Introduction
Venous thromboembolism (VTE), comprising deep vein
thrombosis (DVT) and pulmonary embolism (PE), is a growing public
health problem with an estimated incidence rate of 1.22 per 1,000
person-years (1). It is also the
third most common cardiovascular condition after acute coronary
syndromes and stroke (2). Early
anti-coagulant therapy is necessary for managing the disease, as
untreated VTE may lead to significant morbidity, functional
disability and mortality. In the past two decades,
low-molecular-weight heparin (LMWH) is being increasingly used in
the initial management of VTE with a corresponding decrease in the
use of unfractionated heparin (UFH) (3). As compared to UFH, LMWHs have a longer
half-life and a more predictable anticoagulant response (4). Studies have suggested that LMWH is as
effective as UFH with an advantage of home-based treatment and no
requirement for monitoring the laboratory parameters of the patient
(5). Despite LMWHs being the drug of
choice for acute VTE, there is currently no consensus regarding the
dosing strategy of LMWH for acute VTE (6). In studies evaluating the efficacy of
LMWHs, clinicians have used both once-daily (7) and twice-daily (5) regimens of LMWH and demonstrated good
results.
Several different LMWHs, including enoxaparin,
dalteparin and tinzaparin, are available in the US. The different
LMWHs, however, cannot be used interchangeably, as these drugs
differ in their physicochemical and pharmacologic characteristics
(8). While a once-daily dose of
dalteparin (200 U/kg daily) is equivalent to twice-daily dosing
(100 U/kg twice-daily) on a milligram basis, this does not apply
for enoxaparin. Once-daily enoxaparin (1.5 mg/kg) provides 75% of
the total drug received via twice-daily dosing (1 mg/kg) (9). The administration of 1 mg/kg
twice-daily enoxaparin has been used for in-patient treatment of
DVT with or without PE and outpatient treatment of acute DVT
without PE as a bridge to warfarin (10). However, if a once-daily injection of
enoxaparin is as efficacious as twice-daily dosing, such a regimen
would be more advantageous to patients, as it enables home-based
therapy. A Cochrane review from 2013 last attempted to compare the
efficacy and safety of once-daily and twice-daily LMWH therapy for
the initial treatment of VTE (11).
This review, however, included all types of LMWHs without
specifically focusing on a single drug. Therefore, the purpose of
the present review was to elucidate any difference in efficacy and
safety of once-daily vs. twice-daily enoxaparin when used for the
initial treatment of VTE.
Materials and methods
Study selection and search
strategy
In accordance with the Population, Intervention,
Comparison, Outcome and Study design outline (12), an electronic literature search was
performed for randomized controlled trials (RCTs), quasi-RCTs and
prospective or retrospective cohort studies conducted on adult
patients with acute VTE confirmed by diagnostic imaging
(‘Population’). Studies comparing weight-based once-daily
administration of enoxaparin (‘Intervention’) with weight-based
twice-daily administration of enoxaparin (‘Comparison’) for the
initial treatment of the VTE were included. Studies reporting data
on the recurrence of VTE and hemorrhagic complications (‘Outcomes’)
were included. The definition of recurrence and major/minor
hemorrhage was as specified in the included studies. No
restrictions were applied regarding the location of VTE (DVT or
PE). Studies were excluded if any of the following applied: i)
Studies utilizing LMWHs other than enoxaparin; ii) studies
comparing enoxaparin dosing strategy for VTE prophylaxis; iii)
studies utilizing a fixed dose of the drug; iv) studies comparing
<10 patients; v) studies not reporting relevant outcome data;
vi) studies published in a language other than English; vii) in the
case of duplicate reports, the study with the smaller sample size
was excluded.
The PubMed, Embase, Cochrane Central Register of
Controlled Trials, Science Direct and Google Scholar databases were
searched by two independent reviewers (YS and CL) from inception up
to 1st October 2019 for publications with the following keywords:
‘Low molecular weight heparin’; ‘heparin’; ‘enoxaparin’;
‘anticoagulant’; ‘venous thromboembolism’; ‘thromboembolism’;
‘pulmonary embolism’; ‘deep vein thrombosis’; ‘dosing’; ‘twice
daily’; ‘once daily’; ‘q.d’ and ‘b.i.d’. The references of included
studies were also inspected for the identification of any further
trials. After screening the search results at the title and
abstract level, the full texts of selected papers were extracted
for detailed analysis based on pre-defined inclusion/exclusion
criteria. Any disagreements were resolved by discussion with the
other two reviewers (HR and WZ). The guidelines of the Preferred
Reporting Items for Systematic Reviews and Meta-analyses statement
(12) and Cochrane Handbook for
Systematic Reviews of Intervention (13) were followed during the conduct of
this review.
Risk of bias
For quality assessment of randomized controlled
trials (RCTs), the Cochrane Collaboration risk assessment tool for
RCTs was used (14). Studies were
rated as having low risk, high risk or unclear risk of bias for the
following points: Random sequence generation, allocation
concealment, blinding of participants and personnel, blinding of
outcome assessment, incomplete outcome data and selective
reporting. Other studies were analyzed using the risk of bias
assessment tool for non-randomized studies (15). Studies were rated as having low risk,
high risk or unclear risk of bias for the following points:
Selection of participants, confounding variables, intervention
measures, blinding of outcome assessment, incomplete outcome data
and selective outcome reporting. Two authors conducted the risk of
bias analysis independently (YS and CL). Any disagreements were
resolved by discussion with the other two reviewers (HR and
WZ).
Data extraction and statistical
analysis
A total of two independent reviewers (HR and WZ)
extracted data from the included trials using a data abstraction
form. The following details were extracted: First author name, year
of publication, patient inclusion/exclusion criteria, sample size,
baseline comparability of the two groups, enoxaparin protocol, use
of other anti-coagulants, details for risk of bias analysis,
outcome definition, VTE recurrence, complications and follow-up.
The corresponding authors were e-mailed to request any missing
data. The primary outcome of interest was the recurrence of VTE
assessed by diagnostic imaging. The secondary outcome was the
incidence of major or minor hemorrhage.
All analyses were performed using Review Manager
[RevMan, version 5.3; Nordic Cochrane Centre (Cochrane
Collaboration); 2014]. Outcomes were summarized using the
Mantel-Haenszel odds ratio (OR) with a 95%CI. Considering the
methodological heterogeneity amongst the included studies, a
random-effects model was used to calculate the pooled effect size.
Between-study heterogeneity was calculated using the I2
statistic. I2 values of 25-50% represented low, values
of 50-75% medium and >75% represented substantial heterogeneity.
Furthermore, two sub-group analyses were performed: i) For RCT and
non-RCTs and ii) Depending on the use of enoxaparin as a bridging
therapy for warfarin or as a monotherapy. To assess the outcomes of
once-daily vs. twice-daily enoxaparin in cancer patients, the
results of studies conducted specifically on cancer patients were
pooled separately. A sensitivity analysis was performed to assess
the contribution of each study to the pooled effect size by
sequentially excluding individual studies one at a time and
recalculating the pooled OR estimates for the remaining studies.
Publication bias was not assessed due to the small number of
included studies (<10 studies).
Results
Search results
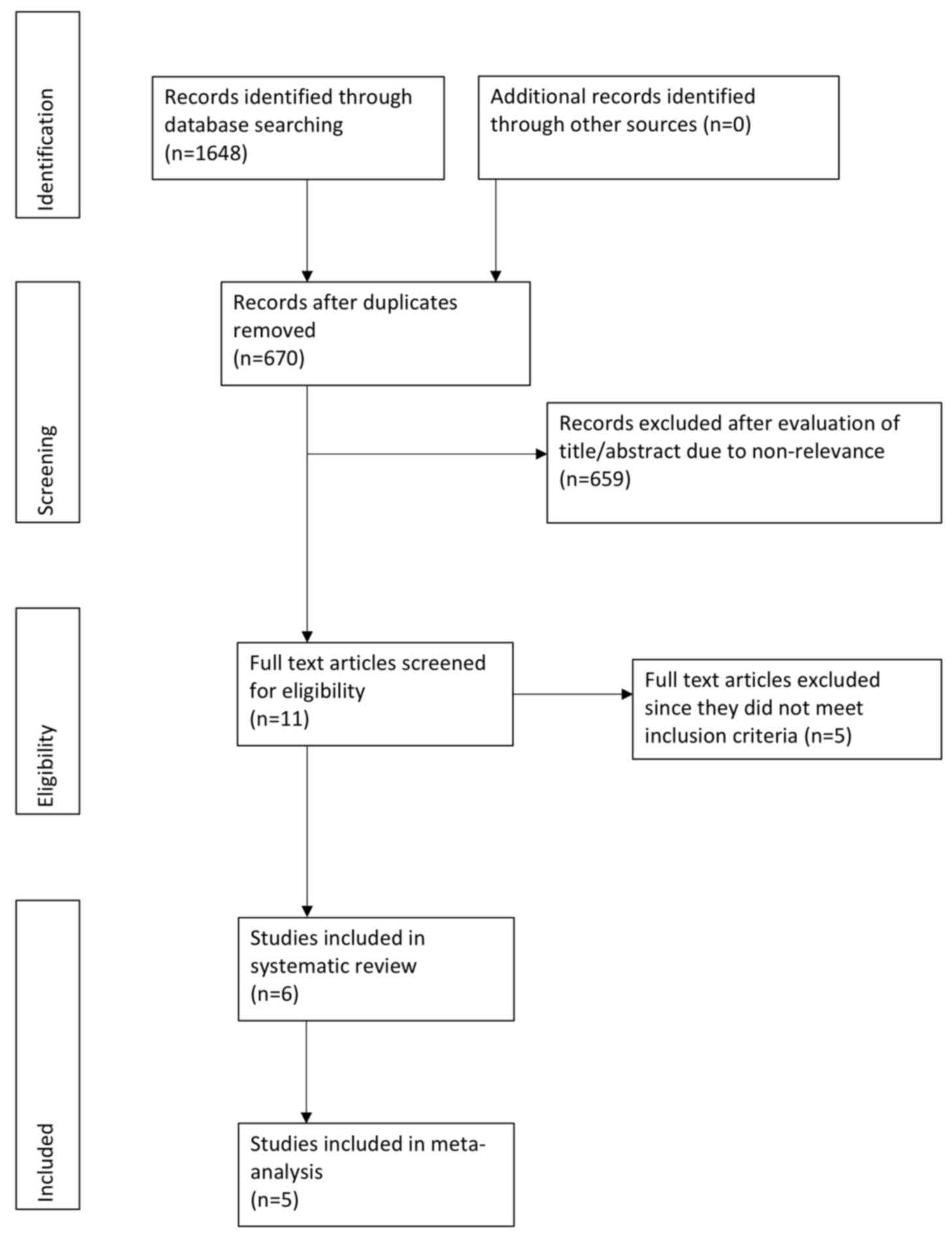
A comprehensive literature search was performed and
a total of 670 unique records were retrieved (Fig. 1). The full texts of 11 studies were
retrieved. Subsequently, 5 studies were excluded (16-20).
In one study, patients were not randomized to once-daily or
twice-daily enoxaparin for the initial treatment of VTE (20), while in four trials, LMWHs other than
enoxaparin were used (16-19).
A total of six studies were finally included in the review
(9,10,21-24).
In one study (21), outcome data
were not extractable and e-mails to the corresponding author did
not elicit a response. This study was not included in the
meta-analysis.
Study characteristics
The characteristics of individual studies are
summarized in Table I. One study was
an RCT (22) and two were
prospective studies with historic control groups (10,23),
while the remaining studies were retrospective studies (9,21,24). A
total of two studies were performed specifically on cancer patients
(9,24). VTE was confirmed by imaging in all
studies. In addition, one study focused only on DVT (10), while another one focused only on PE
(24). DVT and PE were both included
in the definition of VTE for the remaining studies.
Inclusion/exclusion criteria, sample size and follow-up varied
amongst the included studies. The enoxaparin dose was 1.5 mg/kg in
the once-daily group and 1 mg/kg in the twice-daily group in all
studies. The duration of enoxaparin treatment was not reported in
four studies (9,21,23,24). In
three studies (10,22,23),
enoxaparin was used as bridging therapy to oral anti-coagulants. No
major significant differences in baseline characteristics were
reported by the included studies between the two study groups.
There were no significant differences in baseline risk factors for
VTE between the two groups in all studies. In one study (24), the two groups differed significantly
in mean body weight, while in another study (9), the groups differed in their mean body
mass index. One study (21) used
propensity score matching for the two groups but did not report
exact data on the outcome definition and relevant outcomes. A total
of 864 patients on once-daily enoxaparin were matched with 1,407
patients on twice-daily enoxaparin in this study. The authors
reported a similar incidence of recurrent VTE at 15 days [hazard
ratio (HR)=1.26, 95%CI: 0.25-6.36]. A lower rate of major
hemorrhage was seen in patients with once-daily enoxaparin at 15
days (HR=0.30, 95%CI: 0.10-0.88) and at 30 days (HR=0.16, 95%CI:
0.04-0.68). Outcome definitions and data reported by the remaining
five studies are presented in Table
II.
 | Table ICharacteristics of included
studies. |
Table I
Characteristics of included
studies.
| | Sample size | Enoxaparin dose | |
|---|
| Author (year) | Study type | Inclusion
criteria | Exclusion
criteria | Once daily | Twice daily | Once daily | Twice daily | Duration of
prophylaxis and treatment with other anti-coagulant drugs | (Refs.) |
|---|
| Merli (2001) | RCT | Symptomatic DVT or PE
confirmed by imaging | >24 h of previous
treatment with heparin or warfarin, need for thrombolytic therapy,
known hemorrhagic risk, active hemorrhage, angiodysplasia,
eye/spinal/central nervous system surgery in past 1 month,
renal/hepatic insufficiency, allergy to heparin/protamine/ porcine
products/iodine/contrast media, history of heparin-induced
thrombocytopenia/skin necrosis, treatment with other
investigational agents in <4 weeks, inferior vena cava
interruption, pregnant or breastfeeding females | 298 | 312 | 1.5 mg/kg | 1 mg/kg | Duration of
enoxaparin was at least 5 days. Warfarin started within 72 h of
study drug administration and dose was adjusted to maintain INR
between 2 and 3. Enoxaparin discontinued after target INR
achieved. | (22) |
| Hacobian (2010) | Prospective with
historic controls | Symptomatic DVT or PE
confirmed by imaging | >72 h of
hospitalization, PE with enlarged ventricle, high risk of bleeding,
treated without warfarin, creatinine >2 mg/dl, recurrent VTE
with anti-coagulation, life expectancy <3 months, scheduled for
surgery during study period | 40 | 80 | 1.5 mg/kg | 1 mg/kg | Duration of
enoxaparin not reported. Warfarin started on day 1 of study drug
administration and dose adjusted to maintain INR at 2-3. Enoxaparin
discontinued after target INR achieved. | (23) |
| King (2016) | Retrospective | Acute PE in cancer
patients confirmed by imaging, enoxaparin dosing within 20 mg of
body weight, >18 years of age, follow-up >6 months | In or transitioning
to hospice, pregnant patients, patients weighing >190 kg,
patients with ³1 enoxaparin dose held prior to completion of 30
days of drug administration, creatinine clearance <30
ml/min | 48 | 48 | 1.5 mg/kg | 1 mg/kg | NR | (24) |
| Fuller (2018) | Retrospective | Adult cancer
patients receiving enoxaparin for acute VTE event, follow-up >6
months | Creatinine
clearance <30 ml/min, active hemorrhage or fibrinolytic therapy
<3 days prior to enoxaparin initiation, anticoagulation started
>48 h after diagnosis, >5 days treatment with another
anti-coagulant, >15% divergence in the enoxaparin dose when
prescribed according actual body weight, patients switching between
the two study groups, discontinuing the study drug or shifting to
another anti-coagulant | 85 | 38 | 1.5 mg/kg | 1 mg/kg | NR | (9) |
| Trujillo-Santos
(2017) | Retrospective | Symptomatic DVT or
PE confirmed by imaging | NR | 864 | 1,407 | 1.5 mg/kg | 1 mg/kg | NR | (21) |
| Yusuf (2019) | Prospective with
historic controls | Symptomatic DVT
confirmed by imaging | Prolonged
hospitalization for >15 days, PE, high risk of bleeding, renal
impairment, pregnancy, malignancy | 40 | 40 | 1.5 mg/kg | 1 mg/kg | Duration of
enoxaparin was at least 5 days. Warfarin started on day 1 of study
drug administration and dose adjusted to maintain INR at 2-3.
Enoxaparin discontinued after target INR achieved. | (10) |
 | Table IIOutcomes of studies included in the
meta-analysis. |
Table II
Outcomes of studies included in the
meta-analysis.
| | VTE outcomes
(n/N) | | Outcomes (n/N) | |
|---|
| Study | VTE definition | Once daily | Twice daily | Hemorrhage | Once daily | Twice daily | (Refs.) |
|---|
| Merli (2001) | Recurrent DVT or PE
within 3 months of randomization. DVT confirmed with venography,
USG or both. PE confirmed with lung perfusion scanning, pulmonary
angiography or both. | 11/247 | 8/258 | Major hemorrhage
was defined as being associated with at least one of the following:
A decrease in hemoglobin level of at least 20 g/l; need for
transfusion of at least two units of blood; retroperitoneal,
intracranial or intraocular bleeding; other associated serious
clinical event; need for surgical or medical intervention; or
death. Minor hemorrhages were other hemorrhages that were
clinically overt but did not meet the criteria for major
hemorrhage. | Major hemorrhage:
5/298 Minor hemorrhage: 41/298 | Major hemorrhage:
4/312 Minor: hemorrhage 50/312 | (22) |
| Hacobian
(2010) | Definition not
specified. Outcomes assessed at 30 days. | 1/40 | 3/80 | Major hemorrhage
defined by the Global Utilization of Streptokinase and Tissue
Plasminogen Activator for Occluded Coronary Arteries criteria | 0/40 | 3/80 | (23) |
| King (2016) | Recurrent PE was
defined as a new embolism or extension of a current embolism on CT
pulmonary angiography or lung perfusion scanning or symptoms
requiring anticoagulation medication changes such as an increased
strength in dosing, increased frequency of dosing, or a change to
an alternative anticoagulant. Maximum follow-up of 6 months | 6/48 | 3/48 | Major hemorrhage
defined as an intracranial, intraspinal, intraocular,
retroperitoneal, pericardial, intramuscular with compartment
syndrome, or intra-articular bleed, a drop in hemoglobin by ≥2 g/dl
from baseline, a requirement of ≥2 units of packed red blood cells,
or any bleed requiring major medical or surgical intervention. | 7/48 | 3/48 | (24) |
| Fuller (2018) | Recurrent VTE
confirmed by diagnostic imaging. Outcomes evaluated at 30, 90 and
180 days. | 30 day: 7/85 90
day: 5/72 180-day: 0/48 | 30 day: 1/38 90
day: 0/25 180-day: 2/14 | Major bleeding as
defined by the International Society on Thrombosis and Hemostasis,
which includes fatal bleeding, bleeding in a critical area or organ
(intracranial, intraspinal, intraocular, retroperitoneal,
intra-articular or pericardial, or intramuscular with compartment
syndrome), hemoglobin decrease by ≥2 g/dl from baseline as a result
of bleeding, or patients requiring ≥2 units of blood or red cells
as a result of bleeding. Non-major bleed defined as hemorrhage
leading to medical intervention, hospitalization and increased
level of care or necessitating a face-to-face evaluation | Major hemorrhage:
30 days, 1/85; 90 days, 2/72; 180 days, 2/48 Non-major hemorrhage:
30 days, 1/85; 90 days, 4/72; 180 days, 4/48 | Major hemorrhage:
30 days, 1/38; 90 days, 1/25; 180 days, 1/14 Non-major hemorrhage:
30 days, 1/38; 90 days, 1/25; 180 days, 0/14 | (9) |
| Yusuf (2019) | Assessment of DVT
carried out using clinical, radiological and laboratory tests at 30
days | 1/40 | 2/40 | Major hemorrhage
was defined as overt bleeding that required a transfusion of ≥2
units of blood, was retroperitoneal, spinal or intracranial, or was
fatal. | 0/40 | 1/40 | (10) |
Meta-analysis
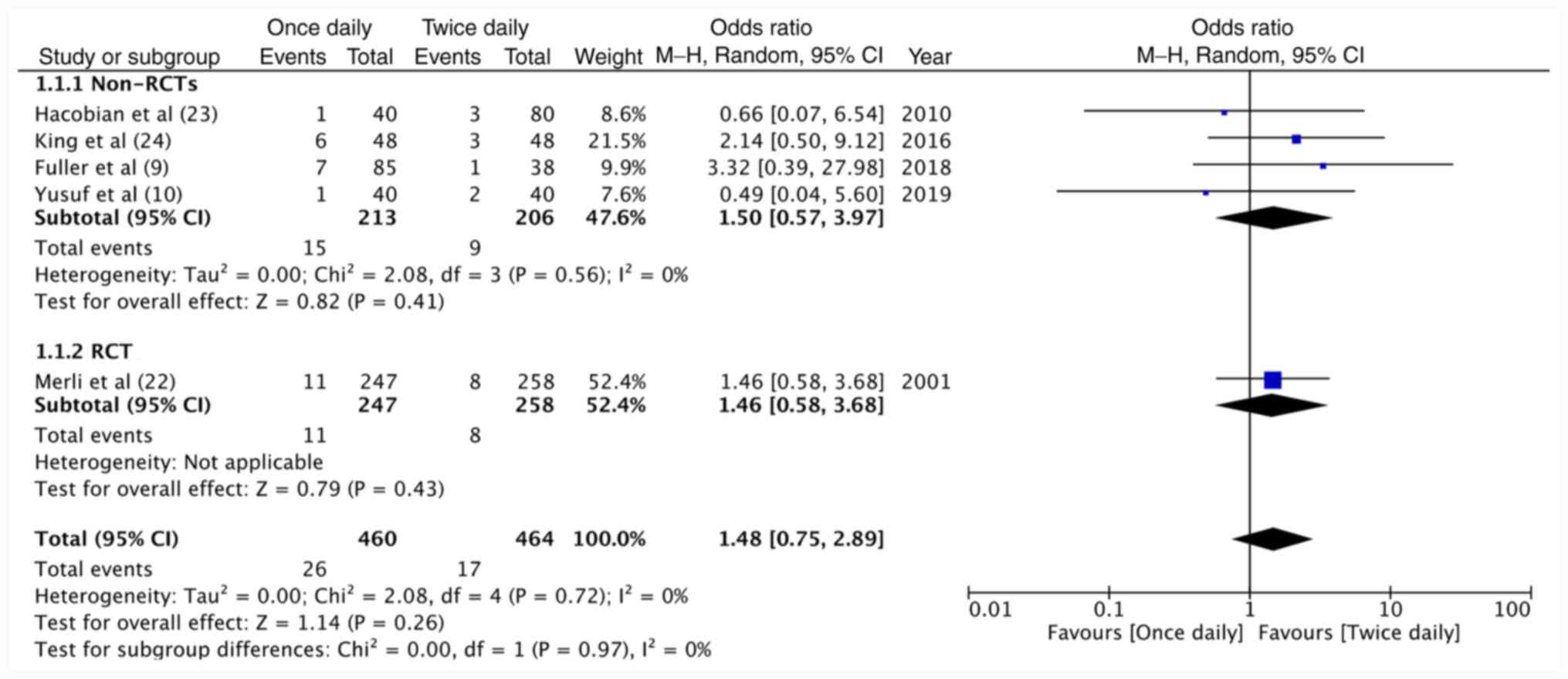
Data of 460 patients receiving once-daily enoxaparin
and 464 patients receiving twice-daily enoxaparin were pooled for
meta-analysis on VTE recurrence. The results indicated no
significant difference between the two dosing regimens in terms of
VTE recurrence (OR=1.48, 95%CI: 0.75-2.89, P=0.26;
I2=0%; Fig. 2). Similar
non-significant results were observed for sub-group analysis of
non-RCTs (OR=1.50, 95%CI: 0.57-3.97, P=0.41; I2=0%) and
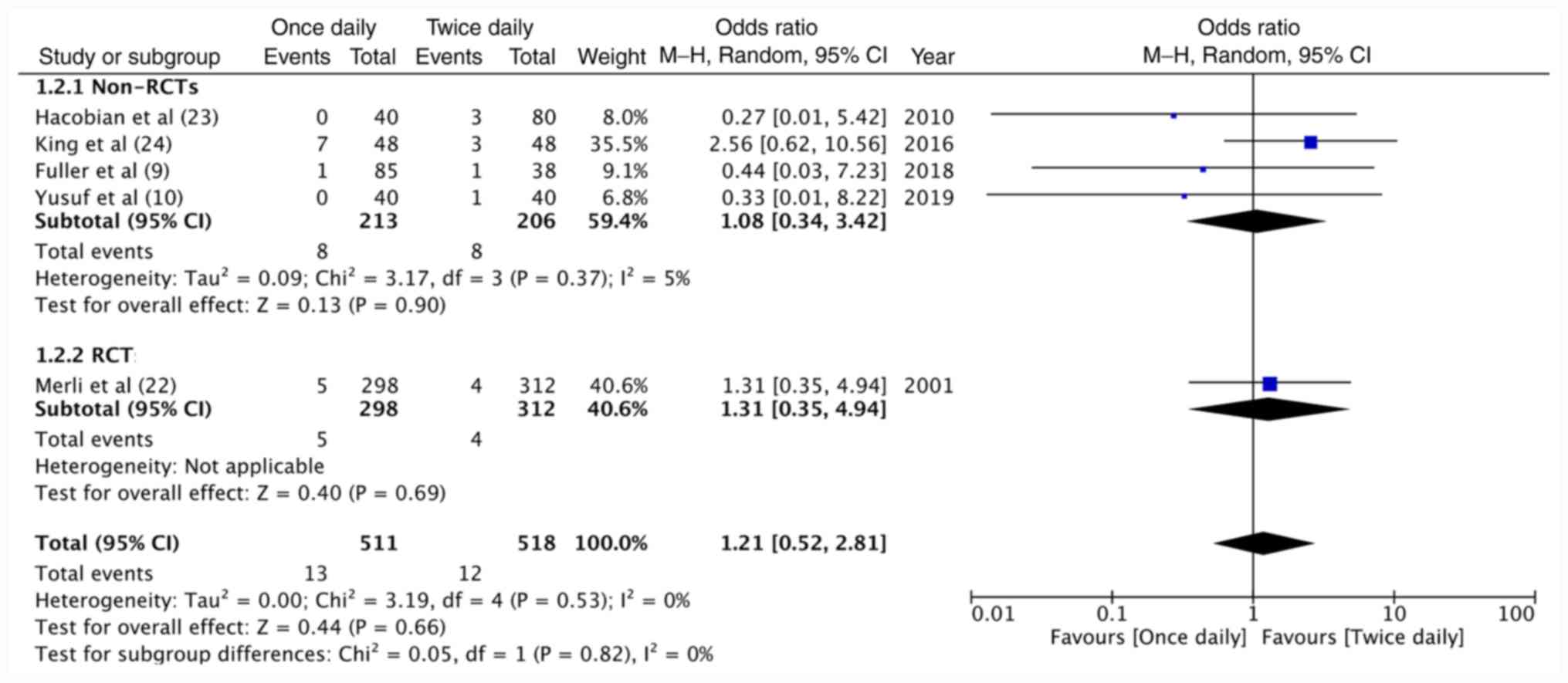
the lone RCT (OR=1.46, 95%CI: 0.58-3.68, P=0.43; Fig. 2). A total of 511 patients on
once-daily enoxaparin and 518 patients on twice-daily enoxaparin
were evaluated in the included studies for major hemorrhage.
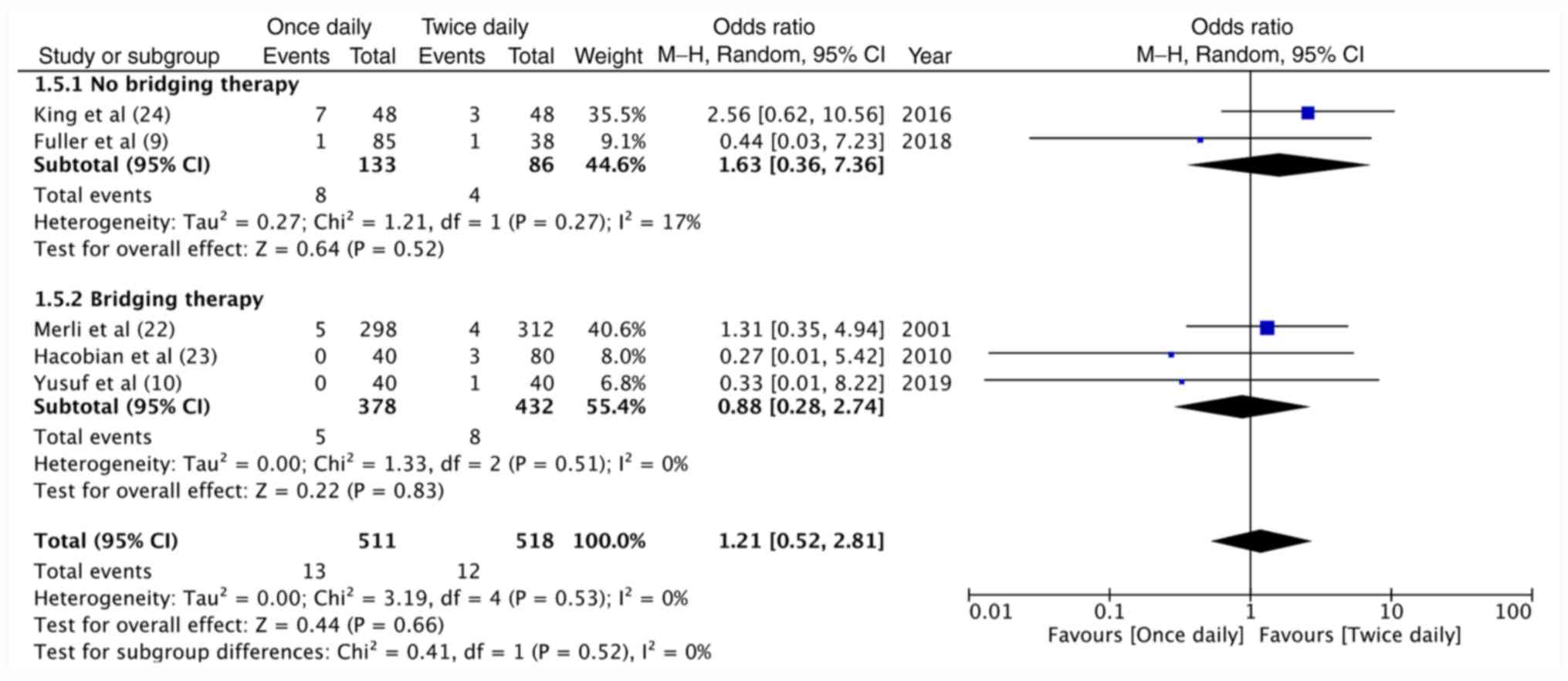
Meta-analysis demonstrated no significant difference in major
hemorrhagic complications between once-daily and twice-daily
enoxaparin (OR=1.21, 95%CI: 0.52-2.81, P=0.66; I2=0%;
Fig. 3). The results were
non-significant for non-RCTs (OR=1.08, 95%CI: 0.34-3.42, P=0.90;
I2=5%) as well as the included RCT (OR=1.31, 95%CI:
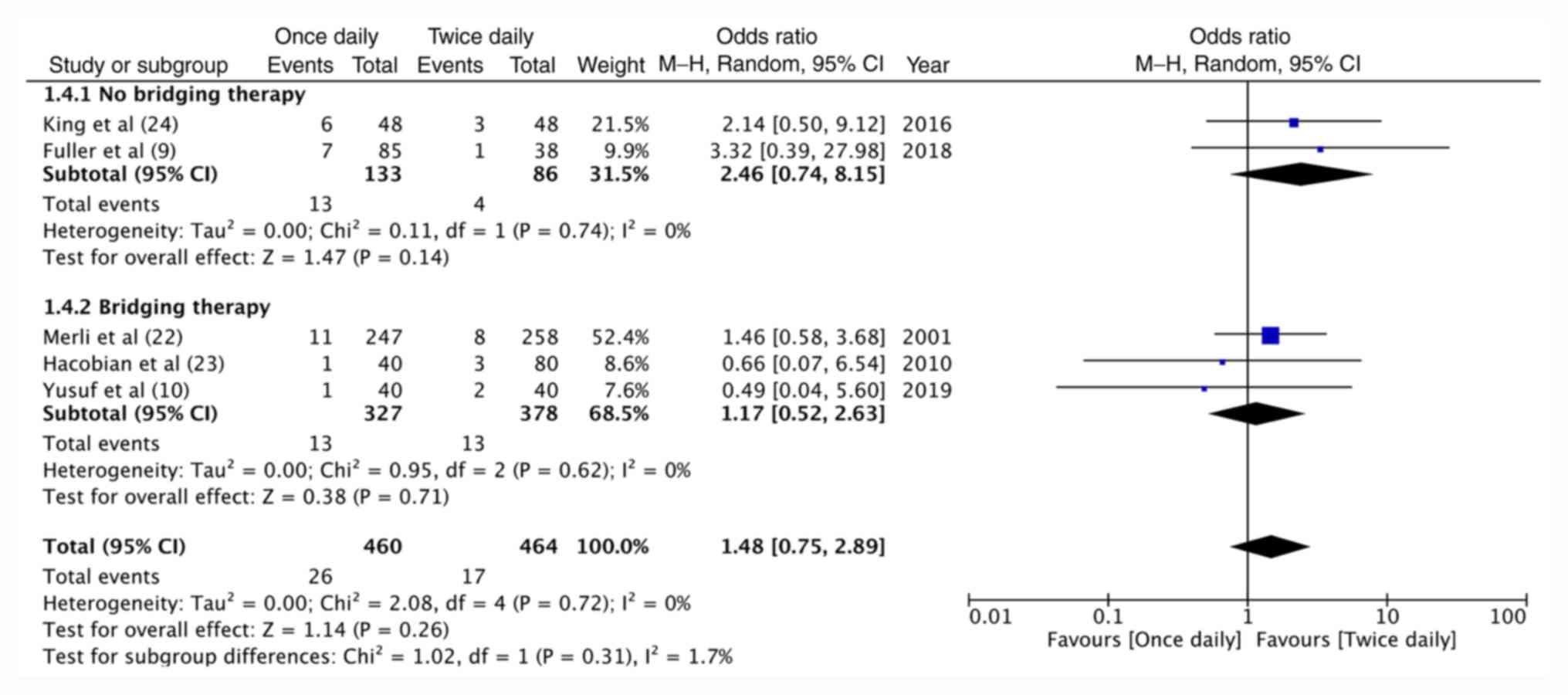
0.35-4.94, P=0.69; Fig. 3). On
grouping studies based on the use of enoxaparin as bridging therapy
for warfarin, no significant difference in recurrent VTE (Fig. 4) and major hemorrhage (Fig. 5) was obtained between once-daily and
twice-daily enoxaparin for both sub-groups (bridging therapy vs.
no-bridging therapy).
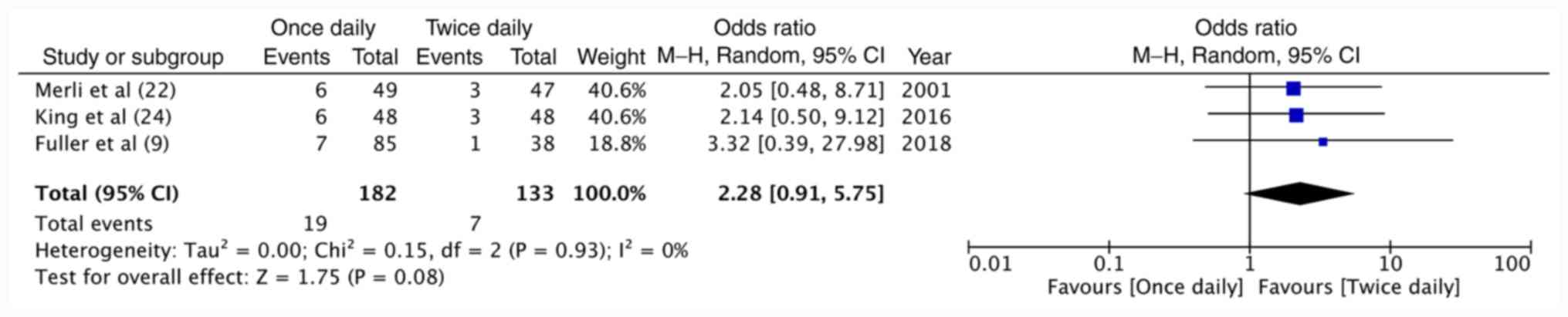
Data of two studies (9,24)
performed specifically on cancer patients and the cancer sub-group
of the RCT (22) were pooled
together for a meta-analysis on recurrent VTE in cancer patients.
The results demonstrated no difference between once-daily and
twice-daily enoxaparin regarding the recurrence of VTE in cancer
patients (OR=2.28, 95%CI: 0.91-5.75, P=0.08; I2=0%;
Fig. 6). Data on hemorrhagic
complications in cancer were not available from the RCT (22); hence, no meta-analysis was conducted
on bleeding complications with just two studies.
The incidence of minor hemorrhage was reported only
by two studies (9,22). While one trial (22) did not report any significant
difference in minor hemorrhage between the two groups, the other
study did not have sufficient statistical power to detect a
significant difference (9).
Sensitivity analysis and risk of bias
assessment
On sensitivity analysis, there was no change in the
results of recurrent VTE and major hemorrhage on the sequential
exclusion of all studies (data not shown). The authors' judgment of
the risk of bias in studies included in the meta-analysis is
presented in Table III. The
included RCT (20) was of high
quality with low risk of bias in all domains.
 | Table IIIRisk of bias assessment. |
Table III
Risk of bias assessment.
| A, Randomized
studies |
|---|
| Study | Random sequence
generation | Allocation
concealment | Blinding of
participants and personnel | Blinding of outcome
assessment | Incomplete outcome
data | Selective
reporting |
|---|
| Merli et al
(22) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| B, Non-randomized
studies |
| Study | Selection of
participants | Confounding
variables | Intervention
measures | Blinding of outcome
assessment | Incomplete outcome
data | Selective outcome
reporting |
| Hacobian et
al (23) | High risk | Unclear risk | Low risk | High risk | Low risk | Low risk |
| King et al
(24) | Low risk | Unclear risk | Low risk | High risk | Low risk | Low risk |
| Fuller et al
(9) | Unclear risk | Unclear risk | Low risk | High risk | Low risk | Low risk |
| Yusuf et al
(10) | High risk | Unclear risk | Low risk | High risk | Low risk | Low risk |
For non-RCTs, only one study (24) had low risk of bias for selection of
participants. None of the studies took into account confounding
factors or had blinded outcome assessment. Therefore, the overall
the quality of included studies was determined to be average.
Discussion
LMWHs are among the most commonly used drugs for the
prevention and management of VTE. In a Moroccan study, >90%
patients with VTE were managed by LMWHs (25). LMWHs are a class of chemically
distinct compounds with products differing in their polysaccharide
chain lengths, mean molecular weights, as well as pharmacological
properties. In the absence of any consensus with regards to the
clinical equivalence of different LMWHs, it is proposed that
clinicians should follow manufacturer-recommended dosing guidelines
when using these drugs (26).
Amongst the three available LMWHs in the US, enoxaparin has the
widest range of FDA-approved indications with established efficacy
and safety in multiple patient populations (2,24,26).
Despite the broad utilization of enoxaparin, there
is no consensus on the optimal dosing strategy of the drug
(11,27). At present, two dosing regimens are
approved by the FDA for the management of DVT with or without PE in
hospitalized patients: 1 mg/kg every 12 h or 1.5 mg/kg every 24 h
(27). Twice-daily administration of
enoxaparin has been historically used for the treatment of VTE,
with initial trials demonstrating equivalence of twice-daily
enoxaparin and UFH (28). In a
recent study, Trujillo-Santos et al (21) demonstrated that the twice-daily
regimen is generally preferred for the treatment of acute VTE with
>70% patients receiving dual injections. Whilst a twice-daily
dosing regimen may theoretically provide a more stable
anti-coagulation profile, the once-daily dose may be preferred by
patients. Such a dosing strategy may halve the number of
injections, reduce treatment costs and promote outpatient
department-based management protocols (10). The once-daily dose may also reduce
hemorrhagic complications due to a reduced dose but may also
potentially increase the recurrence of VTE.
According to the systematic search of the present
study, a total of six studies published to date have performed a
head-to-head comparison of the two dosing strategies of enoxaparin
for the management of acute VTE. In a pooled analysis of five
studies, the present results indicated no difference in the
incidence of recurrent VTE (OR=1.48, 95%CI: 0.75-2.89) and major
bleeding complications (OR=1.21, 95%CI: 0.52-2.81) between
once-daily and twice-daily enoxaparin. The present results concur
with the last meta-analysis of Bhutia and Wong (11), which indicated no difference in terms
of recurrent VTE (OR=1.21, 95% CI: 0.52-2.81) and major hemorrhagic
complications (OR=0.77, 95%CI: 0.40-1.45) with once-daily and
twice-daily LMWHs. It is important to note that while Bhutia and
Wong (11) pooled results of only
RCTs (total of two RCTs for VTE and four RCTs for major
hemorrhage), only one RCT was included in the present analysis.
This is because the present review was focused specifically on
enoxaparin, unlike the past review, which pooled data of different
LMWHs. Furthermore, the confidence interval of the pooled OR was
wider as compared to that of the previous meta-analysis for the
same outcomes. This, along with the inclusion of retrospective
studies whose quality was not high, reduced the quality of evidence
of the present analysis.
Several baseline risk factors are able to influence
outcomes of acute VTE management, including age, history of VTE,
cancer, obesity, trauma, congestive heart failure, pregnancy,
infection, placement of venous catheters and duration of therapy
(27). The use of suitable methods
of randomization in RCTs usually nullifies the influence of such
confounding variables on the study results; however, comparability
is difficult to achieve in retrospective studies. Propensity-score
matching has been used to reduce the bias of observational studies
and was used in one study included in the present review (21). However, due to the absence of
extractable data, the study was not included in the meta-analysis.
Despite the remaining retrospective studies reporting no difference
in the baseline characteristics of their study participants, a
sub-group analysis for the single RCT and non-RCTs was performed to
test the validity of the present results. The sub-group analysis
demonstrated no difference between the two groups for primary or
secondary outcome variables. A similar sub-group analysis of
enoxaparin bridging therapy and monotherapy also yielded a
non-significant result.
Specific sub-groups of patients, e.g. those with
cancer, have an increased risk of developing VTE (9). Despite anti-coagulant therapy, cancer
patients have a three-fold risk of developing recurrent VTE as
compared to patients without malignancy (29). In addition, the risk of hemorrhagic
complications is higher when cancer patients receive
anti-coagulation therapy (24,27). In
the present review, the studies of King et al (24) and Fuller et al (9) were specifically performed on cancer
patients. These two studies individually reported a higher
incidence of recurrent VTE with once-daily enoxaparin compared to
twice-daily enoxaparin; however, the studies were not statistically
powered to detect differences between the two groups. Similarly, a
limited sub-set analysis in the RCT of Merli et al (22) also demonstrated a two-fold increased
incidence of recurrent VTE with once-daily enoxaparin but was
statistically underpowered. On the pooling of data, a higher
incidence of recurrent VTE was obtained with the once-daily
compared to the twice-daily dosing regimen of enoxaparin (10.4 vs.
5.2%). The OR, however, included the null value of 1 with a wide CI
(OR=2.28, 95%CI: 0.91-5.75).
There are certain limitations to the present review
which require to be considered when interpreting the results.
First, a limited number of included studies with only one RCT and
preponderance of retrospective studies are significant drawbacks of
the present review. The inherent drawbacks associated with
retrospective studies, including selection bias and lack of
blinding, may have skewed the results. Furthermore, the majority of
studies were statistically underpowered to detect significant
differences between the two groups. In addition, there were certain
methodological differences between the included studies in terms of
variation in inclusion/exclusion criteria, duration of
anti-coagulant therapy, differences in definition and evaluation of
outcomes, and non-inclusion of DVT or PE in the definition of VTE.
Also, there was a significant difference in follow-up amongst the
studies included in the meta-analysis, with none comparing the
hazard ratio of the two study groups. In addition, long-term
follow-up data were not available for meta-analysis. Furthermore,
outcomes including improvement of thrombus size and incidence of
heparin-induced thrombocytopenia were not reported by the included
trials. Finally, the present meta-analysis did not stratify the
results based on specific risk factors for recurrent VTE. A
sub-group analysis was possible only for cancer patients but with a
limited number of studies.
To the best of our knowledge, the present study was
the first systematic review and meta-analysis comparing once-daily
and twice-daily enoxaparin for the management of VTE. Unlike
previous reviews, the present study focused on a single drug that
was compared using the same dosing protocol in all included
studies. Sub-group and sensitivity analyses were performed to
provide clarity on the overall results of the present review.
To conclude, despite the present results indicating
similar rates of recurrent VTE and major hemorrhagic complications
with once-daily and twice-daily enoxaparin when used for the
treatment of VTE, the overall quality of evidence was not high,
limiting the confidence of the conclusions. Although there was a
tendency favoring twice-daily dosing over once-daily dosing,
particularly for cancer patients, the results on efficacy and
safety of the two dosing regimens of enoxaparin may not be reliable
due to the limited number of available studies. Further
high-quality and adequately powered RCTs are required to
corroborate the present results, particularly in cancer
patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JN conceived and designed the study. YS and CL
performed the literature search. HR and WZ collected the data. YS
and CL assessed the risk of bias of included studies. HR and WZ
were involved in interpretation of results. JN was involved in the
writing of the manuscript. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tagalakis V, Patenaude V, Kahn SR and
Suissa S: Incidence of and mortality from venous thromboembolism in
a Real-world population: The Q-VTE study cohort. Am J Med.
126:832.e13–e21. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Spencer FA, Emery C, Lessard D, Anderson
F, Emani S, Aragam J, Becker RC and Goldberg RJ: The worcester
venous thromboembolism study: A population-based study of the
clinical epidemiology of venous thromboembolism. J Gen Intern Med.
21:722–727. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Huang W, Goldberg RJ, Cohen AT, Anderson
FA, Kiefe CI, Gore JM and Spencer FA: Declining Long-term risk of
adverse events after First-time Community-presenting venous
thromboembolism: The Population-based Worcester VTE study (1999 to
2009). Thromb Res. 135:1100–1106. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hirsh J and Levine MN: Low molecular
weight heparin. Blood. 79:1–17. 1992.PubMed/NCBI
|
|
5
|
Robertson L and Jones LE: Fixed dose
subcutaneous low molecular weight heparins versus adjusted dose
unfractionated heparin for the initial treatment of venous
thromboembolism. Cochrane Database Syst Rev.
2(CD001100)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kearon C, Akl EA, Ornelas J, Blaivas A,
Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, et
al: Antithrombotic therapy for VTE disease: CHEST Guideline and
expert panel report. Chest. 149:315–352. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lindmarker P, Holmström M, Granqvist S,
Johnsson H and Lockner D: Comparison of once-daily subcutaneous
Fragmin with continuous intravenous unfractionated heparin in the
treatment of deep vein thrombosis. Thromb Haemost. 72:186–90.
1994.PubMed/NCBI
|
|
8
|
Collignon F, Frydman A, Caplain H, Ozoux
ML, Le Roux Y, Bouthier J and Thébault JJ: Comparison of the
pharmacokinetic profiles of three low molecular mass
heparins-dalteparin, enoxaparin and nadroparin-administered
subcutaneously in healthy volunteers (doses for prevention of
thromboembolism). Thromb Haemost. 73:630–40. 1995.PubMed/NCBI
|
|
9
|
Fuller K, Malecki S, Anselmo L, Borrego
ME, Jakeman B and Burnett A: Once-daily versus Twice-daily
enoxaparin for the treatment of acute venous thromboembolism in
cancer patients. Ann Pharmacother. 52:257–262. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yusuf M, Gouda M, Herz-Allah A, Alkhouly M
and Samir A: Once-daily versus twice-daily enoxaparin for the
initial treatment of acute deep venous thrombosis: A case-control
study. J Med Sci Res. 2:144–147. 2019.
|
|
11
|
Bhutia S and Wong PF: Once versus twice
daily low molecular weight heparin for the initial treatment of
venous thromboembolism. Cochrane Database Syst Rev.
2013(CD003074)2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Moher D, Liberati A, Tetzlaff J, Altman DG
and PRISMA Group: Preferred reporting items for systematic reviews
and Meta-analyses: The PRISMA statement. PLoS Med.
6(e1000097)2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Higgins JPT and Green S (eds): Cochrane
Handbook for Systematic Reviews of Interventions. Version 5.1.0
(updated March 2011). The Cochrane Collaboration, 2011.
|
|
14
|
Higgins J, Altman D and Sterne J: Cochrane
statistical methods group and the cochrane bias methods group.
Chapter 8: Assessing risk of bias in included studies. In: Cochrane
handbook for systemic reviews of interventions, version 5. The
Cochrane Collaboration, 2011.
|
|
15
|
Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS,
Hahn S, Jang BH and Son HJ: Testing a tool for assessing the risk
of bias for nonrandomized studies showed moderate reliability and
promising validity. J Clin Epidemiol. 66:408–414. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Partsch H, Kechavarz B, Mostbeck A, Köhn H
and Lipp C: Frequency of pulmonary embolism in patients who have
iliofemoral deep vein thrombosis and are treated with once- or
twice-daily low-molecular-weight heparin. J Vasc Surg. 24:774–82.
1996.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Siegbahn A, Y-Hassan S, Boberg J, Bylund
H, Neerstrand HS, Ostergaard P and Hedner U: Subcutaneous treatment
of deep venous thrombosis with low molecular weight heparin. A dose
finding study with LMWH-Novo. Thromb Res. 55:767–78.
1989.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Holmoström M, Berglund MC, Granquist S,
Bratt G, Törnebohm E and Lockner D: Fragmin once or twice daily
subcutaneously in the treatment of deep venous thrombosis of the
leg. Thromb Res. 67:49–55. 1992.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Charbonnier BA, Fiessinger JN, Banga JD,
Wenzel E, d'Azemar P and Sagnard L: Comparison of a once daily with
a twice daily subcutaneous low molecular weight heparin regimen in
the treatment of deep vein thrombosis. FRAXODI group. Thromb
Haemost. 79:897–901. 1998.PubMed/NCBI
|
|
20
|
Narin C, Reyhanoglu H, Tülek B, Onoglu R,
Ege E, Sarigül A, Yeniterzi M and Durmaz I: Comparison of different
dose regimens of enoxaparin in deep vein thrombosis therapy in
pregnancy. Adv Ther. 25:585–594. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Trujillo-Santos J, Bergmann JF, Bortoluzzi
C, López-Reyes R, Giorgi-Pierfranceschi M, López-Sáez JB, Ferrazzi
P, Bascuñana J, Suriñach JM and Monreal M: Once versus twice daily
enoxaparin for the initial treatment of acute venous
thromboembolism. J Thromb Haemost. 15:429–438. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Merli G, Spiro TE, Olsson CG, Abildgaard
U, Davidson BL, Eldor A, Elias D, Grigg A, Musset D, Rodgers GM, et
al: Subcutaneous enoxaparin once or twice daily compared with
intravenous unfractionated heparin for treatment venous
thromboembolic disease. Ann Intern Med. 134:191–202.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Merli H, Shetty R, Niles CM,
Gerhard-Herman M, Vallurupalli N, Baroletti S, McKean SC, Sonis J,
Parasuraman S, Kosowsky JM and Goldhaber SZ: Once daily enoxaparin
for outpatient treatment of acute venous thromboembolism: A
case-control study. Clin Appl Thromb. 16:21–25. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
King AC, Ma MQ, Chisholm G and Toale KM:
Once daily versus twice daily enoxaparin for acute pulmonary
embolism in cancer patients. J Oncol Pharm Pract. 22:265–270.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tazi Mezalek Z, Nejjari C, Essadouni L,
Samkaoui M, Serraj K, Ammouri W, Kanjaa N, Belkhadir Z, Housni B,
Awab M, et al: Evaluation and management of thromboprophylaxis in
Moroccan hospitals at national level: The Avail-MoNa study. J
Thromb Thrombolysis. 46:113–119. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Merli GJ and Groce JB: Pharmacological and
clinical differences between low-molecular-weight heparins:
Implications for prescribing practice and therapeutic interchange.
P T. 35:95–105. 2010.PubMed/NCBI
|
|
27
|
Diaz AH, Rodgers GM and Gilreath JA:
Enoxaparin once daily vs. twice daily dosing for the treatment of
venous thromboembolism in cancer patients: A literature summary. J
Oncol Pharm Pract. 18:264–70. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Levine M, Gent M, Hirsh J, Leclerc J,
Anderson D, Weitz J, Ginsberg J, Turpie AG, Demers C and Kovacs M:
A comparison of low-molecular-weight heparin administered primarily
at home with unfractionated heparin administered in the hospital
for proximal deep-vein thrombosis. N Engl J Med. 334:677–681.
1996.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hutten BA, Prins MH, Gent M, Ginsberg J,
Tijssen JG and Büller HR: Incidence of recurrent thromboembolic and
bleeding complications among patients with venous thromboembolism
in relation to both malignancy and achieved international
normalized ratio: A retrospective analysis. J Clin Oncol.
18:3078–3083. 2000.PubMed/NCBI View Article : Google Scholar
|