regulating RUNX2 and BGLAP expression
levels.
Acknowledgements
We would like to thank Ms Minji Kim (College of
Dentistry, Chosun University, Gwangju 61452, Republic of Korea) for
performing the experiments and providing technical assistance.
Funding
This work was supported by the National Research
Foundation of Korea grant funded by the Korean government (Ministry
of Science and ICT; grant no. 2020R1A2C4001624). The research was
also funded by the Research Fund of Seoul St. Mary's Hospital, The
Catholic University of Korea (Seoul, Korea).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JS, JYT, SKM, YK and JBP collaborated to design the
study; JS, JYT, SKM, YK and JBP were responsible for data access
and analysis; JS, JYT, SKM, YK and JBP performed the experiments;
JS, JYT, SKM, YK and JBP wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Approval was obtained from the Institutional Review
Board at Seoul St Mary's Hospital, College of Medicine, The
Catholic University of Korea (approval no. KC17SESI0290). Informed
consent was obtained from the participant as specified in the
Declaration of Helsinki, and all of the experiments were performed
according to the relevant guidelines as specified in the
Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sohn B, Hwang M, Kim S, Kim HI and Ku Y:
Ridge preservation using basic fibroblast growth factor-2 and
collagenated biphasic calcium phosphate in beagle dogs. J
Periodontal Implant Sci. 47:381–387. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tiong KH, Mah LY and Leong CO: Functional
roles of fibroblast growth factor receptors (FGFRs) signaling in
human cancers. Apoptosis. 18:1447–1468. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nunes QM, Li Y, Sun C, Kinnunen TK and
Fernig DG: Fibroblast growth factors as tissue repair and
regeneration therapeutics. PeerJ. 4(e1535)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Frese L, Dijkman PE and Hoerstrup SP:
Adipose tissue-derived stem cells in regenerative medicine.
Transfus Med Hemother. 43:268–274. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chou YH, Pan SY, Yang CH and Lin SL: Stem
cells and kidney regeneration. J Formos Med Assoc. 113:201–209.
2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Coutu DL and Galipeau J: Roles of FGF
signaling in stem cell self-renewal, senescence and aging. Aging
(Albany NY). 3:920–933. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Boilly B, Vercoutter-Edouart AS,
Hondermarck H, Nurcombe V and Le Bourhis X: FGF signals for cell
proliferation and migration through different pathways. Cytokine
Growth Factor Rev. 11:295–302. 2000.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Henry TD, Grines CL, Watkins MW, Dib N,
Barbeau G, Moreadith R, Andrasfay T and Engler RL: Effects of
Ad5FGF-4 in patients with angina: An analysis of pooled data from
the AGENT-3 and AGENT-4 trials. J Am Coll Cardiol. 50:1038–1046.
2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kapur NK and Rade JJ: Fibroblast growth
factor 4 gene therapy for chronic ischemic heart disease. Trends
Cardiovasc Med. 18:133–141. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jung M, Kern FG, Jorgensen TJ, McLeskey
SW, Blair OC and Dritschilo A: Fibroblast growth factor-4 enhanced
G2 arrest and cell survival following ionizing radiation. Cancer
Res. 54:5194–5197. 1994.PubMed/NCBI
|
|
11
|
Quito FL, Beh J, Bashayan O, Basilico C
and Basch RS: Effects of fibroblast growth factor-4 (k-FGF) on
long-term cultures of human bone marrow cells. Blood. 87:1282–1291.
1996.PubMed/NCBI
|
|
12
|
Wu SM, Chiu HC, Chin YT, Lin HY, Chiang
CY, Tu HP, Fu MM and Fu E: Effects of enamel matrix derivative on
the proliferation and osteogenic differentiation of human gingival
mesenchymal stem cells. Stem Cell Res Ther. 5(52)2014.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Choi SC, Kim SJ, Choi JH, Park CY, Shim WJ
and Lim DS: Fibroblast growth factor-2 and -4 promote the
proliferation of bone marrow mesenchymal stem cells by the
activation of the PI3K-Akt and ERK1/2 signaling pathways. Stem
Cells Dev. 17:725–736. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lee SI, Yeo SI, Kim BB, Ko Y and Park JB:
Formation of size-controllable spheroids using gingiva-derived stem
cells and concave microwells: Morphology and viability tests.
Biomed Rep. 4:97–101. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lee H, Lee SI, Ko Y and Park JB:
Evaluation of the secretion and release of vascular endothelial
growth factor from two-dimensional culture and three-dimensional
cell spheroids formed with stem cells and osteoprecursor cells. Adv
Clin Exp Med. 27:971–977. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lee SI, Ko Y and Park JB: Evaluation of
the maintenance of stemness, viability, and differentiation
potential of gingiva-derived stem-cell spheroids. Exp Ther Med.
13:1757–1764. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jin SH, Lee JE, Yun JH, Kim I, Ko Y and
Park JB: Isolation and characterization of human mesenchymal stem
cells from gingival connective tissue. J Periodontal Res.
50:461–467. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kim BB, Tae JY, Ko Y and Park JB:
Lovastatin increases the proliferation and osteoblastic
differentiation of human gingiva-derived stem cells in
three-dimensional cultures. Exp Ther Med. 18:3425–3430.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lee H, Son J, Na CB, Yi G, Koo H and Park
JB: The effects of doxorubicin-loaded liposomes on viability, stem
cell surface marker expression and secretion of vascular
endothelial growth factor of three-dimensional stem cell spheroids.
Exp Ther Med. 15:4950–4960. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Huang X, Chen X, Chen H, Xu D, Lin C and
Peng B: Rho/Rho-associated protein kinase signaling
pathway-mediated downregulation of runt-related transcription
factor 2 expression promotes the differentiation of dental pulp
stem cells into odontoblasts. Exp Ther Med. 15:4457–4464.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lee H, Lee H, Na CB and Park JB: The
effects of simvastatin on cellular viability, stemness and
osteogenic differentiation using 3-dimensional cultures of stem
cells and osteoblast-like cells. Adv Clin Exp Med. 28:699–706.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kaur G, Valarmathi MT, Potts JD, Jabbari
E, Sabo-Attwood T and Wang Q: Regulation of osteogenic
differentiation of rat bone marrow stromal cells on 2D nanorod
substrates. Biomaterials. 31:1732–1741. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Mmethod. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tae JY, Lee H, Lee H, Ko Y and Park JB:
Osteogenic potential of cell spheroids composed of varying ratios
of gingiva-derived and bone marrow stem cells using concave
microwells. Exp Ther Med. 16:2287–2294. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gao F, Chiu SM, Motan DA, Zhang Z, Chen L,
Ji HL, Tse HF, Fu QL and Lian Q: Mesenchymal stem cells and
immunomodulation: Current status and future prospects. Cell Death
Dis. 7(e2062)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mahla RS: Stem cells applications in
regenerative medicine and disease therapeutics. Int J Cell Biol.
2016(6940283)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lee H, Son J, Yi G, Koo H and Park JB:
Cellular viability and osteogenic differentiation potential of
human gingiva-derived stem cells in 2D culture following treatment
with anionic, cationic, and neutral liposomes containing
doxorubicin. Exp Ther Med. 16:4457–4462. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tomar GB, Srivastava RK, Gupta N,
Barhanpurkar AP, Pote ST, Jhaveri HM, Mishra GC and Wani MR: Human
gingiva-derived mesenchymal stem cells are superior to bone
marrow-derived mesenchymal stem cells for cell therapy in
regenerative medicine. Biochem Biophys Res Commun. 393:377–383.
2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zorin VL, Komlev VS, Zorina AI, Khromova
NV, Solovieva EV, Fedotov AY, Eremin II and Kopnin PB: Octacalcium
phosphate ceramics combined with gingiva-derived stromal cells for
engineered functional bone grafts. Biomed Mater.
9(055005)2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Futrega K, Mosaad E, Chambers K, Lott WB,
Clements J and Doran MR: Bone marrow-derived stem/stromal cells
(BMSC) 3D microtissues cultured in BMP-2 supplemented osteogenic
induction medium are prone to adipogenesis. Cell Tissue Res.
374:541–553. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kang SH, Park JB, Kim I, Lee W and Kim H:
Assessment of stem cell viability in the initial healing period in
rabbits with a cranial bone defect according to the type and form
of scaffold. J Periodontal Implant Sci. 49:258–267. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang ZZ, Zhang HZ and Zhang ZY: 3D
printed poly(ε-caprolactone) scaffolds function with
simvastatin-loaded poly(lactic-co-glycolic acid) microspheres to
repair load-bearing segmental bone defects. Exp Ther Med. 17:79–90.
2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Vahabi S, Amirizadeh N, Shokrgozar MA,
Mofeed R, Mashhadi A, Aghaloo M, Sharifi D and Jabbareh L: A
comparison between the efficacy of Bio-Oss, hydroxyapatite
tricalcium phosphate and combination of mesenchymal stem cells in
inducing bone regeneration. Chang Gung Med J. 35:28–37.
2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Navarro-Tableros V, Gai C, Gomez Y, Giunti
S, Pasquino C, Deregibus MC, Tapparo M, Pitino A, Tetta C, Brizzi
MF, et al: Islet-like structures generated in vitro from adult
human liver stem cells revert hyperglycemia in diabetic SCID mice.
Stem Cell Rev Rep. 15:93–111. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Rao N, Grover GN, Vincent LG, Evans SC,
Choi YS, Spencer KH, Hui EE, Engler AJ and Christman KL: A
co-culture device with a tunable stiffness to understand
combinatorial cell-cell and cell-matrix interactions. Integr Biol.
5:1344–1354. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lawrence LM, Cottrill A, Valluri A,
Marenzi G, Denning KL, Valluri J, Claudio PP and Day JB: Minimally
manipulative method for the expansion of human bone marrow
mesenchymal stem cells to treat osseous defects. Int J Mol Sci.
20(20)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Moritani Y, Usui M, Sano K, Nakazawa K,
Hanatani T, Nakatomi M, Iwata T, Sato T, Ariyoshi W, Nishihara T,
et al: Spheroid culture enhances osteogenic potential of
periodontal ligament mesenchymal stem cells. J Periodontal Res.
53:870–882. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Žigon-Branc S, Markovic M, Van Hoorick J,
Van Vlierberghe S, Dubruel P, Zerobin E, Baudis S and Ovsianikov A:
Impact of hydrogel stiffness on differentiation of human
adipose-derived stem cell microspheroids. Tissue Eng Part A.
25:1369–1380. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Mraz M, Bartlova M, Lacinova Z, Michalsky
D, Kasalicky M, Haluzikova D, Matoulek M, Dostalova I, Humenanska V
and Haluzik M: Serum concentrations and tissue expression of a
novel endocrine regulator fibroblast growth factor-21 in patients
with type 2 diabetes and obesity. Clin Endocrinol (Oxf).
71:369–375. 2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kuroda S, Kasugai S, Oida S, Iimura T,
Ohya K and Ohyama T: Anabolic effect of aminoterminally truncated
fibroblast growth factor 4 (FGF4) on bone. Bone. 25:431–437.
1999.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kubota K, Iseki S, Kuroda S, Oida S,
Iimura T, Duarte WR, Ohya K, Ishikawa I and Kasugai S: Synergistic
effect of fibroblast growth factor-4 in ectopic bone formation
induced by bone morphogenetic protein-2. Bone. 31:465–471.
2002.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Franke Stenport V, Johansson CB, Sawase T,
Yamasaki Y and Oida S: FGF-4 and titanium implants: A pilot study
in rabbit bone. Clin Oral Implants Res. 14:363–368. 2003.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Rath B, Nam J, Knobloch TJ, Lannutti JJ
and Agarwal S: Compressive forces induce osteogenic gene expression
in calvarial osteoblasts. J Biomech. 41:1095–1103. 2008.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Salasznyk RM, Williams WA, Boskey A,
Batorsky A and Plopper GE: Adhesion to vitronectin and collagen I
promotes osteogenic differentiation of human mesenchymal stem
cells. J Biomed Biotechnol. 2004:24–34. 2004.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Guan M, Yao W, Liu R, Lam KS, Nolta J, Jia
J, Panganiban B, Meng L, Zhou P, Shahnazari M, et al: Directing
mesenchymal stem cells to bone to augment bone formation and
increase bone mass. Nat Med. 18:456–462. 2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lee H, Min SK and Park JB: Effects of
demographic factors on adipogenic and chondrogenic differentiation
in bone marrow-derived stem cells. Exp Ther Med. 17:3548–3554.
2019.PubMed/NCBI View Article : Google Scholar
|