Introduction
Depression is a common mental illness that can lead
to upset or anxiety. The disease has a high incidence rate in the
world. Some depressive patients often have suicidal thoughts and
behavior, or experience mental illness symptoms such as fantasy or
delusion (1). World Health
Organization statistics show that more than 300 million people
worldwide suffer from depression, which is equivalent to 4.4% of
the total population in the world. Depression is also the main
cause of disability and suicide worldwide, 7.5% patients suffer
from disability, and nearly 800,000 people die of suicide due to
depression every year (2). It has
been reported that the annual cost of depression treatment in the
United States is close to $23 billion, which places huge economic
burden and loss to patients and society (3).
Many studies have shown that depression is
associated with neurotrophic factors in patients, the most
prominent of which is brain-derived neurotrophic factor (BDNF).
Stress and depression reduce the expression and function of BDNF,
while typical antidepressant therapies increase BDNF expression and
block the growth factor expression deficits caused by stress and
depression (4). Su et al
(5) found that the levels of BDNF
protein and mRNA in hippocampus of depressive mice were
significantly reduced by learned helplessness. Zhou et al
(6) reported that peripheral BDNF
levels will increase during the treatment of antidepressants. BDNF
can play an antidepressant role by mediating downstream signal
transduction through its receptor tyrosine kinase B (Trk B).
Overexpression of Trk B in mice stimulates astrocyte production and
increases the number of young neurons, reduces despair and basal
corticosterone levels (7). It has
been reported that BDNF and Trk B are mainly distributed in the
hippocampus for synaptic transmission (8).
Vortioxetine is a novel multi-target antidepressant
for severe depression. With multi-modal antidepressant mechanism,
it not only induces antidepressant or anti-anxiety activities, but
also improves the cognitive parameters of young adult rodents and
old animal models (9,10). Compared with some common
antidepressants, Vortioxetine has higher remission rate, lower
incidence of adverse events and better tolerance (11-13).
It has been reported that Vortioxetine may have a therapeutic
effect on cognitive aspects such as executive function, attention,
learning and memory that may exceed standard antidepressants
(14,15). However, it is still unclear how
Vortioxetine works on the depression model rats and the expression
of BDNF and Trk B in hippocampus.
Therefore, this study explored the expression of
BDNF and Trk B in hippocampus by observing the behavior and mental
status of Vortioxetine in the treatment of depressive rats, so as
to provide reference for clinical practice.
Materials and methods
General materials
Forty-five healthy adult SD male rats, aged 10-12
weeks and weight of 200-250 gr were used in this study. They were
purchased from Shanghai Branch of Beijing Vitonglihua Laboratory
Animal Technology Co., Ltd., with license number SCXK (Shanghai)
2017-0011. They were randomly divided into model control,
Vortioxetine and normal control group, with 15 rats in each group.
Rats had free access to food and water, 12 h light/12 h dark, and
the temperature kept at 21±2˚C for 7 days, so that the rats could
adapt to the laboratory environment. On the 8th day, depression
models were constructed in the model control and Vortioxetine
group. The normal control group did not receive any treatment for
21 days. On the 29th day, rats in the Vortioxetine group were given
intraperitoneal injection of Vortioxetine (24 mg/kg/day) (15), while rats in the model control and
the normal control group were given normal saline (8 ml/kg/day) for
7 days. The body mass data of rats were collected once a week from
the first week for 5 weeks. The study was approved by the Animal
Ethics Committee of Taian City Central Hospital (Taian, China) (no.
TCH19001B).
Kits and reagents
Vortioxetine (Shantou Jinshi Pharmaceutical Co.,
Ltd. of China Pharmaceutical Group, SFDA Approval No. H44021505),
BDNF enzyme-linked immuno sorbent assay (ELISA) kit (Wuhan
Elabscience Biotechnology Co., Ltd.; E-EL-R1235c), Trk B ELISA kit
(MyBioSource, Inc., MBS161365).
Establishing depression model
rats
Rats randomly received 7 sources of stimulation: i)
Rats had no access to food and water for 24 h, ii) rats were placed
in a light and dark cycle reversed environment, iii) rats were
suddenly placed in 4˚C ice water to force them to swim for 5 min,
and immediately dried in a 30˚C greenhouse. Rectal temperature was
measured before and after the intervention. The rectal temperature
was 36.98±0.30˚C before the intervention and the body temperature
was 37.10±0.22˚C after the intervention, iv) rats were suddenly
forced to swim in warm water at 45˚C for 5 min, and immediately
dried in a greenhouse at 30˚C. Rectal temperature was measured
before and after the intervention. The rectal temperature was
36.98±0.30˚C before the intervention and the body temperature was
37.10±0.22˚C after the intervention, v) 50 V voltage click on sole,
50 sec interval, 10 sec each time, a total of 30 times, vi) tail
clamp for 1 min and vii) cage tilt 45˚ for 24 h. All rats received
different stimuli every day, and the same stimuli did not appear
within 3 days (16,17). The modeling time lasted for 21 days,
and the difference between behavioral experiment and normal control
group was significant, which indicated that the model was
successfully established. The third intervention was performed
twice, and rectal temperature was measured before and after the
intervention. The temperature changes before and after the two
interventions were 0.92±0.26˚C and 0.84±0.30˚C. The fourth
intervention was performed 3 times. Rectal temperature were
measured before and after the intervention. The temperature changes
before and after the intervention were 1.04±0.31˚C, 1.10±0.28˚C,
and 1.14±0.33˚C. None of the rats had excessively high or low body
temperature.
Open field test
A 100x100x50 cm test box was used, with black
perimeter wall, and the bottom of the box was divided into 25
squares of equal area by white lines. The rats were placed in the
center of the test box and measured for 4 min each time. The number
of blocks crossing the ground was horizontal motion score and the
number of upright was vertical motion score. After each rat was
tested, alcohol was used to wipe the test box, and the test process
was recorded by video camera. The test was done once a week at a
fixed time for 5 weeks.
Sugar-water preference experiment
After fasting for 23 h, two bottles containing 1%
sucrose liquid and water were put into each cage. The positions of
the two bottles were replaced 30 min later. The volume of water and
sucrose consumed by each rat within 1 h was measured. Sucrose
preference ratio = the volume of consumed sucrose solution / (the
volume of consumed sucrose solution + the volume of consumed water)
x 100%, determined once a week for 5 weeks.
Morris water maze test
The test was conducted on the 36th day. The
positioning navigation test was performed in the first 4 days. Rats
were placed in water, and the time elapsed from the starting point
to the platform for each rat was recorded as the escape latency
time. If the rat did not find the platform within 60 sec, it was
directed to it and allowed to stay on the platform for 15 sec, and
the escape latency was recorded as 60 sec. On the fifth day, the
platform was removed for space exploration experiment. Rats were
put into the water from the farthest quadrant of the platform. The
swimming time of rats in the original quadrant of the platform was
recorded as the memory time within 60 sec.
Test sample collection
One day after the Morris water maze test, rats were
anesthetized with 5% pentobarbital (50 mg/kg; intraperitoneal
injection), then the whole brain was removed by decapitation, and
the hippocampus tissue was carefully separated on ice, and stored
at -80˚C.
Detection of BDNF and Trk B expression
in hippocampus by ELISA
The hippocampus tissue was weighed and reset in a
homogenizer, and the lysis buffer (50 mM Tris, 150 mM NaCl, 1%
NP-40, 0.5% sodium deoxycholate) was added to centrifuge at 1,500 x
g for 20 min at 4˚C, and the supernatant was collected. The
expression of BDNF and Trk B in supernatant was detected by ELISA.
Blank wells, standard wells and sample wells were tested. Standard
SO with a concentration of 0 was added into blank well and 50 µl of
different concentration standard was added to the standard well.
Sample wells were first added with 10 µl of the sample to be
tested, followed by 40 µl of the sample dilution solution. In
addition to the blank wells, 100 µl of horseradish peroxidase
(HRP)-labeled detection antibody was added to each well of the
standard wells and the sample wells. The reaction well was sealed
with a sealing film, incubated at 37˚C in a water bath for 65 min,
the liquid was discarded, and dried with absorbent paper. Each well
was filled with washing solution and allowed to stand for 2 min,
then washing solution was discarded, patting dry with absorbent
paper, repeated 6 times. Substrate A (50 µl) and B (50 µl) were
added to each well, incubated at 37˚C for 10 min avoiding light. A
total of 50 µl of the termination solution was added to each well,
and the OD value of each well was measured at a wavelength of 450
nm within 15 min. The concentration was calculated.
Observation indicators
The following indicators were observed: Changes of
body mass during the first five weeks; scores of horizontal and
vertical motion; sucrose preference rate; escape latency in the
first four days of Morris water maze and target quadrant residence
time on the last day; and expression levels of BDNF and Trk B in
the hippocampus.
Statistical analysis
SPSS 20.0 (IBM Corp.) medical statistical analysis
software was used to analyze the collected data. GraphPad Prism 7
(San Diego Grapad Software Co., Ltd.) was used to illustrate the
collected data. Chi-square test was used for counting data (%),
expressed as χ2. The normal distributed data were
expressed as mean±standard deviation (mean ± SD). One-way ANOVA
followed by LSD-t test was used for comparison among three or more
groups. Repeated ANOVA test was used for comparison at multiple
time points within the group, and the post hoc test was the
Bonferroni test. F was used for comparison, and P<0.05 was
considered statistically significant.
Results
Changes of body mass in three groups
within 5 weeks
There was no significant difference in body mass
among the three groups in the first week (P>0.05). There was no
significant difference between the Vortioxetine group and the model
control group in the 2nd, 3rd and 4th weeks (P>0.05), and the
body mass was significantly lower than that of the normal control
group (P<0.05). At the 5th week, the body mass of Vortioxetine
group was significantly higher than that of the control group
(P<0.05), and significantly lower than that of normal control
group (P<0.05), and the body mass of the three groups increased
markedly within five weeks (P<0.05), as shown in Table I.
 | Table IBody mass in three groups within 5
weeks (gr). |
Table I
Body mass in three groups within 5
weeks (gr).
| Group | 1st week | 2nd week | 3rd week | 4th week | 5th week | F-value | P-value |
|---|
| Normal control group
(n=15) | 228.63±7.85 |
256.07±11.32c |
278.69±13.48c,d |
297.74±16.58c-e |
321.55±18.34c-f | 99.083 | <0.001 |
| Model control group
(n=15) | 231.65±8.52 |
241.37±9.33a,c |
252.26±10.70Aa,c,d |
264.41±12.04a,c-e |
271.74±13.32a,c-f | 30.557 | <0.001 |
| Vortioxetine group
(n=15) | 227.32±7.38 |
238.69±9.13Aa,c |
253.15±11.25Aa,c,d |
266.28±12.57a,c-e |
293.63±16.32a-f | 57.629 | <0.001 |
| F-value | 1.176 | 13.197 | 23.979 | 27.309 | 35.953 | | |
| P-value | 0.319 | <0.001 | <0.001 | <0.001 | <0.001 | | |
Field test movement in three groups in
5 weeks
Horizontal and vertical movement in 5 weeks in the
three groups was compared. There was no significant difference
between horizontal and vertical movement in the normal control
group (P>0.05). There was no significant difference between
horizontal and vertical movement in three groups in the first week
(P>0.05). Horizontal and vertical motion of Vortioxetine group
and model control group in the 2nd, 3rd and 4th weeks were
significantly lower than those in the first week (P<0.05). There
was no significant difference between Vortioxetine group and model
control group (P>0.05), and the difference was significantly
lower than that of normal control group (P<0.05). In the fifth
week, the horizontal and vertical moves in the Vortioxetine group
were significantly lower than those in the first and second weeks
(P<0.05), significantly higher than those in the 4th week
(P<0.05), and there was no significant difference compared with
those in the 3rd week (P>0.05). In the fifth week, the
horizontal and vertical moves of the model control group were
significantly lower than those of the first, second and third weeks
(P<0.05). There was no significant difference compared with the
fourth week (P>0.05). The horizontal and vertical moves of the
Vortioxetine group were significantly higher than those of the
model control group (P<0.05), and significantly lower than those
of the normal control group (P<0.05) (Tables II and III).
 | Table IIHorizontal motion of field tests in
three groups in 5 weeks. |
Table II
Horizontal motion of field tests in
three groups in 5 weeks.
| Group | 1st week | 2nd week | 3rd week | 4th week | 5th week | F-value | P-value |
|---|
| Normal control
group (n=15) | 27.83±8.21 | 26.79±7.83 | 27.21±7.86 | 27.14±7.59 | 27.57±7.61 | 0.780 | 0.529 |
| Model control group
(n=15) | 27.14±7.73 |
20.84±6.19a,c |
14.88±5.70a,c,d |
8.83±3.94a,c-e |
9.02±4.15a,c-e | 31.926 | <0.001 |
| Vortioxetine group
(n=15) | 26.33±7.52 |
21.27±6.22a,c |
15.75±5.25a,c,d |
8.58±3.71a,c-e |
13.68±5.24a-c,d,f | 21.438 | <0.001 |
| F-value | 0.152 | 3.582 | 17.490 | 58.673 | 40.847 | | |
| P-value | 0.859 | 0.037 | <0.001 | <0.001 | <0.001 | | |
 | Table IIIVertical movement of three groups of
field tests in 5 weeks. |
Table III
Vertical movement of three groups of
field tests in 5 weeks.
| Group | 1st week | 2nd week | 3rd week | 4th week | 5th week | F-value | P-value |
|---|
| Normal control
group (n=15) | 5.13±1.25 | 4.63±1.17 | 4.86±1.20 | 4.87±1.20 | 4.75±1.18 | 0.873 | 0.467 |
| Model control group
(n=15) | 4.88±1.19 |
3.14±0.82a,c |
2.53±0.77a,c,d |
1.79±0.62a,c-e |
1.83±0.63a,c-e | 41.686 | <0.001 |
| Vortioxetine group
(n=15) | 4.74±1.15 |
3.26±0.86a,c |
2.64±0.74a,c,d |
1.84±0.67a,c-e |
3.46±0.87a-c,e,f | 13.600 | <0.001 |
| F-value | 0.408 | 11.088 | 30.992 | 61.595 | 37.843 | | |
| P-value | 0.667 | <0.001 | <0.001 | <0.001 | <0.001 | | |
Sugar and water preference rate in
three groups within 5 weeks
According to the sugar water test, there was no
significant change in the sugar water preference rate within 5
weeks in the normal control group (P>0.05). It was found that
there was no significant difference in the preference rate of sugar
and water among the three groups in the first week (P>0.05), and
the preference rate of sugar and water in the 2nd, 3rd and 4th
weeks of Vortioxetine group and model control group was
significantly lower than that in the previous weeks (P<0.05).
There was no significant difference in sugar preference rate
between Vortioxetine and model control group (P>0.05), and it
was significantly lower than that of normal control group
(P<0.05). Sugar preference rate of Vortioxetine group in the
fifth week was significantly lower than that in the first week
(P<0.05), significantly higher than that in the fourth and fifth
weeks (P<0.05), and there was no difference compared with that
in the second week (P>0.05). Sugar and water preference rate of
week 5 in the model control group was significantly lower than that
of week 1, 2 and 3 (P<0.05), and there was no significant
difference compared with that of week 4 (P>0.05). Sugar and
water preference rate of week 5 in the Vortioxetine group was
significantly higher than that in the model control group
(P<0.05), and significantly lower than that in the normal
control group (P<0.05) (Table
IV).
 | Table IVSugar and water preference rate (%)
within 5 weeks in three groups. |
Table IV
Sugar and water preference rate (%)
within 5 weeks in three groups.
| Group | 1st week | 2nd week | 3rd week | 4th week | 5th week | F-value | P value |
|---|
| Normal control
group (n=15) | 85.13±8.78 | 82.24±7.62 | 83.87±8.69 | 86.37±7.84 | 87.16±7.62 | 2.290 | 0.090 |
| Model control group
(n=15) | 87.43±9.23 |
73.14±8.85a,c |
66.37±7.24a,c,d |
58.21±6.98a,c-e |
60.47±7.36a,c-e | 26.846 | <0.001 |
| Vortioxetine group
(n=15) | 84.57±8.36 |
75.82±9.48a,c |
65.42±7.52a,c,d |
53.58±7.35a,c-e |
71.69±6.24a-c,e,f | 26.527 | <0.001 |
| F-value | 0.445 | 4.349 | 26.326 | 86.305 | 53.461 | | |
| P-value | 0.644 | 0.019 | <0.001 | <0.001 | <0.001 | | |
Morris water maze learning and memory
ability
Morris water maze experiment was used to compare the
escape latency of three groups in positioning navigation test and
the target quadrant residence time in space exploration experiment.
It was found that the escape latency of three groups decreased
significantly within four days (P<0.05). The escape latency of
Vortioxetine group was significantly lower than that of model
control group (P<0.05), but higher than that of normal control
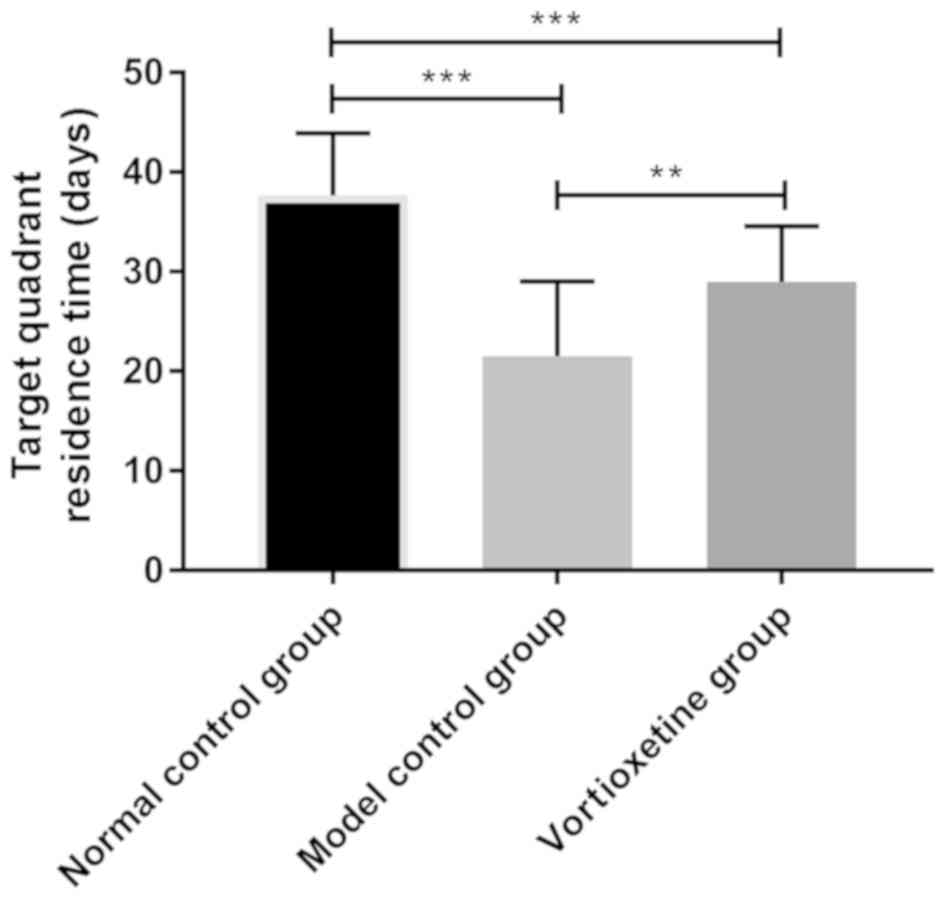
group (P<0.05). The target quadrant dwell time (28.96±5.62 sec)
of Vortioxetine group was significantly lower than that of model
control group (21.54±7.49 sec) (P<0.05), but higher than that of
normal control group (37.64±6.25 sec) (P<0.05) (Table V and Fig.
1).
 | Table VEscape latency (S) of three groups in
positioning and navigation test. |
Table V
Escape latency (S) of three groups in
positioning and navigation test.
| Group | 1st day | 2nd day | 3rd day | 4th day | F-value | P-value |
|---|
| Normal control
group (n=15) | 34.63±3.78 |
26.46±3.62c |
21.48±2.66c,d |
16.79±2.54c-e | 82.141 | <0.001 |
| Model control group
(n=15) |
55.84±6.72a |
51.65±6.49a |
47.26±6.13a,c,d |
42.14±5.46a,c-e | 22.932 | <0.001 |
| Vortioxetine group
(n=15) |
46.83±4.85a |
41.92±4.57a,c |
37.67±4.76a,c,d |
31.73±3.45a-e | 45.955 | <0.001 |
| F-value | 61.458 | 95.411 | 113.507 | 151.694 | | |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 | | |
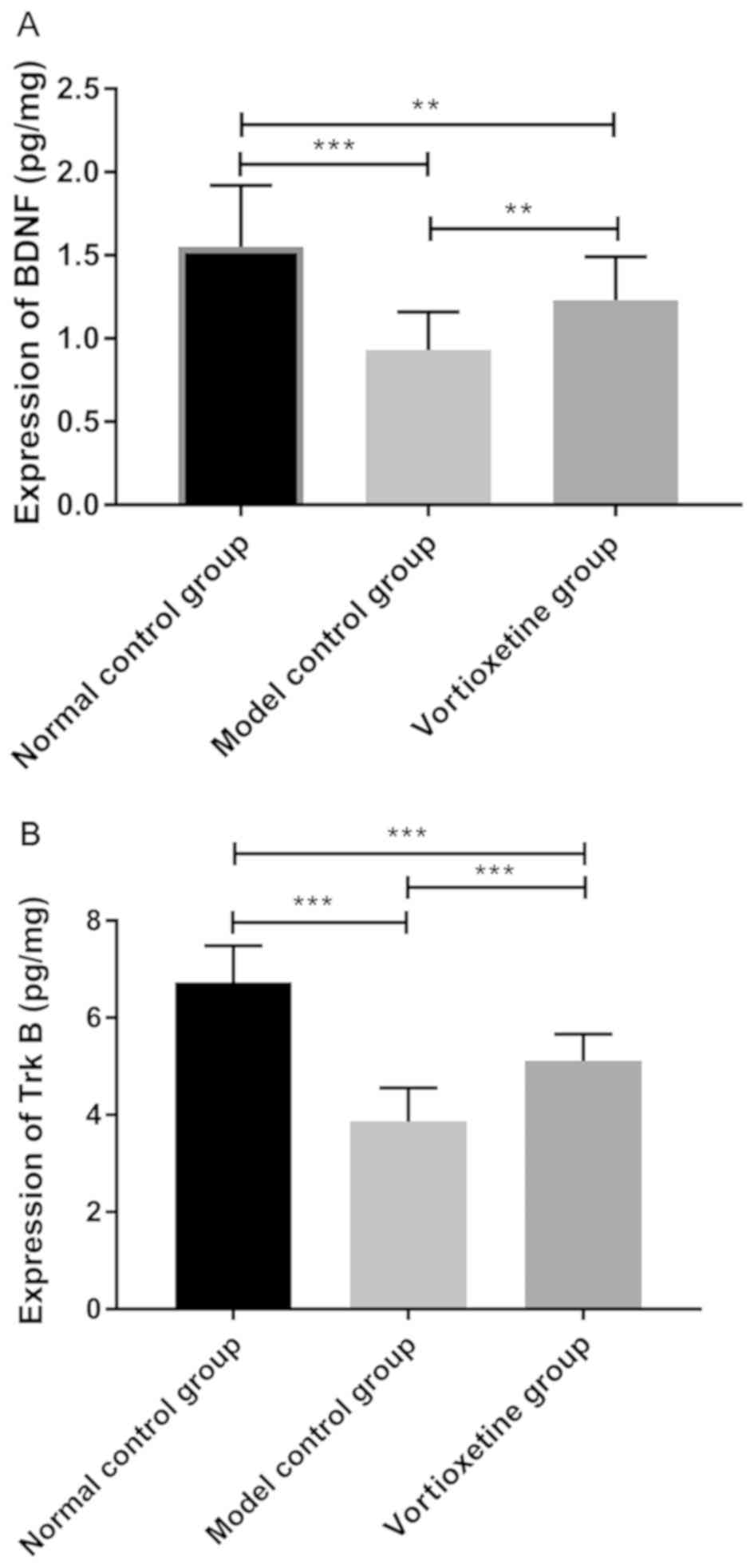
Comparison of expression levels of
BDNF and Trk B
Comparing the expression of BDNF and Trk B in three
groups, showed that the expression of BDNF in Vortioxetine group
(1.23±0.26 pg/mg) was significantly higher than that in the model
control group (0.93±0.23 pg/mg) (P<0.05), but lower than that in
the normal control group (1.55±0.37 pg/mg) (P<0.05). The
expression of Trk B in the Vortioxetine group (5.11±0.55 pg/mg) was
significantly higher than that in the model control group
(3.86±0.70 pg/mg) (P<0.05), but lower than that in the normal
control group (6.73±0.75 pg/mg) (P<0.05) (Fig. 2).
Discussion
It has been mentioned in many studies that serotonin
system is involved in psychological disorders such as depression
and anxiety. As a neurotransmitter, serotonin regulates
physiological processes such as mood, sleep and cognition. The lack
of serotonin in synapses can lead to depression, and serotonin has
to be mediated by corresponding receptors to play its role. Almost
all subtypes of serotonin receptors are involved in antidepressant
or antianxiety effects. Serotonin transporters also involve
behavior, cognition and personality, as well as psychiatric
disorders including depression, which take up serotonin in the
synaptic cleft, leading to a decrease in synaptic serotonin levels
(18-21).
Vortioxetine may have a combined effect on a variety of serotonin
receptors and serotonin transporters. The antidepressant effect is
achieved by activating the relevant serotonin receptor and
inhibiting the serotonin transporter, which is different from
existing antidepressants. Vortioxetine also has memory enhancement
effect (22).
In this study, we used stress to stimulate
depression in rats. It was found that there were no differences in
body mass, preference rate for sugar and water and field test among
the three groups in the first week. However, with stimulation of
source at the 2nd, 3rd and 4th week, there was no significant
difference in body mass, sugar preference rate and field test
exercise between Vortioxetine and model control group, and they
were significantly lower than those of normal control group. At the
5th week, the body mass, sugar preference rate and field test of
Vortioxetine group were significantly higher than those of model
control group, but both groups were lower than the normal control
group. The escape latency in Morris water maze was significantly
lower than that in model control group in the first four days, but
higher than that in normal control group. The target quadrant
memory time in Vortioxetine group was significantly higher than
that in model control group and lower than that in normal control
group. These results indicated that with the occurrence of
depression, rats suffer from loss of pleasure, decrease of
autonomous and exploratory behavior, and decreased learning and
memory. However, the use of Vortioxetine can alleviate the adverse
symptoms of these depressed rats to a certain extent, and reduce
the depression in depressed rats. Mahableshwarkar et al
(23) assessed the effect of
Vortioxetine on cognitive function in adult patients with severe
depression, and found that Vortioxetine could significantly improve
cognitive function and depression, and was well tolerated. This
clinical result combined with our experimental results further
suggested that Vortioxetine has a good therapeutic effect in
depression.
ELISA was used to detect the expression of BDNF and
Trk B in the hippocampus of three groups of rats. It was found that
BDNF and TrkB in Vortioxetine group were significantly higher than
those in model control group and significantly lower than those in
normal control group. It has been reported that BDNF can promote
neuronal differentiation, survival, maintenance and synaptic
plasticity by activating high affinity receptor Trk B. BDNF-TrkB
signal transduction deficiency is the pathogenesis of depression.
The reactivation of BDNF-TrkB signal transduction by
antidepressants can promote the recovery of patients (24). Since TrkB is a high-affinity receptor
for BDNF, the role of BDNF requires TrkB signaling to BDNF to
enhance the ability of synaptic transmission in the hippocampus.
Synaptic transmission depends on the functional Trk B receptor. The
lack of BDNF-TrkB signal due to the inhibitation of TrkB will
reduce the antidepressant effect (25), which suggests that the BDNF-Trk B
signaling pathway will participate in the antidepressant process.
Kozono et al (26) reported
that 5-HT4 receptor coupled with Gs protein can promote dendritic
formation in hippocampal neurons, and increased expression of BDNF
can induce 5-HT4 receptor to play a role by Trk B signal
transduction. The function of voxoxetine is mainly carried out
through the regulation of the serotonin system, which can promote
the level of serotonin. It is 5-HT3, 5-HT7 and 5-HT1D receptor
antagonists, 5-HT1B receptor partial agonists, 5-HT1A receptor
agonists and 5-HT transporters, so increasing serotonin is the key
to futhioxetine. It has been reported (27) that BDNF can increase serotonin by
activating Trk B, and the expression of serotonin increases after
the expression of BDNF and Trk B is increased. According to a
previous study (28), serotonin,
BDNF, and Trk B can promote each other. Therefore, theoretically
Vortioxetine can upregulate the expression of BDNF and Trk B in
depressive rats by regulating the serotonin system, and BDNF and
Trk B can further alleviate the symptoms of depressive rats. Sagud
et al (29) used Vortioxetine
to treat depression patients. The results showed that serotonin
decreased and plasma BDNF increased in depression patients, which
is similar to our results. In the study by Lu et al
(30), it was also found that
futhioxetine can increase the BDNF level in the hippocampus of
depressive rats. However, the effect of futhioxetine on TrkB in the
hippocampus of depressed rats has not been studied previously. It
was found in this study that the expression of Trk B was also
up-regulated.
However, it has been mentioned in some reports that
the effects and side effects of Vortioxetine vary with the dose
(31,32). However, in this study, we only used a
single dose of Vortioxetine, and it is not clear whether this dose
is optimal. Considering the obvious symptoms of the disease and
drug tolerance, the current drug treatment for depression is mainly
individualized, mostly using several common drugs (33). We used Vortioxetine alone, and did
not compare it with other common antidepressants or combination
drugs. How other antidepressants and their combination affect the
expression of BDNF and Trk B still needs to be explored.
Vortioxetine might regulate BDNF-TrkB by regulating the serotonin
system, which needs to be explored.
In conclusion, Vortioxetine can effectively
alleviate the symptoms of depressed rats, such as loss of pleasure,
autonomous and exploratory behavior, and decrease of learning and
memory. The expression of BDNF and Trk B in the hippocampus of
depressive rats was significantly lower than that of normal rats.
Vortioxetine increased the expression of BDNF and Trk B in
depressive rats and reduced their depressive behavior.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
BS, YL and HX conceived and designed the study, and
drafted the manuscript. BS, CQ, CL and PL collected, analyzed and
interpreted the experimental data. BS revised the manuscript for
important intellectual content. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Animal Ethics
Committee of Taian City Central Hospital (Taian, China) (no.
TCH19001B).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ahmed R, Khan NA, Waseem M and Khan ZJ:
Holistic approach in the management of depression: A Review. J
Integ Comm Health. 6:10–14. 2017.
|
|
2
|
World Health Organization (WHO):
Depression and other common mental disorders. Global Health
Estimates (GHE), Geneva, Switzerland pp24, 2017.
|
|
3
|
Siu AL, Bibbins-Domingo K, Grossman DC,
Baumann LC, Davidson KW, Ebell M, García FA, Gillman M, Herzstein
J, Kemper AR, et al: US Preventive Services Task Force (USPSTF).
Screening for depression in adults: US Preventive Services Task
Force recommendation statement. JAMA. 315:380–387. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Duman RS, Aghajanian GK, Sanacora G and
Krystal JH: Synaptic plasticity and depression: New insights from
stress and rapid-acting antidepressants. Nat Med. 22:238–249.
2016.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Su CL, Su CW, Hsiao YH and Gean PW:
Epigenetic regulation of BDNF in the learned helplessness-induced
animal model of depression. J Psychiatr Res. 76:101–110.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhou C, Zhong J, Zou B, Fang L, Chen J,
Deng X, Zhang L, Zhao X, Qu Z, Lei Y, et al: Meta-analyses of
comparative efficacy of antidepressant medications on peripheral
BDNF concentration in patients with depression. PLoS One.
12(e0172270)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
De Vry J, Vanmierlo T, Martínez-Martínez
P, Losen M, Temel Y, Boere J, Kenis G, Steckler T, Steinbusch HWM,
Baets M, et al: TrkB in the hippocampus and nucleus accumbens
differentially modulates depression-like behavior in mice. Behav
Brain Res. 296:15–25. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lapchak PA, Araujo DM and Hefti F: BDNF
and trkB mRNA expression in the rat hippocampus following
entorhinal cortex lesions. Neuroreport. 4:191–194. 1993.PubMed/NCBI View Article : Google Scholar
|
|
9
|
David DJ, Tritschler L, Guilloux JP,
Gardier AM, Sanchez C and Gaillard R: Pharmacological properties of
vortioxetine and its pre-clinical consequences. Encephale.
42(1S12-1S23)2016.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
10
|
Frampton JE: Vortioxetine: A review in
cognitive dysfunction in depression. Drugs. 76:1675–1682.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Thase ME, Danchenko N, Brignone M, Florea
I, Diamand F, Jacobsen PL and Vieta E: Comparative evaluation of
vortioxetine as a switch therapy in patients with major depressive
disorder. Eur Neuropsychopharmacol. 27:773–781. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Baune BT, Sluth LB and Olsen CK: The
effects of vortioxetine on cognitive performance in working
patients with major depressive disorder: A short-term, randomized,
double-blind, exploratory study. J Affect Disord. 229:421–428.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nishimura A, Aritomi Y, Sasai K, Kitagawa
T and Maha-bleshwarkar AR: Randomized, double-blind,
placebo-controlled 8-week trial of the efficacy, safety, and
tolerability of 5, 10, and 20 mg/day vortioxetine in adults with
major depressive disorder. Psychiatry Clin Neurosci. 72:64–72.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Connolly KR and Thase ME: Vortioxetine: A
new treatment for major depressive disorder. Expert Opin
Pharmacother. 17:421–431. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Harrison JE, Lophaven S and Olsen CK:
Which cognitive domains are improved by treatment with
vortioxetine? Int J Neuropsychopharmacol.
19(pyw054)2016.10.1093/ijnp/pyw054. PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lee B and Lee H: Systemic administration
of curcumin affect anxiety-related behaviors in a rat model of
posttraumatic stress disorder via activation of serotonergic
systems. Evid Based Complement Alternat Med. 2018(9041309)2018.
View Article : Google Scholar : https://doi.org/10.1155/2018/9041309.
|
|
17
|
Pooley AE, Benjamin RC, Sreedhar S, Eagle
AL, Robison AJ, Mazei-Robison MS, Breedlove SM and Jordan CL: Sex
differences in the traumatic stress response: The role of adult
gonadal hormones. Biol Sex Differ. 9(32)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hatherall L, Sánchez C and Morilak DA:
Chronic vortioxetine treatment reduces exaggerated expression of
conditioned fear memory and restores active coping behavior in
chronically stressed rats. Int J Neuropsychopharmacol. 20:316–323.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Żmudzka E, Sałaciak K, Sapa J and Pytka K:
Serotonin receptors in depression and anxiety: Insights from animal
studies. Life Sci. 210:106–124. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yohn CN, Gergues MM and Samuels BA: The
role of 5-HT receptors in depression. Mol Brain.
10(28)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fisher PM, Ozenne B, Svarer C, Adamsen D,
Lehel S, Baaré WF, Jensen PS and Knudsen GM: BDNF val66met
association with serotonin transporter binding in healthy humans.
Transl Psychiatry. 7(e1029)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Katona CL and Katona CP: New generation
multi-modal antidepressants: Focus on vortioxetine for major
depressive disorder. Neuropsychiatr Dis Treat. 10:349–354.
2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mahableshwarkar AR, Zajecka J, Jacobson W,
Chen Y and Keefe RS: A randomized, placebo-controlled,
active-reference, double-blind, flexible-dose study of the efficacy
of vortioxetine on cognitive function in major depressive disorder.
Neuropsychopharmacology. 40:2025–2037. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Talebian A, Robinson-Brookes K and Meakin
SO: TrkB regulates N-Methyl-D-aspartate receptor signaling by
uncoupling and recruiting the brain-specific guanine nucleotide
exchange factor, RasGrf1. J Mol Neurosci. 67:97–110.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fukuda M, Takatori A, Nakamura Y, Suganami
A, Hoshino T, Tamura Y and Nakagawara A: Effects of novel small
compounds targeting TrkB on neuronal cell survival and
depression-like behavior. Neurochem Int. 97:42–48. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kozono N, Ohtani A and Shiga T: Roles of
the serotonin 5-HT4 receptor in dendrite formation of the rat
hippocampal neurons in vitro. Brain Res. 1655:114–121. 2017.
View Article : Google Scholar
|
|
27
|
Rumajogee P, Madeira A, Vergé D, Hamon M
and Miquel MC: Up-regulation of the neuronal serotoninergic
phenotype in vitro: BDNF and cAMP share Trk B-dependent mechanisms.
J Neurochem. 83:1525–1528. 2002.PubMed/NCBI View Article : Google Scholar
|
|
28
|
McMorris T: Developing the catecholamines
hypothesis for the acute exercise-cognition interaction in humans:
Lessons from animal studies. Physiol Behav. 165:291–299.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sagud M, Nikolac Perkovic M, Vuksan-Cusa
B, Maravic A, Svob Strac D, Mihaljevic Peles A, Zivkovic M, Kusevic
Z and Pivac N: A prospective, longitudinal study of platelet
serotonin and plasma brain-derived neurotrophic factor
concentrations in major depression: Effects of vortioxetine
treatment. Psychopharmacology (Berl). 233:3259–3267.
2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lu Y, Ho CS, McIntyre RS, Wang W and Ho
RC: Effects of vortioxetine and fluoxetine on the level of Brain
Derived Neurotrophic Factors (BDNF) in the hippocampus of chronic
unpredictable mild stress-induced depressive rats. Brain Res Bull.
142:1–7. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Thase ME, Mahableshwarkar AR, Dragheim M,
Loft H and Vieta E: A meta-analysis of randomized,
placebo-controlled trials of vortioxetine for the treatment of
major depressive disorder in adults. Eur Neuropsychopharmacol.
26:979–993. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li Y, Pehrson AL, Oosting RS, Gulinello M,
Olivier B and Sanchez C: A study of time- and sex-dependent effects
of vortioxetine on rat sexual behavior: Possible roles of direct
receptor modulation. Neuropharmacology. 121:89–99. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
McIntyre RS: The role of new
antidepressants in clinical practice in Canada: A brief review of
vortioxetine, levomilnacipran ER, and vilazodone. Neuropsychiatr
Dis Treat. 13:2913–2919. 2017.PubMed/NCBI View Article : Google Scholar
|