Introduction
Long-term hyperglycemia, which causes diabetic
nephropathy (DN), is a frequent and principal cause of death and
morbidity among patients with diabetes (1). DN is one of the most common diabetic
complications that often occurs in patients with type 1 and 2
diabetes (2), and is also major risk
factor for chronic kidney diseases (3). Moreover, patients with DN are highly
likely to develop end-stage renal disease, which ultimately
requires renal replacement (4);
therefore, the current treatments, such as intensive blood pressure
regulation focused on blockage of the renin-angiotensin system, and
the regulation of hyperglycaemia, dyslipidaemia and albuminuria,
are not effective (5).
Previous studies have demonstrated that factors,
such as mesangial extracellular matrix deposition, thickening of
the basement membrane, glomerular pericytes, and decreased numbers
of podocytes and microvascular alterations, are closely related to
renal dysfunction in DN (6-9).
Terminally differentiated podocytes are specialized glomerular
visceral endothelial cells that exert critical effects on the
formation of the glomerular filtration barrier and inhibition of
urinary protein loss (10). Damages
to the glomerular filtration barrier increase the glomerular
filtration rate and albuminuria (11). Podocytes damage contribute to
proteinuria and the development of glomerulosclerosis (11). Collectively, the aforementioned
findings suggested that protecting against podocyte injury may
serve as a potential therapeutic strategy for DN progression. In
addition, podocyte injury includes loss of podocytes in the
glomerulus (12), which is
attributed to hyperglycemia-induced apoptosis, and directly leads
to proteinuria and glomerular sclerosis (13). Therefore, the potential therapeutic
application of mitigating podocyte apoptosis for the progression of
DN requires further investigation.
Lycopene (Lyc), a nutraceutical, is a natural
pigment that is rich in tomatoes and other plants (14). Lyc not only displays remarkable
active oxygen scavenging abilities and antioxidation activities
against quenching singlet oxygen and lipid peroxidation, but also
displays anticancer, anti-inflammatory and antiapoptotic effects
(15-17).
Moreover, Li et al (18)
demonstrated that Lyc can improve DN progression in diabetic model
rats, and Ni et al (19)
reported that Lyc can enhance autophagy and attenuate apoptosis to
protect against high glucose (HG)-induced podocyte injury.
Although the effects of Lyc on podocyte injury and
apoptosis have attracted increasing attention, the exact mechanism
underlying how Lyc exerts its protective effect on HG-induced
podocyte apoptosis is not completely understood. Therefore, the
present study explored the protective effect of Lyc on HG-induced
MPC5 podocyte apoptosis and the underlying mechanism.
Materials and method
Cell culture
Conditionally immortalized mouse podocytes (MPC5)
were purchased from American Tissue Culture Collection. MPC5
podocytes were cultured and induced for cell proliferation and
differentiation as previously described (20). MPC5 podocytes were cultured in RPMI
1640 medium (Beijing Solarbio Science & Technology Co., Ltd.)
containing 10% FBS (Sigma-Aldrich; Merck KGaA) and 10 U/ml
recombinant mouse interferon-γ (IFNγ; Peprotech, Inc.) at 33˚C with
5% CO2 and 95% relative humidity.
To stimulate cell differentiation, MPC5 podocytes
were subcultured in RPMI-1640 containing 10% FBS without mouse IFNγ
for 10-14 days at 37˚C with 5% CO2 to reach 80-90%
confluence. Prepared MPC5 podocytes were used for subsequent
experiments.
Cell viability assay
The viability of differentiated MPC5 podocytes was
determined using an MTT assay (Sigma-Aldrich; Merck KGaA),
according to the manufacturer's protocol. MPC5 podocytes were
seeded (1x104 cells/well) into 96-well plates and
incubated with RPMI-1640 supplemented with 10% FBS for 24 h at
37˚C. As previously described (21),
MPC5 podocytes were divided into seven groups: i) Normal group (NG;
5.5 mM glucose); ii) hypertonic group [HP; 5.5 mM glucose and 19.5
mM mannitol (Sigma-Aldrich; Merck KGaA)]; iii) HG (25 mM glucose);
iv) HG and low-concentration Lyc treatment group [HG + L-Lyc; 25 mM
glucose + 3.125 mM Lyc (Sigma-Aldrich; Merck KGaA)]; v) HG and
high-concentration Lyc treatment group (HG + H-Lyc; 25 mM glucose +
12.5 mM Lyc); vi) low-concentration Lyc treatment group (L-Lyc;
3.125 mM Lyc); and vii) high-concentration Lyc treatment group
(H-Lyc; 12.5 mM Lyc). All groups were treated at 37˚C for 48 h.
Subsequently, 20 µl MTT solution (5 mM) was added to each well and
incubated for another 4 h at 37˚C. The absorbance of each well was
measured at a wavelength of 570 nm using a microplate reader. The
cell viability in individual groups of cells was calculated as the
optical density (OD) value of experiments/the OD values of control
cells.
Western blotting
MPC5 podocyte protein expression was assessed via
western blotting as previously described (11). Briefly, MPC5 podocytes were washed
twice with PBS. Subsequently, total protein was extracted from MPC5
podocytes using RIPA buffer (Thermo Fisher Scientific, Inc.) with a
complete protease inhibitor cocktail (Roche Diagnostics GmbH) on
ice for 30 min. Subsequently, the supernatants were collected by
centrifugation at 12,000 x g for 10 min at 4˚C and total protein
was quantified using the BCA protein assay kit (Beijing Solarbio
Science & Technology Co., Ltd.).
Subsequently, protein (30 µg/lane) was separated via
12% SDS-PAGE and transferred onto PVDF membranes (EMD Millipore),
which were then blocked with 5% non-fat milk in TBST (0.1%
Tween-20) for 1 h at room temperature. The membranes were incubated
at 4˚C overnight with the following primary antibodies:
Anti-nephrin (rabbit; 1:400; cat. no. ab58968; Abcam), anti-podocin
(rabbit; 1:1,300; cat. no. ab50339; Abcam), anti-Bcl-2 (rabbit;
1:1,000; cat. no. ab59348; Abcam), anti-Bax (rabbit; 1:1,000; cat.
no. ab32503; Abcam), anti-cleaved (C) caspase-3 (rabbit; 1:500;
cat. no. ab2302; Abcam), anti-phosphorylated (p)-PI3K (rabbit;
1:1,000; cat. no. 4228; Cell Signaling Technology, Inc.), anti-PI3K
(rabbit; 1:1,000; cat. no. 4292; Cell Signaling Technology, Inc.),
anti-p-AKT (rabbit; 1:1,000; cat. no. 9271; Cell Signaling
Technology, Inc.), anti-AKT (rabbit; 1:1,000; cat. no. 9272; Cell
Signaling Technology, Inc.), anti-Beclin-1 (rabbit; 1:200; cat. no.
ab62557; Abcam), anti-microtubule associated protein 1 light chain
3 (LC3)I (rabbit; 1:1,000; cat. no. 12741; Cell Signaling
Technology, Inc.), anti-LC3II (rabbit; 1:1,000; cat. no. 12741;
Cell Signaling Technology, Inc.) and anti-GAPDH (rabbit; 1:10,000;
cat. no. ab181602; Abcam). Following primary incubation, the
membranes were incubated with a horseradish peroxidase-conjugated
goat anti-rabbit secondary antibody (1:2,000; cat. no. ab205718;
Abcam) at room temperature for 2 h. Subsequently, the membranes
were washed three times with TBST [50 mM Tris-HCl (pH 8.0); 150 mM
NaCl; 0.05% Tween-20] for 10 min. Protein bands were visualized
using an ECL detection kit (Promega Corporation). Protein
expression levels were quantified using ImageJ software (version
1.8.0; National Institute of Health) with GAPDH as the loading
control.
Flow cytometry analysis
MPC5 podocytes (1x105 cells/well) were
cultured in 24-well plates and FBS-starved for 24 h at 37˚C. To
investigate the effects of Lyc on HG-induced MPC5 podocyte injury,
cells were then divided into five groups: i) NG; ii) HG; iii) HG +
L-Lyc; iv) HG + H-Lyc; and v) H-Lyc. Podocytes were treated with
the aforementioned treatment for 48 h at 37˚C. MPC5 podocytes
(1x105 cells/well) were cultured in 24-well plates and
FBS-starved for 24 h at 37˚C. To investigate the effects of Lyc on
HG-induced MPC5 podocyte injury, and the PI3K/AKT pathway, cells
were divided into five groups: i) NG; ii) HG; iii) HG + H-Lyc; iv)
HG + LY; and v) HG + H-Lyc + LY. Podocytes were treated with the
normal glucose, high glucose, high glucose with H-Lyc, high glucose
with the PI3K inhibitor 20 µM LY (cat. no. S1105; Selleck
Chemicals) or 20 µM LY for 48 h at 37˚C, respectively. MPC5
podocytes (1x105 cells/well) were cultured in 24-well
plates and FBS-starved for 24 h at 37˚C. To investigate whether Lyc
has an effect on HG-induced MPC5 podocyte injury through autophagy,
the cells were divided into five groups: i) NG; ii) HG; iii) HG +
H-Lyc; iv) HG + 3-MA; and v) HG + H-Lyc + 3-MA. MPC5 podocytes in
the HG + 3-MA and HG + H-Lyc + 3-MA groups were pretreated with 5
mM 3-MA (cat. no. M9281; Sigma-Aldrich; Merck KGaA), an inhibitor
of autophagy initiation, for 2 h at 37˚C, then treated with high
glucose or H-Lyc additively for 48 h at 37˚C. The cells of NG, HG
and HG + H-Lyc groups were treated with normal glucose, high
glucose and high glucose + H-Lyc for 48 h at 37˚C, respectively.
Subsequently, treated MPC5 podocytes were collected in PBS and
stained using the Annexin V-FITC Apoptosis Detection kit (cat. no.
CA1020; Beijing Solarbio Science & Technology Co., Ltd.). After
washing, MPC5 podocyte apoptosis was determined by flow cytometry
using the Accuri™ C6 flow cytometer (BD Biosciences) and Cell Quest
software (version 3.3; BD Biosciences). The apoptotic rate was
calculated as follows: Apoptotic rate=early + late apoptosis.
Statistical analysis
Data are presented as the mean ± SD. Comparisons
among multiple groups were conducted using one-way ANOVA followed
by Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference. Statistical analyses were
performed using SPSS software (version 17.0; SPSS, Inc.). All
experiments were performed in triplicate.
Results
Lyc mitigates HG-induced MPC5 podocyte
injury
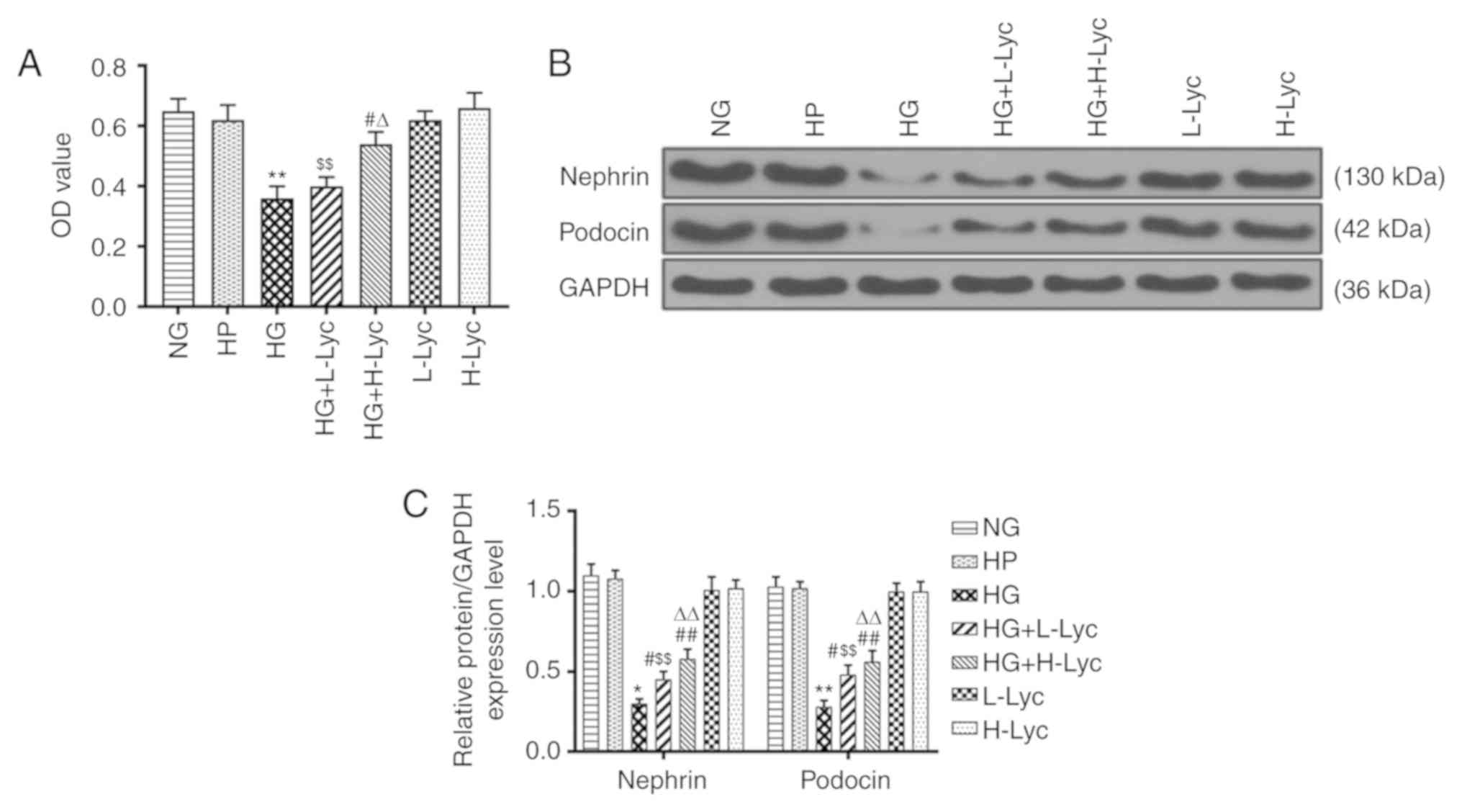
Cell viability was not significantly different
between the NG and HP groups, which indicated that HP had a limited
effect on MPC5 podocyte viability (Fig.
1A; P>0.05). However, cell viability in the HG group was
significantly reduced compared with the NG group (Fig. 1A; P<0.01). Compared with the HG
group, cell viability in the HG + L-Lyc group was not significantly
increased (Fig. 1A; P>0.05),
whereas cell viability was significantly increased in the HG +
H-Lyc group (Fig. 1A; P<0.01),
which indicated that Lyc may increase MPC5 podocyte viability under
HG conditions.
Compared with the NG group, the relative protein
expression levels of nephrin and podocin were significantly reduced
in the HG group (Fig. 1B and
C; P<0.05 and P<0.01,
respectively); however, L-Lyc or H-Lyc significantly reversed
HG-mediated effects on nephrin and podocin expression (Fig. 1B and C; P<0.05 and P<0.01,
respectively).
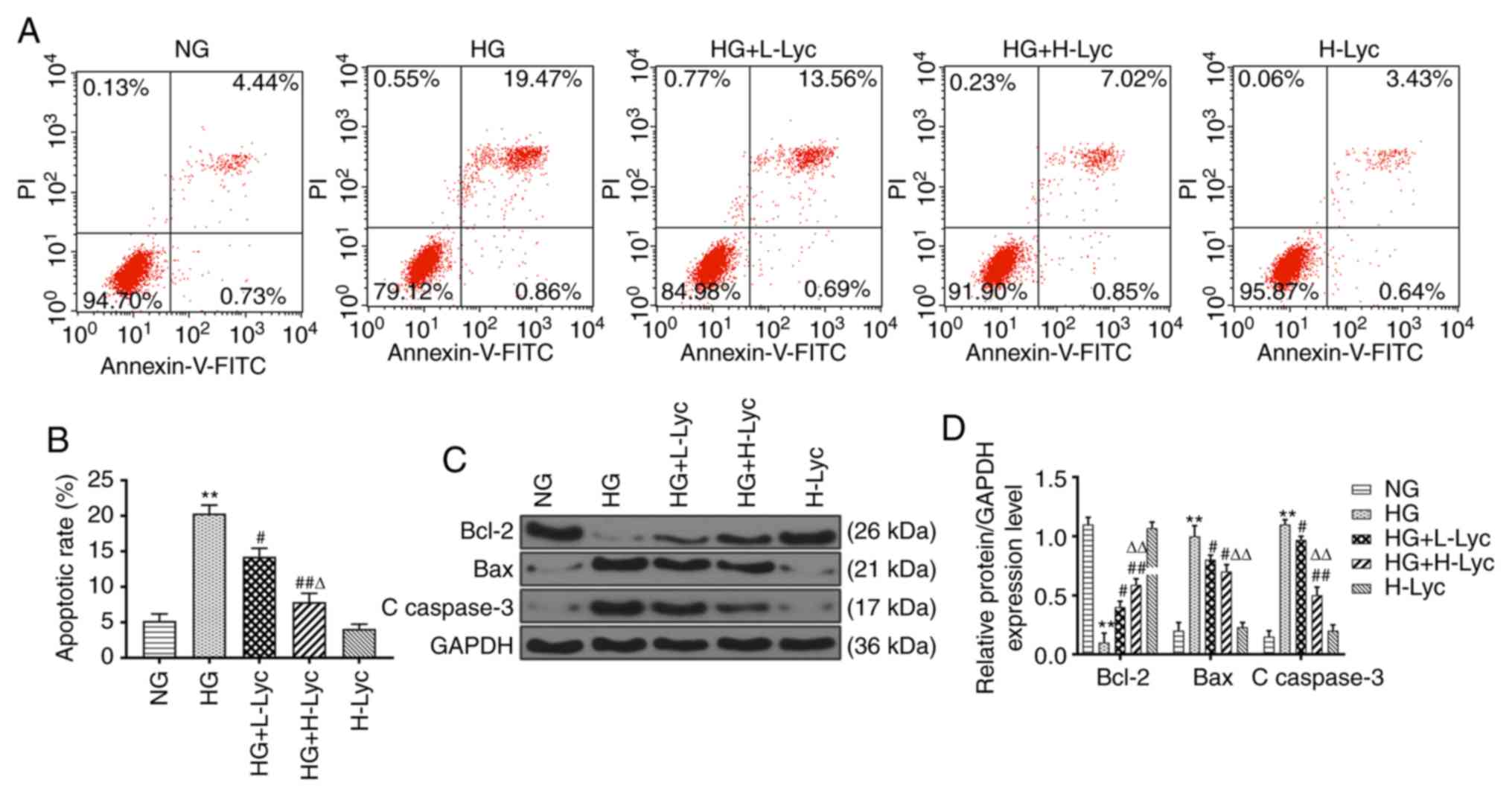
Lyc attenuates HG-induced MPC5
podocyte apoptosis
The effect of HG and Lyc on MPC5 podocyte apoptosis
was assessed by flow cytometry (Fig.
2A and B). The HG group
displayed a significantly increased rate of apoptosis compared with
the NG group (P<0.01), which was significantly reduced by the
addition of L-Lyc or H-Lyc (Fig. 2A
and B; P<0.05 and P<0.01,
respectively). Subsequently, the expression levels of
apoptosis-related proteins (Bcl-2, Bax and C caspase-3) in the
different groups were measured. Compared with the NG group, the
protein expression levels of Bax and C caspase-3 were significantly
increased (Fig. 2C and D; P<0.01), whereas the protein
expression levels of Bcl-2 were significantly decreased in the HG
group (Fig. 2C and D; P<0.01). However, the presence of
L-Lyc or H-Lyc significantly reversed HG-mediated effects on the
expression of apoptosis-related proteins (Fig. 2C and D; all P<0.05).
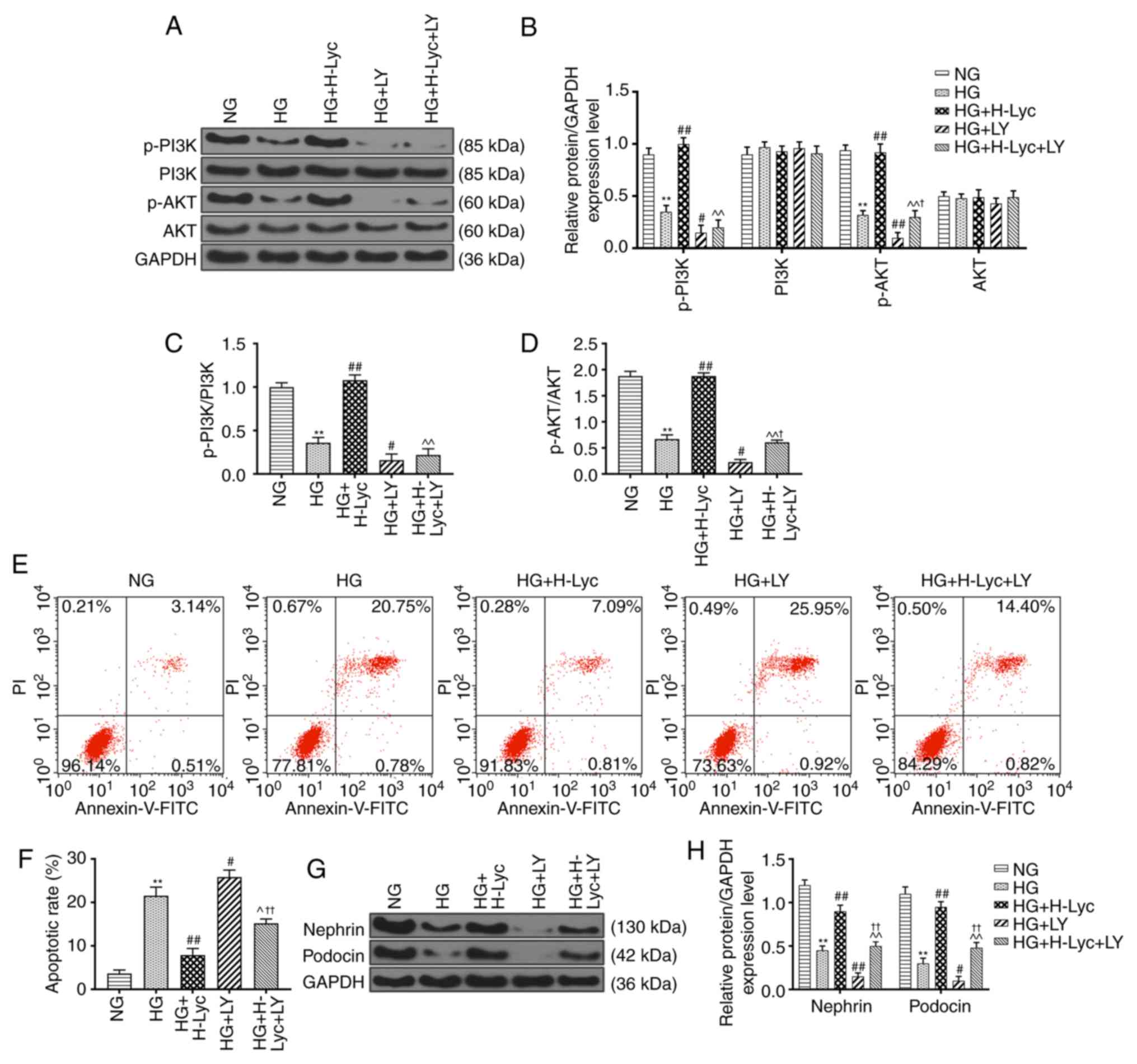
Lyc attenuates HG-induced MPC5
podocyte apoptosis via activation of the PI3K/AKT signaling
pathway
In order to investigate the mechanism underlying
Lyc-mediated inhibition of HG-induced MPC5 podocyte apoptosis, the
expression levels of PI3K/AKT signaling pathway-related proteins
were measured via western blotting. Compared with the NG group, the
protein expression levels of p-PI3K and p-AKT were significantly
downregulated in the HG group (Fig.
3A-D; P<0.01). By contrast, compared with the HG group, the
protein expression levels of p-PI3K and p-AKT were significantly
upregulated in the HG + H-Lyc group (Fig. 3A-D; P<0.01), but significantly
downregulated in the HG + LY group (Fig.
3A-D; P<0.05 and P<0.01, respectively). Moreover,
compared with the HG + H-Lyc group, the expression levels of p-PI3K
and p-AKT were significantly downregulated in the HG + H-Lyc + LY
group (Fig. 3A-D; P<0.01). In
addition, HG-mediated downregulation of p-PI3K and p-AKT expression
levels was enhanced by LY, with the HG + LY group displaying
significantly decreased p-PI3K and p-AKT expression levels compared
with the HG group (Fig. 3A-D;
P<0.05 and P<0.01, respectively). However, under HG
conditions, Lyc increased the expression levels of p-PI3K and p-AKT
in MPC5 podocytes but LY inhibited Lyc-mediated upregulation
(Fig. 3A-D).
Flow cytometry analysis indicated that the HG group
displayed significantly increased rates of MPC5 podocyte apoptosis
compared with those in the NG group, whereas the HG + H-Lyc + LY
group displayed a significantly reduced rate of MPC5 podocyte
apoptosis compared with that in the HG + LY group (Fig. 3E and F; P<0.01). HG + H-Lyc + LY group
exhibited significantly increased rates of MPC5 podocyte apoptosis
compared with those in the HG + H-Lyc group (Fig. 3E and F; P<0.05). In addition the expression
levels of nephrin and podocin were significantly reduced in the HG
group compared with the NG group. Compared with those in the HG
group, the expression levels of nephrin and podocin were
significantly increased in the HG + H-Lyc group (Fig. 3G and H; P<0.01), but were significantly
reduced in the HG + LY group compared with HG group (Fig. 3G and H; P<0.05, respectively). However, the HG
+ H-Lyc + LY group displayed significantly increased nephrin and
podocin expression levels compared with the HG + LY group (Fig. 3G and H; P<0.01).
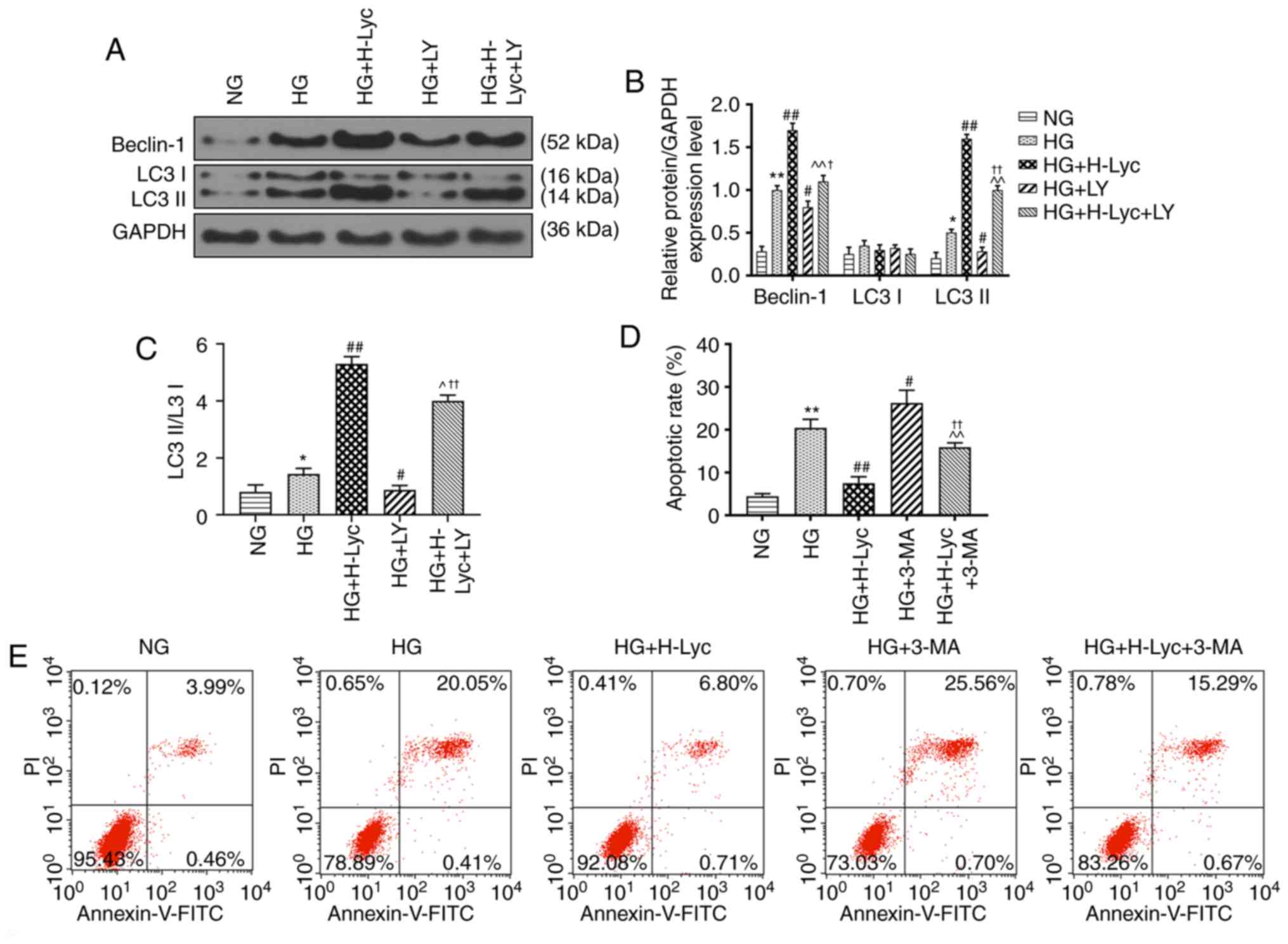
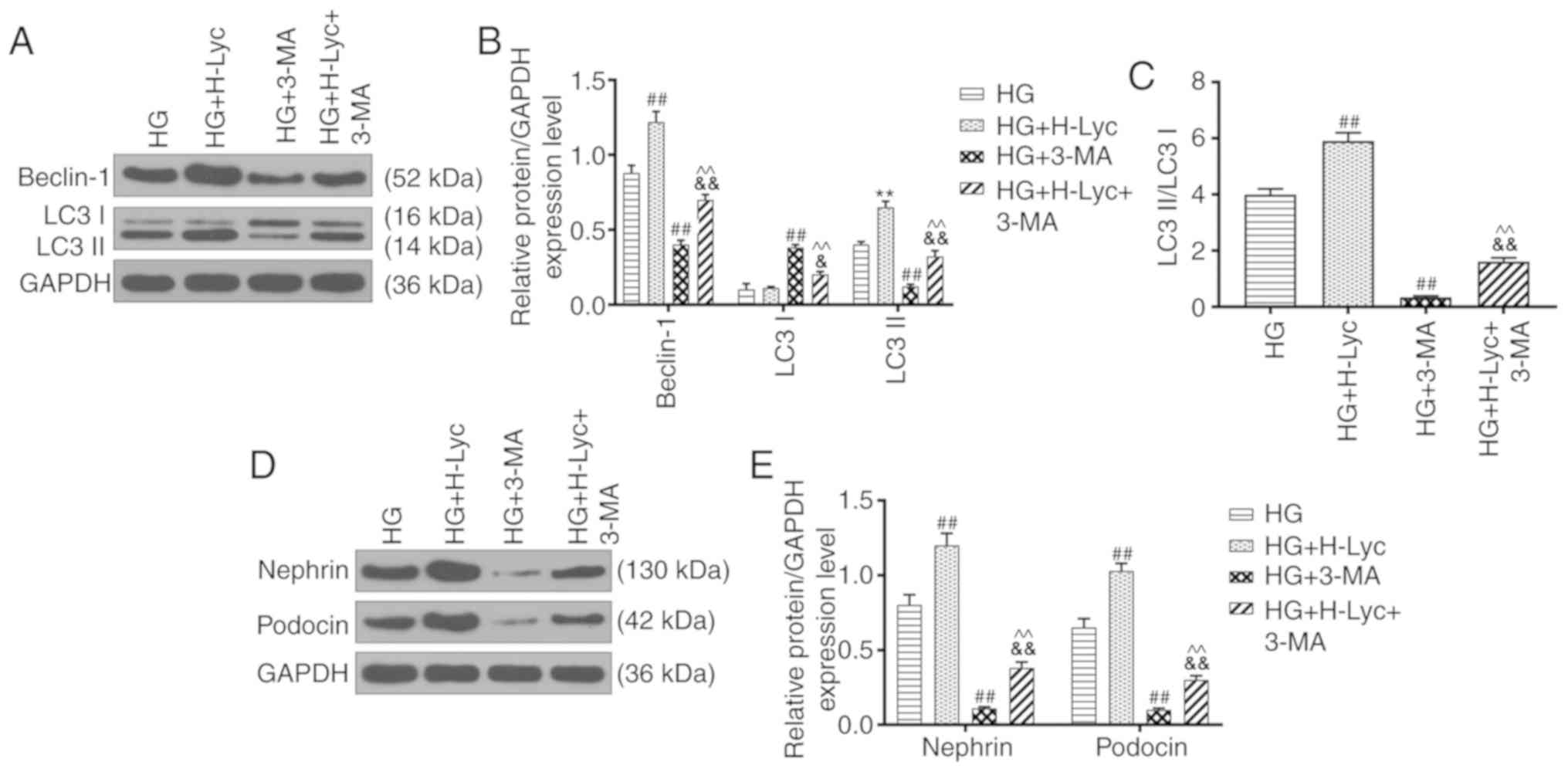
Lyc mitigates HG-induced MPC5 podocyte
apoptosis by promoting autophagy
The expression levels of autophagy-related proteins
were measured via western blotting. The protein expression levels
of LC3II/LC3I and Beclin-1 were significantly upregulated in the HG
group compared with the NG group (Fig.
4A-C; P<0.05 and P<0.01, respectively). Compared with the
HG group, the protein expression levels of LC3II/LC3I and Beclin-1
were significantly increased in the HG + H-Lyc group (P<0.01),
but significantly decreased in the HG + LY group (P<0.05).
Moreover, the effects of LY on Beclin-1 and LC3II/LC3I expression
levels were significantly inhibited in the presence of Lyc under HG
conditions (Fig. 4A-C; P<0.05 and
P<0.01, respectively).
Autophagy is associated with cell apoptosis
(22), so whether 3-methyladenine
(3-MA), an autophagy inhibitor, altered the protective effect of
Lyc on HG-induced MPC5 podocyte apoptosis was investigated. The HG
+ 3-MA group displayed significantly increased rates of MPC5
podocyte apoptosis compared with the HG group (Fig. 4D and E; P<0.05). Pretreament with 3-MA
inhibited the protective effect of Lyc on HG-induced MPC5 podocyte
apoptosis (Fig. 4D and E; P<0.01). In addition, compared with
the HG group, the protein expression levels of LC3II/LC3I and
Beclin-1 were significantly increased in the HG + H-Lyc group, but
significantly reduced in the HG + 3-MA group (Fig. 5A-C; P<0.01). Moreover, 3-MA
partially reversed H-Lyc-mediated effects on autophagy-related
protein expression under HG conditions (Fig. 5A-C; P<0.01). Furthermore, compared
with the HG group, the HG + H-Lyc group displayed significantly
increased podocin and nephrin expression levels (P<0.01),
whereas the HG + 3-MA group displayed significantly decreased
expression levels of podocin and nephrin (Fig. 5D-E; P<0.01). In addition, 3-MA
weakened H-Lyc-induced podocin and nephrin expression (Fig. 5D-E; P<0.01).
Therefore, the results suggested that HG and Lyc
significantly enhanced MPC5 podocyte autophagy, which may be
crucial for the protective effects of Lyc against HG-induced MPC5
podocyte apoptosis.
Discussion
The function of a podocyte depends on the
cytoskeleton and certain marker proteins of podocytes, such as
nephrin and podocin (23). Nephrin
and podocin proteins function as glomerular filtration barriers,
and their expression affects the permeability of the glomerular
matrix membrane, which in turn affects kidney function (24,25).
Abnormal expression of nephrin and podocin is a marker of podocyte
injury (26). Consistent with
previous study (21), the present
study demonstrated that Lyc significantly reversed the inhibitory
effects of HG on cell viability, and nephrin and podocin expression
in podocytes.
Podocyte loss is one of the most important
pathological alterations that occurs in DN (27). As a key event during DN progression,
HG-induced apoptosis is often accompanied by podocyte loss, which
is an early feature of DN and is highly predictive of the disease
progression (28-30).
Apoptosis-defined programmed cell death is also caused by external
killers in certain circumstances (31). For example, irradiation or drugs used
for cancer chemotherapy results in DNA damage in some cells, which
can lead to apoptotic death (32).
In the present study, the results indicated that HG promoted MPC5
podocyte apoptosis, and Lyc mitigated MPC5 podocyte apoptosis under
HG conditions. Apoptosis can be regulated via the PI3K/AKT
signaling pathways (33) and
PI3K/AKT can be activated by a number of different cellular stimuli
or toxic insults (34,35). Previous studies have reported that
Lyc can regulate PI3K/AKT signaling pathways in prostate cancer and
human mesenchymal stem cells (36,37). To
further explore the mechanism underlying the effects of Lyc on
HG-induced MPC5 podocyte apoptosis, a PI3K inhibitor was used. Lyc
activated the PI3K/AKT signaling pathway, which inhibited
HG-mediated MPC5 podocyte apoptosis. Similarly, a previous study
reported that Lyc reduced tert-Butyl hydroperoxide-induced cell
apoptosis, which is possibly related to activation of the PI3K/AKT
signaling pathway (38). However,
Chan et al (39) reported
that Lyc can inhibit the PI3K/AKT signaling pathway during
platelet-derived growth factor-BB-induced retinal pigment
epithelial cell migration. In addition, the results of the present
study suggested that Lyc treatment had no significant effect on
cell viability or the expression of nephrin and podocin in MPC5
podocytes compared with the NG and HG groups; however, under HG
conditions, Lyc treatment promoted MPC5 podocyte cell viability,
and nephrin and podocin protein expression. Li et al
(40) reported that Lyc treatment
had no significant effect on ameloblast apoptosis, whereas Lyc
attenuated fluoride-induced ameloblasts apoptosis. Collectively,
the aforementioned findings indicated that Lyc may serve a role
under certain pathological conditions.
As the inhibition of autophagy is highly associated
with triggering cell apoptosis, and both apoptosis and autophagy
are involved in cell growth, differentiation, and death (41), whether autophagy regulated the
protective effect of Lyc on HG-induced MPC5 podocyte apoptosis was
investigated. Autophagy is a natural process related to numerous
human diseases, which can protect the human body from cell injury
and death (42). Therefore,
autophagic activity of podocytes serves a protective role in renal
injury and could delay the progression of podocytopathies (43). Cell apoptosis and autophagy are
complex regulatory processes that involve a number of upstream
regulatory signaling pathways, including the PI3K/AKT signaling
pathway (44). A previous study
demonstrated that enhancing autophagy protects against palmitic
acid-mediated podocyte apoptosis (45), and progranulin alleviates HG-induced
human podocyte injury by regulating CAMKK/AMPK-mediated autophagy
(46). Therefore, promoting
autophagy could attenuate HG-induced podocyte apoptosis (47). Moreover, resveratrol protected
against podocyte apoptosis by activating autophagy in DN model mice
(48). Consistently, another study
reported that Lyc protected against apoptosis by increasing
autophagy in hypoxia/reoxygenation-induced H9C2 myocardioblast
cells (49). However, Kobayashi
et al (50) reported that
suppression of autophagy exerted protective effects against
HG-mediated cardiomyocyte injury. In the present study, the results
also indicated that the HG group displayed increased apoptosis
compared with the NG group, and promotion of autophagy promotes
apoptosis, which is a stress phenomenon (51).
In the present study, 3-MA, an autophagy inhibitor,
was used to explore the mechanism underlying the effect of Lyc on
autophagy. The results indicated that promoting autophagy by Lyc
attenuated HG-mediated MPC5 podocyte apoptosis, which was
associated with activation of the PI3K/AKT signaling pathway. It
has been reported that Lyc relieved HG-mediated podocyte injury by
promoting MPC5 podocyte autophagy, which may involve the PI3K/AKT
signaling pathway (21). Similarly,
a previous study reported that notoginsenoside R1 attenuated
glucose-mediated podocyte injury by reducing apoptosis and
promoting autophagy via activation of the PI3K/AKT/mTOR signaling
pathway (11). However, in
colorectal cancer cells, autophagy and apoptosis were promoted by
Grape seed procyanidin B2 via regulation of the PI3K/AKT signaling
pathway (44).
In conclusion, the present study indicated that Lyc
protected against HG-induced MPC5 podocyte injury and attenuated
HG-induced MPC5 podocyte apoptosis by promoting autophagy via
activation of the PI3K/AKT signaling pathway. The present study
demonstrated a potential mechanism underlying the therapeutic
effect of Lyc on DN, which may serve as a potential therapeutic
target for DN progression.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QW substantially contributed to the conception and
design of the study. RL, ZX and CH acquired, analyzed and
interpreted the data. QW drafted the manuscript and critically
revised it for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jin Y, Liu S, Ma Q, Xiao D and Chen L:
Berberine enhances the AMPK activation and autophagy and mitigates
high glucose-induced apoptosis of mouse podocytes. Eur J Pharmacol.
794:106–114. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hazman Ö and Bozkurt MF: Anti-inflammatory
and antioxidative activities of safranal in the reduction of renal
dysfunction and damage that occur in diabetic nephropathy.
Inflammation. 38:1537–1545. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Aso Y: Cardiovascular disease in patients
with diabetic nephropathy. Curr Mol Med. 8:533–543. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lee JH, Kim D, Oh YS and Jun HS:
Lysophosphatidic acid signaling in diabetic nephropathy. Int J Mol
Sci. 20(2850)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Vervoort G: Treatment Goals in Diabetic
Nephropathy. Pathophysiology and Clinical. Aspects. In: Diabetic
Nephropathy. Roelofs J and Vogt L (eds). Springer, Cham, pp435-450,
2019.
|
|
6
|
Najafian B, Alpers CE and Fogo AB:
Pathology of human diabetic nephropathy. Contrib Nephrol.
170:36–47. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ziyadeh FN, Hoffman BB, Han DC,
Iglesias-De La, Cruz MC, Hong SW, Isono M, Chen S, McGowan TA and
Sharma K: Long-term prevention of renal insufficiency, excess
matrix gene expression, and glomerular mesangial matrix expansion
by treatment with monoclonal antitransforming growth factor-beta
antibody in db/db diabetic mice. Proc Natl Acad Sci USA.
97:8015–8020. 2000.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yu SM and Bonventre JV: Acute kidney
injury and progression of diabetic kidney disease. Adv Chronic
Kidney Dis. 25:166–180. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tharaux PL and Huber TB: How is
proteinuric diabetic nephropathy caused by disturbed proteostasis
and autophagy in podocytes? Diabetes. 65:539–541. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Marusugi K, Nakano K, Sasaki H, Kimura J,
Yanobu-Takanashi R, Okamura T and Sasaki N: Functional validation
of tensin2 SH2-PTB domain by CRISPR/Cas9-mediated genome editing. J
Vet Med Sci. 78:1413–1420. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Huang G, Zou B, Lv J, Li T, Huai G, Xiang
S, Lu S, Luo H, Zhang Y, Jin Y and Wang Y: Notoginsenoside R1
attenuates glucose-induced podocyte injury via the inhibition of
apoptosis and the activation of autophagy through the PI3K/Akt/mTOR
signaling pathway. Int J Mol Med. 39:559–568. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Barisoni L, Schnaper HW and Kopp JB:
Advances in the biology and genetics of the podocytopathies:
Implications for diagnosis and therapy. Arch Pathol Lab Med.
133:201–216. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shankland SJ: The podocyte's response to
injury: Role in proteinuria and glomerulosclerosis. Kidney Int.
69:2131–2147. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Agarwal S and Rao AV: Tomato lycopene and
its role in human health and chronic diseases. CMAJ. 163:739–744.
2000.PubMed/NCBI
|
|
15
|
Fan S, Sun JB, Li R, Song X and Li J:
Lycopene protects myocardial ischemia injury through anti-apoptosis
and anti-oxidative stress. Eur Rev Med Pharmacol Sci. 23:3096–3104.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sahin K, Orhan C, Sahin N and Kucuk O:
Anticancer Properties of Lycopene. Springer, Cham, 2019.
|
|
17
|
Kawata A, Murakami Y, Suzuki S and
Fujisawa S: Anti-inflammatory activity of β-Carotene, Lycopene and
Tri-n-butylborane, a scavenger of reactive oxygen species. In Vivo.
32:255–264. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li W, Wang G, Lu X, Jiang Y, Xu L and Zhao
X: Lycopene ameliorates renal function in rats with
streptozotocin-induced diabetes. Int J Clin Exp Pathol.
7:5008–5015. 2014.PubMed/NCBI
|
|
19
|
Ni L, Yang XL, Qiao H and Lin J: Effects
of lycopene on high glucose-induced autophagy and apoptosis of
podocytes. Clin Nephrol. 18:48–53. 2018.(In Chinese).
|
|
20
|
Liu WT, Peng FF, Li HY, Chen XW, Gong WQ,
Chen WJ, Chen YH, Li PL, Li ST, Xu ZZ and Long HB: Metadherin
facilitates podocyte apoptosis in diabetic nephropathy. Cell Death
Dis. 7(e2477)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ni L, Qiao H, Lin J and Chang-xuan L:
Lycopene relieves the damage of mouse podocytes induced by high
glucose and its mechanism. Southeast Univ (Med Sci Edi).
37:229–234. 2018.(In Chinese).
|
|
22
|
Wang K: Autophagy and apoptosis in liver
injury. Cell Cycle. 14:1631–1642. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gao F, He X, Liang S, Liu S, Liu H, He Q,
Chen L, Jiang H and Zhang Y: Quercetin ameliorates podocyte injury
via inhibition of oxidative stress and the TGF-β1/Smad pathway in
DN rats. RSC Adv. 8:35413–35421. 2018.
|
|
24
|
Wei M, Li Z, Xiao L and Yang Z: Effects of
ROS-relative NF-KB signaling on high glucose-induced TLR4 and MCP-1
expression in podocyte injury. Mol Immunol. 68:261–271.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gong Q, He LL, Wang ML, Ouyang H, Gao HW,
Feng YL, Yang SL, Du LJ, Li J and Luo YY: Anemoside B4 protects rat
kidney from adenine-induced injury by attenuating inflammation and
fibrosis and enhancing podocin and nephrin expression. Evid Based
Complement Alternat Med. 2019(8031039)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tesch GH: Review: Serum and urine
biomarkers of kidney disease: A pathophysiological perspective.
Nephrology (Carlton). 15:609–616. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Szrejder M and Piwkowska A: AMPK
signalling: Implications for podocyte biology in diabetic
nephropathy. Biol Cell. 111:109–120. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Reidy K, Kang HM, Hostetter T and Susztak
K: Molecular mechanisms of diabetic kidney disease. J Clin Invest.
124:2333–2340. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Li W, Wang Q, Du M, Ma X, Wu L, Guo F,
Zhao S, Huang F, Wang H and Qin G: Effects of overexpressing FoxO1
on apoptosis in glomeruli of diabetic mice and in podocytes
cultured in high glucose medium. Biochem Biophys Res Commun.
478:612–617. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Saurus P, Kuusela S, Dumont V, Lehtonen E,
Fogarty CL, Lassenius MI, Forsblom C, Lehto M, Saleem MA, Groop PH
and Lehtonen S: Cyclin-dependent kinase 2 protects podocytes from
apoptosis. Sci Rep. 6(21664)2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nagata S: Apoptosis by death factor. Cell.
88:355–365. 1997.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Fulda S: Therapeutic exploitation of
necroptosis for cancer therapy. Semin Cell Dev Biol. 35:51–56.
2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Dey JH, Bianchi F, Voshol J, Bonenfant D,
Oakeley EJ and Hynes NE: Targeting fibroblast growth factor
receptors blocks PI3K/AKT signaling, induces apoptosis, and impairs
mammary tumor outgrowth and metastasis. Cancer Res. 70:4151–4162.
2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Song H, Fu Y, Wan D, Xia W, Lyu F, Liu L
and Shen L: Mytoxin B and myrothecine A induce apoptosis in human
hepatocarcinoma cell line SMMC-7721 via PI3K/Akt signaling pathway.
Molecules. 24(2291)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Porta C and Figlin RA:
Phosphatidylinositol-3-Kinase/Akt signaling pathway and kidney
cancer, and the therapeutic potential of
phosphatidylinositol-3-Kinase/Akt inhibitors. J Urol.
182:2569–2577. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chen J, O'Donoghue A, Deng YF, Zhang B,
Kent F and O'Hare T: The effect of lycopene on the PI3K/Akt
signalling pathway in prostate cancer. Anticancer Agents Med Chem.
14:800–805. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ji YK, Lee JS, Han YS, Lee JH, Bae I, Yoon
YM, Kwon SM and Lee SH: Pretreatment with lycopene attenuates
oxidative Stress-Induced apoptosis in human mesenchymal stem cells.
Biomol Ther (Seoul). 23:517–524. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Huang C, Wen C, Yang M, Gan D, Fan C, Li
A, Li Q, Zhao J, Zhu L and Lu D: Lycopene protects against
t-BHP-induced neuronal oxidative damage and apoptosis via
activation of the PI3K/Akt pathway. Mol Biol Rep. 46:3387–3397.
2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chan CM, Fang JY, Lin HH, Yang CY and Hung
CF: Lycopene inhibits PDGF-BB-induced retinal pigment epithelial
cell migration by suppression of PI3K/Akt and MAPK pathways.
Biochem Biophys Res Commun. 388:172–176. 2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Li W, Jiang B, Cao X, Xie Y and Huang T:
Protective effect of lycopene on fluoride-induced ameloblasts
apoptosis and dental fluorosis through oxidative stress-mediated
Caspase pathways. Chem Biol Interact. 261:27–34. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Fiandalo MV and Kyprianou N: Caspase
control: Protagonists of cancer cell apoptosis. Exp Oncol.
34:165–175. 2012.PubMed/NCBI
|
|
42
|
Murrow L and Debnath J: Autophagy as a
stress-response and quality-control mechanism: Implications for
cell injury and human disease. Annu Rev Pathol. 8:105–137.
2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zeng C, Fan Y, Wu J, Shi S, Chen Z, Zhong
Y, Zhang C, Zen K and Liu Z: Podocyte autophagic activity plays a
protective role in renal injury and delays the progression of
podocytopathies. J Pathol. 234:203–213. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhang R, Yu Q, Lu W, Shen J, Zhou D, Wang
Y, Gao S and Wang Z: Grape seed procyanidin B2 promotes the
autophagy and apoptosis in colorectal cancer cells via regulating
PI3K/Akt signaling pathway. Onco Targets Ther. 12:4109–4118.
2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Jiang XS, Chen XM, Wan JM, Gui HB, Ruan XZ
and Du XG: Autophagy protects against palmitic acid-induced
apoptosis in podocytes in vitro. Sci Rep. 7(42764)2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zhou D, Zhou M, Wang Z, Fu Y, Jia M, Wang
X, Liu M, Zhang Y, Sun Y, Zhou Y, et al: Progranulin alleviates
podocyte injury via regulating CAMKK/AMPK-mediated autophagy under
diabetic conditions. J Mol Med (Berl). 97:1507–1520.
2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Dong C, Zheng H, Huang S, You N, Xu J, Ye
X, Zhu Q, Feng Y, You Q, Miao H, et al: Heme oxygenase-1 enhances
autophagy in podocytes as a protective mechanism against high
glucose-induced apoptosis. Exp Cell Res. 337:146–159.
2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Huang SS, Ding DF, Chen S, Dong CL, Ye XL,
Yuan YG, Feng YM, You N, Xu JR, Miao H, et al: Resveratrol protects
podocytes against apoptosis via stimulation of autophagy in a mouse
model of diabetic nephropathy. Sci Rep. 7(45692)2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chen F, Sun ZW, Ye LF, Fu GS, Mou Y and Hu
SJ: Lycopene protects against apoptosis in
hypoxia/reoxygenation-induced H9C2 myocardioblast cells through
increased autophagy. Mol Med Rep. 11:1358–1365. 2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kobayashi S, Xu X, Chen K and Liang Q:
Suppression of autophagy is protective in high glucose-induced
cardiomyocyte injury. Autophagy. 8:577–592. 2012.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Chiarelli R, Martino C, Agnello M, Bosco L
and Roccheri MC: Autophagy as a defense strategy against stress:
Focus on paracentrotus lividus sea urchin embryos exposed to
cadmium. Cell Stress Chaperones. 21:19–27. 2016.PubMed/NCBI View Article : Google Scholar
|