Introduction
Cardiac ischemic disease and myocardial infarction
are disabling conditions that result in the appearance of scar
fibrosis and loss of myocardial contractile function. The chronic
intermittent cardiac ischemia following this event is one of the
main causes of death among human population (1-3).
Until recent times, cardiac ischemic diseases were
treated only by drug therapy, associated with surgical
revascularization procedures and with reperfusion treatments
(4,5). In the last decade, modern modalities of
treatment of ischemic myocardium, involving gene therapy,
stimulation of interstitial stem cells and heart transplant, have
also been investigated (6).
In this situation, a new dynamic view considers that
cell death and cell restoration in the heart are a part of organ
homeostasis, although the rate of myocyte renewal/turnover is very
low (7,8).
In the heart regeneration process, important
information could be obtained by detection and characterization of
a subpopulation of cardiac progenitor cells (CPC) and cardiac stem
cells (CSC), which are immature, but already committed stem cells.
These heterogenic group of cells are concentrated in specific areas
of the heart, such as the atria or pericardium (8) and express mesenchymal stem cell
markers, such as c-kit, CD34, CD90 and CD105 and sometimes
extracellular markers, such as Rex1, Nanog and Sox-2 (9,10).
Apart from this, CSCs have also been identified
based on the expression of early cardiogenesis markers such as
platelet-derived growth factor receptor-α, and fetal liver
kinase-1(11).
Patients and methods
Case selection for human tissue
specimens
From a study group of 30 cases with various ischemic
heart diseases, in an interval of one year, a series of cases
consisting of 3 males (aged 69, 75 and 82 years) and 2 females
(aged 58 and 88 years) were selected for histopathology, in order
to determine the presence of CSC.
They all died of cardiac arrhythmia secondary to
scarring myocardial infarctions (~4-6 weeks). The lesions were
located subendocardial and intramural.
The old myocardial infarctions were associated with
variable myocardium fibrosis, subsequent to a long standing
ischemic cardiopathy and atherosclerosis. The study was performed
according to the World Medical Association Declaration of Helsinki
and the tissue specimens were collected according to national
legislation, using a protocol approved by the local Bioethics
Committee of ‘Sf. Pantelimon’ Emergency Clinical Hospital
(Bucharest, Romania). All patients provided a signed informed
consent regarding hospitalization, treatment and the possible
future publication of data.
Tissue sampling and stains
Tissue samples from the heart were taken for
microscopy investigation. The fragments were harvested from the
anterior and lateral wall of the left ventricle.
The selected tissue samples were fixed in 10%
neutral buffered formalin (pH 7.0) for 24-48 h and
paraffin-embedded. Sections were cut at 5 µm and stained with
standard H&E and van Gieson.
Tissue samples have been divided into
appropriate-sized slices for conventional microscopy and
immunohistochemistry. Multiple series of histological sections were
also performed and examined.
Semi-thin sections (~1 µm) were stained with
Toluidine blue and examined under light microscopy, in order to
determine morphometric analysis in the interstitial area.
Immunohistochemistry
Immunohistochemical analysis (IHC) was done using
sections displayed on slides treated first with poly-L-lysine. IHC
was performed on 3 µm thick sections from formalin-fixed
paraffin-embedded specimens.
The method used was an indirect tristadial
avidin-biotin-complex technique, with a NovoLink Polymer detection
system which utilizes a novel control polymerization technology to
prepare polymeric HRP-linker antibody conjugates, according to the
manufacturer's instructions (Novocastra).
Briefly, the procedure comprised: deparaffination in
toluene and rehydration in alcohol series, washing in
phosphate-buffered saline (PBS), blocking with normal serum, 5-min
incubation with primary antibody 60-min incubation with
post-primary block for 30 min, then with NovoLink Polymer for 30
min. Sections are further incubated with the substrate/chromogen
3,3'-DAB and counterstained with Meyers' hematoxylin.
The antibodies used for IHC were: CD56/N-CAM (clone:
1 B6, RTU, Novocastra), CD117/c-kit (T595, RTU, Novocastra) and
CD34 (QBend/10, RTU, Novocastra).
Antigen retrieval techniques (thermal or enzymatic
pre-treatment) for the aforementioned antibodies were done,
according to the producer's specifications.
Negative control was made by using a primary
irrelevant antibody or by replacing the secondary antibody with
PBS. Positive control was made comparatively with the expression of
antibody investigated in specific cells or tissue structures
(positive internal control on slides).
To ensure the reliability of the experimental study,
internal quality control of histopathologic and IHC techniques were
performed as part of an implemented and certified quality assurance
system (ISO 9001/2018).
All slides were examined and photographed on an
AccuScope Imager microscope. Digital images acquired with an
incorporated software program were processed and analyzed with
Microsoft Office Picture Manager, running under Windows 10.
IHC assessment and statistics
The distribution of marker- positivity was assessed
using the modified Quick score method (12), which takes into account the intensity
and distribution of the IHC reaction: 0, negative (no staining); 1,
weak (only visible at high magnification); 2, moderate (readily
visible at low magnification); 3, strong (strikingly positive at
low magnification).
Statistical analysis was carried out for the
obtained data, using the Student's t-test, along with descriptive
statistics for mean, median and standard error (SE). P<0.05 was
considered to indicate a statistically significant difference.
Results
We identified the cells in the proximity of the
myocardial collagenous scar, in close contact with the residual
ischemic cardiomyocytes. CSCs were located peri-fibrillar and
interstitial, adjacent to the plasma membrane (sarcolemma) of the
cardiac muscle fibers.
The distribution along the muscular fibers, showed
that they were oriented parallel or perpendicular to the
longitudinal axis of the cardiomyocytes.
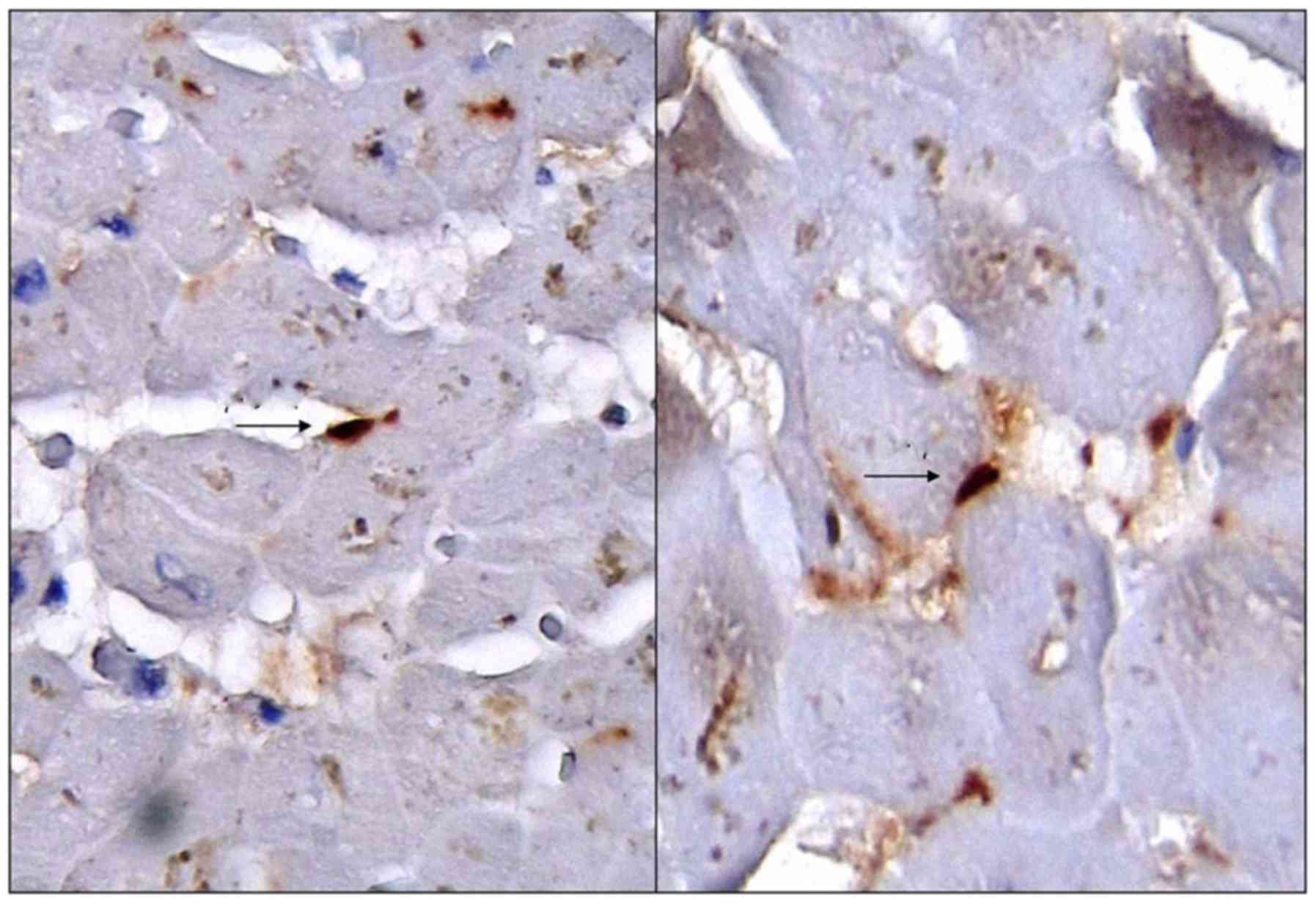
From the morphological point of view, CSC are small,
plump and ovoid, mononuclear cells, with well defined borders,
scarce cytoplasm and centrally located nuclei, sometimes hardly
noticeable (depending upon the incidence of the cut section) and
with a high nuclear to cytoplasm volume ratio.
The morphometry analysis showed that these cells,
measured at x400 magnification, had a minimum diameter of 6 µm and
a maximum diameter of 16 µm, with a mean of 11 µm and SE, ± 3
µm.
CSC stained positive for CD117 (Fig. 1), but stained negative for CD56 and
CD34. Positive intern control was used for correct IHC assessment
in mast cells for CD117, in neural fibers for CD56 and in capillary
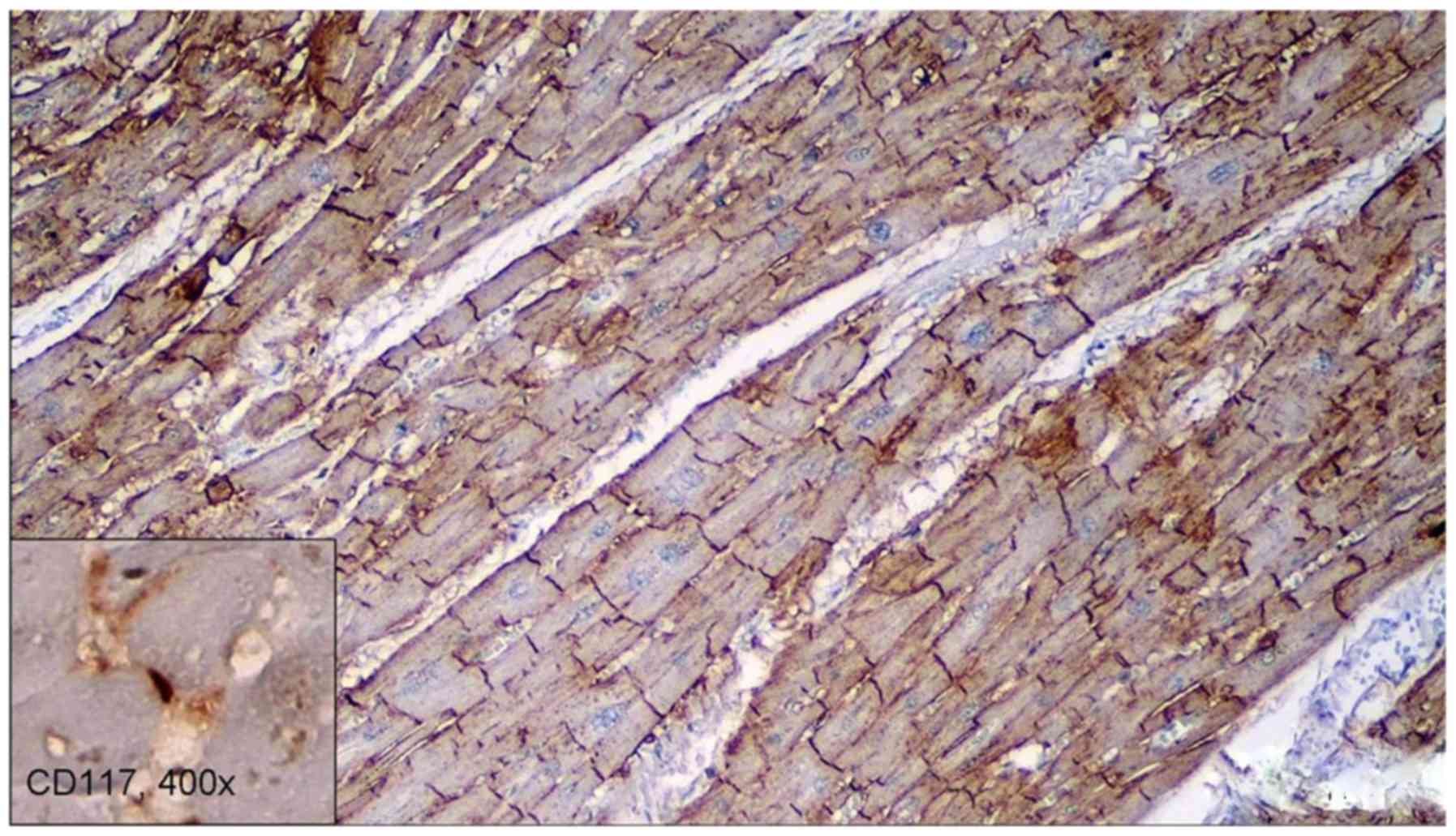
vessels for CD34. CD56 stained positive in the gap junctions of the
cardiomyocytes (Fig. 2).
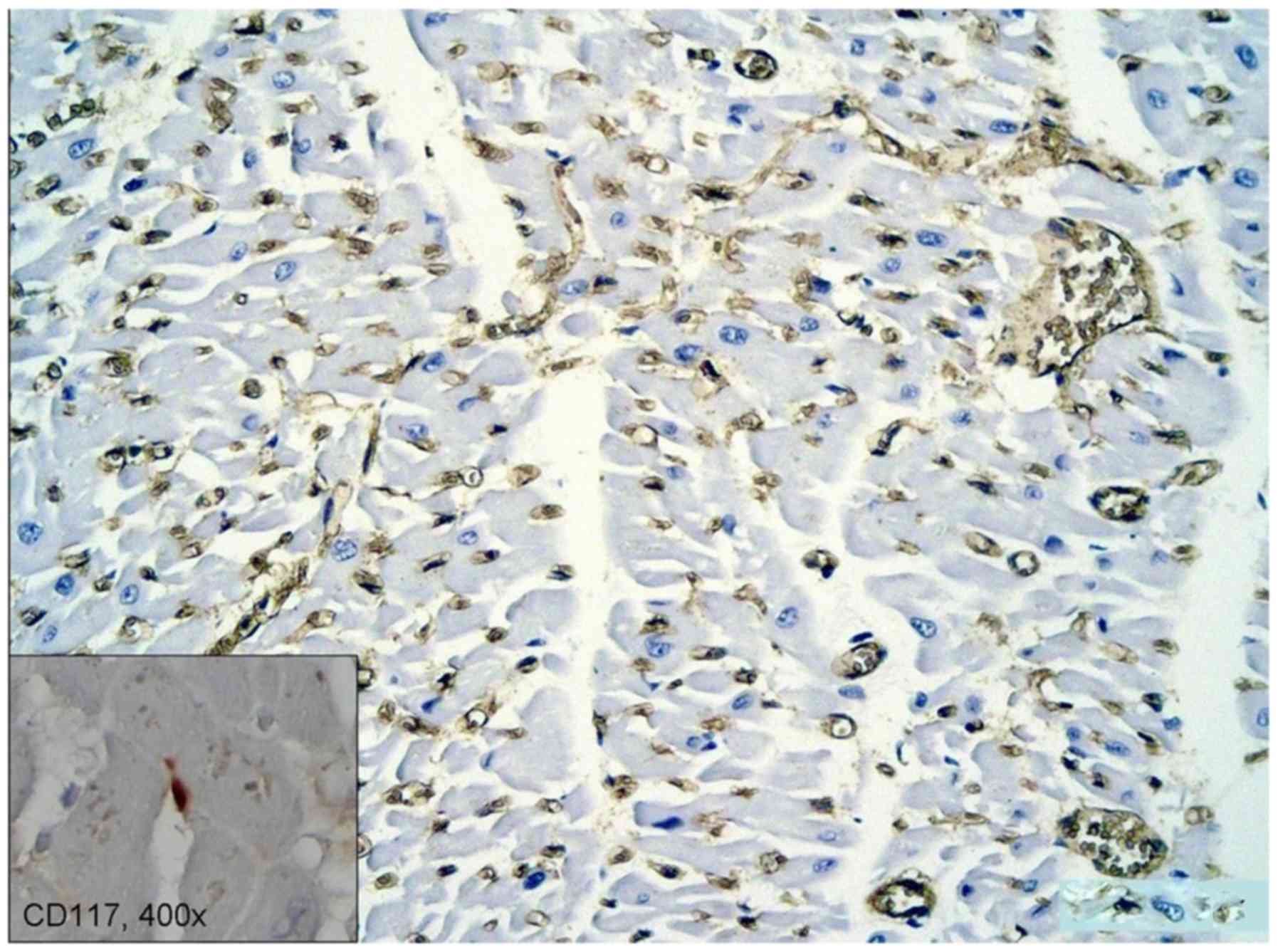
The microvascular density in the adjacent area of
the myocardium infarction, assessed by CD34 revealed a high density
capillary network, with activated CSC (Fig. 3 inset). The size and shape of these
cells are influenced by the vascularization of the surrounding
micro-environment and possibly by other stromal cells.
Discussion
Various new potential therapeutics have been shown
in stem cells. Stem cells have been discovered in various tissues:
icluding in small bowel (intestinal stem cells), in the skin
(epidermal stem cells), skeletal muscle (satellite stem cells) and
liver (oval cells). The small bowel mucosa is an example of a
rapidly self-renewing tissue. Its origin is represented by
dedicated stem cells, located at the crypt base (13). Intestinal stem cells provide by
differentiation mature functional cells. They follow a migratory
path from the base of the crypt toward the villi. In skeletal
muscle they were identified up to 17 days in humans and up to 14
days in animal experimental models (mice) post-mortem (14) and they were also demonstrated in
human cadavers up to six days after the estimated time of death
(15). Stem cells exist not only in
skeletal muscle, but also in the myocardium.
Activated CSC are pluripotent and are involved in
regeneration of the myocardium. In normal myocardium they are
quiescent and adopt a dormant state (retaining the regenerative
capacity), but they become activated in stress conditions,
particularly in chronic ischemia. They grow through symmetrical
cell division, being influenced also by the nearby stromal
cells.
It has been shown (16) that they are in close contact with
telocytes, which may play a role in their activation. According to
the study, CSC and telocytes form a ‘tandem’, both morphological
and functional, advancing the idea that this binary unit might be
useful in cell transplantation procedures.
In the animal kingdom, heart can be seen as a
self-renewing organ, in some metazoans. For instance, in zebrafish,
within 2 months after a significant heart injury, it can be
assisted to cardiac regeneration, facilitated by proliferation and
subsequent dedifferentiation of cardiomyocytes (17,18).
In humans, the neonatal heart is associated with
considerable growth and cellular proliferation of cardiomyocytes.
The heart of newborn continues to grow and proliferate in their
first week of life. The post-natal period is marked by increased
apoptosis, active cardiac remodelling and modest cellular turn-over
(19).
In adults, regarding the cardiomyocytes, the only
response to stress is hypertrophy, secondary to an increased
workload or death, after acute ischemic injury. In the second
situation, the necrotic cardiomyocytes decay and are slowly removed
by macrophages (which phagocytose the cellular debris) and is later
replaced by collagenous tissue with scar formation. In long
standing ischemia after a devastating event such myocardium
infarction, CSC from cardiac niches are activated.
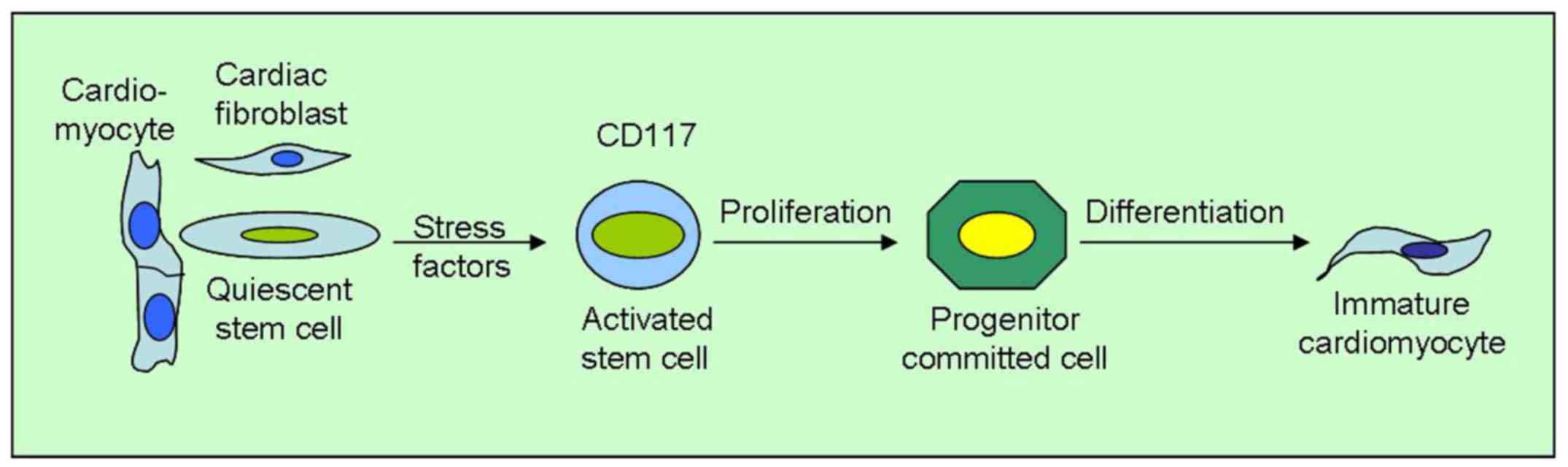
The quiescent CSC become active stem cells, which
proliferate, turning into committed progenitor cells and finally
giving rise to immature cardio-myocytes and new vascular structures
(Fig. 4). The process, however, is
slow and requires a long time for renewal in vivo (20).
Cardiac committed progenitor cells obtained from
adult hearts adhere in culture to form a three-dimensional
spherical structure named a cardio-sphere. These cardio-spheres (up
to 150 microns in size) have an enormous proliferative capacity,
generating one million cardio-spheres in a period of one month.
They represent a heterogeneous cell population, consisting of an
outer layer of proliferating cells (c-kit-positive) and an inner
layer of differentiated, contractile cardiomyocytes
(desmin-positive) (21,22).
CSC possess growth factor-receptor systems that
after activation regenerate the infarcted myocardium, improving
ventricular function and long-term survival (23).
CSC originating from the infarction area had a
higher proliferative potential and a greater propensity to migrate
in comparison to the cells originated from a healthy myocardial
area. Also, the expression level of several specific markers of
cardiogenic differentiation was higher in the cells from the
infarction area than in cells from the healthy myocardium (24).
In vitro, CSC have self-renewal capacities
and in vivo they are able to differentiate into three cell
types: cardiomyocytes, endothelial cells and vascular smooth muscle
cells. It is thought that the cardiac stem/progenitor cells express
a variety of markers, the most known and used being CD117/c-kit,
CD34, integrin β-1 (CD29), Islet-1, SCF (stem cell factor also
known as c-kit Ligand or steel factor). CD117 and CD34 are also
markers for telocytes (Cajal cells) from intestinal epithelium and
other tissues (25).
Stem cell factor provides proliferation,
differentiation and survival of stem cells. It assists the recovery
of cardiac function after myocardial infarction by increasing the
number of cardiomyocytes and vascular channels.
In addition, in vivo lineage tracing studies
suggest that another population of CPC expressing TBX18 and WT1
reside in the epicardium (26).
Furthermore, freshly isolated heart specimens taken
by myocardial endo-biopsy could add significant and valuable
information into understanding of myocardial biology of
development, injury, and aging with a great impact on therapy
(27).
In conclusion, we consider that chronic intermittent
myocardial ischemia activates the intrinsic regenerative potential
of dormant CSC, these being influenced by the capillary
microvascular density and the interstitial micro-environment
conditions. In these conditions, the cells seem to turn themselves
from dormant quiescent cells into activated progenitor committed
cells.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZC and MCe performed the histological examinations
and IHC, and had major contributions in the writing of the
manuscript. BS, DP and VDC analyzed and interpreted the patient
data. MCo, MCTD, NB and CC performed the literature research and
contributed to the writing of the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was conducted according to the World
Medical Association Declaration of Helsinki and the protocol used
was approved by the local Bioethics Committee of ‘Sf. Pantelimon’
Emergency Clinical Hospital (Bucharest, Romania). All patients
provided a signed informed consent regarding hospitalization,
treatment and the possible future publication of data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Manea M, Marcu D, Pantea Stoian A, Gaman
MA, Gaman AM, Socea B, Neagu TP, Stanescu AMA, Bratu OG and Diaconu
CC: Heart failure with preserved ejection fraction and atrial
fibrillation: A review. Rev Chim. 69:4180–4184. 2018.
|
|
2
|
Abela GS, Kalavakunta JK, Janoudi A,
Leffler D, Dhar G, Salehi N, Cohn J, Shah I, Karve M, Kotaru VPK,
et al: Frequency of cholesterol crystals in culprit coronary artery
aspirate during acute myocardial infarction and their relation to
inflammation and myocardial injury. Am J Cardiol. 120:1699–1707.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Diaconu C: Midaortic syndrome in a young
man: Case presentation. Cor Vasa. 59:e171–e173. 2017.
|
|
4
|
Tica OA, Tica O, Antal L, Hatos A, Popescu
MI, Pantea Stoian A, Bratu OG, Gaman MA, Pituru SM and Diaconu CC:
Modern oral anticoagulant treatment in patients with atrial
fibrillation and heart failure: Insights from the clinical
practice. Farmacia. 66:972–976. 2018.
|
|
5
|
Laslo C, Pantea Stoian A, Socea B,
Paduraru D, Bodean O, Socea L, Neagu TP, Stanescu AMA, Marcu D and
Diaconu C: New oral anticoagulants and their reversal agents. J
Mind Med Sci. 5:195–201. 2018.
|
|
6
|
Rebouças JS, Santos-Magalhães NS and
Formiga FR: Cardiac regeneration using growth factors: Advances and
challenges. Arq Bras Cardiol. 107:271–275. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Prasad M, Corban MT, Henry TD, Dietz AB,
Lerman LO and Lerman A: Promise of autologous CD34+
stem/progenitor cell therapy for treatment of cardiovascular
disease. Cardiovasc Res: Feb 5, 2020 (Epub ahead of print). doi:
10.1093/cvr/cvaa027.
|
|
8
|
Nadal-Ginard B, Kajstura J, Leri A and
Anversa P: Myocyte death, growth, and regeneration in cardiac
hypertrophy and failure. Circ Res. 92:139–150. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dixit P and Katare R: Challenges in
identifying the best source of stem cells for cardiac regeneration
therapy. Stem Cell Res Ther. 6(26)2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tateishi K, Ashihara E, Honsho S, Takehara
N, Nomura T, Takahashi T, Ueyama T, Yamagishi M, Yaku H, Matsubara
H, et al: Human cardiac stem cells exhibit mesenchymal features and
are maintained through Akt/GSK-3beta signaling. Biochem Biophys Res
Commun. 352:635–641. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chong JJ, Reinecke H, Iwata M, Torok-Storb
B, Stempien-Otero A and Murry CE: Progenitor cells identified by
PDGFR-alpha expression in the developing and diseased human heart.
Stem Cells Dev. 22:1932–1943. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lee H, Douglas-Jones AG, Morgan JM and
Jasani B: The effect of fixation and processing on the sensitivity
of oestrogen receptor assay by immunohistochemistry in breast
carcinoma. J Clin Pathol. 55:236–238. 2002.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tan DW and Barker N: Intestinal stem cells
and their defining niche. Curr Top Dev Biol. 107:77–107.
2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Latil M, Rocheteau P, Châtre L, Sanulli S,
Mémet S, Ricchetti M, Tajbakhsh S and Chrétien F: Skeletal muscle
stem cells adopt a dormant cell state post mortem and retain
regenerative capacity. Nat Commun. 3(903)2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ceausu M, Hostiuc S and Dermengiu D:
Skeletal muscle satellite stem cells at different postmortem
intervals. Rev Med Leg. 24:23–27. 2016.
|
|
16
|
Popescu LM, Curici A, Wang E, Zhang H, Hu
S and Gherghiceanu M: Telocytes and putative stem cells in ageing
human heart. J Cell Mol Med. 19:31–45. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bettencourt-Dias M, Mittnacht S and
Brockes JP: Heterogeneous proliferative potential in regenerative
adult newt cardiomyocytes. J Cell Sci. 116:4001–4009.
2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jopling C, Sleep E, Raya M, Marti M, Raya
A and Belmonte JC: Zebrafish heart regeneration occurs by
cardiomyocyte dedifferentiation and proliferation. Nature.
464:606–609. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rasmussen TL, Raveendran G, Zhang J and
Garry DJ: Getting to the heart of myocardial stem cells and cell
therapy. Circulation. 123:1771–1779. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Anversa P, Kajstura J, Leri A and Bolli R:
Life and death of cardiac stem cells: A paradigm shift in cardiac
biology. Circulation. 113:1451–1463. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Messina E, De Angelis L, Frati G, Morrone
S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV,
Coletta M, et al: Isolation and expansion of adult cardiac stem
cells from human and murine heart. Circ Res. 95:911–921.
2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Smith RR, Barile L, Cho HC, Leppo MK, Hare
JM, Messina E, Giacomello A, Abraham MR and Marbán E: Regenerative
potential of cardiosphere-derived cells expanded from percutaneous
endomyocardial biopsy specimens. Circulation. 115:896–908.
2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Urbanek K, Rota M, Cascapera S, Bearzi C,
Nascimbene A, De Angelis A, Hosoda T, Chimenti S, Baker M, Limana
F, et al: Cardiac stem cells possess growth factor-receptor systems
that after activation regenerate the infarcted myocardium,
improving ventricular function and long-term survival. Circ Res.
97:663–673. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Docshin PM, Karpov AA, Eyvazova SD,
Puzanov MV, Kostareva AA, Galagudza MM and Malashicheva AB:
Activation of cardiac stem cells in myocardial infarction. Cell
Tissue Biol. 12:175–182. 2018.
|
|
25
|
Constantin VD, Socea B, Popa F, Carâp AC,
Popescu G, Vlădescu T, Ceauşu Z, Berteşteanu ŞV and Ceauşu MC: A
histopathological and immunohistochemical approach of surgical
emergencies of GIST. An interdisciplinary study. Rom J Morphol
Embryol. 55 (Suppl):619–627. 2014.PubMed/NCBI
|
|
26
|
Zeng B, Ren XF, Cao F, Zhou XY and Zhang
J: Developmental patterns and characteristics of epicardial cell
markers Tbx18 and Wt1 in murine embryonic heart. J Biomed Sci.
18(67)2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sussman MA: Cardiac nonmyocyte
subpopulations: A secular congregation. Regen Med. 14:489–494.
2019.PubMed/NCBI View Article : Google Scholar
|