Introduction
Pulmonary arterial hypertension (PAH) is a disease
mainly characterized by pulmonary vascular remodeling and elevated
pulmonary arterial pressure (PAP) (1). The abnormal cardiac structure in
patients with congenital heart disease (CHD), a common cardiac
disease in clinical practice, may cause aggravation of the cardiac
load, resulting in elevated blood flow in the pulmonary circulation
and increased pulmonary pressure, leading to the development of PAH
(2,3). Congenital heart disease associated
pulmonary arterial hypertension (CHD-PAH) is one of the most common
types of PAH. Once PAH occurs in CHD patients, it will further
increase the blood flow to the heart and aggravate the cardiac
load, thus seriously affecting the quality of life of patients and
even leading to cardiac failure or death (4,5).
Moreover, since there are no typical clinical symptoms in early
CHD-PAH, seeking a specific marker is of great significance for its
diagnosis and treatment.
MicroRNAs (miRNAs, miRs) have been reported to play
an important role in the pathogenesis of PAH, which regulate not
only the proliferation and apoptosis of vascular endothelial cells
(VECs), but also angiogenesis, proliferation and apoptosis of
vascular smooth muscle cells (VSMCs) (6,7). miR-27b
is a miRNA differentially expressed in CHD-PAH. Previous studies
have only detected the expression of miR-27b, its clinical
significance and mechanism in CHD-PAH has rarely been discussed
(8). miR-451 is down-regulated in
the lungs of PAH animal models, and its reduction in serum may
affect the function of VECs or VSMCs (9). There is research highlighting the role
of miR-451 in the development of heart disease (10), which leads us to speculate that
miR-451 is also closely related to the pathogenesis of CHD-PAH.
This study analyzed the expressions and clinical
significance of miR-27b and miR-451 in CHD-PAH and explored its
risk factors, in order to provide new targets and more theoretical
basis for the diagnosis and treatment of CHD-PAH.
Patients and methods
General data
A total of 114 patients with CHD admitted to the
First Affiliated Hospital of the University of South China
(Hengyang, China) from July 2016 to January 2019 were prospectively
analyzed, including 63 males and 51 females, with an average age of
12.29±1.15 years. They were allocated into a study group (61
patients with PAH) and a control group (53 patients without PAH).
Inclusion criteria: All patients enrolled were diagnosed with CHD,
and those in the study group were diagnosed with CHD-PAH by PAP
measurements. Exclusion criteria: Patients with other malignant
tumors, severe immune system diseases, liver and kidney
dysfunction, or lung diseases. The study was approved by the Ethics
Committee of The First Affiliated Hospital of the University of
South China. Patients who participated in this research had
complete clinical data. The patients and their families agreed to
participate in the experiment and signed written informed consents
were obtained from the parents of the child patients.
Index detection
Fasting venous blood (5 ml) collected from the
patients in the morning following admission was added with heparin
for anticoagulation, then centrifuged at 1,500 x g for 5 min at
4˚C. The serum was obtained for testing.
Detection of miR-27b and miR-451 by reverse
transcription-polymerase chain reaction (RT-PCR). Fasting venous
blood (5 ml) collected from all the patients was centrifuged at
1,500 x g for 10 min at 4˚C, and the supernatant was obtained.
TRIzol reagent was added to serum to extract total RNA, and the
purity and concentration of the RNAs were tested by an ultraviolet
spectrophotometer. The total RNAs were reverse transcribed using
SYBR-Green Real-time PCR Master Mix in strict accordance with the
instruction of the kit. Then PCR amplification was carried out with
the PrimeScript RT Master Mix Kit (Takara Bio). Amplification
system: 10 µl SYBR qPCR Mix, 0.8 µl each upstream and downstream
primers, 2 µl cDNA product, 0.4 µl 50X ROX reference dye, made up
to 20 µl with RNase-free water. PCR reaction condition:
Pre-denaturation at 95˚C for 35 sec, followed by 40 cycles of
denaturation at 94˚C for 30 sec, annealing at 60˚C for 40 sec, and
extension at 72˚C for 30 sec. The primers were all synthesized at
Sangon Bioengineering Co., Ltd. (Table
I). U6 was used as an internal reference and the relative mRNA
expression was calculated by 2-ΔΔCt.
 | Table IRelated primer sequences. |
Table I
Related primer sequences.
| Gene | Upstream primers | Downstream
primers |
|---|
| miR-27b |
5'-TTCACAGTGGCTAAGT-3' |
5'-CAGTGCGTGTCGTGGAGT-3' |
| miR-451 |
5'-CACCTATCGTGGTGAAGTT-3' |
5'-GAATGCACTGCACAATATT-3' |
| U6 |
5'-GCTTCGGCAGCACATATACTAAAAT-3' |
5'-CGCTTCACGAATTTGCGTGTCAT-3' |
Detection of other biochemical indexes. The levels
of total cholesterol (TC), triglyceride (TG), alanine transaminase
(ALT), blood urea nitrogen (BUN) and serum creatinine (SCr) were
measured by the DXC800 automatic biochemical analyzer (Beckman
Coulter). The levels of brain natriuretic peptide (BNP) and
asymmetric dimethylarginine (ADMA) were detected by enzyme-linked
immunosorbent assay (ELISA). The kits were all purchased from Wuhan
MSK Biotechnology Co., Ltd. and the operation was carried out
strictly in accordance with the instructions of the kits.
Measurement of mPAP by right heart
catheterization
Right heart catheterization (RHC) is the gold
standard for diagnosis of CHD-PAH (11). The patients underwent femoral venous
puncture after local anesthesia. Next, a 6-F vascular sheath was
inserted, and heparin was injected intravenously for
anticoagulation. Right heart catheter was inserted into right
ventricle and pulmonary artery through the vascular sheath to
measure the mean pulmonary artery pressure (mPAP).
Statistical analysis
In this study, SPSS 18.0 [Boyi Zhixun (Beijing)
Information Technology Co., Ltd.] was used to carry out statistical
analysis. Chi-square test was employed for counting data and mean ±
standard deviation for measurement data. The comparison between the
two groups was conducted by t-test. Pearson was used for
correlation analysis and receiver operating characteristics (ROC)
for assessment of the predictive value of single and combined
miR-27b and miR-451 for CHD-PAH. Multivariate Logistic regression
was performed to analyze the risk factors of CHD-PAH. A value of
P<0.05 was considered statistically significant.
Results
General data
There was no significant difference in sex, age and
body mass index (BMI) between the two groups (P>0.05) (Table II).
 | Table IIGeneral data [n (%)]. |
Table II
General data [n (%)].
| Factor | Study group
(n=61) | Control group
(n=53) | t/χ2
value | P-value |
|---|
| Sex | | | 0.012 | 0.913 |
|
Male | 34 (55.74) | 29 (54.72) | | |
|
Female | 27 (44.26) | 24 (45.28) | | |
| Age, years | | | 0.237 | 0.626 |
|
≤12 | 35 (57.38) | 28 (52.83) | | |
|
>12 | 26 (42.62) | 25 (47.17) | | |
| BMI,
kg/m2 | | | 0.058 | 0.809 |
|
≤15 | 29 (47.54) | 24 (45.28) | | |
|
>15 | 32 (52.46) | 29 (54.72) | | |
| TC (mmol/l) | 4.59±0.55 | 4.62±0.57 | 0.286 | 0.776 |
| TG (mmol/l) | 0.81±0.17 | 0.79±0.19 | 0.593 | 0.554 |
| ALT (U/l) | 29.11±4.32 | 28.16±5.24 | 1.061 | 0.291 |
| BUN (mmol/l) | 4.98±1.14 | 5.03±1.22 | 0.226 | 0.822 |
| SCr (µmol/l) | 61.64±9.37 | 59.96±10.01 | 0.925 | 0.357 |
| CHD type | | | 6.705 | 0.035 |
|
VSD | 25 (40.98) | 10 (18.87) | | |
|
ASD | 15 (24.59) | 23 (43.39) | | |
|
ADS+VSD | 21 (34.43) | 20 (37.74) | | |
| mPAP (mmHg) | 43.96±7.68 | 15.32±3.78 | 24.67 | <0.001 |
| BNP (pg/ml) | 221.54±51.46 | 110.37±25.94 | 14.23 | <0.001 |
| ADMA (µmol/l) | 0.58±0.13 | 0.30±0.10 | 12.74 | <0.001 |
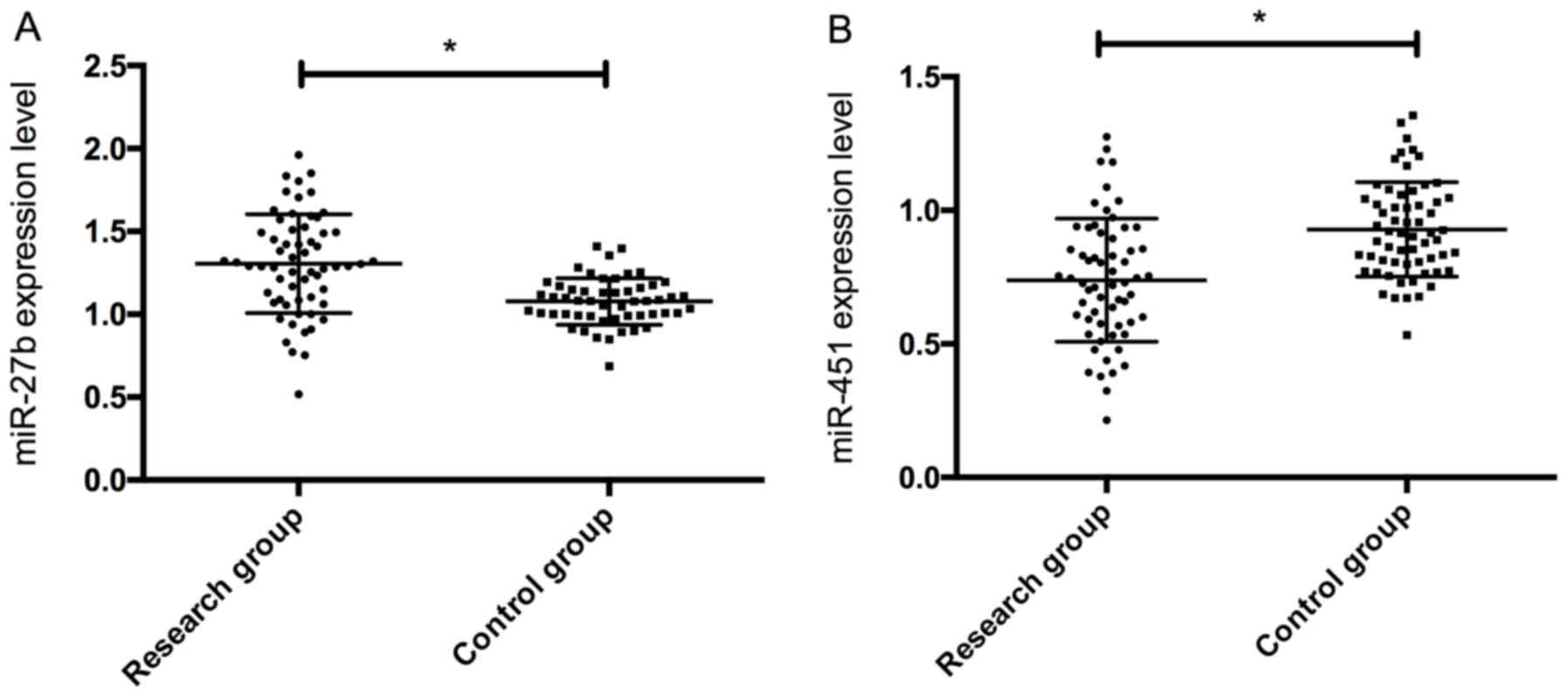
Expression of serum miR-27b and
miR-451
Expression of serum miR-27b and miR-451 in the study
group was 1.32±0.28 and 0.73±0.24, respectively, while those in the
control group 1.03±0.13 and 0.95±0.18, respectively. Therefore, the
expression of serum miR-27 in the study group was significantly
higher than that in the control group, and the expression of
miR-451 was significantly lower than that in the control group
(P<0.05) (Fig. 1).
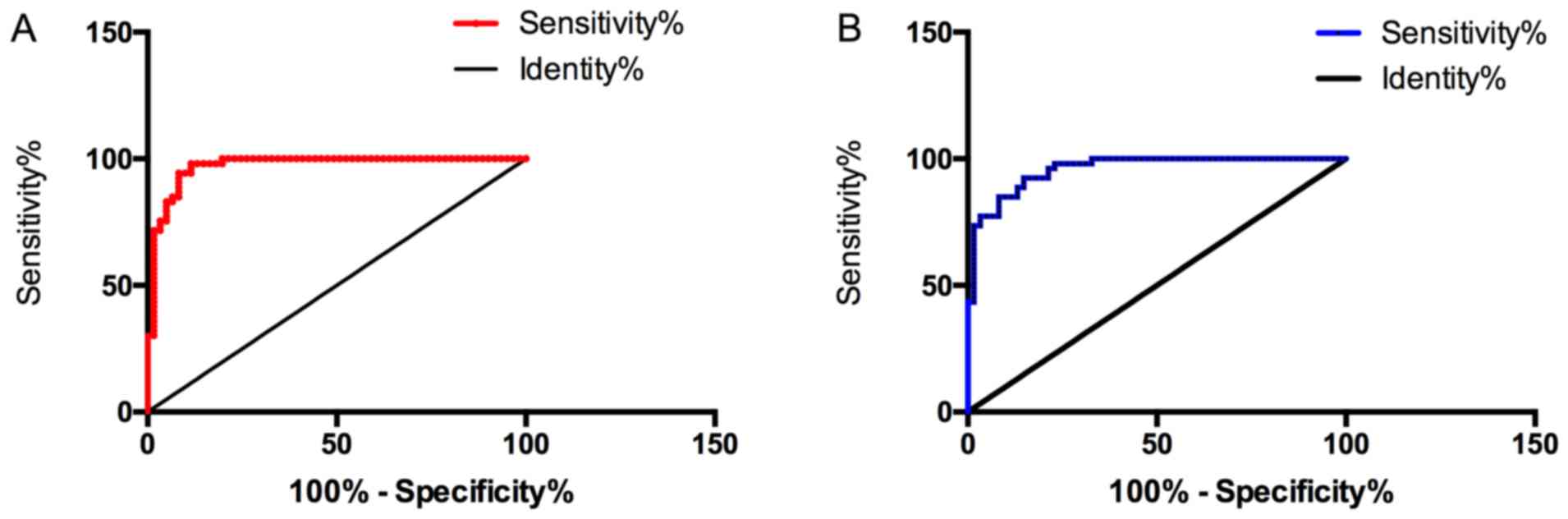
Predictive value of BNP and ADMA in
CHD-PAH
The sensitivity, specificity, and area under the
curve (AUC) of serum BNP in predicting CHD-PAH were 96.23%, 88.52%
and 0.971, respectively, and those of ADMA were 94.34%, 78.69% and
0.959, both of which were of high value. Therefore, BNP and ADMA
were selected to carry out correlation analysis with miR-27b and
miR-451 to evaluate the correlation between miR-27b, miR-451 and
CHD-PAH (Fig. 2).
Correlation of serum miR-27b and
miR-451 with mPAP, BNP and ADMA
Serum miR-27b was positively correlated with mPAP,
BNP and ADMA (r=0.690, P<0.05; r=0.688, P<0.05; r=0.658,
P<0.05), and serum miR-451 was negatively correlated with mPAP,
BNP and ADMA (r =-0.736, P<0.05; r=-0.761, P<0.05; R=-0.730,
P<0.05) (Fig. 3).
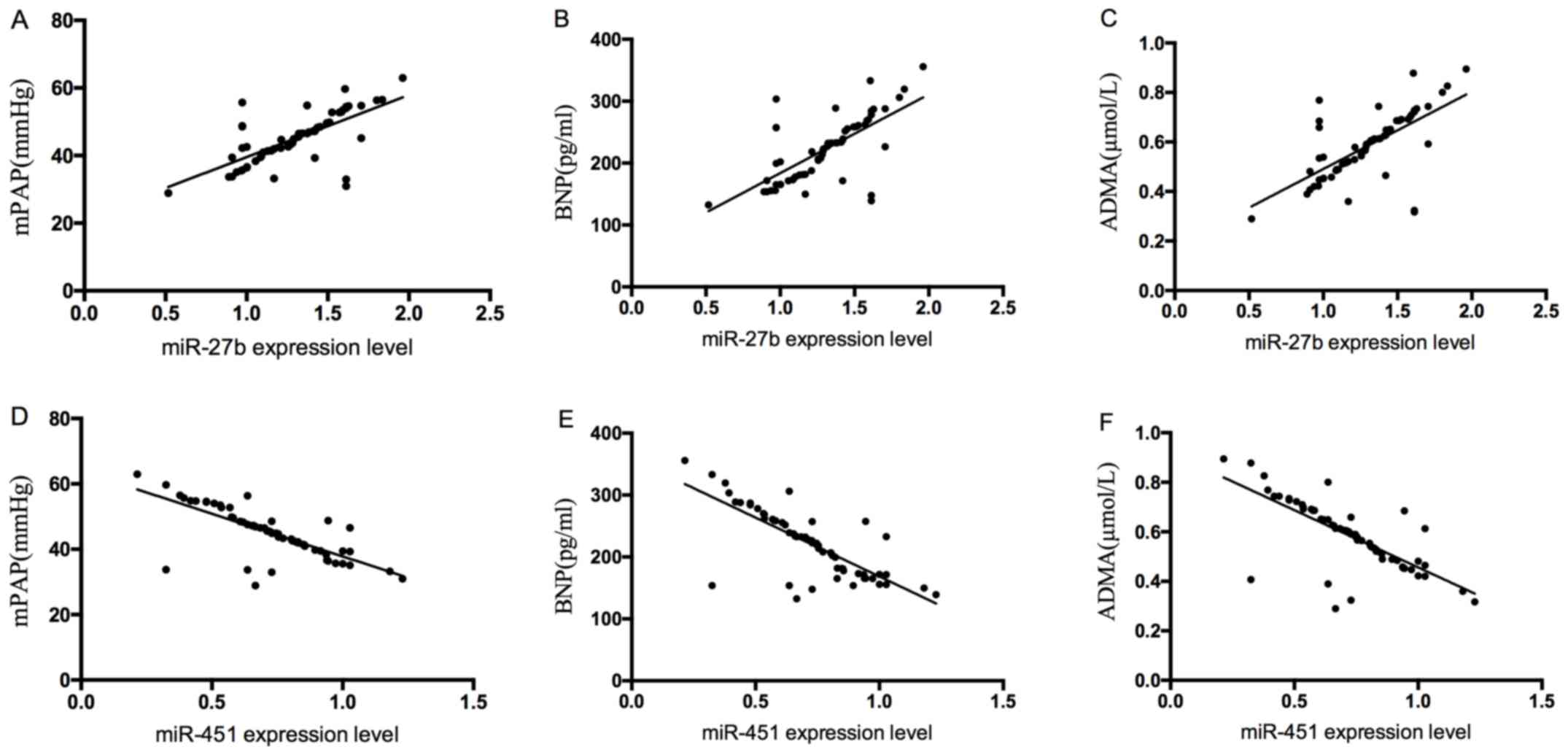 | Figure 3Correlation of serum miR-27b and
miR-451 with mPAP, BNP and ADMA. (A) Serum miR-27b was positively
correlated with mPAP (r=0.690, P<0.05). (B) Serum miR-27b was
positively correlated with BNP (r=0.688, P<0.05). (C) Serum
miR-27b was positively correlated with ADMA (r=0.658, P<0.05).
(D) Serum miR-451 was negatively correlated with mPAP (r=-0.736,
P<0.05). (E) Serum miR-451 was negatively correlated with BNP
(r=-0.761, P<0.05). (F) Serum miR-451 was negatively correlated
with ADMA (r=-0.730, P<0.05). mPAP, mean pulmonary artery
pressure; BNP, brain natriuretic peptide; ADMA, asymmetric
dimethylarginine. |
Predictive value of single and
combined miR-27b and miR-451 in CHD-PAH
The sensitivity, specificity and AUC of serum
miR-27b in CHD-PAH prediction were 73.58%, 70.49% and 0.757,
respectively, while those of miR-451 were 71.70%, 60.66% and 0.737,
respectively. Binary Logistic regression analysis was conducted
with miR-27b and miR-451 as independent variables. Logistic
regression model: Logit (P) = 0.884+1.673 miR-27b+-2.960 miR-451.
The AUC, sensitivity and specificity of this model in diagnosing
CHD-PAH were 0.840, 86.79% and 68.85%, respectively. The combined
detection of miR-27b and miR-451 was more valuable in the diagnosis
of CHD-PAH than the single detection (Fig. 4).
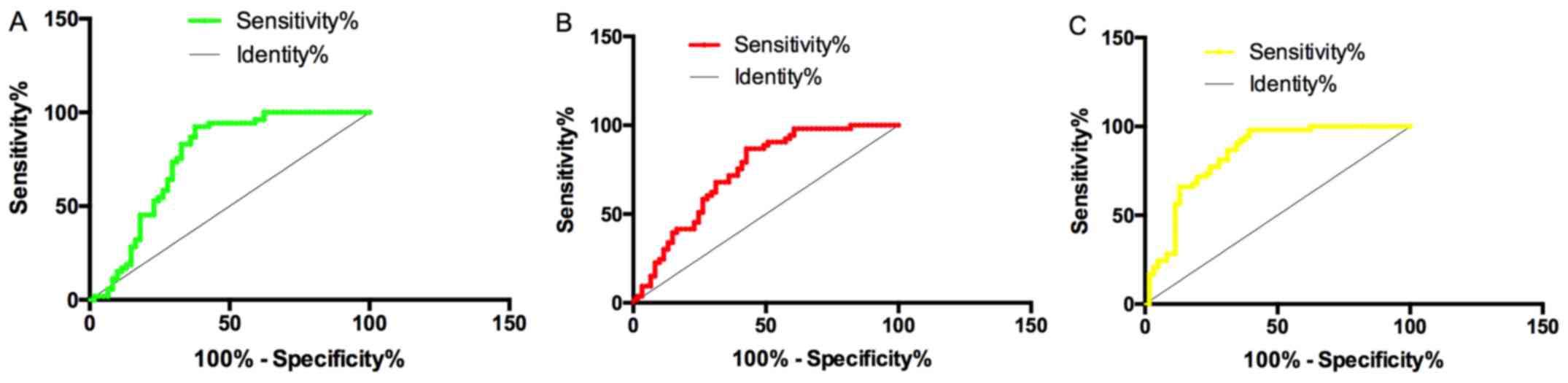 | Figure 4Predictive values of single and
combined miR-27b and miR-451 for CHD-PAH. (A) Sensitivity,
specificity and AUC of serum miR-27b in CHD-PAH prediction were
73.58%, 70.49% and 0.757, respectively. (B) Sensitivity,
specificity and AUC of miR-451 in CHD-PAH prediction were 71.70%,
60.66% and 0.737, respectively. (C) AUC, sensitivity and
specificity of combined detection of miR-27b and miR-451 in CHD-PAH
prediction were 0.840, 86.79% and 68.85%, respectively. CHD-PAH,
congenital heart disease associated pulmonary arterial
hypertension. |
Risk factors of PAH in patients with
CHD
Factors with differences between CHD and CHD-PAH
patients were classified as independent variables, including BNP,
ADMA, ventricular septal defect (VSD), miR-27b and miR-451. After
assignments (Table III), logistic
regression was used to analyze whether these factors were
independent risk factors of COPD-PH. The results showed that
increased BNP, ADMA, miR-27b, decreased miR-451, as well as the
presence of VSD were independent risk factors of CHD-PAH (Table IV).
 | Table IIIAssignments. |
Table III
Assignments.
| Factor | Assignment |
|---|
| BNP | The data is a
continuous variable and analyzed with raw data. |
| ADMA | The data is a
continuous variable and analyzed with raw data. |
| VSD | Yes =1, No =2 |
| miR-27b | The data is a
continuous variable and analyzed with raw data. |
| miR-451 | The data is a
continuous variable and analyzed with raw data. |
 | Table IVMultivariate analysis. |
Table IV
Multivariate analysis.
| Factor | β | SE | Wald | OR | 95% CI | P-value |
|---|
| BNP | 3.167 | 0.649 | 2.227 | 6.097 | 3.124-5.011 | <0.01 |
| ADMA | 2.876 | 0.422 | 1.970 | 2.286 | 1.019-4.211 | <0.01 |
| VSD | 3.146 | 0.605 | 2.191 | 2.578 | 1.116-4.778 | <0.01 |
| miR-27b | 3.455 | 0.729 | 2.554 | 3.783 | 1.226-5.103 | <0.01 |
| miR-451 | 3.145 | 0.608 | 2.139 | 2.686 | 1.189-4.856 | <0.01 |
Discussion
PAH, a common complication of CHD, is one of the
main causes of death in patients with CHD (12). PAH-CHD is a disease with high
incidence posing a serious threat to the physical and mental health
of the majority of children (13).
As a highly stable single-stranded small molecule, miRNA has been
widely studied in recent years and it has been found that the
differential expression of several miRNAs may be closely related to
the pathogenesis of PAH (14,15).
In this study, the expression and clinical
significance of miR-27b and miR-451 in PAH-CHD were explored. Some
scholars proved that miR-27b is differentially expressed in PAH-CHD
using gene chip technology (16).
Moreover, miR-451 is found to be closely related to the
pathogenesis of PAH (17), but its
clinical significance in CHD-PAH has not been not discussed yet. In
this study, expressions of serum miR-27b and miR-451 in patients
with PAH and CHD-PAH were detected. It turned out that serum
miR-27b in patients with CHD-PAH was significantly higher than that
in patients with PAH, and miR-451 was significantly lower than that
in patients with PAH. Previously scholars also stated that miR-27b
is highly expressed in PAH-CHD by screening the differential
expression of miRNAs (18). Animal
experiments showed that miR-451 is down-regulated in PH rats, which
was consistent with our conclusion (19). Subsequently, in order to explore the
clinical significance of miR-27b and miR-451 in CHA-PAH, we
conducted analysis on the correlation of miR-27b and miR-451 with
mPAP, BNP and ADMA. BNP is one of the most sensitive indicators for
predicting heart failure clinically. Damage of myocardial cells
increases the synthesis of BNP rapidly and greatly, which can
better reflect the cardiac function (20). ADMA, a newly discovered inhibitory
cytokine, is found to inhibit the release of NO by binding with the
active site of NOS, and it is considered to be one of the
predictors of PAH (21). MPAP is the
most direct indicator to reflect PAP and the severity of PAH
(22). This study showed that serum
miR-27b was positively correlated with mPAP, BNP and ADMA, and
serum miR-451 was negatively correlated with them, which suggested
that both miR-27b and miR-451 might be used as evaluation
indicators of CHD-PAH.
At present, RHC is the gold standard for the
diagnosis of CHD-PAH (23), but it
is still of great significance to seek effective noninvasive
biomarkers for clinical practice. Therefore, in this study, we
analyzed the diagnostic value of single and combined miR-27b and
miR-451 in CHD-PAH diagnosis. The results showed that the AUC of
miR-27b and miR-451 was 0.757 and 0.737, respectively, indicating
their low predictive value. However, the AUC of the combined
detection increased to 0.840, which suggested that miR-27b and
miR-451 may be used as reference indicators for the diagnosis of
CHD-PAH. A study noted that the AUC of miR-451 in PAH diagnosis was
~0.710, which was similar to our conclusion (24). However, the predictive value of
miR-27b for CHD-PAH has been poorly explored. In addition for
timely diagnosis and treatment, it is equally important to identify
the risk factors to effectively prevent CHD-PAH. Therefore, the
etiologic factors of CHD-PAH were also analyzed in this study.
Increased BNP, ADMA and miR-27b, and decreased miR-451 as well as
the presence of VSD were shown as independent risk factors of
CHD-PAH. A previous study (25)
indicated that the untimely repair of severe VSD results in
irreversible remodeling of pulmonary vessels, and spontaneous PAH
may occur if surgery is performed at this time. Besides, another
study showed that VSD is more likely to cause PAH due to its
special hemodynamics (26). At
present, there is no related research reported on the mechanism of
miR-27b and miR-451 in CHD-PAH. Therefore, the conclusion that
miR-27b and miR-451 are independent risk factors of CHA-PAH remains
to be further investigated by follow-up research.
To sum up, miR-27b is highly expressed and miR-451
expression is low in patients with CHD-PAH. miR-27b and miR-451 are
significantly correlated with the severity of the disease, BNP and
ADMA. The combination of miR-27b and miR-451 has a high diagnostic
value and can be used as a biological marker for diagnosis and
assessment of CHD-PAH. However, we failed to access enough
literature to explain the mechanism of miR-27b and miR-451 in
CHD-PAH, which is to be further elaborated by in vivo and
in vitro experiments. Analysis of risk factors of CHD-PAH
was not comprehensive. Therefore, further study is required on the
risk factors of CHD-PAH.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL designed the study. YX was responsible for the
detection of the indices. XY contributed with new reagents and
analytic tools. SG analyzed the data. LZ and HL were responsible
for the measurement of mPAP by right heart catheterization and
wrote the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The First Affiliated Hospital of the University of South China
(Hengyang, China). Patients who participated in this research had
complete clinical data. Signed written informed consents were
obtained from the parents of the child patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chew JD, Loyd JE and Austin ED: The
genetics of pulmonary arterial hypertension. Semin Respir Crit Care
Med. 38:585–595. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
van der Feen DE, Bartelds B, de Boer RA
and Berger RM: Pulmonary arterial hypertension in congenital heart
disease: Translational opportunities to study the reversibility of
pulmonary vascular disease. Eur Heart J. 38:2034–2041.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Roberts KE, McElroy JJ, Wong WP, Yen E,
Widlitz A, Barst RJ, Knowles JA and Morse JH: BMPR2 mutations in
pulmonary arterial hypertension with congenital heart disease. Eur
Respir J. 24:371–374. 2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sun ML, Cheng CY, Wu DC, Zhao QH, Liu QQ,
Jiang R, Jiang X and Jing ZC: Long-term survival of patients with
repaired congenital heart disease associated pulmonary arterial
hypertension (CHD-PAH) in the modern management era. Eur Heart J.
34 (Suppl 1)(P287)2013.
|
|
5
|
Sun ML, Cheng CY, Wu DC, Zhang R, Zhang X,
Xu XQ, Sun K, Zhao QH, Wang X-J, Jiang R, et al: Survival of
patients with repaired CHD-PAH in the modern management era. J Am
Coll Cardiol. 63(A1484)2014.
|
|
6
|
Boucherat O, Potus F and Bonnet S:
microRNA and pulmonary hypertension. Adv Exp Med Biol. 888:237–252.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Grant JS: The role of microRNA in the
development of pulmonary arterial hypertension: Studies in cell
culture and animal models. University of Glasgow, p263, 2014.
|
|
8
|
Hailong D and Yin XL: GW25-e2158. The
changes of plasma miR-18a, miR-27b, miR-130a, miR-204 in patients
with pulmonary arterial hypertension due to congenital heart
disease. J Am Coll Cardiol. 64 (Suppl 16):C190. 2014.
|
|
9
|
Caruso P, Dempsie Y, Stevens HC, McDonald
RA, Long L, Lu R, White K, Mair KM, McClure JD, Southwood M, et al:
A role for miR-145 in pulmonary arterial hypertension: Evidence
from mouse models and patient samples. Circ Res. 111:290–300.
2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang X, Zhu H, Zhang X, Liu Y, Chen J,
Medvedovic M, Li H, Weiss MJ, Ren X and Fan GC: Loss of the
miR-144/451 cluster impairs ischaemic preconditioning-mediated
cardioprotection by targeting Rac-1. Cardiovasc Res. 94:379–390.
2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Grymuza M, Małaczyńska-Rajpold K,
Jankiewicz S, Siniawski A, Grygier M, Mitkowski P, Kałużna-Oleksy
M, Lesiak M, Mularek-Kubzdela T and Araszkiewicz A: Right heart
catheterization procedures in patients with suspicion of pulmonary
hypertension - experiences of a tertiary center. Postepy Kardiol
Interwencyjnej. 13:295–301. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Stephan R, Ghofrani H, Beghetti M, Ivy D,
Fritsch A, Weimann G, Saleh S, Apitz C and Frey R: Riociguat for
pulmonary arterial hypertension (PAH) associated with congenital
heart disease (CHD): A subgroup analysis from the PATENT studies.
BMC Pharmacol Toxicol. 16 (Suppl 1)(A79)2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen IC and Dai ZK: Insight into pulmonary
arterial hypertension associated with congenital heart disease
(PAH-CHD): Classification and pharmacological management from a
pediatric cardiological point of view. Acta Cardiol Sin.
31:507–515. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen W and Li S: Circulating microRNA as a
novel biomarker for pulmonary arterial hypertension due to
congenital heart disease. Pediatr Cardiol. 38:86–94.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhao H, Guo Y, Sun Y, Zhang N and Wang X:
miR-181a/b-5p ameliorates inflammatory response in
monocrotaline-induced pulmonary arterial hypertension by targeting
endocan. J Cell Physiol. 235:4422–4433. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Thum T, Galuppo P, Wolf C, Fiedler J,
Kneitz S, van Laake LW, Doevendans PA, Mummery CL, Borlak J,
Haverich A, et al: MicroRNAs in the human heart: A clue to fetal
gene reprogramming in heart failure. Circulation. 116:258–267.
2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Grant JS, Morecroft I, Dempsie Y, van
Rooij E, MacLean MR and Baker AH: Transient but not genetic loss of
miR-451 is protective in the development of pulmonary arterial
hypertension. Pulm Circ. 3:840–850. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Ma K, Zhao Q, Chen W, Zhang H, Li S, Pan X
and Chen Q: Human lung microRNA profiling in pulmonary arterial
hypertension secondary to congenital heart defect. Pediatr
Pulmonol. 50:1214–1223. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wei C, Henderson H, Spradley C, Li L, Kim
IK, Kumar S, Hong N, Arroliga AC and Gupta S: Circulating miRNAs as
potential marker for pulmonary hypertension. PLoS One.
8(e64396)2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
McLellan J, Heneghan CJ, Perera R,
Clements AM, Glasziou PP, Kearley KE, Pidduck N, Roberts NW, Tyndel
S, Wright FL, et al: B-type natriuretic peptide-guided treatment
for heart failure. Cochrane Database Syst Rev: Dec 22, 2016 (Epub
ahead of print). doi: 10.1002/14651858.CD008966.pub2.
|
|
21
|
Pruneda-Alvarez LG, Ruíz-Vera T,
Ochoa-Martínez AC, Pérez-Vázquez FJ, González Palomo AK,
Ilizaliturri-Hernández CA and Pérez-Maldonado IN: Plasma asymmetric
dimethylarginine (ADMA) levels in Mexican women exposed to
polycyclic aromatic hydrocarbons (PAHs): A preliminary study. Sci
Total Environ. 572:1195–1202. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Krowka MJ, Plevak DJ, Findlay JY, Rosen
CB, Wiesner RH and Krom RA: Pulmonary hemodynamics and
perioperative cardiopulmonary-related mortality in patients with
portopulmonary hypertension undergoing liver transplantation. Liver
Transpl. 6:443–450. 2000.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sr GY: Evaluation of acute hemodymamic
effect of inhaled iloprost in pulmonary artery hypertension with
right heart catheterization. Cardiovasc Revasc Med. 10:197.
2009.
|
|
24
|
Song XW, Zou LL, Cui L, Li SH, Qin YW,
Zhao XX and Jing Q: Plasma miR-451 echocardiography can be used as
a reference for the diagnosis of pulmonary hypertension. Acta
Pharmacol Sin. 39:1208–1216. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Friedman WF: Proceedings of the National
Heart, Lung, and Blood Institute Pediatric Cardiology Workshop:
Pulmonary Hypertension. Pediatr Res. 20:811–824. 1986.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Schuijt MT, Blok IM, Zwinderman AH, van
Riel AC, Schuuring MJ, de Winter RJ, Duijnhouwer AL, van Dijk AP,
Mulder BJ and Bouma BJ: Mortality in pulmonary arterial
hypertension due to congenital heart disease: Serial changes
improve prognostication. Int J Cardiol. 243:449–453.
2017.PubMed/NCBI View Article : Google Scholar
|