Introduction
Osteoarthritis (OA) is a degenerative joint disease
affecting millions globally. OA is one of the primary types of
arthritis and is predicted to become the fourth leading cause of
disability by 2020(1). The
pathological features of knee osteoarthritis (KOA) result from the
chronic degeneration of chondrocytes (2,3). Since
individuals >65 years of age suffer from different degrees of
KOA, its prevalence has steadily increased. KOA-related cartilage
destruction, synovitis and osteophyte hyperplasia have become the
main causes of disability in adults, especially in the elderly
(1). As a progressive degenerative
joint disease, the continual degeneration of articular cartilage
promotes bone-to-bone friction, resulting in severe pain, stiffness
and disability. A close correlation between inflammatory and
catabolic changes and the occurrence and development of OA has been
reported. However, the pathogenetic mechanisms of OA are yet to be
fully elucidated (4). Therefore,
current OA treatment options are limited, and there are no
treatments that can effectively prevent its progression. With the
recent development of biological agents, the TNF-α inhibitor
etanercept has become an effective agent that provides biological
support for tissue regeneration and repair (5). Total knee arthroplasty can only be used
to treat patients with advanced OA, while those with mild disease
are largely treated with drugs, including joint nutrients,
non-steroidal anti-inflammatories, opioid analgesics and
antipyretic analgesics. To a certain extent, patient joint mobility
and clinical symptoms can be improved, but these treatments cannot
slow or reverse the degeneration process, and the side effects of
long-term use are significant (6).
Therefore, the identification of safe and efficient treatments for
OA is paramount.
At present, increasing numbers of natural plant
compounds are being used to treat diseases and their associated
complications. Astilbe chinensis is a traditional Chinese
medicine which is commonly used to treat rheumatoid and skeletal
muscle diseases, of which astilbin (AST) is one of the primary
dihydroflavonol glycoside constituents (7,8).
Previous studies have shown that AST can significantly decrease the
levels of serum interleukin (IL)-1, tumor necrosis factor-α (TNF-α)
and IL-6 (as well as the activities of their associated protein
targets) in rats with rheumatoid arthritis (9).
Previous studies have demonstrated that the NF-κB
inhibitor α and PI3K/AKT signaling pathways serve key roles in
cartilage degeneration (10). In
addition, TNF-α and IL-6 may be used as downstream regulators of
the nuclear PI3K/AKT/NF-κB signaling, and thus mediate the
inflammatory response. Therefore, inhibiting the functions of such
inflammatory pathways is an acknowledged option for the treatment
of OA (11). Since AST downregulates
the expression of TNF-α, we hypothesized that it may also serve a
preventive role in OA. In the present study, papain-induced rats
were used to evaluate the therapeutic effects of AST on OA, and to
investigate its potential anti-inflammatory mechanisms.
Materials and methods
AST and its primary reagents
Pure AST was provided by Shanghai Yuanye
Biotechnology Co., Ltd. For experiments, AST was dissolved in
phosphate buffered saline (PBS) and diluted with normal saline.
Pentobarbital sodium, papain and cysteine were purchased from
Guangzhou Chemical General Factory, and hematoxylin, water-soluble
eosin Y, toluidine blue O, paraformaldehyde and EDTA were acquired
from Takara Bio, Inc. Anti-TNF (cat. no. ab1793), IL-1 (cat. no.
ab9722), β-actin (cat. no. ab8227), AKT (cat. no. ab8805), NF-κB
(cat. no. ab28849), PI3K (cat. no. ab140307) and IL-6 (cat. no.
ab6672) antibodies were all purchased from Abcam. The quantitative
PCR detection and Prime Script RT kits were purchased from Bao
Biological Engineering Co. Ltd., and Takara Biotechnology Co. Ltd.,
respectively.
Animals
A total of 24 male Sprague-Dawley rats (age, 12
weeks; weight, 200-250 g) were purchased from and raised in the
Experimental Animal Center of Guiyang College of Traditional
Chinese Medicine (Guiyang, China), in line with the specific
pathogen-free conditions of the College Animal Care and Use
Committee. The rats were allowed free access to food and water.
All experiments conducted in the present study were
approved by the Ethics Committee of Guiyang College of Traditional
Chinese Medicine.
Animal model establishment and
intervention
The OA model was constructed using 12-week-old rats
that were randomly divided into the following 4 groups (n=6 per
group): i) AST; ii) PBS; iii) OA; and iv) control. AST was
dissolved in PBS, thus the solvent was used as a control to
eliminate interference factors. Rats in the AST, PBS and OA groups
were injected with a mixed solution (0.25 ml/kg) of 4% (w/w) papain
and 0.3 mol/l cysteine at the knee joint on days 1, 3 and 5. The
control group did not receive surgical intervention. After
successful establishment of the OA model, 3 mg/kg AST was
administered to the AST group by gavage, and the PBS group received
an equal volume of PBS only; the other two groups were treated with
normal saline once a day for 4 weeks. At 4 weeks post-surgery, the
rats were euthanized with pentobarbital sodium, and the knee joint
tissues were collected for further analysis.
Hematoxylin and eosin (H&E)
staining
Cartilage tissues were collected from the knee
joints of each rat, fixed in 4% paraformaldehyde for 24 h, and then
dehydrated using a graded alcohol series. The tissue were embedded
in paraffin and sliced into sections of 4 μm thickness.
After routine dewaxing, the sections were stained with hematoxylin
for 8 min, washed with tap water for 1 min, and stained for 3 min
in eosin solution. The sections were then sequentially sealed by
graded alcohol dehydration, transparent xylene and neutral gum.
Toluidine blue staining
After routine dewaxing, the tissue sections were
stained with 0.5% toluidine blue solution for 30 min and washed
with tap water. After separation for 5 sec in 0.5% glacial acetic
acid solution, the sections were washed with distilled water and
subsequently sealed by gradient alcohol dehydration, transparent
xylene and neutral gum (as aforementioned). The average optical
density of toluidine blue was then analyzed.
Western blot analysis
The articular cartilage was placed in a mortar,
liquid nitrogen was added, and the samples were immediately ground.
After grinding, the proteins were extracted using 1 g/4 ml RIPA
protein lysis buffer at 4˚C. After 2 h with vibration at 30 min
intervals, the lysates were centrifuged at 219,128 x g at 4˚C for
30 min and quantified using a BCA assay. The supernatants (200
μl each) were transferred to a fresh centrifuge tube and 50
μl protein loading buffer was added before boiling for 5
min. Equal amounts of protein were separated using 10% SDS-PAGE
gels, and then transferred onto PVDF membranes for 2 h. After
transfer, the membranes were blocked with 5% skimmed milk at room
temperature for 2 h, and then β-catenin antibody, AKT (1:1,000),
PI3K (1:1,000), TNF-α (1:1,500), IL-6 (1:1,000) and IL-1β (1:1,000)
primary antibodies were added for overnight incubation at 4˚C. The
membranes were washed 3 times with TBST buffer (10 min each), 4 ml
secondary goat anti-rabbit IgG/HRP antibody (1:10) was added, and
the membranes were incubated at room temperature for 1 h. The
membranes were then washed as aforementioned prior to exposure and
image development. The grayscale value was analyzed using Image Lab
software (Bio-Rad Laboratories, Inc.) and the relative protein
expression levels were calculated. The relative expression of
β-actin = actual protein gray value/internal reference gray
value.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from the rat cartilage
tissues using TRIzol® reagent. According to the
manufacturer's instructions, mRNA was reverse transcribed using the
PrimeScript RT kit (Takara Bio, Inc.) and qPCR was conducted using
the Thermal Cycler Dice™ real-time system (Takara Bio, Inc.) with
SYBR® Green I dye. Sequence-specific primers were designed to
produce products with lengths between 120 and 436 bp (listed in
Table I). The average Ct values were
standardized to that of β-actin, and the results were quantified
using the 2-ΔΔCq method. All experiments were repeated
three times.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Genes | Forward | Reverse |
|---|
| TNF |
CTCACCACAAAGGAGAAGCCT |
GGTAAGGGAAAGAGGTCGGC |
| IL-6 |
CTGATGCTGCCTATTGCCCA |
TGCTCAGACTCTCCCTTCTGA |
| IL-1β |
CCTTGTCGAGAATGGGCAGT |
CAGGGAGGGAAACACACGTT |
| AKT |
CACTCCCGGTGAACTCTGAC |
CTAAAGGCCGCCCTACACAA |
| PI3K |
ACCTGGGAGTGGAGAAACAGA |
GTGGGCCACATCACTTAGACA |
| β-actin |
GTGTGGTCAGCCCTGTAGTT |
CCTAGAAGCATTTGCGGTGC |
Statistical analysis
The data were obtained from ≥3 independent
experiments and are expressed as the mean ± standard deviation.
Single factor analysis of variance followed by the LSD test was
used for comparisons between groups. Data analysis was performed
using the SPSS 20.0 statistical software package (IBM Corp.) and
P<0.05 was considered to indicate a statistically significant
difference.
Results
H&E staining analysis
H&E staining revealed that the surface layers of
the control group tissues were intact and smoother than those of
the AST group, where the cartilage layer was clearly discernible
and the tidal line was intact (Fig.
1D). In the PBS group, the articular cartilage was damaged with
a rough surface; the cartilage layer was narrower, and the
chondrocytes were exposed with a disordered arrangement (Fig. 1B). In the OA group, severe
destruction of the cartilage, an obvious decrease in cell numbers,
a decrease in the cartilage layer and proteoglycan loss were
observed (Fig. 1C). In the AST
group, the cartilage surface was smooth, and the structure was
normal. The cytoplasm and cartilage matrix were pink and evenly
stained, the chondrocytes were neatly arranged and the tide line
was intact (Fig. 1A). The AST group
exhibited decelerated cartilage destruction and the lowest degree
of joint damage, which was similar to the results of the control
group. The chondrocytes were more neatly arranged than those in the
PBS and OA groups, with decreased levels of cytoclasis.
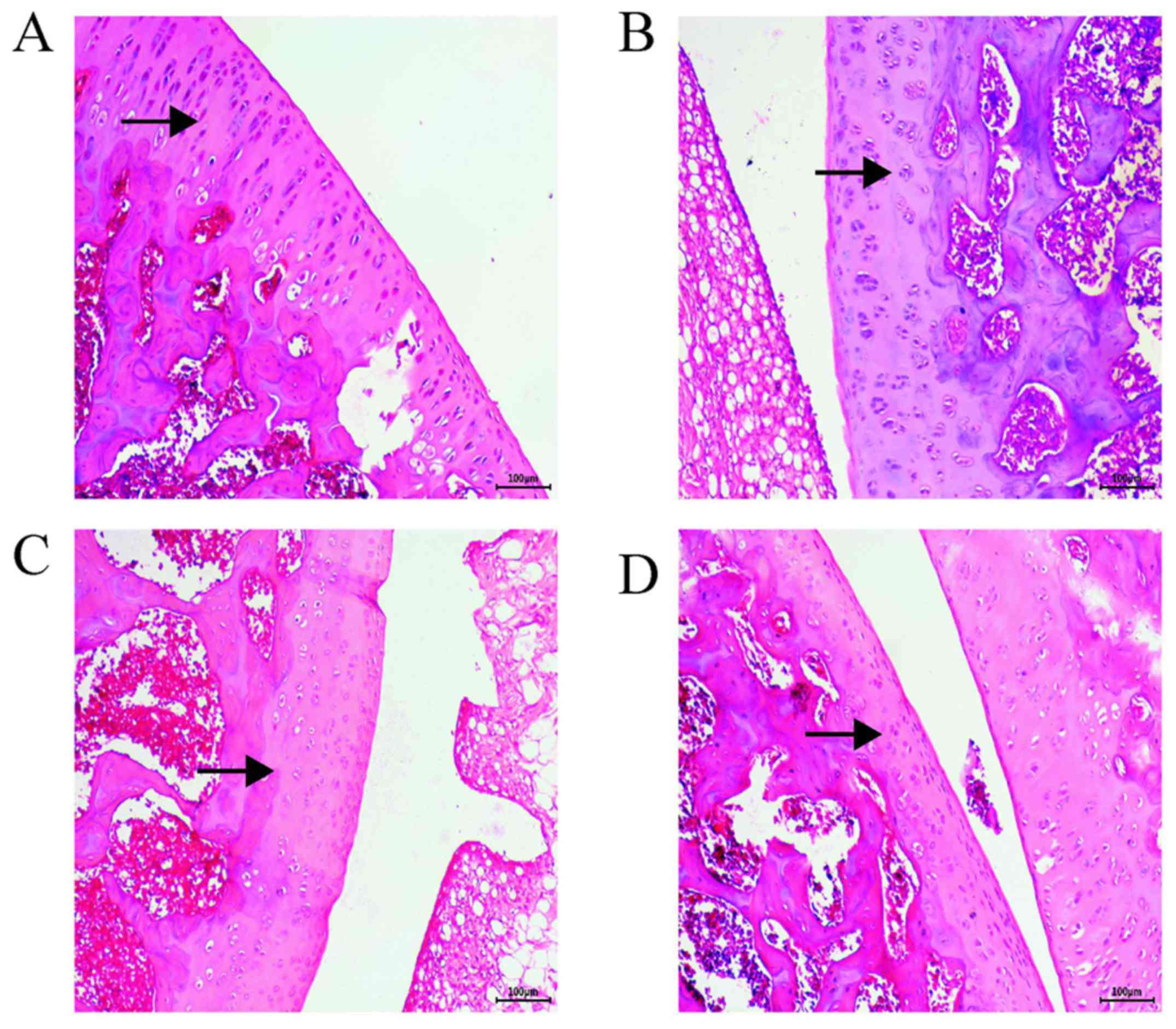 | Figure 1Hematoxylin and eosin staining map of
rat cartilage tissue sections. Cartilage matrix in the OA and the
PBS groups was lightly stained; arrows indicate chondrocytes, and
the cartilage matrix. In the OA and PBS groups, the cartilage was
severely damaged, the cartilage layer and cell numbers were
significantly reduced, and proteoglycan was lost. In the AST group,
the cytoplasm and cartilage matrix were pink and evenly stained,
and the chondrocytes were neatly arranged. Surface layers of the
control group tissues were smoother than those of the AST group;
the cartilage layer was clearly discernible and the tidal line was
largely complete. Magnification, x100. (A) AST, (B) PBS, (C) OA and
(D) control groups. OA, osteoarthritis; AST, astilbin. |
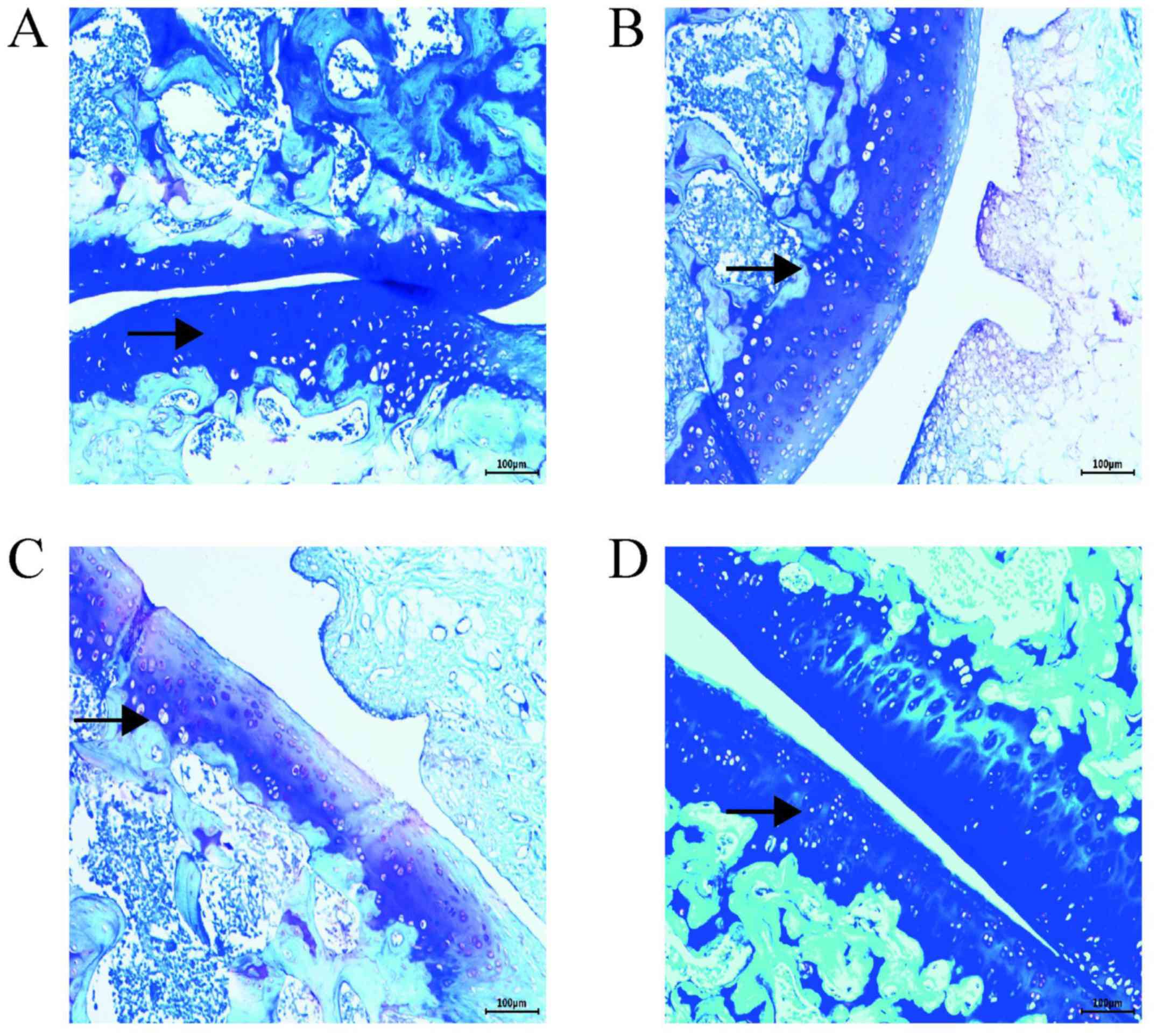
Toluidine blue staining
Toluidine blue staining revealed that in the control
group, the superficial layer of the cartilage was deeply stained
with a blueish-purple color; the stained area was large with a
uniform color distribution (Fig.
2D). In the PBS group, the staining depth and area were
decreased, and large unstained areas were present (Fig. 2B). Tissues in the OA group also
possessed large unstained areas, a light blue color, and a
significantly lower proteoglycan content than those of the other
groups (Fig. 2C). Compared with the
OA and PBS groups, the AST group exhibited relatively uniform
blueish-purple staining of a higher color depth (Fig. 2A).
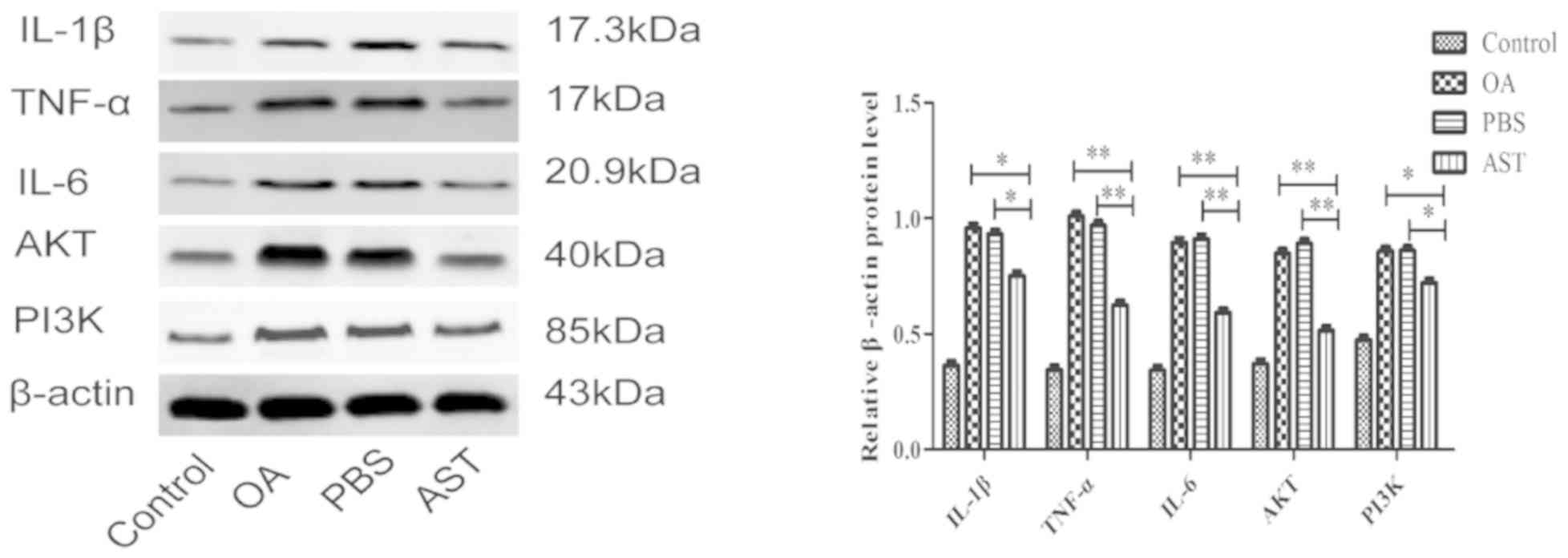
Western blot analysis
Western blot detection demonstrated that TNF-α, IL-6
and AKT protein expression was significantly lower in the cartilage
of the AST group rats than in those of the PBS and OA groups
(P<0.001). The levels of IL-1β and PI3K in the AST group were
lower than those in the PBS and OA groups, though the difference
was still significant (P<0.05). Compared with the control group,
the relative expression level of each protein was higher in the PBS
and OA groups (Fig. 3).
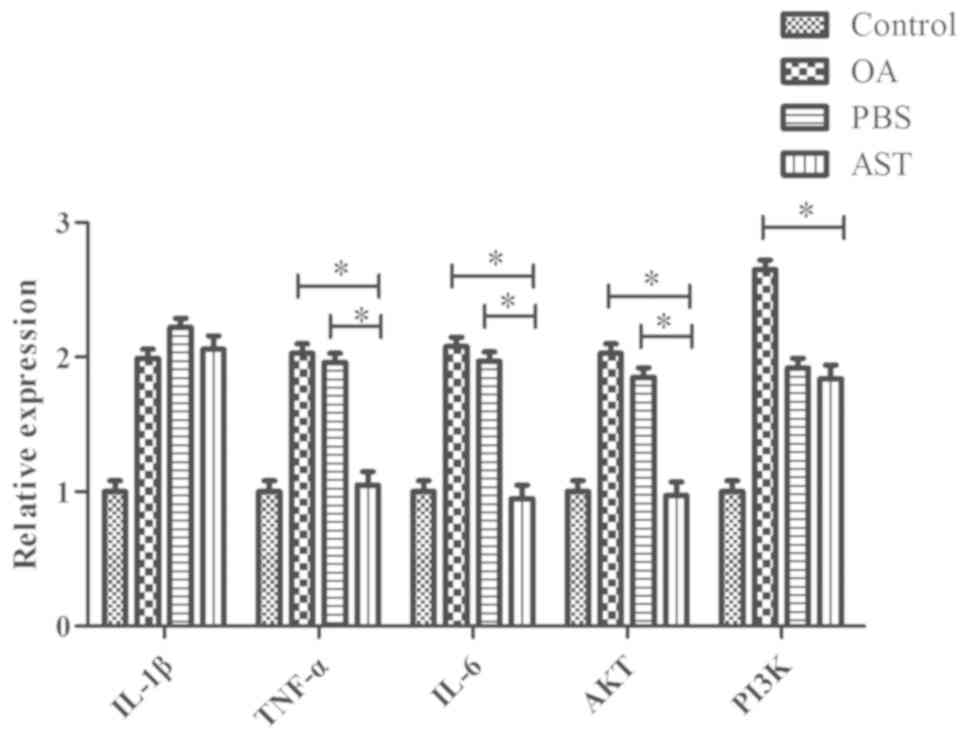
RT-qPCR
The results of RT-qPCR indicate that when compared
with the PBS and OA groups, the mRNA levels of TNF-α, IL-6 and AKT
were significantly lower in the cartilage of rats in the AST group.
Relative to the OA group, the PI3K levels in the AST group tissues
were also significantly lower (P<0.05). There were no
significant differences in IL-1β expression between the groups, and
the control group displayed the lowest expression levels of all
five genes (Fig. 4).
Discussion
OA is the most common joint disease and the primary
cause of joint-associated disability worldwide. As the population
ages, the prevalence of KOA is expected to continue to rise, and to
place a heavy burden on the social health care system (12). Although a variety of risk factors
have been associated with the onset of OA in recent decades, the
therapeutic effects of physiotherapy remain limited. As such,
non-steroidal anti-inflammatory drugs are the primary treatment
options for OA (13); however, these
compounds only temporarily ameliorate the clinical symptoms, rather
than slow the development of OA. Therefore, there is an urgent need
for drugs that specifically target the molecular components of
OA-associated cartilage degradation.
Research into the efficacy and mechanisms of Chinese
herbal medicines for the treatment of OA are critical. Previously,
Astilbe chinensis was mainly used to treat rheumatism and
relieve swelling and pain. Astilbe chinensis contains a
series of bioactive components, including flavonoids,
mannose-binding lectins and terpenes. In the clinic, it is often
used to treat nephritis, rheumatoid arthritis, leptospirosis,
bacillary dysentery, urethral infections and various other diseases
(11,14). Studies have shown that the rhizome
extract of Astilbe chinensis can inhibit the activity of
specific inflammatory cells via immunomodulation. However, its
mechanism is yet to be elucidated. AST is a dihydroflavonol
derivative isolated from the rhizome of Astilbe chinensis,
which exerts a variety of pharmacological effects, including
antioxidant, anti-inflammatory, anti-diabetic and nephrotic
properties (15,16). Evidence suggests that AST can reduce
collagen-induced arthritis-associated dysfunction by selectively
inhibiting lymphocyte function (17)
and decreasing the production of matrix metalloproteinases (MMPs)
and nitric oxide. Moreover, AST attenuates contact allergies by
stimulating IL-10 in lymphocytes (18), and alleviates disease progression in
rats prone to systemic lupus erythematosus by inhibiting the
functionally activated T and B cells (19). Although AST has been reported to
inhibit inflammatory factors and suppress the immune response,
there are no reports addressing the application of AST in OA. In
addition, the anti-inflammatory mechanisms of AST in the treatment
of OA remain to be elucidated. To the best of our knowledge, the
present study is the first to report the use of AST in the
treatment of OA.
The release of TNF-α and IL-6 serves a key role in
the synovial fluid and serum of patients with OA (20). IL-6 is the primary mediator of
cartilage and bone degeneration, the accumulation of inflammatory
cells, the persistence of inflammation, and the emergence of
rheumatoid factors (21). IL-8
functions to promote the infiltration of immune cells and the
recruitment of white blood cells (22). In patients with OA, TNF-α is largely
produced by activated macrophages and is believed to be a major
contributor to inflammation and joint destruction (23). The results of the present study
indicate that AST possesses potential anti-inflammatory properties
and can decrease OA-associated cartilage damage. The PI3K/AKT
pathway is associated with the pathogenesis of OA, as well as
changes in the extracellular matrix (24). Activation of the PI3K/AKT pathway can
induce the phosphorylation of IκBα and p65, activating the
downstream NF-κB pathway in chondrocytes, thus increasing the
production of MMP and cyclooxygenase-2(25). Hence, inhibition of the PI3K/AKT
pathway has been considered as an option for the treatment of
OA.
In the present study, H&E staining indicated
that AST may protect the articular surface and reduce OA-associated
damage. According to western blot analysis, AST was found to
inhibit the phosphorylation of PI3K and AKT (25). At the same time, the mRNA expression
levels of TNF-α, IL-6, PI3K and AKT were downregulated in the
cartilage tissue of rats in the AST group, suggesting that the
inflammatory response was retarded, and that inhibiting the
inflammatory pathways may provide a favorable environment for
articular cartilage repair.
The results of the present study indicate that AST
significantly inhibits the expression of inflammation-associated
factors by targeting the PI3K/AKT pathway, which enables it to play
a significant role in the treatment of OA. AST is believed to
regulate and prevent OA by downregulating IL-1β and TNF-α
expression, which delays cartilage degeneration and promotes
cartilage repair. The present study provides experimental evidence
and a theoretical basis, expounding the mechanisms of this
traditional Chinese medicine in the treatment of OA. However, there
are limitations to the current study. It is necessary to further
investigate the potential synergism between NF-κB and other related
factors (including Toll-like receptors and mitogen-activated
protein kinases), and the relevant molecular pathways associated
with their inflammatory mechanisms. This will be the focus of
subsequent studies.
Acknowledgements
Not applicable.
Funding
The study was supported by the Science and
Technology Cooperation Program of the Department of Science and
Technology of Guizhou Province and the First Affiliated Hospital of
Guizhou University of Traditional Chinese Medicine (fund nos.
LH[2016] and 7508).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CC and MY conceived the study and wrote the
manuscript. YC established the animal model. YW and KW were
responsible for H&E staining and Toluidine blue staining. TL
and QH were responsible for western blot analysis. WZ and JX
performed real-time fluorescence quantitative PCR. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Guiyang College of Traditional Chinese Medicine (Guiyang,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Thielen NGM, van der Kraan PM and van Caam
APM: TGFβ/BMP signaling pathway in cartilage homeostasis. Cells.
8(E969)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Neogi T: Structural correlates of pain in
osteoarthritis. Clin Exp Rheumatol. 35 (Suppl 107):75–78.
2017.PubMed/NCBI
|
|
3
|
Zhao Y, Li Z, Wang W, Zhang H, Chen J, Su
P, Liu L and Li W: Naringin protects against cartilage destruction
in osteoarthritis through repression of NF-κB signaling pathway.
Inflammation. 39:385–392. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ahn H, La JH, Chung JM, Miao H, Zhong C,
Kim M, An K, Lyon D, Choi E and Fillingim RB: The relationship
between β-endorphin and experimental pain sensitivity in older
adults with knee osteoarthritis. Biol Res Nurs. 21:400–406.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mehana EE, Khafaga AF and El-Blehi SS: The
role of matrix metalloproteinases in osteoarthritis pathogenesis:
An updated review. Life Sci. 234(116786)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fei J, Liang B, Jiang C, Ni H and Wang L:
Luteolin inhibits IL-1β-induced inflammation in rat chondrocytes and
attenuates osteoarthritis progression in a rat model. Biomed
Pharmacother. 109:1586–1592. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
He X, Yi T, Tang Y, Xu J, Zhang J, Zhang
Y, Dong L and Chen H: Assessing the quality of Smilacis Glabrae
Rhizoma (Tufuling) by colormetrics and UPLC-Q-TOF-MS. Chin Med.
11(33)2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hao G, Zheng J, Huo R, Li J, Wen K, Zhang
Y and Liang G: Smilax glabra Roxb targets
Aktp-Thr308 and inhibits Akt-mediated signaling pathways
in SGC7901 cells. J Drug Target. 24:557–565. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dong L, Zhu J, Du H, Nong H, He X and Chen
X: Astilbin from Smilax glabra Roxb. Attenuates inflammatory
responses in complete Freund's adjuvant-induced arthritis rats.
Evid Based Complement Alternat Med. 2017(8246420)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Huang X, Xi Y, Mao Z, Chu X, Zhang R, Ma
X, Ni B, Cheng H and You H: Vanillic acid attenuates cartilage
degeneration by regulating the MAPK and PI3K/AKT/NF-κB pathways.
Eur J Pharmacol. 859(172481)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lu CL, Zhu W, Wang M, Xu XJ and Lu CJ:
Antioxidant and anti-inflammatory activities of phenolic-enriched
extracts of Smilax glabra. Evid Based Complement Alternat
Med. 2014(910438)2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bijlsma JW, Berenbaum F and Lafeber FP:
Osteoarthritis: An update with relevance for clinical practice.
Lancet. 377:2115–2126. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nguyen PT and Marquis RE: Antimicrobial
actions of α-mangostin against oral streptococci. Can J Microbiol.
57:217–225. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Wang M, Yang XB, Zhao JW, Lu CJ and Zhu W:
Structural characterization and macrophage immunomodulatory
activity of a novel polysaccharide from Smilax glabra Roxb.
Carbohydr Polym. 156:390–402. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lu CL, Zhu YF, Hu MM, Wang DM, Xu XJ, Lu
CJ and Zhu W: Optimization of astilbin extraction from the rhizome
of Smilax glabra, and evaluation of its anti-inflammatory
effect and probable underlying mechanism in
lipopolysaccharide-induced RAW264.7 macrophages. Molecules.
20:625–644. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yan R and Xu Q: Astilbin selectively
facilitates the apoptosis of interleukin-2-dependent
phytohemagglutinin-activated Jurkat cells. Pharmacol Res.
44:135–139. 2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cai Y, Chen T and Xu Q: Astilbin
suppresses collagen-induced arthritis via the dysfunction of
lymphocytes. Inflamm Res. 52:334–340. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fei M, Wu X and Xu Q: Astilbin inhibits
contact hypersensitivity through negative cytokine regulation
distinct from cyclosporin A. J Allergy Clin Immunol. 116:1350–1356.
2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Guo L, Liu W, Lu T, Guo W, Gao J, Luo Q,
Wu X, Sun Y, Wu X, Shen Y, et al: Decrease of functional activated
T and B cells and treatment of glomerulonephitis in lupus-prone
mice using a natural flavonoid astilbin. PLoS One.
10(e0124002)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kaneko S, Satoh T, Chiba J, Ju C, Inoue K
and Kagawa J: Interleukin-6 and interleukin-8 levels in serum and
synovial fluid of patients with osteoarthritis. Cytokines Cell Mol
Ther. 6:71–79. 2000.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Szekanecz Z and Koch AE: Successes and
failures of chemokine-pathway targeting in rheumatoid arthritis.
Nat Rev Rheumatol. 12:5–13. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Guha M and Mackman N: LPS induction of
gene expression in human monocytes. Cell Signal. 13:85–94.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mateen S, Zafar A, Moin S, Khan AQ and
Zubair S: Understanding the role of cytokines in the pathogenesis
of rheumatoid arthritis. Clin Chim Acta. 455:161–171.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen J, Crawford R and Xiao Y: Vertical
inhibition of the PI3K/Akt/mTOR pathway for the treatment of
osteoarthritis. J Cell Biochem. 114:245–249. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yuan FL, Xu RS, Jiang DL, He XL, Su Q, Jin
C and Li X: Leonurine hydrochloride inhibits osteoclastogenesis and
prevents osteoporosis associated with estrogen deficiency by
inhibiting the NF-κB and PI3K/Akt signaling pathways. Bone.
75:128–137. 2015.PubMed/NCBI View Article : Google Scholar
|